Abstract
We describe the first detailed histological description of an excised calcified Taenia solium granuloma from a patient who developed recurrent seizures associated with perilesional edema surrounding a calcified cysticercus (PEC). The capsule, around a degenerated cysticercus, contained marked mononuclear infiltrates that extended to adjacent brain, which showed marked astrocytosis, microgliosis, and inflammatory perivascular infiltrates. The presence of large numbers of mononuclear cells supports an inflammatory cause of PEC. Immunosuppression or anti-inflammatory measures may be able to treat and prevent PEC and recurrent seizures.
Introduction
Neurocysticercosis (NCC), the most common cause of adult onset epilepsy worldwide, is caused by the cystic larvae of the human tapeworm, Taenia solium.1 Seizures, the most frequent manifestation of NCC, arise when viable cysts degenerate during the course of natural infection2 or as a consequence of treatment with anthelminthic medication.3 Seizures commonly occur in the presence of calcified granulomas4–9 and are frequently localized to them.10–12 Moreover, recurrent, transient episodes of perilesional edema (PEC) and enhancement, both maximally centered around the calcifications, have been described.13,14 In a recent prospective study of patients with only calcified granulomas and a history of remote seizures, PEC was found in 50% of those patients with recurrent seizures and 8.7% of asymptomatic controls from the same population.13 Because calcified granulomas are the most common radiological finding in endemic populations, PEC is likely frequent in those patients with epilepsy.8
Understanding the pathophysiology of PEC is hampered by the inability to directly study the implicated calcified foci. We report the first complete histological description of a calcified lesion that was surgically removed after repeated episodes of perilesional edema. The lesion contains intense regions of mononuclear infiltrates, suggesting that the perilesional edema is inflammatory in nature.
Case report
A 23-year-old Brazilian male, who immigrated to the United States at the age of 18 years, presented with generalized tonic–clonic seizures in December 2004. Neuroimaging showed three cystic lesions in the right posterior frontal, left frontal, and left posterior occipital lobes consistent with NCC (image not shown). A Western blot assay for cysticercosis antibodies was positive. The lesion in the left frontal lobe showed ring enhancement with edema consistent with a degenerating cyst. Administration of phenytoin sodium and oxacarbazepine led to control of seizures.
Eight months later, in August 2005, he experienced seizures of a different semiology, consisting of focal left-sided motor seizures initially involving the tongue and then shoulder followed by speech arrest. Magnetic resonance imaging (MRI) of the brain showed resolution of the left frontal lobe lesion, but a new area of perilesional edema had developed around the ring enhancing non-calcified lesion in the right posterior frontal lobe (not shown). Despite attempts to optimize anti-epileptic drugs (AED) and treatment with corticosteroids, he continued to experience focal seizures.
He was transferred to Lahey Clinic in August of 2006. Computed tomography (CT) and MRI imaging showed a calcified lesion with surrounding edema in the right frontal lobe (RFL) (Figure 1A–C). He was treated for 3 weeks with albendazole and 14 days with corticosteroids, resulting in marked improvement of the perilesional edema 1 month after discharge (Figure 1D). He was seizure-free until March 2007, when he again developed recurrent focal seizures and marked increase in the edema around the lesion in the RFL (Figure 1E), despite continued treatment with anti-seizure medication. Intractable seizures with recurrent episodes of status epilepticus persisted over the next 6 months, despite maximal AED. MRI imaging showed varying amounts of edema that waxed and waned over time (Figure 1F). After extensive evaluation, including mapping of the seizure focus to the RFL, the lesion was excised in July of 2007. The patient was seizure-free for a period of 2 years while on levetiracetam (1,000 mg/day) and valproate (1,000 mg/day) until he again presented with seizures associated with perilesional edema around the third cyst in the left frontal lobe that had evolved into a calcified granuloma.
Figure 1.
