Abstract
We herein report a case of a young Japanese female who was confirmed to have cystic echinococcosis (CE) 1 stage based on the World Health Organization Informal Working Group on Echinococcosis pathological classification of CE, and she was also suspected to be infected with eggs of the G1 Echinococcus granulosus sensu stricto during her stay in the United Kingdom and therefore, suffered from synchronous pulmonary and hepatic CE. Oral albendazole was administered initially, but rupture of a lung hydatid cyst was observed. To avoid additional rupture, we performed two surgeries. CE is very rare in Japan; all CE cases in Japan during the past two decades have been confirmed to be imported, and almost all cases are hepatic CE. This case is the first case report of a Japanese patient who had concomitant giant lung and liver CE with early-stage CE1 and was successfully treated by surgery and pharmacotherapy with a serological follow-up.
Introduction
Cystic echinococcosis (CE) caused by Echinococcus granulosus is a parasitosis that is still endemic in the sheep-raising areas of the world.1–3 It has rarely been reported in Japan, but all CE cases confirmed in Japan during the past two decades were exclusively imported cases.4–7 The lungs are the most common site of infection in children, and the liver is the most common site of infection in adults.2 According to the World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) pathological classification, CE liver cysts are divided into six (CL, CE1, CE2, CE3, CE4, and CE5) stages with sonographical findings.2,3 Synchronous pulmonary and hepatic CE may occur in from 4% to 25% of cases.2 We herein present a case of synchronous right lung and liver CE with CE1 stage (based on the WHO-IWGE classification) that occurred in a healthy Japanese female after her stay in the United Kingdom.
Case Report
The patient was a 39-year-old Japanese female. She was born in Japan and had no remarkable past medical history. She visited several sightseeing areas near sheep ranges in the Cotswols two times, namely in 2001 and 2002, but she never touched any sheep or dogs directly. She began her stay at Wimbledon in southwest London in May of 2002. She also visited Edinburgh, Scotland, for a short trip in 2004. During her stay in the United Kingdom, she also made short tour trips to Rome, Florence, and Paris in 2002, but she never visited any rural areas. She remembered that she had developed hives in July of 2002 on the day after her welcome party at Wimbledon, which took place 3 months after her stay there. The hives lasted for 1 day and disappeared without treatment. She consulted a doctor and was told that the hives were likely from an allergy to food or because of stress. However, she had no history of food or drug allergies and had never developed hives in the past.
Beginning in 2006, she started to cough and experienced weak epigastralgia in December of 2006. The cough was not sputum-producing but gradually increased its intensity. At 8 months after the initial start of these symptoms, she experienced sudden strong right back pain in July of 2007. She visited the local hospital's emergency room. Although several laboratory tests and abdominal X-rays were performed, no abnormal findings were detected. The physician told her that she did not have a serious health condition. She was, therefore, discharged from the emergency room with pain medication.
In November 2007, she returned to Japan, where she visited a clinic for her symptoms. Her chest X-ray revealed a giant round shadow in the right lung field (Figure 1A). An abdominal echogram and computed tomography (CT) scan showed a large cyst in the right lower lobe of her lung and the right lobe of her liver (Figure 1B and C). These cysts were diagnosed at first as simultaneous occurrences of a large bronchial cyst and benign large liver cyst. However, it was indicated that there was a possibility that it might be caused by a parasite. For the purpose of additional investigation, she visited our hospital. Antibody responses to recombinant AgB8/1 (rAgB)8–10 by Western blotting (WB) were positive on March 12, 2008 (before the first operation), but they later became negative on January 8, 2009 (6 months after the second operation). Enzyme-linked immunosorbent assay (ELISA) values on March 12, 2008, June, 2008, and January 8, 2009, were 0.152, 0.076, and 0.019, respectively, with a cut-off value of 0.084 optical density (OD) value at 405 nm.
Figure 1.
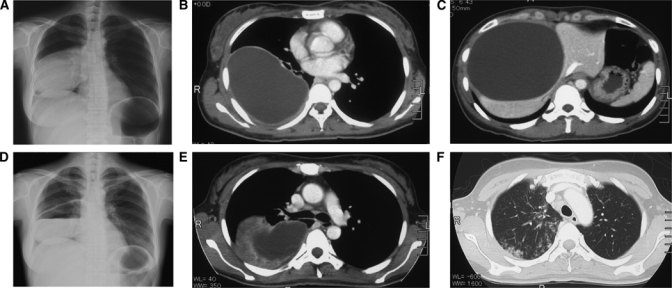
Chest radiography and abdominal tomography of this CE case. (A) A posterolateral chest radiograph revealed a well-circumscribed mass of 13 × 10 cm with homogenous density located on the right hemithorax. (B) On a CT scan of the thorax, a right lung lesion of 13 × 10 × 10 cm was noted. (C) Abdominal tomography showed a hepatic lesion with fluid density of 9 × 10 × 10 cm. (D) The wall of the lung cyst had started to degenerate and rupture at 14 days after the initiation of treatment. A water-fluid level was observed inside the cyst. (E and F) Eosinophilic infiltration appeared in both lung fields.
