Abstract
The purpose of this study was to conduct an entomological analysis, determination of feeding sources, and molecular identification of triatomines in five communities of the Istmo de Tehuantepec, Oaxaca. The only found species in two of five searched communities (San Mateo del Mar and Tehuantepec City) was Triatoma phyllosoma. Colonization indices were high in both communities. In San Mateo del Mar, the insects were found indoors and in Tehuantepec City in peridomestic areas. The Trypanosoma cruzi infection indices were 2.1% in San Mateo del Mar and 39.4% in Tehuantepec City. This difference could be related to the high numbers of triatomine feeding on hens in the former community. In contrast, in Tehuantepec, dogs were the principal triatomine feeding sources. All nymphs and adults that were genetically analyzed belonged to the species T. phyllosoma. Low levels of genetic variation were found between vectors from both communities.
Introduction
The blood-sucking insects of the subfamily Triatominae are vectors of Trypanosoma cruzi, the causal agent of Chagas disease, one of the most important vector-transmitted diseases in Latin America.1 In Mexico, 33 species of triatomines have been reported2,3 and many of these species are endemic,4,5 including the species of the Phyllosoma complex: Triatoma bassolsae, Triatoma longipennis, Triatoma mazzottii, Triatoma mexicana, Triatoma pallidipennis, Triatoma phyllosoma, and Triatoma picturata.6–8 These species have been considered of epidemiological importance because of their domestic habits and high levels of infection with T. cruzi.9–12
Triatomine classification is based on the morphological characteristics of adult specimens. However, this type of classification is complicated for closely related species because of the similarity of some of their characters. Recent studies using molecular markers have helped to solve this problem. For example, Martínez and others8 reported that it is possible to identify each species of the Phyllosoma complex and to exclude Triatoma dimidiata from this complex using the sequences of internal transcribed spacer 2 (ITS-2) and cytochrome B (CytB). This finding is important because a high percentage of individuals collected are nymphs that cannot be classified because of morphological similarities between the species of the Phyllosoma complex and many are infected with T. cruzi.9,13,14 Thus, the use of molecular markers to identify nymphs could be a useful tool.15
It is also important to understand the entomological features of the T. cruzi transmission cycle in this southern Mexican area because several species of triatomine have been reported in the region. In particular, identifying feeding sources is a way to understand the relationship between insects and their hosts, especially humans and could contribute to our knowledge of natural hosts and their role in T. cruzi transmission. There have been several studies of host feeding in different ecotopes; for example, Triatoma infestans collected in Chuquisaca, Bolivia, were found to have fed on multiple peridomestic hosts including dogs, pigs, chickens, and guinea pigs.16 Rhodnius pallescens collected from peridomestic palm trees had fed on opossums, whereas insects collected inside houses showed increased anthropophagy; however, no significant difference in the infection index was observed between the two habitats.17 Belminus herreri was positive for only one feeding source, Blattidae.18 In localities of Jalisco, Mexico, T. longipennis had different preferences depending on the habitat. For example, in Tepehuaje de Morelos, its blood meals were mainly from chicken, opossum, and pig, whereas in Los Guerreros, rats were the principal source.19,20
In Mexico, Chagas disease was first reported in the mid-1940s in the State of Oaxaca.21 In this state, eight species of triatomines have been reported.22 One of the most important regions of the state is the Istmo de Tehuantepec, where autochthonous cases of dilated cardiomyopathy associated with T. cruzi infection have been diagnosed.23 In this particular region of the state, the presence of three species of triatomines has been reported: T. dimidiata, T. mazzottii, and T. phyllosoma.22 Of these, T. phyllosoma has been reported only in Oaxaca.2,6 Several years ago, this species was reported in 22 villages, presenting a low colonization index (0.3%) and a moderate level of T. cruzi infection (27.2%).22 These villages are situated in the southern Zapotecan Sierra east of the Istmo de Tehuantepec. Given their endemic distribution, infection with T. cruzi rate and near contact with the human population T. phyllosoma could be considered the most important vector in Chagas disease transmission in the southern part of the state. Nevertheless, little information is available about entomological patterns in infested villages, feeding sources, habitats, and seasonal variation. These data are important because they will contribute to a better understanding of the vector and to the control of Chagas disease transmission in this part of southern Mexico.
To contribute to our understanding of the species T. phyllosoma in the state of Oaxaca, we assessed entomological indices, distribution, feeding sources, and indoor and outdoor ecotopes associated with the vector in five communities in the Istmo de Tehuantepec. We also conducted a molecular identification of nymphs and adults using the molecular markers ITS-2 and CytB.
Materials and Methods
Study area.
The Istmo de Tehuantepec region is located in the state of Oaxaca in southern Mexico. This study was conducted in five localities: 1) Santa María Petapa (16°49′N, 95°07′W) at an altitude of 260 m; 2) Magdalena Tequisistlan (16°24′N, 95°37′W) at 190 m; 3) San Miguel Ecatepec (16°13′N, 96′43′W) at 699 m; 4) Tehuantepec City (16°17′N, 95°25′W) at 99 m; and 5) San Mateo del Mar (16°12′N, 94°59′W) at 10 m (Figure 1). The average annual temperature ranges from 24 to 36°C and the average annual precipitation is 3,960 mm. With the exception of Tehuantepec City (urban area), the study areas were considered rural communities (http://www.inegi.gov.mx).
Figure 1.
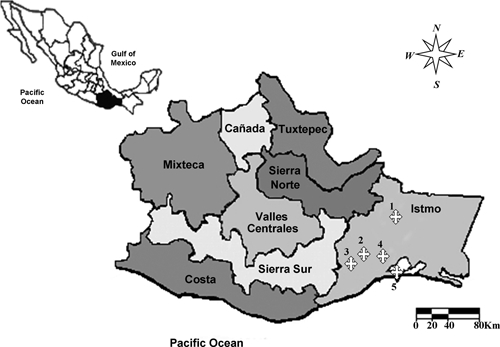
The smaller map indicates the Oaxaca state in Mexico (dark area), whereas the main map represents the eight socioeconomic regions of state, showing with crosses the studied communities in the Istmo de Tehuantepec region. 1) Santa María Petapa, 2) Magdalena Tequisistlan, 3) San Miguel Ecatepec, 4) Tehuantepec City, and 5) San Mateo del Mar.