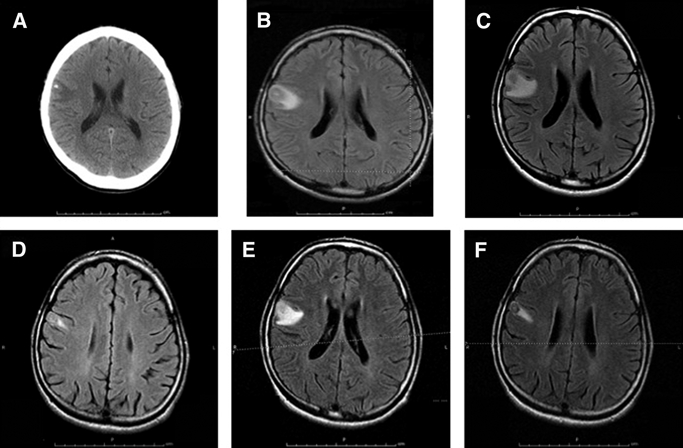
Serial MRI fluid-attenuated inversion recovery (FLAIR) imaging of the patient showing change in perilesional edema over time. (A) CT scan of the patient at presentation (August 11, 2006) to the treating hospital. (B) MRI on August 8, 2006 at presentation. (C) MRI on August 11, 2006 at presentation. (D) MRI on September 5, 2006. (E) MRI on March 1, 2007. (F) MRI on May 11, 2007. A–C show a central area of calcification with surrounding edema. The edema around the lesion changed over time depending on the clinical state.
Discussion
This case report is the first complete histological description of a calcified T. solium granuloma associated with episodes of perilesional edema, and it suggests that inflammation is directly or indirectly involved (Figures 2A and B, 3, and 4). A previously published report of the histology of a calcified granuloma was limited to identification of calcareous corpuscles,15 characteristic bodies of unknown function but specific to cestodes.16
Figure 2.
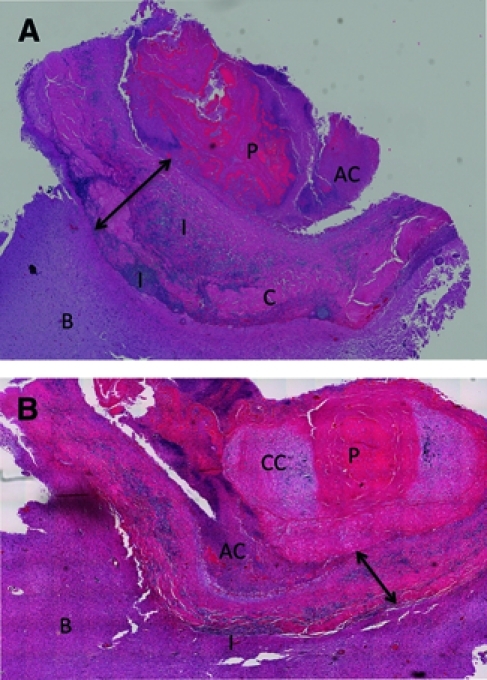
(A and B) Photograph of two histological sections of the excised lesion. P = degenerated parasite; I = inflammation; AC = amorphous material with calcification; C = collagen; CC = calcareous corpuscles; B = brain. The arrowed line shows the extent of the capsule. The lesion is roughly organized into concentric layers (A) consisting of centrally located eosinophilic material containing thick, bright ribbons of membranous-like tissue, which is most likely a degenerated cysticercus. A granular calcified layer, often adjacent to the host capsule, partially surrounds the core. A tissue section from another region (B) contains a dense mass of concentrated calcified calcareous corpuscles in the core, which are characteristic of cestodes. The degenerated parasite mass is surrounded by a dense collagenous wall making up the host capsule. A mononuclear infiltrate consisting primarily of lymphocytes, macrophages, and plasma cells courses through the capsule but is particularly dense adjacent to the brain. Eosinophilis are present in relatively small numbers and do not predominate. The surrounding brain is markedly abnormal with reactive gliosis, which is denoted by glial fibrillary acid protein immunostaining (not shown), CD3-positive T cells (Figure 4C), and histiocytosis and microgliosis by KP1 reactivity (Figure 4D). There is extensive inflammatory perivascular cuffing in adjacent brain tissue.
Figure 3.
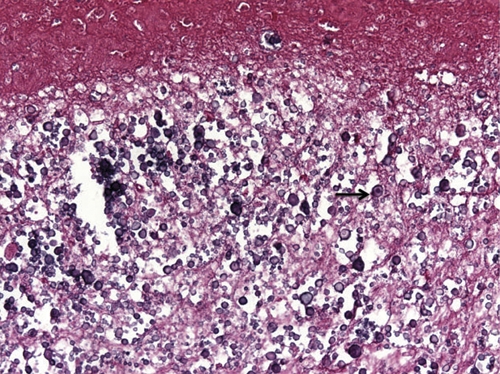
Higher power view of calcareous corpuscles, which are characteristic of cestodes. The very dense and large mass likely is a cause of calcification. The arrow points to one of many calcareous corpuscles. This figure appears in color at www.ajtmh.org.
