Abstract
The aim of this study is to investigate the changes in clinical pattern and therapeutic measures in leptospirosis-associated acute kidney injury; a retrospective study with 318 patients in Brazil. Patients were divided according to the time of admission: 1985–1996 (group I) and 1997–2010 (group II). Patients were younger in group I (36 ± 13 versus 41 ± 16 years, P = 0.005) and the numbers of oliguria increased (21% versus 41% in group II, P = 0.014). Higher frequency of lung manifestations was observed in group II (P < 0.0001). Although increased severity, there was a significant reduction in mortality (20% in group I versus 12% in group II, P = 0.03). Mortality was associated with advanced age, low diastolic blood pressure, oliguria, arrhythmia, and peritoneal dialysis, besides a trend to better mortality with penicillin administration. Leptospirosis is occurring in an older population, with a higher number of oliguria and lung manifestations. However, mortality is decreasing and can be the result of changes in treatment.
Introduction
Leptospirosis is the most important zoonose in the world and its frequency is higher in tropical countries.1–3 The disease is caused by spirochaete bacteria of Leptospira genus, which results from the exposure to urine of infected animals.4,5 Higher rates of incidence occur during the rainy season.2,5,6
The disease begins suddenly with headache, high-degree fever, malaise, myalgia, conjunctival suffusion, and a transient rash. The severe form is characterized by jaundice, acute kidney injury (AKI), and hemorrhage, known as Weil's disease, and it is mainly caused by serovars Icterohaemorrhagiae, Copenhageni, Lai, and others.7 Renal involvement is characterized by AKI, associated with oliguria and dialysis requirement in severe cases.8,9 Pulmonary involvement can predominate, and it is the main cause of death.10
In Brazil, leptospirosis is endemic, and outbreaks occur during the rainy season, coinciding with localized flooding.11 The real impact of the disease might be underestimated because many patients with leptospirosis are misdiagnosed as suffering from other infectious diseases, such as dengue or influenza.
Early diagnosis is necessary to institute adequate therapy. Therapy is mainly supportive and many doubts persist in some aspects of treatment, such as antibiotic administration, whereas other uncertain topics have been clarified: early dialysis initiation was associated with a better outcome in leptospirosis-associated AKI.12
Associated with treatment evolution, a clinical pattern of leptospirosis seems also to change over time and there are few data on this subject.13 The aim of this study is to investigate the changes in the clinical pattern of leptospirosis-associated AKI and the effects of general guidelines in management of severe leptospirosis.
Methods
Study population.
This is a retrospective study with patients admitted to the São José Infectious Diseases Hospital and Walter Cantídio University Hospital in Fortaleza city, Northeast of Brazil, between May 1985 and December 2010, with confirmed diagnosis of leptospirosis (Weil Syndrome) and presence of renal injury by RIFLE criteria.14 All patients had clinical and epidemiological data suggestive of leptospirosis, and a positive laboratory test for leptospirosis (microscopic agglutination test, higher than 1:800). Patients with diagnosis performed only in the convalescence phase were not included because it was unlikely the result had influenced physician practice.
Definitions.
Acute kidney injury was defined according to the RIFLE classification—risk, injury, failure, loss, and end-stage kidney disease.14 Systolic blood pressure and diastolic blood pressure (DBP) at admission were analyzed. Oliguria was considered to be present when the urinary volume was < 400 mL/day. Dialysis was indicated for those patients who remained oliguric after effective hydration, in those cases where uremia was associated with hemorrhagic or severe respiratory failure, in severe cases or refractory metabolic acidosis and severe or refractory hyperkalemia. Lung manifestation was considered if patients present with dyspnea, pulmonary crackle, hemoptysis, or pO2 < 60 mmHg in arterial blood gas. Hemorrhagic manifestation was considered if patient presented with any petechiae, hematemesis, or hemoptysis.
Patients groups.
The patients were divided into two groups according to the years: group I (1985–1996) and group II (1997–2010), and according to the year of admission. These two periods were chosen because there were significant changes in physicians' orientation about patients' treatment after 1996. The main changes were as follows:
-
1.
Early diagnosis: all physicians were oriented to consider leptospirosis diagnosis in patients with fever, jaundice associated with respiratory failure, and/or AKI.
2. Supportive therapy: patients receive crystalloid solutions to restore volume with caution, especially if there were signs of respiratory failure, lung crackles, or hemoptysis.
3. Antibiotic therapy: physicians were oriented to administer penicillin to patients even in later disease presentation.
4. Dialysis therapy: hemodialysis therapy was the preferred method instead of peritoneal dialysis and it was intended to be initiated early after intensive care unit admission and performed daily.
All clinical manifestations and laboratory tests were evaluated. A comparison of clinical and laboratory data was performed between the two groups to investigate if there was any difference in the pattern of leptospirosis presentation in the two periods analyzed. A comparison between survivors and non-survivors in the two periods was also done.