Triatomine collection.
Collections were performed in the following periods: January–April (before the rains) and September–December (after the rains). The houses examined were selected randomly in each locality and the local authorities were informed of the purpose of the study. Collections were conducted using the man/hour method24 in domestic (on walls, beds, and floors) and peridomestic (the surrounding areas of the houses that included henhouses, wood piles, and stone walls) areas. Insect searches were done manually using flashlights. The same man/hour effort (70 hours) was done in each community in the different collect periods. Collected insects were placed in separate labeled plastic flasks containing folded cardboard sheets and transported to the laboratory for morphological and genetic identification.
Entomological indices.
Each insect was evaluated for sex and developmental stage. Four entomological indices were calculated according to World Health Organization (WHO)25 guidelines: infestation index (houses positive for triatomine/number of houses examined × 100); colonization index (houses with triatomine nymphs/number of houses positive for triatomine × 100); crowding index (triatomine captured/number of houses positive for triatomine × 100); and natural infection index (triatomine positive for T. cruzi/number of triatomine examined × 100). For the infestation and colonization indices the vectors of the peridomestic area, such as the surrounding areas of houses, where the inhabitants stay for a long period of time during the day and frequently sleep during the night, were also considered as has been reported in other regions of Mexico.14,26 For the infection index, feces were obtained from each insect by abdominal pressure and diluted in phosphate buffered saline. The presence of T. cruzi was determined by direct microscopic observation at 400× magnification.9
Antibody production.
Guinea pig antibodies against serum proteins from rat, mouse, rabbit, opossum, and human were produced in the laboratory for the identification of feeding sources. Briefly, animals were immunized three times at 15-day intervals with 200 μg of serum protein from the animals listed previously. A serum fraction depleted of bovine serum albumin by the Hitrap Blue System (Amersham Pharmacia Biotech, Sweden) was used for the last two immunizations.27 Immunizations were administered subcutaneously, the first with complete Freund's adjuvant and the last two with incomplete Freund's adjuvant. The presence of specific antibodies was determined by enzyme-linked immunosorbent assay (ELISA). Anti-bovine, anti-cat, anti-chicken, anti-dog, anti-goat, anti-horse, and anti-pig anti-sera were commercially purchased. Dilutions were determined by ELISA with homologous and heterologous sera: 1:500 for anti-rat, anti-mouse, anti-rabbit, anti-opossum, and anti-human; 1:20,000 for anti-chicken; 1:40,000 for anti-dog, and 1:60,000 for anti-bovine, anti-cat, anti-goat, anti-horse, and anti-pig. At these dilutions, maximal reactivity with homologous sera and minimal cross-reactivity were observed.28,29
Feeding sources.
The midgut of each triatomine was macerated with a sterile plastic stick in sterile phosphate buffered saline. After two centrifugations at 4°C (15,000 × g for 5 min), the supernatant was collected and the protein concentration was determined. Samples were then maintained at −20°C until use. To determine feeding sources, ELISAs were performed on each sample against 12 different antibodies.28 For each sample, the highest value of absorption detected was considered positive and values below half this value were considered negative.
Morphological and molecular identification.
Morphological identification of adult insects was performed according to Lent and Wygodzinsky.6 Additionally, four adults and 10% of the collected nymphs were genetically identified. The insects were chosen arbitrarily from different community and collection periods. Briefly, DNA extraction was performed according to the phenol/chloroform technique8,30 using a leg of each insect. The primers used to amplify the ITS-2 and CytB sequences were reported previously.8,31 The polymerase chain reaction fragment obtained was subcloned into the cloning vector pCR 2.1 (Invitrogen, Carlsbad, CA) and sequenced using vector-specific primers (T7 promoter and M13 reverse). Refer to Martínez and others8 for a detailed description of the methodology used. Sequencing was performed using an ABI prism sequencer (Applied Biosystem, Model 310, Faster City, CA). The obtained sequences were deposited in GenBank (see Table 1).
Table 1.
Localities, stages, and codes of triatomines
| Localities | Developmental stage | Code | GeneBank accession no. mtCytB/ITS-2 |
|---|---|---|---|
| San Mateo del Mar | Adult | SMMOAX15 | HQ185141/ HQ185167 |
| 1st instar nymph | SMMOAX54 | HQ185146/HQ185172 | |
| 1st instar nymph | SMMOAX145 | HQ185150/HQ185176 | |
| 2nd instar nymph | SMMOAX1 | HQ185140/HQ185166 | |
| 2nd instar nymph | SMMOAX112 | HQ185149/HQ185175 | |
| 3rd instar nymph | SMMOAX21 | HQ185142/HQ185168 | |
| 3rd instar nymph | SMMOAX90 | HQ185148/HQ185174 | |
| 4th instar nymph | SMMOAX22 | HQ185143/HQ185169 | |
| 4th instar nymph | SMMOAX37 | HQ185145/HQ185171 | |
| 5th instar nymph | SMMOAX23 | HQ185144/HQ185170 | |
| 5th instar nymph | SMMOAX80 | HQ185147/HQ185173 | |
| Tehuantepec | Adult | TEHOAX1 | HQ185152/HQ185178 |
| Adult | TEHOAX4 | HQ185153/HQ185179 | |
| Adult | TEHOAX28 | HQ185156/HQ185182 | |
| 1st instar nymph | TEHOAX40 | HQ185158/HQ185184 | |
| 1st instar nymph | TEHOAX41 | HQ185159/HQ185185 | |
| 2nd instar nymph | TEHOAX45 | HQ185160/HQ185186 | |
| 2nd instar nymph | TEHOAX27 | HQ185155/HA185181 | |
| 3rd instar nymph | TEHOAX33 | HQ185157/HQ185183 | |
| 3rd instar nymph | TEHOAX86 | HQ185164/HQ185190 | |
| 4th instar nymph | TEHOAX50 | HQ185161/HQ185187 | |
| 4th instar nymph | TEHOAX55 | HQ185162/HQ185188 | |
| 5th instar nymph | TEHOAX25 | HQ185154/HQ185180 | |
| 5th instar nymph | TEHOAX71 | HQ185163/HQ185189 | |
| Salina Cruz* | Adult | SCOAX1 | HQ185139/HQ185165 |
| Tlacolulita* | Adult | TLAOAX1 | HQ185151/HQ185177 |
Triatomines from communities near Tehuantepec City.