Figure 4.
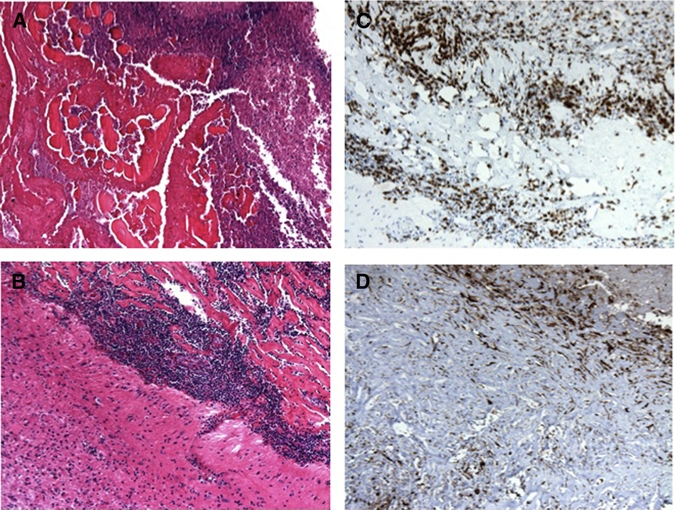
(A) Higher power view of degenerated parasite and amorphous calcified material. (B) Higher power view of the capsule wall with mononuclear infiltrate. (C) CD3-positive T cells. (D) KP1-positive macrophages and microglia. This figure appears in color at www.ajtmh.org.
Few prior histological descriptions of calcified granulomas are available. These describe nodules with a fibrous capsule without notable inflammation, a hyalinized or necrotic parasite that may or may not contain recognizable remnants or calcifications.17–21 These reports and similar earlier descriptions led to the concept that calcified lesions are inactive, have no accompanying inflammation, and play little role in the pathophysiology of disease.
Not all calcified granulomas seem to be the same. The evolution of the cellular granulomas to inert calcifications is not instantaneous and may be slow,22 hindered, or incapable of further evolution, which results in varying levels of calcification in a cellular granulomatous milieu. Additionally, MRIs show enhancement in a subset of calcified lesions,7,23 suggesting dysfunction of the blood–brain barrier and the presence of on-going inflammation. The phenomenon of PEC itself and the pathology of the present calcified granuloma also support an inflammatory etiology for PEC, which may be caused by episodic failure of inhibition of an ongoing inflammation,24 the periodic release of antigen leading to initiation,7,25,26 aggravation of an ongoing inflammatory response,19,25,26 a generalized hyperimmunity,14,27 or a combination of these processes. An inflammatory etiology of PEC suggests that anti-inflammatory and/or immunosuppressive measures28,29 may be efficacious in the treatment and prevention of recurrences. In fact, one person with recurrent perilesional edema and seizures caused by calcified foci responded dramatically to methotrexate therapy.28 However, no therapy has been shown to be efficacious, and it is premature to recommend methotrexate use as a policy. Because calcified foci, whether associated with perilesional edema (as shown in the present report) or not, consist of dead calcified granulomas, anti-parasitic treatment is not indicated. Anti-epileptic drugs should be administered, although formal studies showing efficacy are lacking.
Surgical removal of epileptogenic foci is accepted treatment of some forms of epilepsy.30 Presumably, removal of calcified cysticercal foci after similar criteria might be beneficial. However, the literature documenting control of seizures in neurocysticercosis by surgical removal of the foci is scant. Surgical excision should rest on the individual findings and circumstances of a particular patient.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Grace Lee of Lahey Clinic for her help and support in preparing this manuscript.
Footnotes
Financial support: Support for this study was received from the intramural National Institutes of Allergy and Infectious Diseases at the National Institutes of Health.
Authors' addresses: Winnie W. Ooi, Department of Infectious Diseases, Lahey Clinic, Burlington, MA, E-mail: Winnie.W.Ooi@lahey.org. Subhashie Wijemanne, Department of Neurology, Lahey Clinic, Burlington, MA. Christine B. Thomas, Department of Clinical Pathology, Lahey Clinic, Burlington, MA. Martha Quezado, Laboratory of Pathology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD. Charles R. Brown, Laboratory of Molecular Microbiology, National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD. Theodore E. Nash, Laboratory of Parasitic Diseases, National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, E-mail: tnash@niaid.nih.gov.