To avoid the development of liver dysfunction, 400 mg/day of oral albendazole (ABZ) were started after admission to our hospital. Five days after treatment, urticaria was seen in her lower extremities. After she was treated with anti-allergic drugs, her hives were relieved within 1 week. Because she showed no signs of liver dysfunction or any other allergic reaction, the dosage of oral ABZ was increased up to 600 mg/day, which is the generally recommended dose to treat echinococcosis in Japan. On day 9, the peripheral blood eosinophilic leukocyte counts were 2,066 cells/μL, and the serum total immunoglobulin E (IgE) titer was elevated to 5,200 IU/mL. There were many eosinophils in her sputum samples. She complained of bitterness in her mouth after approximately 3 weeks of treatment. At this time, her serum eosinophils had increased to 4,950 cells/μL, and a chest X-ray showed an air-fluid level inside the lung cyst (Figure 1D). Her chest CT showed new infiltration and wall thickening around the lung cyst (Figure 1E and F), whereas no remarkable change was seen in the liver cyst. Repeated chest CT scans showed a lung cyst with decreased size and air density inside the cyst.
Because of the risk of further rupture, surgery was considered for these cysts. We planned a two-stage surgical approach from a safety standpoint, because simultaneous resection of the lung and the liver cyst might cause a serious physical burden for the patient. In the first operation, we performed a right lower lung lobectomy, including total removal of the lung CE lesion and open abdominal puncture of the liver CE lesion. Aspiration, injection of ethanol and hypertonic saline, and reaspiration was considered for the liver cyst. The first operation was performed by the surgery unit after 40 days of medical treatment. ABZ was given 2 hours before and 8 hours after the incision was made through a naso-gastric tube after intubation.
Because the right diaphragm was severely displaced by the liver cyst, drainage of the liver cyst was first performed. After liver exposure, 20% saline-irrigated gauze packs were settled around the liver to avoid the risk of spilling the cyst contents into the peritoneal cavity. An 18-gauge elastic needle was inserted into the cyst under guidance with sonography. The initial pressure of the cyst was 26–28 mmHg. A percutaneous transhepatic catheter drainage (PTCD) tube was inserted through the elastic needle. Then, we started aspiration of the intracystic fluid. The hydatid cyst fluid (HCF) was transparent and nonviscous. Microscopically, there were numerous hooks and protoscolices unique to the Echinococcus species (Figure 2A). Protoscolices kept in 70% ethanol were analyzed for mitochondrial cox1 gene11 and confirmed to be E. granulosus sensu stricto (G1, the sheep strain; data not shown). Detachment of the inner wall from outer membrane was observed, and about 400 mL intracystic fluid were aspirated. After aspiration, 20% hypertonic saline was injected and followed by injection of 80% ethanol for 15 minutes to kill protoscolices. After injection, the fluid inside the liver cyst was carefully aspirated again, and a PTCD tube was settled in place.
Figure 2.
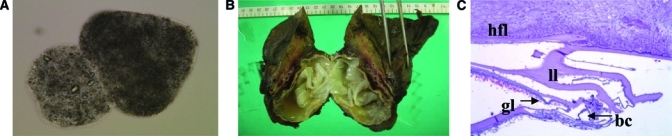
Parasitologic evidence of CE. (A) Protoscolices (0.2% trypan blue stain, 400×) in the aspirated fluid of both the lung and the hepatic cyst. (B) The lung CE cyst with a white outer membrane in the resected right lower lung lobe. (C) Microscopically, the cyst wall was composed of a brood capsule (bc), germinal layer (gl), laminated layer (ll), and hydatid fibrotic layer (hfl), (hematoxylin/eosin stain, 100x).