Sequence analysis.
Twenty-four sequences from San Mateo del Mar and Tehuantepec City were analyzed. Multiple alignments were performed with the CLUSTAL W program version 1.8.V,32 and Modeltest version 3.733 was used to determine the appropriate model of molecular evolution. The Hasegawa-Kishino-Yano model with gamma distribution (HKY+G)34 was used for both markers. Phylograms were created using Bayesian inference with MrBayes 3.1.2.35 Analyses were run for 10,000,000 generations, with tree sampling every 1,000 generations. Trees with scores lower than those at stationary (burn-in) were discarded from the analysis. The remaining trees were used to build a consensus tree. TreeView was used to visualize trees.36 The following CytB and ITS-2 sequences from GenBank were used to construct the trees: T. phyllosoma (AY859411, AJ286881); Triatoma picturata (AY859413, AY860404); T. pallidipennis (AY859419, AY860403); T. mazzottii (AY859421, AY860393); T. longipennis (AY859412, AY860398); T. mexicana (DQ118976; AM286728); T. bassolsae (AY859410, AY860402); Triatoma lecticularia (AY859414, AY860407), and T. infestans (DQ118975, AY860388). In addition, a distance matrix was constructed using the Kimura-two parameter model with the program MEGA version 3.37
Statistics.
A Mann-Whitney test was realized to compare the number of insects collected in all months between communities. Differences were considered to be significant when P < 0.05.
Results
Field collections of triatomine.
Triatomines were found in only two of the five communities investigated: Tehuantepec City and San Mateo del Mar. A total of 243 insects were collected, with the most abundant being fifth instars and adults. Even though the number of insects collected in both communities was different, this difference was not statistically significant (P = 0.699).
All insects collected in the adult stage were morphologically identified as T. phyllosoma according to Lent and Wygodzinsky.6
Triatomine habitats.
The presence of triatomines was investigated in houses and peridomestic areas. In San Mateo del Mar, 60 dwellings were randomly selected; > 60% of these dwellings were built of palm leaves. Insects were found inside seven houses, specifically between the palm-leaf walls of the bedroom near the bed (Figure 2A). Occasionally, insects were also found in henhouses in the peridomestic area of the same houses. The infested houses were located in a recent settlement in the northern part of the community. Ninety-two dwellings were analyzed in Tehuantepec City, where > 80% of the houses was built of concrete. In nine houses located in the outskirts of the city, insects were found outdoors in the peridomestic area. These triatomines were found in piles of stone and stone walls (Figure 2B).
Figure 2.

Localization of triatomines in two communities of the Istmo de Tehuantepec. (A) Typical palm-leaf house in San Mateo del Mar; the vectors were found inside the palm wall of the bedroom (indoors). (B) Peridomestic area of typical outskirts house from Tehuantepec City, triatomine were found in piles of stone and bricks.
Entomological indices.
Entomological indices were calculated for each community. The infestation indices were of 11.7% in San Mateo del Mar and 9.8% in Tehuantepec City. The colonization indices were 88.8% in San Mateo del Mar and 100% in Tehuantepec City. The crowding indices were 21.7% for San Mateo del Mar and 11.4% for Tehuantepec City. Feces of 137 bugs were microscopically examined. The T. cruzi prevalence was 39.4% for Tehuantepec City and 2.1% for San Mateo del Mar. In Tehuantepec City, high levels of infection with T. cruzi were observed in fifth-instar nymphs (62.5% of infected individuals), whereas in San Mateo del Mar no predominance in any instar stage was observed.
Feeding source.
Seventy-five samples were analyzed and several different blood-feeding sources were detected in insects collected in both communities. A single feeding source was found in 49.8% of the samples from Tehuantepec City and 59.8% of the samples from San Mateo del Mar; of importance, the remaining samples contained blood proteins from two or more species. In Tehuantepec City, the principal feeding source was dog (36.6%), although samples containing blood from more than two different feeding sources were the second most abundant (30%). Interestingly, human blood was not found in the samples (Figure 3A). In San Mateo del Mar, chicken was the principal feeding source (28.8%), followed by human (15.5%), and blood from more than two different feeding sources (15.5%) (Figure 3B). Samples with more than one feeding source were found in both communities, cats and dogs were the more abundant feeding sources in Tehuantepec City (Figure 3). None of the samples tested were positive for horse, pig, mouse, or opossum. The distribution of feeding sources found in the samples was in accordance with the abundance of animals observed in the communities.
Figure 3.