Ethics.
The protocol of this study was approved by the Ethical Committee of the Walter Cantídio University Hospital and São José Infectious Diseases Hospital.
Statistical analysis.
The results were expressed through tables and summary measures (mean ± standard deviation) in the cases of quantitative variables. All data were analyzed with the programs SPSS version 10.0 (SPSS Inc., Chicago, IL) and Epi Info version 6.04b (Centers for Disease Control and Prevention, Atlanta, GA). Comparison of parameters was done with Student's t test, Mann-Whitney, and Fisher's exact test when appropriated. Mann-Whitney test was used for the parameters with a non-normal distribution. Stepwise backward elimination multivariate analysis was performed for the investigation of factors associated with death. All variables presented were considered and it included the factors that presented a significance level < 10% in the univariate analysis. Allocation into the two defined groups was considered an independent variable. P value < 0.05 was considered as statistically significant in all other cases.
Results
A total of 374 patients were evaluated initially. Three hundred eighteen patients were included because they presented with AKI. There were 94 patients in group I and 224 in group II, with an increasing number of cases in the last years (Figure 1). There was a male predominance in both groups (77% and 84.8%, respectively). Patients in group I were younger (36 ± 13 years versus 41 ± 16 years, P = 0.001). Diabetes mellitus and hypertension was present in 13 (4.1%) and 27 (8.5%) of all patients with no difference between groups (3.1 versus 4.5%, P = 0.600 and 7.4 versus 8.0%, P = 0.860, respectively).
Figure 1.
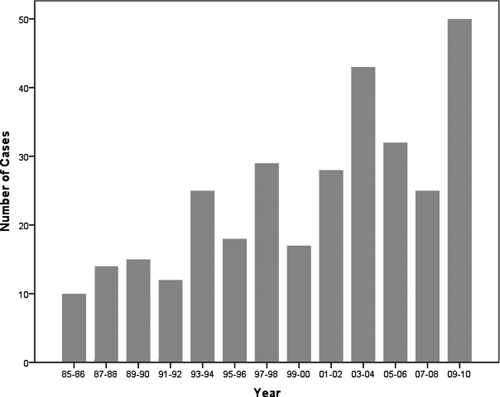
Number of patients with leptospirosis-associated acute kidney injury.
Serogroup was Icterohaemorrhagiae (100%) with serovar Icterohaemorrhagiae I 100% of cases and Copenhageniin in 59%. No significant difference was observed between the groups. The cases were predominant during the months from March to July. During this period there occurred 76 (81%) cases in group I and 176 (78.5%) in group II. The analysis of the clinical manifestations showed a higher frequency of headache in group II (77% versus 65%, P < 0.02), as well as lung (59% versus 26.6%, P < 0.0001) and hemorrhagic (46.9% versus 23.4%, P < 0.0001) manifestations. Patients in group I had a higher frequency of arrhythmias (20% versus 11.6%, P = 0.04) and need of renal replacement therapy (75% versus 24%, P < 0.0001), as can be seen in Table 1. The comparison between survivors and non-survivors according to groups can be seen in Tables 2 and 3.
Table 1.
Clinical characteristics of patients with leptospirosis-associated acute kidney injury*
| Group I (N = 94) | Group II (N = 224) | P | |||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| Age | 36 ± 13 | 41 ± 16 | 0.001 | ||
| SBP (mm of Hg) | 109 ± 19 | 111 ± 20 | 0.378 | ||
| DBP (mm of Hg) | 66 ± 15 | 70 ± 14 | 0.020 | ||
| n | % | n | % | ||
| Gender | |||||
| Female | 22 | 23 | 34 | 15.2 | 0.06 |
| Male | 72 | 77 | 190 | 84.8 | |
| Symptoms and signs | |||||
| Headache | 61 | 64.8 | 173 | 77.2 | 0.002 |
| Fever | 92 | 97.6 | 213 | 95.1 | 0.207 |
| Myalgia | 86 | 91.8 | 194 | 86.6 | 0.179 |
| Vomiting | 65 | 69.4 | 149 | 66.5 | 0.600 |
| Dehydration | 57 | 60.7 | 139 | 62.0 | 0.768 |
| Jaundice | 93 | 98.8 | 211 | 94.2 | 0.569 |
| Hepatomegaly | 37 | 40.0 | 85 | 37.9 | 0.874 |
| Hemorrhagic manifestations | 22 | 23.4 | 105 | 46.9 | 0.001 |
| Respiratory infection | 8 | 9.5 | 18 | 8.0 | 0.896 |
| Calf pain | 70 | 82.4 | 94 | 41.8 | 0.001 |
| Arrhythmias | 19 | 20.2 | 26 | 11.6 | 0.04 |
| Pancreatitis | 0 | 0.0 | 1 | 0.4 | 1.0 |
| Meningitis | 2 | 2.4 | 1 | 0.4 | 0.162 |
| Secondary infections | 11 | 11.8 | 15 | 6.7 | 0.177 |
| Lung manifestation | 25 | 26.6 | 132 | 59 | < 0.0001 |
| Convulsion | 0 | 0.0 | 3 | 1.3 | 1.0 |
| Splenomegaly | 0 | 0.0 | 6 | 2.6 | 1.0 |
| Obtundation | 18 | 22.0 | 8 | 3.6 | < 0.0001 |
| Contact with rats | 37 | 39.8 | 58 | 25.8 | 0.06 |
| Oliguria | 25 | 26.5 | 92 | 41.1 | 0.014 |
| Need of dialysis | 70 | 75.0 | 54 | 24.1 | < 0.0001 |
| Death | 19 | 20.2 | 27 | 12.1 | 0.039 |
SBP = systolic blood pressure; DBP = dyastolic blood pressure. Student t test and Fisher's exact test. Significant P < 0.05.