After the liver cyst drainage, a right thoracotomy was performed. Protecting the surgical field with 20% NaCl irrigated gauze pads, the lung hydatid cyst was punctured with an 18-gauge elastic needle. The cyst pressure was 8 mmHg, and a total of 1,130 mL intracystic fluid was aspirated. The color of the pulmonary cyst fluid was turbid and yellow. The right lower lung was resected with the lung cyst, and the right thoracic space was washed with warmed normal saline. The lung cyst had a white outer membrane (Figure 2B), and a cross-section of the cyst showed two layered capsules (Figures 2C). We did not find any tears in the outer membrane. A 24-Fr thoracic drainage tube was settled in her right thorax. No significant surgical complication was observed during the first operation or when the PTCD tube was removed 2 weeks after the operation.
One month later, the patient developed a fever, and her abdominal CT scan revealed that the hepatic cyst had grown larger. The second operation was performed to remove the hepatic cyst completely in June of 2008. After resection of the liver cyst was carried out, intracystic fluid was submitted for laboratory culture and pathological examination. The results of the cultivation test of intracystic fluid showed exclusive infection with Pseudomonas aeruginosa. We could not detect any hooks or protoscolices in the hepatic cyst fluid. After treatment with antibiotics against bacterial infection, the patient was finally discharged from the hospital in July of 2008. Oral treatment with ABZ was continued before and after surgery with no cessation for a total duration of 7 months (from February 2008 to September 2008).
Discussion
Human CE caused by E. granulosus is very rare in Japan,4 and all cases diagnosed in Japan during the past two decades were not indigenous,3,4,6 although CE is an endemic parasitic disease in various parts of the world.1–3 Infection usually takes place by ingestion of the eggs of E. granulosus sensu lato. Humans become intermediate hosts and harbor cysts, which are most commonly found in the liver (70%) and lungs (20%).1–3,7,10 For treatment, medical management through the oral administration of antihelminthic agents has been the preferred and established method.12 However, this treatment may be associated with serious complications, such as anaphylactic shock and death.13 In this case, we observed a marked increase in peripheral blood eosinophils, urticaria, and pulmonary eosinophilic infiltration. The patient's cough was increased in intensity about 2 weeks after treatment, and a bitter taste was noted. These symptoms suggested a rupture of the lung cyst.
It has been reported that rupture of a lung cyst into an adjacent bronchus may be manifested by vigorous coughing and expectoration of a salty sputum consisting of mucous, HCF, and in some cases, fragments of the laminated membrane.14,15 CE cysts in lungs can reach very large sizes because of the relatively higher elasticity of the lung tissue compared with other organs. Surgical procedures to protect the lung parenchyma are frequently used.16 A single-stage transthoracic approach for right lung and liver CE is performed in the countries where this disease is endemic.14 In this case, we planned to perform this method at first, but we ended up having to perform the thoracotomy for the lung cyst. We opened abdomen for drainage of the liver cyst during the first operation, because the liver cyst was too large to enucleate.
Figure 1 clearly indicates this case to have CE1 stage based on the WHO-IWGE classification of CE.2,3 Percutaneous puncture of a hydatid cyst is performed under ultrasound or CT guidance followed by aspiration of substantial amounts of cystic fluid and injection of a protoscolicidal agent into the cyst cavity (usually hypertonic saline or ethanol). This procedure, called puncture, aspiration, injection, and reaspiration (PAIR), is recommended for uncomplicated unilocular cysts (CE1).2,3 However, with this method, there might be a risk of abdominal leakage of intracystic fluid that contains numerous live protoscolices of E. granulosus, especially in cases where a cyst is too large and close to the wall of a liver. We, therefore, performed liver cyst puncture and drainage under direct observation with sonography by opening the abdomen.
Adjunctive chemotherapy before and after surgery seems to reduce the risk of recurrence by killing protoscolices and lessening the tension of the cysts, thus allowing for easier cyst removal.17–19 However, the optimal duration of chemotherapy before and after surgery has not been determined. Therapy generally should be started at least 4 days before surgery, and the WHO recommends between 4 days and 1 month. We observed air density inside the lung cyst beginning at 2 weeks after the oral ABZ was started. Because the lung cyst itself is fragile compared with the liver cyst and the risk of rupture after pharmacotherapy is high, surgical therapy for lung cysts caused by CE is generally recommended.20,21
The patient had no history of travel outside of Japan except for her 4-year stay in the United Kingdom (from May of 2002 to November of 2007) and very short visits to Paris, Florence, and Rome on a single trip. Because she became sick during her stay in the United Kingdom and CE is not indigenous in Japan,5 we suspected that she had likely been infected with eggs of G1 E. granulosus11 during her stay in the United Kingdom, perhaps either in 2001 in Cotswols, a rural area in the United Kingdom where there were many sheep and sheep dogs, or in 2002 at the welcome party in Wimbledon. She might have ingested eggs of this parasite on contaminated vegetables.