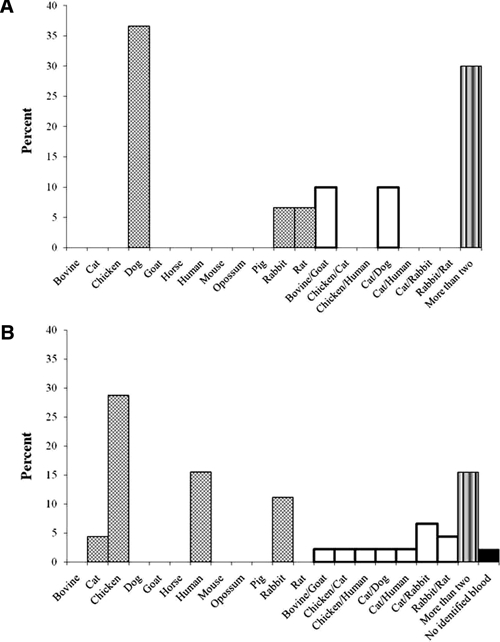
Feeding sources. Percentages of each type of feeding source detected in intestinal samples from insect of the species Triatoma phyllosoma from (A) Tehuantepec City and (B) San Mateo del Mar. The feeding sources were as follow: only one source (crossed bars); two different sources (white bars); more than two (vertical lines bars); feeding source no identified (dark bar).
Molecular identification.
Twenty nymphs and four adults were analyzed (Table 1). Additionally, two T. phyllosoma individuals that had been collected in two communities near Tehuantepec City were also included in the study. For each individual, the sequences of ITS-2 and CytB were amplified. The size of the amplified ITS-2 fragment was 900 bp, whereas for CytB it was 330 bp. Each obtained sequence was compared with sequences found in GenBank. The sequences of the two markers had high percentages of identity (up to 98%) with T. phyllosoma sequences.
With the sequences obtained from the genetic analysis, a distance matrix was built using the Kimura 2-parameter model. The individuals from both communities presented small genetic distances. In the case of ITS-2, the distances were < 0.01. For CytB, the distances ranged from 0.02 to 0.2 (data not shown).
With the results obtained in Modeltest, a phylogenetic tree was constructed using the program MrBayes (Figure 4). Triatoma infestans was used as the outgroup.8 In the tree obtained with the sequences of CytB, all of the samples analyzed were grouped within the T. phyllosoma clade. Inside this clade, it was possible to distinguish two principal groups. The first group included the majority of individuals analyzed and the T. phyllosoma sequences from the GenBank. The second group included the sequences SMMOAX37, SMMOAX112, TEHOAX25, and TEHOAX86, and two sequences from individuals from other communities in Oaxaca State (SCOAX1, from Salina Cruz and TLAOAX1, from Tlacolulita). In contrast, the ITS-2 tree showed a single clade that included the sequences of the majority of the individuals analyzed, as well as the T. phyllosoma and T. mazzottii sequences from GenBank. Only the individual TEHOAX86 fell outside this clade, along with other species of the Phyllosoma complex (Figure 4B).
Figure 4.
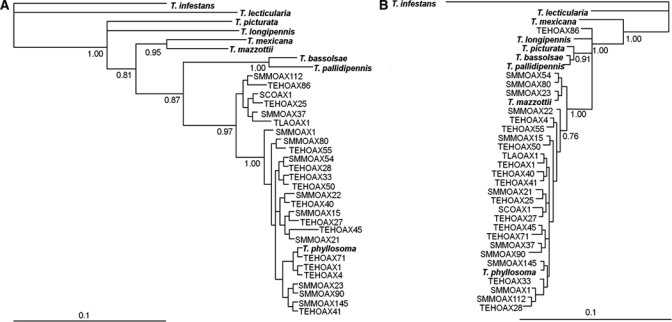
Phylogenetics trees constructed with Triatoma phyllosoma sequences using two different markers: (A) CytB and (B) ITS-2. Majority-rule phylogram resulting from probabilistic analysis under the HKY+G model. The values of the nodes indicate the values of posterior probability. Other Triatomine from the Phyllosoma Complex in bold were used for comparison, Triatoma infestans was used as an outgroup.
Discussion
The first cases of Chagas disease in Mexico were found in Oaxaca State21 and several studies have been conducted in the state since that time. A seroepidemiological survey conducted in 1971 in 60 rural communities of the Pacific side of Oaxaca State showed high rates of human T. cruzi infection, particularly in three communities: Chila, Tataltepec, and Cerro del Aire with 76%, 58%, and 51%, respectively (all these communities belong to the Costa region, Figure 1).38 Further studies in the latter community showed that the infection caused significant electrocardiographic abnormalities in seropositive persons.39 In other communities of the state 9.17% of anti-T. cruzi antibodies have been reported.40 The Istmo de Tehuantepec is one of the more extensive regions in the state of Oaxaca where Chagas disease is endemic.41 In a serological and clinical study carried out in this region on dilated cardiomyopathy patients, 81% of the analyzed samples presented anti-T. cruzi antibodies.23 In addition, three triatomine species have been reported in the area: T. dimidiata, T. mazzottii, and T. phyllosoma.22 However, the entomological characteristics of these vector populations have been only partially described. Knowledge of these characteristics is a crucial factor to implement vector control strategies.
In this study, five communities were analyzed in the Istmo de Tehuantepec. In three communities (Santa Maria Petapa, San Miguel Ecatepec, and Magdalena Tequisistlan), no insects were found. The absence of insects in peridomestic and domestic habitats in these communities could be explained for several reasons: 1) the use of pesticides for agricultural crops; 2) insects are only in nearby sylvatic areas where they can get food; 3) small population densities during collection periods. A better understanding of the role that these insects play in sylvatic areas and their relationship with the communities in this region is still necessary. In contrast, insects were collected in San Mateo del Mar and Tehuantepec City, which are close to the communities where no insects were found (~60 km away). This situation has also been found in other parts of Mexico; for example, Phyllosoma complex triatomine collected in villages in the eastern part of the country were not found in neighboring villages.42
In this study, the only species found was T. phyllosoma. Several years ago, this species was reported in several areas close to the areas analyzed in this study.22 We found nymphs and adults insects in the studied periods of the year in both communities with a higher abundance of adults and fifth-instar nymphs. There was no significant difference in the numbers of collected vectors between periods or between communities found. More studies are necessary in these communities to identify possible reproductive periods in which to apply control measures.