Table 2.
Clinical characteristics of patients with leptospirosis-associated acute kidney injury*
| Group I (N = 94) | P | Group II (N = 107) | P | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survivors (n-75) | Non-survivors (N = 19) | Survivors (n-197) | Non-survivors (N = 27) | |||||||
| Median [25th–75th] | Median [25th–75th] | Mean ± SD | Mean ± SD | |||||||
| Age | 32 [24–45] | 44 [32–53.5] | 0.003 | 40 ± 15 | 48 ± 17 | 0.001 | ||||
| SBP (mm of Hg) | 110 [100–120] | 100 [90–112.5] | 0.038 | 111 ± 25 | 111 ± 19 | 0.896 | ||||
| DBP (mm of Hg) | 70 [60–80] | 60 [50–65] | 0.006 | 71 ± 13 | 66 ± 13 | 0.144 | ||||
| n | % | n | % | n | % | n | % | |||
| Gender | ||||||||||
| Female | 11 | 16.7 | 8 | 42.1 | 0.029 | 26 | 13.2 | 8 | 29.6 | 0.041 |
| Male | 55 | 83.3 | 11 | 57.9 | 171 | 86.8 | 19 | 70.4 | ||
| Symptoms and signs | ||||||||||
| Headache | 48 | 72.7 | 8 | 42.1 | 0.026 | 146 | 74.1 | 21 | 77.7 | 0.791 |
| Fever | 64 | 97.0 | 19 | 100.0 | 1.000 | 190 | 96.4 | 23 | 85.2 | 0.031 |
| Myalgia | 63 | 95.5 | 15 | 78.9 | 0.041 | 172 | 87.3 | 22 | 81.5 | 0.376 |
| Vomiting | 43 | 65.2 | 16 | 84.2 | 0.159 | 131 | 66.5 | 18 | 66.7 | 1.0 |
| Dehydration | 42 | 63.6 | 9 | 50.0 | 0.415 | 78 | 39.6 | 12 | 44.4 | 0.678 |
| Jaundice | 65 | 98.5 | 19 | 100.0 | 1.000 | 144 | 73.1 | 21 | 77.8 | 0.816 |
| Hepatomegaly | 25 | 37.9 | 9 | 47.4 | 0.596 | 48 | 24.4 | 10 | 37.0 | 1.0.166 |
| Hemorrhagic manifestations | 20 | 26.7 | 2 | 10.5 | 0.138 | 89 | 45.2 | 16 | 59.2 | 0.330 |
| Respiratory infection | 5 | 7.6 | 3 | 15.7 | 0.360 | 14 | 7.1 | 4 | 14.8 | 0.167 |
| Calf pain | 57 | 86.4 | 13 | 68.4 | 0.091 | 86 | 43.5 | 8 | 29.6 | 0.143 |
| Arrhythmias | 9 | 13.8 | 8 | 42.1 | 0.019 | 16 | 8.1 | 10 | 37.0 | 0.001 |
| Pancreatitis | 0 | 0.0 | 0 | 0.0 | – | 1 | 0.50 | 0 | 0.0 | 1.000 |
| Meningitis | 2 | 3.0 | 0 | 0.0 | 1.000 | 1 | 0.50 | 0 | 0.0 | – |
| Secondary infections | 10 | 15.2 | 0 | 0.0 | 0.108 | 13 | 6.6 | 2 | 7.4 | 0.699 |
| Lung manifestations | 18 | 24.0 | 7 | 36.8 | 0.258 | 118 | 59.9 | 14 | 51.8 | 0.496 |
| Convulsion | 0 | 0.0 | 0 | 0.0 | – | 2 | 1.0 | 1 | 3.7 | 0.254 |
| Splenomegaly | 0 | 0.0 | 0 | 0.0 | – | 6 | 3.0 | 0 | 0.0 | 1.000 |
| Obtundation | 14 | 21.5 | 4 | 23.5 | 1.000 | 5 | 2.5 | 3 | 11.1 | 0.02 |
| Contact with rats | 27 | 65.9 | 3 | 25.0 | 0.019 | 54 | 27.4 | 4 | 14.8 | 0.161 |
| Oliguria | 12 | 18.2 | 13 | 68.4 | < 0.0001 | 80 | 40.6 | 12 | 44.4 | 0.704 |
| Need of dialysis | 55 | 74.3 | 15 | 75 | 1.00 | 39 | 19.8 | 15 | 55.6 | < 0.001 |
SBP = systolic blood pressure; DBP = dyastolic blood pressure. Student t test and Fisher's exact test. Significant P < 0.05.