References
- 1.International League Against Epilepsy Relationship between epilepsy and tropical diseases. Commission on Tropical Diseases of the International League Against Epilepsy. Epilepsia. 1994;35:89–93. [PubMed] [Google Scholar]
- 2.MacArthur W. Cysticercosis as seen in the British Army, with special reference to the production of Epilepsy. Trans R Soc Med. 1933;27:343–363. [Google Scholar]
- 3.Spina-Franca A, Nobrega JP, Livramento JA, Machado LR. Administration of praziquantel in neurocysticercosis. Z Tropenmed Parasitol. 1982;33:1–4. [PubMed] [Google Scholar]
- 4.Park SY, Barkovich AJ, Weintraub PS. Clinical implications of calcified lesions of neurocysticercosis. Pediatr Infect Dis J. 2000;19:581–583. doi: 10.1097/00006454-200006000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Del Brutto OH, Santibanez R, Noboa CA, Aguirre R, Diaz E, Alarcon TA. Epilepsy due to neurocysticercosis: analysis of 203 patients. Neurology. 1992;42:389–392. doi: 10.1212/wnl.42.2.389. [DOI] [PubMed] [Google Scholar]
- 6.White AC., Jr Neurocysticercosis: a major cause of neurological disease worldwide. Clin Infect Dis. 1997;24:101–113. doi: 10.1093/clinids/24.2.101. [DOI] [PubMed] [Google Scholar]
- 7.Nash TE, Patronas NJ. Edema associated with calcified lesions in neurocysticercosis. Neurology. 1999;53:777–781. doi: 10.1212/wnl.53.4.777. [DOI] [PubMed] [Google Scholar]
- 8.Nash TE, Del BH, Butman JA, Corona T, Delgado-Escueta A, Duron RM, Evans CAW, Gilman RH, Gonzalez AE, Loeb JA, Medina MT, Pietsch-Escueta S, Pretell EJ, Takayanagui OM, Theodore W, Tsang VCW, Garcia HH. Calcific neurocysticercosis and epileptogenesis. Neurology. 2004;62:1934–1938. doi: 10.1212/01.wnl.0000129481.12067.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoniuk SA, Bruck I, Dos Santos LH, Pintarelli VL, Navolar FB, Brackmann PC, Jr, de Morais RL. Seizures associated with calcifications and edema in neurocysticercosis. Pediatr Neurol. 2001;25:309–311. doi: 10.1016/s0887-8994(01)00324-1. [DOI] [PubMed] [Google Scholar]
- 10.Singh G, Sachdev MS, Tirath A, Gupta AK, Avasthi G. Focal cortical-subcortical calcifications (FCSCs) and epilepsy in the Indian subcontinent. Epilepsia. 2000;41:718–726. doi: 10.1111/j.1528-1157.2000.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 11.Cukiert A, Puglia P, Scapolan HB, Vilela MM, Marino Junior R. Congruence of the topography of intracranial calcifications and epileptic foci. Arq Neuropsiquiatr. 1994;52:289–294. doi: 10.1590/s0004-282x1994000300001. [DOI] [PubMed] [Google Scholar]
- 12.Murthy JM, Reddy VS. Clinical characteristics, seizure spread patterns and prognosis of seizures associated with a single small cerebral calcific CT lesion. Seizure. 1998;7:153–157. doi: 10.1016/s1059-1311(98)80072-1. [DOI] [PubMed] [Google Scholar]
- 13.Nash TE, Pretell EJ, Lescano AG, Bustos JA, Gilman RH, Gonzalez AE, Garcia HH. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol. 2008;7:1099–1105. doi: 10.1016/S1474-4422(08)70243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheth TN, Lee C, Kucharczyk W, Keystone J. Reactivation of neurocysticercosis: Case report. Am J Trop Med Hyg. 1999;60:664–667. doi: 10.4269/ajtmh.1999.60.664. [DOI] [PubMed] [Google Scholar]
- 15.Klotz P, Tappe D, Abele-Horn M, Warmuth-Metz M, Sorensen N, Speer CP, Girschick HJ. Cerebral mass in a 13-year-old girl following long-term sojourn in the tropics. J Med Microbiol. 2006;55:345–347. doi: 10.1099/jmm.0.46381-0. [DOI] [PubMed] [Google Scholar]