The habitat of this species of triatomine has been only partially studied in some areas and remains unknown in others. In this study, T. phyllosoma was found in two different habitats. In Tehuantepec City, insects were only collected outdoors, mainly associated with piles of stone and bricks and the inhabitants observed the insects indoors only rarely. Most houses were built from concrete, which does not provide a good habitat for insects; therefore, triatomines were only found in peridomestic areas. In contrast, in San Mateo del Mar, insects were collected indoors (in domestic areas) between the palm leaves of bedrooms. These insects were likely feeding on humans and hens that also slept in the bedrooms. The palm-leaf houses in San Mateo del Mar provide an excellent refuge for the insects, where they can be protected from predators and are close to feeding sources. Our data show that this species can survive successfully in domestic and peridomestic habitats. In addition, our data suggest that the improvement of traditional dwellings and the implementation of pens for domestic animals could be effective against T. phyllosoma domestic infestation in this region, particularly because chickens, cats, dogs, and other animals often live together with the human population inside the houses. The location of houses could also be important because the houses with insects were located only on the periphery of the communities. Furthermore, the growth of human populations and the invasion of wild areas have destroyed the natural habitats of many insects, prompting them to colonize the dwellings built by poor people.
In this work, high colonization indices for T. phyllosoma were observed in the two communities. This result is in contrast with the early reported domiciliary indices for the same species in Oaxaca,22 indicating that this species has been successful in colonizing this region. In general, the percentage of T. cruzi infection in Tehuantepec City was higher than that reported before for different triatomine species in Oaxaca (values ranging from 4.1% to 9.1%)43 and also for domiciliary T. phyllosoma in Oaxaca (27.2%).22
In Mexico, few feeding source studies have been carried out on triatomine. Two studies of T. longipennis showed different feeding sources in peridomestic habitats in two communities of Jalisco.19,20 However, ours is the first report on feeding sources of T. phyllosoma, a species with a restricted distribution that has only been described in Oaxaca State.2 In Tehuantepec City, where higher percentages of infection with T. cruzi were observed, dogs were the principal feeding source of triatomine. It had been reported that T. infestans that fed on humans, dogs, or guinea pigs had high rates of T. cruzi infection.16 In Tehuantepec City, dogs and cats are the principal pets and they usually remain on patios where insects can easily feed on them. The presence of infected bugs that feed on dogs and other pets represents a risk factor for transmission of the parasite to humans in this community.
According to our observations, the principal feeding source in San Mateo del Mar was hens, followed by humans. In addition, multiple feeding sources were observed. Our results are in accordance with other studies of feeding sources showing that domestic insects often feed on multiple hosts.44 For example, Panstrongylus lutzi was found to show a feeding preference for hens, rodents, humans, cats, and dogs, among others.29 Several studies of triatomine feeding sources have found that in areas where the insects feed on hens, there is a reduction in the prevalence of T. cruzi and in its clinical manifestation in humans.45,46 In addition, it has been demonstrated that birds are the only animals that do not get infected with the parasite.47,48 Human blood was the second most prevalent feeding source in San Mateo del Mar, indicating the close relationship between insects and humans and the potential for T. cruzi transmission. The diversity of feeding sources observed for T. phyllosoma shows their ability to feed on any animal that could increase their success in adapting to a certain habitat. Similar studies in other areas are necessary to assess the principal feeding sources and potential reservoirs that maintain T. cruzi infection in triatomine in Mexico.
Triatoma phyllosoma is a member of the Phyllosoma complex, this is a group of species endemic to Mexico that has recently been analyzed with several genetic markers.7,8,31,49–51 In this work, we used ITS-2 and CytB for the identification of both nymphs and adults. In our analysis, all individuals were assigned to the T. phyllosoma clade. The intraspecific variation in ITS-2 observed between the two populations (San Mateo del Mar and Tehuantepec City) was < 1%, which is in accordance with previously described levels of variation in this marker.8 In the case of CytB, the variation was < 2%. Phylogenetic trees were also constructed with both markers. For the CytB tree, all sequences analyzed fell within the T. phyllosoma clade; for the tree obtained with ITS-2, T. mazzottii was included in the T. phyllosoma clade, along with the sequences analyzed in this study. The possibility exists that this T. mazzottii individual was erroneously classified, however hybridization between sympatric species has been demonstrated recently by our group and these could be the case for the results observed with the ITS-2 analysis.49 Introgression can result from hybridization but in this case more experiments must be conducted to clarify this point. This contradictory result points out the importance of reviewing the validity of the taxonomic level of the species between the individuals of this complex; this discussion has been previously initiated.8,51 It also made clear the need for other markers that help to understand the relationship between these species. These data indicate that little variation exists among the T. phyllosoma populations from Tehuantepec City and San Mateo del Mar, which could be caused by the proximity of the communities. Another important contribution of this work was the use of molecular markers in the identification of species in the nymph state. Molecular identification of nymphs shows that T. phyllosoma is the only vector associated with the transmission of T. cruzi in these two communities. This method of nymphs identification will help to differentiate between multiple species in sympatric areas and to identify the species with epidemiological importance.15,49 Control programs for this local species can be carried out easily than in other regions where more than one species are present.
In conclusion, Mexico has a great diversity of triatomines and studies focused on understanding their biology, distribution, habitats, and relationship with human dwellings are necessary to establish vector control measures and decrease domestic transmission of T. cruzi. The results presented here will help to reach this goal.
ACKNOWLEDGMENTS
We are grateful for the facilities provided to accomplish the present work with the Municipal Governments Authorities of Magdalena Tequisistlan, San Mateo del Mar, San Miguel Ecatepec, Santa María Petapa, and Tehuantepec City. We also acknowledge Ignacio Martínez for his technical help, Saúl López Alcaide for his help in the statistics analysis, and Alejandro Martínez-Ibarra for the gift of the opossum blood. Deyanira Morales.-Pérez, Cenia Almazán-Marín and Janet García-Pillado help in the collection of some Triatomines. We also thank Isabel Perez-Montfort for help in the English review.