Table 3.
Laboratory characteristics of patients with leptospirosis-associated acute kidney injury, according survivors and non-survivors in both groups*
| Group I (N = 94) | Group II (N = 107) | |||||
|---|---|---|---|---|---|---|
| Survivors (N = 75) | Non-survivors (N = 19) | P | Survivors (N = 197) | Non-survivors (N = 27) | P | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| Namax (mEq/L) | 132 ± 0.8 | 131 ± 1.6 | 0.807 | 138 ± 0.42 | 135 ± 1.4 | 0.007 |
| Namin (mEq/L) | 132 ± 0.8 | 131 ± 1.6 | 0.875 | 130 ± 0.43 | 131 ± 0.9 | 0.320 |
| Kadmission (mEq/L) | 3.9 ± 0.1 | 4.1 ± 0.2 | 0.489 | 3.9 ± 0.07 | 3.7 ± 0.14 | 0.484 |
| Kmin (mEq/L) | 3.1 ± 0.07 | 3.6 ± 0.192 | 0.023 | 3.3 ± 0.04 | 3.4 ± 0.14 | 0.421 |
| Urmax (mg/dL) | 216 ± 11 | 207 ± 19 | 0.541 | 133 ± 6.5 | 158 ± 20 | 0.215 |
| Crmax (mg/dL) | 6.4 ± 0.3 | 6.7 ± 0.6 | 0.620 | 4.1 ± 0.2 | 4.4 ± 0.4 | 0.534 |
| Arterial pH | 7.39 ± 0.01 | 7.32 ± 0.04 | 0.123 | 7.37 ± 0.01 | 7.36 ± 0.07 | 0.708 |
| Arterial pO2 | 84 ± 22 | 79 ± 20 | 0.784 | 85 ± 16 | 86 ± 24 | 0.937 |
| Arterial HCO3 (mEq/L) | 20 ± 1.2 | 16 ± 1.7 | 0.126 | 19 ± 0.5 | 17 ± 0.91 | 0.268 |
| Hb (g/dL) | 10.3 ± 0.25 | 10.4 ± 0.36 | 0.758 | 11.1 ± 0.1 | 9.97 ± 0.48 | 0.010 |
| White blood count (×103/mm3) | 16 ± 14 | 15 ± 17 | 0.752 | 11 ± 0.3 | 12 ± 1.3 | 0.347 |
| LDHmax (UI/L) | 568 ± 53 | 747 ± 103 | 0.118 | 760 ± 68 | 767 ± 151 | 0.972 |
| CKmax (UI/L) | 136 ± 36 | 400 ± 205 | 0.283 | 268 ± 54 | 356 ± 50 | 0.412 |
| AST (UI/L) | 69 ± 6.8 | 94 ± 18 | 0.097 | 89 ± 9 | 88 ± 12 | 0.978 |
| Direct bilirubin (mg/dL) | 14 ± 1.4 | 16 ± 2.3 | 0.620 | 9.5 ± 0.7 | 11.8 ± 2.7 | 0.364 |
| Indirect bilirubin (mg/dL) | 5.5 ± 0.6 | 6.2 ± 1.0 | 0.359 | 4.9 ± 0.4 | 4.1 ± 1.0 | 0.878 |
| PT (%) | 68 ± 4.0 | 75 ± 9.1 | 0.468 | 68 ± 7.7 | 70 ± 15 | 0.789 |
Max = maximum values during hospital stay; min = minimum values during hospital stay; Na = sodium; K = potassium; Ur = urea; Cr = creatinine; HCO3 = bicarbonate; Hb = hemoglobin; LDH = lactate dehydrogenase; AST = aspartate aminotransferase; CK = creatine kinase; PT = prothrombin time.
According to the RIFLE classification there was 75 (23.6%) patients in each “Risk” and “Injury” categories, whereas 168 (52.8%) of the patients were classified as “Failure”. Mortality was higher in group I in all the classes, but not statistically significant (Table 4). The number of patients in “Failure” was higher in group I (77% versus 42.4%, P < 0.0001). The number of patients with oliguria was higher in group II. In group I there were 25 cases of oliguria (26%) and in group II there were 92 patients with oliguria (41.1%), P = 0.01.