Based on her clinical background, she might have been exposed to eggs just before she got the hives in 2002. If this prediction is the case, such allergic responses might be a new clinical marker for the initial stage of infection with eggs of E. granulosus. Experimental infection with eggs of Echinococcus spp. in animals may provide additional information on the early or initial phases of infection.
In conclusion, we herein reported the treatment of a young Japanese female who had concomitant right lung and liver hydatid cysts caused by G1 E. granulosus with a right lower lung lobectomy, including total removal of the lung cyst, drainage of the liver cyst by opening the abdomen, and oral ABZ therapy. Based on our experience, physicians must take care to prevent the risk of rupture of lung hydatid cysts before starting medical treatment. This case is the first case of a synchronous giant lung and liver hydatid cyst caused by E. granulosus that was successfully treated by surgery and medical therapy with serological follow-up in Japan.
ACKNOWLEDGMENTS
The authors thank Dr. Kimiaki Yamano of Hokkaido Institute of Public Health for the suggestion for serology in the present case. We also thank Dr. Tadaoki Uezato for the total surgical excision of the liver cyst and Drs. Shin Yamashiro, Yasushi Ihama, Tatsuji Maeshiro, Futoshi Higa, and Masao Tateyama, Faculty of Medicine, University of the Ryukyus, for their clinical contributions. Mrs. Sonoyo Ito, Asahikawa Medical University, is also thanked for the serological analysis.
Footnotes
Financial support: Serological and molecular studies on this case report were supported by Grant-in-Aid 21256003 for Scientific Research from the Japan Society for Promotion of Science (JSPS) and the Special Coordination Funds for Promoting Science and Technology (MEXT; to A.I.).
Authors' addresses: Kiwamu Nakamura, Department of Internal Medicine, University of Texas Medical Branch, Galveston, TX, E-mail: kiwamu1204@hotmail.com. Akira Ito and Yasuhito Sako, Department of Parasitology, Asahikawa Medical University, Midorigaoka Higashi, Asahikawa, Hokkaido, Japan, E-mails: akiraito@asahikawa-med.ac.jp and yasusako@asahikawa-med.ac.jp. Satomi Yara, Shusaku Haranaga, Kenji Hibiya, and Jiro Fujita, Department of Infectious, Respiratory, and Digestive Medicine, Control and Prevention of Infectious Diseases, Faculty of Medicine, University of the Ryukyus, Nishihara, Okinawa, Japan, E-mails: f040621@med.u-ryukyu.ac.jp, f014936@med.u-ryukyu.ac.jp, kenjihibiya@gmail.com, and fujita@med.u-ryukyu.ac.jp. Tsuneo Hirayasu, Division of Thoracic and Cardiovascular Surgery, Department of Bioregulatory Medicine, Faculty of Medicine, University of the Ryukyus, Nishihara, Okinawa, Japan, E-mail: t7696713h@yahoo.co.jp.
Reprint requests: Kiwamu Nakamura, University of Texas Medical Branch, Department of Internal Medicine, 301 University Boulevard, Galveston, TX 77555-0435, E-mail: kiwamu1204@hotmail.com.
References
- 1.Eckert J, Conraths FJ, Tackmann K. Echinococcosis: an emerging or re-emerging zoonosis? Int J Parasitol. 2000;30:1283–1294. doi: 10.1016/s0020-7519(00)00130-2. [DOI] [PubMed] [Google Scholar]
- 2.Eckert J, Gemmell MA, Meslin FX, Pawlowski ZS. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 3.Brunetti E, Kern P, Vuitton DA. Writing Panel for the WHO-IWGE, 2010. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 114:1–16. doi: 10.1016/j.actatropica.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Hatakeyama Y, Sato N, Koyama Y, Inoue N, Takenosita S, Takeuchi S, Ito A. One surgical case of hepatic cystic echinococcosis. Shujutsu. 2002;56:819–823. [Google Scholar]
- 5.Doi R, Ito A, Watanabe H, Morishima H. Cystic hydatidosis—its occurrence and prevention in Japan. Nippon Koshu Eisei Zasshi. 2003;50:1066–1078. [PubMed] [Google Scholar]