Footnotes
Financial support: This work was support by grant no. 229209 from PAPIIT-DGAPA Universidad Nacional Autónoma de México. GV and FMH thank to CONACYT, Mexico, for the scholarships during their graduate studies.
Authors' addresses: Guiehdani Villalobos, Patricia de la Torre, Juan Pedro Laclette, and Bertha Espinoza, Departamento de Inmunología, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, México, Distrito Federal, 3° Circuito, Ciudad Universitaria, México, D.F., E-mails: guiehda@yahoo.com.mx, pdltorre@servidor.unam.mx, laclette@servidor.unam.mx, and besgu@biomedicas.unam.mx. Fernando Martínez-Hernández, Departamento de Epidemiología Molecular, Hospital General Doctor Manuel Gea González, Calzada de Tlalpan, México, D.F., E-mail: fherxyz@yahoo.com.
Reprint requests: Bertha Espinoza, Departamento de Inmunología, Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, México, Distrito Federal, 3° Circuito, Ciudad Universitaria, P.C. 04510, México, D.F., Tel: (525) 56 22 89 43, Fax: (525) 56 22 91 98, E-mail: besgu@biomedicas.unam.mx.
References
- 1.Rodríguez Coura J, Albajar Viñas P. Chagas disease: a new worldwide challenge. Nature. 2010;465:S6–S7. doi: 10.1038/nature09221. doi:10.1038/nature09221. [DOI] [PubMed] [Google Scholar]
- 2.Zárate L, Zárate RJ. A checklist of the Triatominae (Hemiptera: Reduviidae) of Mexico. Int J Entomol. 1985;27:102–127. [Google Scholar]
- 3.Galvão C, Carcavallo R, Da Silva Rocha D, Jurberg J. A checklist of the current valid species of the subfamily Triatominae Jeannel, 1919 (Hemiptera: Reduviidae) and their geographical distribution with nomenclatural and taxonomic notes. Zootaxa. 2003;202:1–36. [Google Scholar]
- 4.Velasco-Castrejón O, Valdespino JL, Tapia-Conyer R, Salvatierra B, Guzmán-Bracho C, Magos C, Llausás A, Gutiérrez G, Sepúlveda J. Seroepidemiology Chagas disease in Mexico. Salud Publica Mex. 1992;34:186–196. [PubMed] [Google Scholar]
- 5.Guzmán-Bracho C. Epidemiology of Chagas disease in México: an update. Trends Parasitol. 2001;17:372–376. doi: 10.1016/s1471-4922(01)01952-3. [DOI] [PubMed] [Google Scholar]
- 6.Lent H, Wygodzinsky P. Revision of Triatominae (Hemiptera: Reduviidae) and their significance as vector of Chagas disease. Bull Am Mus Nat Hist. 1979;163:125–520. [Google Scholar]
- 7.Martínez F, Alejandre-Aguilar R, Hortelano-Moncada Y, Espinoza B. Molecular taxonomic study of Chagas disease vectors from the Phyllosoma, Lecticularia and Rubrofasciata complexes. Am J Trop Med Hyg. 2005;73:321–325. [PubMed] [Google Scholar]
- 8.Martínez F, Villalobos G, Cevallos AM, de la Torre P, Laclette JP, Alejandre-Aguilar R, Espinoza B. Taxonomic study of the Phyllosoma complex and other triatomine (Insecta: Hemiptera: Reduviidae) species of epidemiological importance in the transmission of Chagas disease: using ITS-2 and mtCytB sequences. Mol Phylogenet Evol. 2006;41:279–287. doi: 10.1016/j.ympev.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Flores A, Magallón-Gastélum E, Bosseno MF, Ordoñez R, Lozano Kasten F, Espinoza B, Ramsey J, Breniére SF. Isoenzime variability of five principal triatomine vector species of Chagas disease in Mexico. Infect Genet Evol. 2001;1:21–28. doi: 10.1016/s1567-1348(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 10.Martínez-Ibarra JA, Bárcenas-Ortega NM, Nogueda-Torres B, Alejandre-Aguilar R, Lino Rodríguez M, Magallón-Gastélum E, López-Martínez V, Romero-Nápoles J. Role of two Triatoma (Hemiptera: Reduviidae: Triatominae) species in the transmission of Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae) to man in the west coast of Mexico. Mem Inst Oswaldo Cruz. 2001;96:141–144. doi: 10.1590/s0074-02762001000200001. [DOI] [PubMed] [Google Scholar]
- 11.Cohen JM, Wilson ML, Cruz-Celis A, Ramsey JM. Infestation by Triatoma pallidipennis (Hemiptera: Reduviidae: Triatominae) is associated with housing characteristics in rural Mexico. J Med Entomol. 2006;43:1252–1260. doi: 10.1603/0022-2585(2006)43[1252:ibtphr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Walter A, Lozano-Kasten F, Bosseno MF, Ruvalcaba EG, Gutierrez MS, Luna CE, Baunaure F, Phélinas P, Magallón-Gastélum E, Brenière SF. Peridomicilary habitat and risk factors for Triatoma infestation in a rural community of the Mexican occident. Am J Trop Med Hyg. 2007;76:508–515. [PubMed] [Google Scholar]
- 13.Magallón-Gastélum E, Magdaleno Peñaloza NC, Katthain Duchateau G, Trujillo Contreras F, Lozano-Kasten FJ, Hernández-Gutiérrez R. Distribución de los vectores de la enfermedad de Chagas (Hemiptera: Reduviidae: Triatominae), en el estado de Jalisco, México. Distribution of Chagas disease vectors(Hemiptera: Rediviidae: Triatominae) in Jalisco, Mexico. Rev Biomed. 1998;9:151–157. [Google Scholar]