- 16.Chacko G, Rajshekhar V, Chandy MJ, Chandi SM. The calcified intracorporeal vacuole: an aid to the pathological diagnosis of solitary cerebral cysticercus granulomas. J Neurol Neurosurg Psychiatry. 2000;69:525–527. doi: 10.1136/jnnp.69.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escobar A. In: Cysticercosis of the Central Nervous System. Palacios E, Rodriguez-Carbajal J, Taveras JM, editors. Springfield, IL: Charles Thomas; 1983. pp. 27–54. (Pathology of the nervous system). [Google Scholar]
- 18.Escobar A, Weidenheim K. In: Taenia solium Cysticercosis—From Basics to Clinical Science. Singh G, Prabhakar S, editors. Wallingford, Oxon, United Kingdom: CABI Publishing; 2002. pp. 289–305. (The pathology of neurocysticercosis). [Google Scholar]
- 19.Hennenberg R. In: Handbuch der Neurologie. Lewandowsky M, editor. Verlag Von Julius Springer; Berlin: 1912. pp. 642–683. (Die tierischen Parasiten des Zentralnervensystems). [Google Scholar]
- 20.Marquez-Monter H. In: Pathology of Protozoal and Helminthic Diseases. Marcial-Rojas R, editor. Baltimore, MD: Williams & Wilkins Company; 1971. pp. 592–617. (Cysticercosis). [Google Scholar]
- 21.Raliela-Cervantes MTR, Rivas-Hernandez A, Rodriguez-Ibarra J, Castillo-Medina S, Cancino FDM. In: Cysticercosis: Present State of Knowledge and Perspectives. Flisser A, Willms K, Juan Pedro Laclette, Larralde C, Ridaura C, Beltran F, editors. New York, NY: Academic Press; 1982. pp. 179–200. (Anatomopathological aspects of human brain cysticercosis). [Google Scholar]
- 22.Rajshekhar V. Rate of spontaneous resolution of a solitary cysticercus granuloma in patients with seizures. Neurology. 2001;57:2315–2317. doi: 10.1212/wnl.57.12.2315. [DOI] [PubMed] [Google Scholar]
- 23.Sheth TN, Pillon L, Keystone J, Kucharczyk W. Persistent MR contrast enhancement of calcified neurocysticercosis lesions. AJNR Am J Neuroradiol. 1998;19:79–82. [PMC free article] [PubMed] [Google Scholar]
- 24.Wang IC, Fan PC, Lu SC, Fan CK, Su KE. Suppression of host Th1-type granulomatous inflammation by Taenia solium metacestodes is related to down-regulation of osteopontin gene expression. Int J Parasitol. 2008;38:239–248. doi: 10.1016/j.ijpara.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Gupta RK, Kumar R, Chawla S, Pradhan S. Demonstration of scolex within calcified cysticercus cyst: its possible role in the pathogenesis of perilesional edema. Epilepsia. 2002;43:1502–1508. doi: 10.1046/j.1528-1157.2002.21302.x. [DOI] [PubMed] [Google Scholar]
- 26.Pradhan S, Kathuria MK, Gupta RK. Perilesional gliosis and seizure outcome: a study based on magnetization transfer magnetic resonance imaging in patients with neurocysticercosis. Ann Neurol. 2000;48:181–187. [PubMed] [Google Scholar]
- 27.Poeschl P, Janzen A, Schuierer G, Winkler J, Bogdahn U, Steinbrecher A. Calcified neurocysticercosis lesions trigger symptomatic inflammation during antiparasitic therapy. AJNR Am J Neuroradiol. 2006;27:653–655. [PMC free article] [PubMed] [Google Scholar]
- 28.Mitre E, Talaat KR, Sperling MR, Nash TE. Methotrexate as a corticosteroid-sparing agent in complicated neurocysticercosis. Clin Infect Dis. 2007;44:549–553. doi: 10.1086/511040. [DOI] [PubMed] [Google Scholar]
- 29.Nash TE, Singh G, White AC, Rajshekhar V, Loeb JA, Proano JV, Takayanagui OM, Gonzalez AE, Butman JA, DeGiorgio C, Del Brutto OH, Delgado-Escueta A, Evans CA, Gilman RH, Martinez SM, Medina MT, Pretell EJ, Teale J, Garcia HH. Treatment of neurocysticercosis: current status and future research needs. Neurology. 2006;67:1120–1127. doi: 10.1212/01.wnl.0000238514.51747.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]