Table 4.
RIFLE classification in patients with leptospirosis-associated acute kidney injury, according groups*
| Group I (N = 94) | Group II (N = 224) | P | |||
|---|---|---|---|---|---|
| Total | Non-survivors | Total | Non-survivors | ||
| Risk (N = 75) | 4 | 1 (25%) | 71 | 4 (5.6%) | 0.24 |
| Injury (N = 75) | 17 | 2 (11.7%) | 56 | 6 (10.7%) | 0.98 |
| Failure (N = 168) | 73 | 16 (21.9%) | 95 | 17 (17.9%) | 0.51 |
Fisher's exact test. Significant P < 0.05.
Antibiotic therapy with Penicillin G was administered in 230 (65%) of the patients, 38 (44%) of them in group I and 192 (86%) in group II (P < 0.0001). Jarisch-Herxheimer's reaction was not seen in these patients.
Dialysis was required for 124 cases (39%). The mean number of dialysis sessions was 3.8 ± 2.1 sessions. Among the patients submitted to dialysis, intermittent peritoneal dialysis (IPD) was performed in 63 (50.8%) and daily hemodialysis (DHD) in 61 (49.2%). Mortality was high in patients under IPD versus hemodialysis (22.2% versus 8.2%, P = 0.03). The IPD was the predominant method in group I and DHD in group II. Hemodialysis was instituted early (in the first 48 h after admission), whereas peritoneal dialysis was started later (48 h after admission).
Overall mortality was 13%. Mortality was significantly higher in group I, and there was a clear reduction in years since 1996 as illustrated in Figure 2. In group I there were 19 deaths (20%) and in group II there were 27 deaths (12%), P = 0.039. Factors associated with death were age > 60 years old, DBP below 60 mm of Hg, arrhythmia, and oliguria; whereas hemodialysis was associated with better mortality in relation to peritoneal dialysis. Furthermore, there was a tendency for penicillin to be a protective factor (Table 5).
Figure 2.
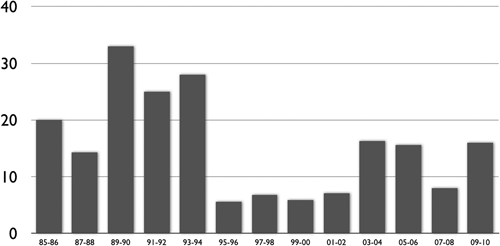
Mortality of patients with leptospirosis-associated acute kidney injury throughout years.
Table 5.
Risk factors for death in patients with leptospirosis-associated acute kidney injury*
| OR | Adjusted OR | 95% CI | P | |
|---|---|---|---|---|
| Advanced age (> 60 years) | 1.697 | 5.456 | 1.115–26.706 | 0.036 |
| Diastolic blood pressure (< 60 mmHg) | 2.257 | 9.555 | 1.523–59.941 | 0.016 |
| Oliguria | 2.325 | 10.231 | 1.930–54.223 | 0.006 |
| Arrhythmia | 1.683 | 5.383 | 1.026–28.243 | 0.047 |
| Hemodialysis (vs. peritoneal dialysis) | −2.630 | 0.072 | 0.006–0.811 | 0.033 |
| Penicillin use | −2.194 | 0.112 | 0.018–1.332 | 0.092 |
Out of the model: sex, RIFLE classification, inclusion according group, headache, lung, and hemorrhagic manifestations.
OR = odds ratio; CI = confidence interval.
Discussion
This study is one of the few to investigate the changes in the presentation and evolution of leptospirosis during a large period of time. Patients are getting older and presenting with more lung involvement. Although this greater severity, after implementation of a program mainly based in vigilant hydratation, penicillin administration, early renal substitutive therapy, and preferable hemodialysis instead of peritoneal dialysis, there was an improvement in mortality rates. In multivariate analysis, hemodialysis instead of peritoneal dialysis was associated with decreased mortality, whereas penicillin administration presented a statistical trend to it.