- 14.Brenière SF, Bosseno MF, Magallón-Gastelúm E, Castillo-Ruvalcaba EG, Soto-Gutiérrez M, Montaño-Luna EC, Tejeda-Basulto J, Mathieu-Daudè F, Walter A, Lozano-Kasten F. Peridomestic colonization of Triatoma longipennis (Hemiptera: Reduviidae) and Triatoma barberi (Hemiptera: Reduviidae) in a rural community with active transmission of Trypanosoma cruzi in Jalisco state, Mexico. Acta Trop. 2007;101:249–257. doi: 10.1016/j.actatropica.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Justi SA, Noireau F, Cortez MR, Monteiro FA. Infestation of peridomestic Attalea phalerata palms by Rhodnius stali, a vector of Trypanosoma cruzi in the Alto Beni, Bolivia. Trop Med Int Health. 2010;15:727–732. doi: 10.1111/j.1365-3156.2010.02527.x. [DOI] [PubMed] [Google Scholar]
- 16.Pizarro JC, Stevens L. A new method for forensic DNA analysis of the blood meal in Chagas disease vectors demonstrated using Triatoma infestans from Chuquisaca, Bolivia. PLoS ONE. 2008;3:e3585. doi: 10.1371/journal.pone.0003585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pineda V, Montalvo E, Alvarez D, Santamaría AM, Calzada JE, Saldaña A. Feeding sources and trypanosome infection index of Rhodnius pallescens in a Chagas disease endemic area of Amador County, Panama. Rev Inst Med Trop Sao Paulo. 2008;50:113–116. doi: 10.1590/s0036-46652008000200009. [DOI] [PubMed] [Google Scholar]
- 18.Sandoval CM, Duarte R, Gutíerrez R, da Silva Rocha D, Angulo VM, Esteban L, Reyes M, Jurberg J, Galvão C. Feeding sources and natural infection of Belminus herreri (Hemiptera: Reduviidae: Triatominae) from dwellings in Cesar, Colombia. Mem Inst Oswaldo Cruz. 2004;99:137–140. doi: 10.1590/s0074-02762004000200004. [DOI] [PubMed] [Google Scholar]
- 19.Brenière SF, Pietrokovsky S, Magallón-Gastelúm E, Bosseno MF, Soto MM, Ouaissi A, Lozano Kasten F, Wisnivesky Coll C. Feeding patterns of Triatoma longipennis Usinger (Hemiptera: Reduviidae) in peridomestic habitats of a rural community in Jalisco, Mexico. J Med Entomol. 2004;41:1015–1020. doi: 10.1603/0022-2585-41.6.1015. [DOI] [PubMed] [Google Scholar]
- 20.Bosseno MF, García LS, Baunaure F, Gastelúm EM, Gutierrez MS, Kasten FL, Dumontiel E, Brenière SF. Identification in triatomine vectors of feeding sources and Trypanosoma cruzi variants by heteroduplex assay and a multiplex miniexon polymerase chain reaction. Am J Trop Med Hyg. 2006;74:303–305. [PubMed] [Google Scholar]
- 21.Mazzotti L. Dos casos de enfermedad de Chagas en el estado de Oaxaca. Two case of Chagas disease in the state of Oaxaca. Gac Med Mex. 1940;70:417–420. [Google Scholar]
- 22.Ramsey JM, Ordoñez R, Cruz-Celis A, Alvear AL, Chávez V, López R, Pintor JR, Gama F, Carrillo S. Distribution of domestic triatominae and stratification of Chagas Disease transmission in Oaxaca, Mexico. Med Vet Entomol. 2000;14:19–30. doi: 10.1046/j.1365-2915.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- 23.Moreno-López R, Sánchez PL, Muñoz JL, Monteón V, Reyes LP. Chagasic cardiopathy in Tehuantepec. Preliminary report. Arch Cardiol Mex. 2001;71:43–49. [PubMed] [Google Scholar]
- 24.Oliveira FA. Uso de nuevas herramientas para el control de triatóminos en diferentes situaciones entomológicas en el con-tinente americano. New tools for controling triatomines in different entomological situations in American continet. Rev Soc Bras Med Trop. 1997;30:41–46. doi: 10.1590/s0037-86821997000100008. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization . Control of Chagas Disease. Second report of the WHO Expert Committee; Geneva: 2002. [Google Scholar]
- 26.Villagrán ME, Marín C, Hurtado A, Sánchez-Moreno M, de Diego JA. Natural infection and distribution of triatomines (Hemiptera: Reduviidae) in the state of Querétaro, Mexico. Trans R Soc Trop Med Hyg. 2008;102:833–838. doi: 10.1016/j.trstmh.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Molina MC, Cattán P, Canals M, Cruzat L, Aguillón JC, Ferreira A. A simple immunometric assay to assess the feeding habits of Meprai spinolai, a Trypanosoma cruzi vector. Parasitol Res. 2004;92:375–379. doi: 10.1007/s00436-003-1011-6. [DOI] [PubMed] [Google Scholar]
- 28.Farfán AE, Gutiérrez R, Angulo VM. ELISA for the identification of food patterns of Triatominae in Colombia. Rev Salud Publica (Bogota) 2007;9:602–608. doi: 10.1590/s0124-00642007000400013. [DOI] [PubMed] [Google Scholar]
- 29.Caranha L, Seixas Lorosa E, da Silva Rocha D, Jurberg J, Galvão C. Feeding sources evaluation of Panstrongylus lutzi (Neiva & Pinto, 1923) (Hemiptera: Reduviidae: Triatominae) in the state of Ceará. Rev Soc Bras Med Trop. 2006;39:347–351. doi: 10.1590/s0037-86822006000400006. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Second edition. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Marcilla A, Bargues MD, Ramsey J, Magallon-Gastelum E, Salazar-Schettino PM, Abad-Franch F, Dujardin JP, Schofield CJ, Mas-Coma S. The ITS-2 of the nuclear rADN as a molecular marker for populations, species, and phylogenetic relationships in Triatominae (Hemiptera: Reduviidae), vectors of Chagas disease. Mol Phylogenet Evol. 2001;18:136–142. doi: 10.1006/mpev.2000.0864. [DOI] [PubMed] [Google Scholar]