- 6.Kimura M, Nakamura T, Iwamoto A, Nishimura Y, Egawa T, Ito A. Cystic echinococcosis in a Jordanian patient: albendazole in a short-term immigrant. J Travel Med. 1999;6:249–253. doi: 10.1111/j.1708-8305.1999.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 7.Ito A, Okamoto M, Ishiguro T, Ma L, Suzuki H, Yasui A, Shigeta H, Matsuura T, Hosokawa T, Chai JJ. Short report: an imported case of cystic echinococcosis in Japan diagnosed by imaging and serology with confirmation of Echinococcus granulosus-specific DNA sequences. Am J Trop Med Hyg. 1998;58:790–792. doi: 10.4269/ajtmh.1998.58.790. [DOI] [PubMed] [Google Scholar]
- 8.Mamuti W, Yamasaki H, Sako Y, Nakao M, Xiao N, Nakaya K, Sato N, Vuitton DA, Piarroux R, Lightowlers MW, Craig PS, Ito A. Molecular cloning, expression, and serological evaluation of an 8-kilodalton subunit of antigen B from Echinococcus multilocularis. J Clin Microbiol. 2004;42:1082–1088. doi: 10.1128/JCM.42.3.1082-1088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li T, Ito A, Chen X, Sako Y, Qiu J, Xiao N, Qiu D, Nakao M, Yanagida T, Craig PS. Specific IgG responses to recombinant antigen B and em18 in cystic and alveolar echinococcosis in china. Clin Vaccine Immunol. 2010;17:470–475. doi: 10.1128/CVI.00466-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito A, Nakao M, Sako Y. Echinococcosis: serological detection of patients and molecular identification of parasites. Future Microbiol. 2007;2:439–449. doi: 10.2217/17460913.2.4.439. [DOI] [PubMed] [Google Scholar]
- 11.Nakao M, McManus DP, Schantz PM, Craig PS, Ito A. A molecular phylogeny of the genus Echinococcus inferred from complete mitochondrial genomes. Parasitology. 2007;134:713–722. doi: 10.1017/S0031182006001934. [DOI] [PubMed] [Google Scholar]
- 12.Mawhorter S, Temeck B, Chang R, Pass H, Nash T. Nonsurgical therapy for pulmonary hydatid cyst disease. Chest. 1997;112:1432–1436. doi: 10.1378/chest.112.5.1432. [DOI] [PubMed] [Google Scholar]
- 13.Kurkcuoglu IC, Eroglu A, Karaoglanoglu N, Polat P. Complications of albendazole treatment in hydatid disease of lung. Eur J Cardiothorac Surg. 2002;22:649–650. doi: 10.1016/s1010-7940(02)00396-2. [DOI] [PubMed] [Google Scholar]
- 14.Dhaliwal RS, Kallat MS. One-stage surgical procedure for bilateral lung and liver cysts. Ann Thorac Surg. 1997;64:338–341. doi: 10.1016/S0003-4975(97)00475-X. [DOI] [PubMed] [Google Scholar]
- 15.Santivanez S, Garcia HH. Pulmonary cystic echinococcosis. Curr Opin Pulm Med. 2010;16:257–261. doi: 10.1097/MCP.0b013e3283386282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurkcuoglu IC, Eroglu A, Karaoglanoglu N, Polat P. Complications of albendazole treatment in hydatid disease of lung. Eur J Cardiothorac Surg. 2002;22:649–650. doi: 10.1016/s1010-7940(02)00396-2. [DOI] [PubMed] [Google Scholar]
- 17.Smego RA, Jr, Bhatti S, Khaliq AA, Beg MA. Percutaneous aspiration-injection-reaspiration drainage plus albendazole or mebendazole for hepatic cystic echinococcosis: a meta-analysis. Clin Infect Dis. 2003;37:1073–1083. doi: 10.1086/378275. [DOI] [PubMed] [Google Scholar]
- 18.Aktan AO, Yalin R. Preoperative albendazole treatment for liver hydatid disease decreases the viability of the cyst. Eur J Gastroenterol Hepatol. 1996;8:877–879. [PubMed] [Google Scholar]
- 19.Erzurumlu K, Hökelek M, Gönlüsen L, Tas K, Amanvermez R. The effect of albendazole on the prevention of secondary hydatidosis. Hepatogastroenterology. 2000;47:247–250. [PubMed] [Google Scholar]
- 20.Celik M, Senol C, Keles M, Halezeroglu S, Urek S, Haciibrahimoglu G, Ersev AA, Arman B. Surgical treatment of pulmonary hydatid disease in children: report of 122 cases. J Pediatr Surg. 2000;35:1710–1713. doi: 10.1053/jpsu.2000.19219. [DOI] [PubMed] [Google Scholar]
- 21.Doğan R, Yüksel M, Cetin G, Süzer K, Alp M, Kaya S, Unlü M, Moldibi B. Surgical treatment of hydatid cysts of the lung: report on 1055 patients. Thorax. 1989;44:192–199. doi: 10.1136/thx.44.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]