The epidemiology of leptospirosis is changing, reemerging in the large cities in developing countries, where there are proliferations of slums and lack of sanitation.15 The disease is more frequent among young males, as demonstrated previously in other studies.16 In our study, we observed an increased number of cases in older individuals that can be a reflection of the aging population, which is a worldwide trend.17,18 Predominance of male is a known characteristic of leptospirosis and data of the Brazilian Ministry of Health, a total of 4,539 cases were registered in 2006, and 73.7% were male.19
There was an increase in the severe forms of the disease, evidenced by the higher frequency of oliguria and symptoms related to lung involvement, such as dyspnea and pulmonary crackles, which can reflect lung involvement and a complication of AKI. There was also a higher frequency of hemorrhagic phenomena, including the appearance of petechiae, hemoptysis, and hematemesis. The higher frequency of manifestations such as oliguria, lung involvement, and hemorrhagic manifestations in the last decade characterizes the more frequent incidence of severe forms of leptospirosis. This severity can be associated with the intensity of immune response on a possible reinfection20 or inoculum size.3
The use of RIFLE classification showed a higher incidence of severe forms of AKI (patients classified in “Failure”) in the first decade, which can be responsible for the higher mortality observed in the first group of patients. In a recent study including critically ill patients with infections, disease-associated AKI (of which 11.6% had leptospirosis), the RIFLE classification was significantly associated with mortality.21 The mortality was higher in the “Failure” group in the two studied groups in comparison to the “Injury” group. Patients in “Risk” had an even higher mortality in the first group (25%) but because of a small number of patients it is not possible to draw any conclusion.
There was a significant decrease in mortality in the last group (from 20% to 12%), and this can be attributed to a more adequate treatment. Several studies have described a mortality rate near 20%,22,23 which was similar to what we observed in the first group in our cohort. The risk factors for death in these studies were age > 40 years, dyspnea, oliguria, elevated white blood cell count, repolarization abnormalities on electrocardiograms, and alveolar infiltrates on chest radiographs.23,24 In this study, we found oliguria, arrhythmias, lower DBP, and age as independent risk factors for death. We believe that those factors associated with mortality reflect severity of the disease evolution. Arrhythmias, for instance, was more frequent in group I and it was associated with mortality, suggesting it is a marker of severity as demonstrated by others.24 The higher mortality observed in the first group can be caused by differences in treatment protocols as evidenced by the protective effect of hemodialysis in comparison with peritoneal dialysis and the trend in association between survival and penicillin usage. In the second group, these factors were implemented more frequently and the higher survival rate can be attributed to this clinical approach.
An important phase in the treatment of leptospirosis-associated AKI is hydration. Hypotension and hypovolemia are important factors that lead to renal dysfunction in leptospirosis and venous hydration is crucial to patients' improvement. Recent studies show that hydration should be done with caution, because there is a high risk for pulmonary congestion and deterioration of lungs, which are generally involved in severe leptospirosis with hemorrhagic manifestations.25 A change in the approach we used for our patients was done at the end of the 1990s decade, with a cautious hydration. Even with a vigilant hydratation approach, fewer patients needed dialysis. This is in accordance with recent observations that overzealous fluid therapy is associated with worse renal function.26 Unfortunately, it is difficult and imprecise to measure fluid balance in a retrospective study.
Early dialysis is of huge importance in leptospirosis and is associated with a significant decrease in mortality.12 Recent studies show that the early institution of dialysis and daily dialysis is beneficial and decreases mortality.12 Dialysis was required for a lower number of patients in the second group, which contradicts the severity of disease, but this can be caused by an adequate and efficacious institution of conservative treatment of leptospirosis associated-AKI and correct indication of dialysis. We observed a change from peritoneal dialysis to hemodialysis in the second period. Hemodialysis was done earlier (in the first 48 h after admission), and this could be responsible for the reduction in mortality. Antibiotic use was administered to a higher percentage of cases in the second group (86% versus 44%), which could also have influenced the mortality reduction. Antibiotic use is still controversial in leptospirosis, but some recent studies reported favorable outcomes in leptospirosis patients treated with penicillin.27,28
This study has several limitations. First, its retrospective nature does not permit a conclusion on which intervention changes were really associated with a better outcome, although multivariate analysis suggests it. Second, there is a lack of data about diuretic usage in oliguric patients. However, we know the lack of impact in mortality of transforming an oliguric AKI into a non-oliguric one.29 Third, it is the inability to access fluid balance and its association with renal function, a need of dialysis and mortality.
In summary, leptospirosis is occurring in an older population, with an increase in severity, as evidenced by an increase in oliguric cases, pulmonary, and hemorrhagic manifestations. Risk factors for death were oliguria, lower DBP, arrhythmia, and age. Of importance, we detected that therapeutic measures are associated with better survival.
ACKNOWLEDGMENTS
We are very grateful to the team of physicians, residents, medical students, and nurses from the Hospital São José de Doenças Infecciosas for providing technical support to the development of this research and for the exceptional assistance provided to the patients.
Footnotes
Financial support: This research was supported by CNPq – Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazilian Research Council).