- 32.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity and progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posada D, Crandall KA. Modeltest: testing the model of ADN substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa M, Iida Y, Yano T, Takaiwa F, Iwabuchi M. Phylogenetic relationships among eukaryotic kingdoms inferred from ribosomal RNA sequences. J Mol Evol. 1985;22:32–38. doi: 10.1007/BF02105802. [DOI] [PubMed] [Google Scholar]
- 35.Ronquis F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;9:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 36.Page RD. An application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 37.Kumar S, Tamura K, Nei M. MEGA 3: integrated software for Molecular Evolutionary Genetics Analysis and sequences alignment. Brief Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- 38.Goldsmith RS, Kagan IG, Zarate R, Reyes-González MA, Cedeño-Ferreira J. Epidemiologic studies of Chagas disease in Oaxaca, Mexico. Bull Pan Am Health Organ. 1978;12:236–250. [PubMed] [Google Scholar]
- 39.Goldsmith RS, Zárate RJ, Zárate LG, Kagan IG, Jacobson LB. Clinical and epidemiological studies of Chagas disease in rural communities in Oaxaca State, Mexico, and a seven-year follow-up: I. Cerro del Aire. Bull Pan Am Health Organ. 1985;19:120–138. [PubMed] [Google Scholar]
- 40.Salazar-Schettino PM, Tay J, Ruíz-Hernández AL, De Haro I, Bucio MI, Jímenez J, García-Yáñez Y, Gutiérrez M. Seropositividad a Trypanosoma cruzi en cuatro grupos de población del estado de Oaxaca. Trypanosoma cruzi seropositivity in four populations groups of the State of Oaxaca. Salud Publica Mex. 1984;26:589–595. [PubMed] [Google Scholar]
- 41.Mazzotti L. Triatomideos de México y su infección natural por Trypanosoma cruzi Chagas. Triatomines of Mexico and its natural infections by Trypanosoma cruzi. Rev Med (Mex) 1940;20:95–109. [Google Scholar]
- 42.Magallón-Gastélum E, Lozano Kasten F, Soto Gutiérrez M, Flores Pérez A, Sánchez B, Espinoza B, Bosseno MF, Brenière SF. Epidemiological risk for Trypanosoma cruzi transmission by species of Phyllosoma complex in the occidental part of Mexico. Acta Trop. 2006;97:331–338. doi: 10.1016/j.actatropica.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Vidal-Acosta V, Ibáñez-Bernal S, Martínez-Campos C. Natural infections of Triatominae bugs associated with Trypanosoma cruzi in human dwellings in México. Salud Publica Mex. 2000;42:496–503. [PubMed] [Google Scholar]
- 44.Sasaki H, Rosales R, Tabaru Y. Host feeding profiles of Rhodnius prolixus and Triatoma dimidiata in Guatemala (Hemiptera: Reduviidae: Triatominae) Med Entomol Zool. 2003;54:283–289. [Google Scholar]
- 45.Cecere MC, Gürtler RE, Canale D, Chuit R, Cohen JE. The role of the peridomiciliary area in the elimination of Triatoma infestans from rural Argentine communities. Rev Panam Salud Publica. 1997;1:273–279. doi: 10.1590/s1020-49891997000400003. [DOI] [PubMed] [Google Scholar]
- 46.Calderón-Arguedas O, Chinchilla M, García F, Vargas M. Biologic variation of Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae) associated with ingestion of different types of blood by the vector Triatoma dimidiata (Hemiptera: Reduviidae) Parasitol Latinoam. 2003;58:3–10. [Google Scholar]
- 47.DaMatta R, Seabra S, Manhães L, de Souza W. Nitric oxide is not involved in the killing of Trypanosoma cruzi by chicken macrophages. Parasitol Res. 2000;86:239–243. doi: 10.1007/s004360050037. [DOI] [PubMed] [Google Scholar]
- 48.Teixeira A, Nascimento R, Sturm N. Evolution and pathology in Chagas disease: a review. Mem Inst Oswaldo Cruz. 2006;101:463–491. doi: 10.1590/s0074-02762006000500001. [DOI] [PubMed] [Google Scholar]
- 49.Martínez-Hernández F, Martínez-Ibarra JA, Villalobos G, De la Torre P, Laclette JP, Alejandré-Aguilar R, Catalá S, Espinoza B. Natural crossbreeding between sympatric species of the Phyllosoma complex (Insecta: Hemiptera: Reduviidae) indicate the existence of one species with morphologic and genetic variations. Am J Trop Med Hyg. 2010;82:74–82. doi: 10.4269/ajtmh.2010.09-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfeiler E, Bitler BG, Ramsey JM, Palacios-Cardiel C, Markow TA. Genetic variation, population structure, and phylogenetic relationships of Triatoma rubida and Triatoma recurva (Hemiptera: Reduviidae: Triatominae) from the Sonoran Desert, insect vectors of the Chagas disease parasite Trypanosoma cruzi. Mol Phylogenet Evol. 2006;41:209–221. doi: 10.1016/j.ympev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Marcilla A, Bargues MD, Abad-Franch F, Panzera F, Carcavallo RU, Noireau F, Galvão C, Jurberg J, Miles MA, Dujardin JP, Mas-Coma S. Nuclear rADN ITS-2 sequences reveal polyphyly of Panstrongylus species (Hemiptera: Reduviidae: Triatominae), vectors of Trypanosoma cruzi. Infect Genet Evol. 2002;1:225–235. doi: 10.1016/s1567-1348(02)00029-1. [DOI] [PubMed] [Google Scholar]