Authors' addresses: Elizabeth F. Daher, Department of Internal Medicine, Federal University of Ceara, Fortaleza, Ceara, Brazil, E-mail: ef.daher@uol.com.br. Geraldo B. Silva Junior, School of Medicine, University of Fortaleza, and Department of Internal Medicine, Federal University of Ceara, Fortaleza, Ceara, Brazil, E-mail: geraldobezerrajr@unifor.br. Rafael S. A. Lima, School of Medicine of Ribeirao Preto, University of Sao Paulo, Ribeirao Preto, Sao Paulo, Brazil, E-mail: rafaelsiqueiraufc@gmail.com. Rosa M. S. Mota, Department of Statistics, Federal University of Ceara, Fortaleza, Ceara, Brazil, E-mail: rosa@ufc.br. Hermano A. L. Rocha, Department of Internal Medicine, Federal University of Ceara, Fortaleza, Ceara, Brazil, E-mail: hermanoalexandre@yahoo.com.br. Krasnalhia Livia S. Abreu, Department of Internal Medicine, Federal University of Ceara, Fortaleza, Ceara, Brazil, E-mail: kras_livinha@yahoo.com.br. Adller G. C. Barreto, Department of Internal Medicine, Federal University of Ceara, Fortaleza, Ceara, Brazil, E-mail: adller.md@gmail.com. Eanes D. B. Pereira, Department of Internal Medicine, Federal University of Ceara, Fortaleza, Ceara, Brazil, E-mail: eanes@fortalnet.com.br. Sônia M. H. A. Araújo, Department of Internal Medicine, Federal University of Ceara, Fortaleza, Ceara, Brazil, E-mail: sonia.holanda@gmail.com. Alexandre Braga Libório, Post-Graduation Program in Public Health, University of Fortaleza, Fortaleza, Ceara, Brazil, E-mail: alexandreliborio@yahoo.com.br.
References
- 1.Adler B, de la Peña Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. 2010;140:287–296. doi: 10.1016/j.vetmic.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Victoriano AF, Smythe LD, Gloriani-Barzaga N, Cavomta LL, Kasai T, Limpakarnjanarat K, Ong BL, Gongal G, Hall J, Coulcombe CA, Yanagihara Y, Yoshida S, Adler B. Leptospirosis in the Asia Pacific region 2009. BMC Infect Dis. 2009;9:147. doi: 10.1186/1471-2334-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, Lovett MA, Gilman RH, Willig MR, Gotuzzo E, Vinetz JM. Leptospirosis: a zoonotic disease of global importance. Lancet Infect Dis. 2003;3:757–771. doi: 10.1016/s1473-3099(03)00830-2. Reru-United States Leptospirosis Consortium. [DOI] [PubMed] [Google Scholar]
- 4.Yanagihara Y, Villanueva SY, Yoshida S, Okamoto Y, Masuzawa T. Current status of leptospirosis in Japan and Philippines. Comp Immunol Microbiol Infect Dis. 2007;30:399–413. doi: 10.1016/j.cimid.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Vinetz JM, Glass GE, Flexner CE, Mueller P, Kaslow DC. Sporadic urban leptospirosis. Ann Intern Med. 1996;125:794–798. doi: 10.7326/0003-4819-125-10-199611150-00002. [DOI] [PubMed] [Google Scholar]
- 6.Sejvar J, Tangkanakul W, Ratanasang P, Dowell SF, Sangjun N, Bragg S, Ashford D, Tappero J. An outbreak of leptospirosis, Thailand–the importance of the laboratory. Southeast Asian J Trop Med Pub Hlth. 2005;36:289–295. [PubMed] [Google Scholar]
- 7.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrade L, Daher EF, Seguro AC. Leptospiral nephropathy. Semin Nephrol. 2008;28:383–394. doi: 10.1016/j.semnephrol.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Daher EF, Silva Junior GB, Karbage NN, Carvalho PC, Jr, Kataoka RS, Silva EC, Magalhães MM, Avaújo SM, Gutiérrez-Adruabzén OA, Libório AB. Predictors of oliguric acute kidney injury in leptospirosis: a retrospective study on 196 consecutive patients. Nephron Clin Pract. 2009;112:c25–c30. doi: 10.1159/000210571. [DOI] [PubMed] [Google Scholar]
- 10.Roca B. Leptospirosis. Rev Med Univ Navarra. 2006;50:3–6. [PubMed] [Google Scholar]
- 11.Ko AI, Galvao RM, Ribeiro DC, Johnson WD, Jr, Riley LW. Urban epidemic of severe leptospirosis in Brazil. Salvador Leptospirosis Study Group. Lancet. 1999;354:820–825. doi: 10.1016/s0140-6736(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 12.Andrade L, Cleto S, Seguro AC. Door-to-dialysis time and daily hemodialysis in patients with leptospirosis: impact on mortality. Clin J Am Soc Nephrol. 2007;2:739–744. doi: 10.2215/CJN.00680207. [DOI] [PubMed] [Google Scholar]
- 13.Daher EF, Zanetta DM, Cavalcante MB, Abdulkader RC. Risk factors for death and changing patterns in leptospirosis acute renal failure. Am J Trop Med Hyg. 1999;61:630–634. doi: 10.4269/ajtmh.1999.61.630. [DOI] [PubMed] [Google Scholar]
- 14.Bellomo R, Kellum JA, Ronco C. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med. 2007;33:409–413. doi: 10.1007/s00134-006-0478-x. [DOI] [PubMed] [Google Scholar]
- 15.Ko AI, Galvao RM, Ribeiro DC, Johnson WD, Jr, Riley LW. Urban epidemic of severe leptospirosis in Brazil. Salvador Leptospirosis Study Group. Lancet. 1999;354:820–825. doi: 10.1016/s0140-6736(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 16.Esen S, Sunbul M, Leblebicioglu H, Eroglu C, Turan D. Impact of clinical and laboratory findings on prognosis in leptospirosis. Swiss Med Wkly. 2004;134:347–352. doi: 10.4414/smw.2004.10436. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . What are the main risk factors for disability at old age and how can disability be prevented? Europe: Regional Office for Europe's Health Evidence Network (HEN); 2003. [Google Scholar]
- 18.U.S. Census Bureau Projections of the Population by Selected Age Groups and Sex for the United States: 2010 to 2050. 2010. http://www.census.gov/population/www/projections/summarytables.html Available at. Accessed May 14, 2010.
- 19.Minisério da Saúde do Brasil = Brazilian Ministry of Health Leptospirose – Casos confirmados notificados no Sistema de Informação de Agravos de Notificação – Sinan. Leptospirosis – Confirmed cases, registered by the Notifiable Diseases System – Sinan. 2010. http://dtr2004.saude.gov.br/sinanweb/tabnet/dh?sinan/lepto/bases/leptobr.def Available at. Accessed June 6, 2010.
- 20.Abdulkader RC, Daher EF, Camargo ED, Spinosa C, Silva MV. Leptospirosis severity may be associated with the intensity of humoral immune response. Rev Inst Med Trop Sao Paulo. 2002;44:79–83. doi: 10.1590/s0036-46652002000200005. [DOI] [PubMed] [Google Scholar]
- 21.Daher EF, Marques CN, Lima RS, Silva Júnior GB, Barbosa AS, Barbosa ES, Mota RM, Leite da Silva S, Araújo SM, Libório AB. Acute kidney injury in an infectious disease intensive care unit – an assessment of prognostic factors. Swiss Med Wkly. 2008;138:128–133. doi: 10.4414/smw.2008.12062. [DOI] [PubMed] [Google Scholar]
- 22.Spichler AS, Vilaça PJ, Athanazio DA, Albuquerque JO, Buzzar M, Catro B, Seguro A, Vinetz JM. Predictors of lethality in severe leptospirosis in urban Brazil. Am J Trop Med Hyg. 2008;79:911–914. [PMC free article] [PubMed] [Google Scholar]
- 23.Dupont H, Dupont-Perdrizet D, Perie JL, Zehner-Hansen S, Jarrige B, Daijardin JB. Leptospirosis: prognostic factors associated with mortality. Clin Infect Dis. 1997;25:720–724. doi: 10.1086/513767. [DOI] [PubMed] [Google Scholar]
- 24.Sacramento E, Lopes AA, Costa E, Passos OL, Costa YA, Matos ED. Electrocardiographic alterations in patients hospitalized with leptospirosis in the Brazilian city of Salvador. Arq Bras Cardiol. 2002;78:267–270. doi: 10.1590/s0066-782x2002000300002. [DOI] [PubMed] [Google Scholar]
- 25.Siriwanij T, Suttinont C, Tantawichien T, Chusil S, Kanjanabuch T, Sitprija V. Haemodynamics in leptospirosis: effects of plasmapheresis and continuous venovenous hemofiltration. Nephrology (Carlton) 2005;10:1–6. doi: 10.1111/j.1440-1797.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 26.Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010;6:107–115. doi: 10.1038/nrneph.2009.213. [DOI] [PubMed] [Google Scholar]
- 27.Michalopoulos A, Pappas G, Papadakis E, Chirtoforatos T, Malamos P, Koumoudiou C, Chalevelakis G. Leptospirosis in a European intensive care unit. Scand J Infect Dis. 2010;42:69–71. doi: 10.3109/00365540903302861. [DOI] [PubMed] [Google Scholar]
- 28.Shah I. Non-oliguric renal failure – a presentation of leptospirosis. Ann Trop Med Parasitol. 2009;103:53–56. doi: 10.1179/136485909X384947. [DOI] [PubMed] [Google Scholar]
- 29.Joannidis M, Druml W, Forni LG, Groeneveld AB, Honore P, Oudemans-van Straaten HM, Ronco C, Schetz MR, Woittiez AJ. Prevention of acute kidney injury and protection of renal function in the intensive care unit. Expert opinion of the Working Group for Nephrology. Intensive Care Med. 2010;36:392–411. doi: 10.1007/s00134-009-1678-y. [DOI] [PubMed] [Google Scholar]


