Abstract
The presence of a single EGF receptor in Drosophila is contrasted by multiple ligands activating it. This work explores the role of two ligands, Spitz and Vein, in the embryonic ventral ectoderm. Spitz is a potent ligand, whereas Vein is an intrinsically weak activating ligand. We show that secreted Spitz emanating from the midline, triggers expression of vein in the ventral-most cell rows, by inducing expression of the ETS domain transcription factor Pointed P1. In the absence of Vein, lateral cell fates are not induced when Spitz levels are compromised. The positive feedback loop of Vein generates a robust mechanism for patterning the ventral ectoderm.
Keywords: EGF receptor, Spitz, Vein, embryonic patterning, Drosophila
The epidermal growth factor (EGF) receptor pathway in Drosophila (DER/EGFR) emerges as a highly pleiotropic signaling cascade, involved in many aspects of development (Perrimon and Perkins 1997; Schweitzer and Shilo 1997). This pathway utilizes a conserved signaling module in the different scenarios in which it functions. A very tight regulation of the pathway in time and space is essential. Whereas the receptor itself and the components downstream to it (e.g., the Ras/MAP kinase cascade) are expressed during development ubiquitously, intricate regulatory mechanisms have been uncovered at the level of the ligands.
The presence of a single EGF receptor is contrasted by the multiplicity of activating ligands, which provide elaborate regulation of the pathway. One of the ligands, Gürken, is utilized only during oogenesis. It encodes a TGFα-like protein with a single EGF domain, a signal peptide, and a transmembrane domain. Gürken localization is tightly regulated. This is achieved primarily by localization of the transcript to the vicinity of the oocyte nucleus (Neuman-Silberberg and Schüpbach 1993). Gürken protein localization follows that of the mRNA (Neuman-Silberberg and Schüpbach 1996).
A ligand that is used more broadly is Spitz. It too is similar in structure to TGFα. In contrast to Gürken, the Spitz precursor is expressed broadly (Rutledge et al. 1992). However, in its transmembrane form Spitz is inactive. Only the secreted, cleaved form of Spitz is active as an EGF receptor ligand (Schweitzer et al. 1995b; Freeman 1994). Processing of Spitz appears to be regulated by Rhomboid (Rho) and Star, two novel proteins containing multiple or a single transmembrane domains, respectively (Bier et al. 1990; Kolodkin et al. 1994). This is suggested by similarity of phenotypes, epistatic relationships and the nonautonomous activity of Rho and Star (Mayer and Nüsslein-Volhard 1988; Schweitzer et al. 1995b; Golembo et al. 1996a; Sapir et al. 1998).
Finally, Vein represents another ligand of the EGF receptor. It is produced as a secreted ligand containing a single EGF module and an immunoglobulin domain. The presence of an immunoglobulin domain makes Vein more similar to Neuregulin, another vertebrate ligand of the EGF receptor family (Schnepp et al. 1996). In contrast to Spitz, which must be processed to an active ligand, Vein is constitutively active. However, the activity of Vein is intrinsically weaker than that of Spitz. This is reflected by its reduced capacity to induce activated MAP kinase in cells and embryos, and the limited ability to induce ectopic expression of DER target genes (Schnepp et al. 1998; Yarnitzky et al. 1998).
Activation of the EGF receptor in the embryo appears to represent the sum of activations induced by Spitz and Vein. Thus, only double mutants for spitz and vein give rise to a cuticle phenotype that resembles that of mutations in DER (Schnepp et al. 1996). Vein is expressed in a highly dynamic pattern in the embryo and larval imaginal discs (Schnepp et al. 1996; Simcox et al. 1996; Yarnitzky et al. 1997). Analysis of vein mutant phenotypes reveals two different modes of activity. In one case, Vein functions independently in tissues where no activity of Spitz is required. Accordingly, the vein mutant phenotype in these tissues is severe and comparable to that of loss of function of the receptor. For example, proliferation of cells in the wing imaginal disc is driven by Vein-induced DER activation. In vein mutants reduced proliferation is observed in these discs (Simcox et al. 1996; Simcox 1997). In the embryo, the migrating muscle fibers approach the ectodermal muscle attachment (EMA) cells, produce Vein, and activate DER on the EMA cells. This activation is essential for the induction of specific genes in the EMA cells (e.g., β1 tubulin). In vein mutant embryos muscle fibers do not associate properly with the EMA cells (Yarnitzky et al. 1997). Mechanisms for ligand concentration in the vicinity of the EMA cells may compensate for the weak activity of Vein (Strumpf and Volk 1998).
In another set of tissues, Vein functions in parallel to Spitz, such that the combined activity of both ligands gives rise to proper activation of the receptor. Activation of the DER pathway in the neuroectoderm prior to gastrulation or immediately following it, is responsible for proper patterning of medial neuroblasts. Only simultaneous elimination of Spitz and Vein results in a phenotype that is comparable to that of receptor loss (Skeath 1998). In the induction of dorsal muscle cells of the embryo, an interplay between the two ligands was also observed (Yarnitzky et al. 1998).
This work provides another paradigm for the utilization of Spitz and Vein. In the embryonic ventral ectoderm following gastrulation, a group of ∼8 cells on each side of the ventral midline are designated as neuroectodermal cells. Within this region, different rows of cells assume distinct fates, through activation of the EGF receptor pathway (Raz and Shilo 1993; Schweitzer et al. 1995b). The capacity of the pathway to induce graded fates stems from the fact that the activating signal, secreted Spitz, emanates from a single row of cells positioned at the ventral midline, the midline glial cells (Golembo et al. 1996a). Diffusion of the ligand to neighboring cells generates graded activation of the pathway, as can be visualized by the distribution of activated MAP kinase (Gabay et al. 1997).
The ectodermal cells respond differently to the varying levels of EGF receptor activation. In the ventral-most cells, high levels of DER induce pointed f1 transcription. A central target gene for Pointed P1 is argos, encoding a secreted protein containing an EGF repeat, which functions as an antagonist of the EGF receptor pathway (Freeman et al. 1992; Schweitzer et al. 1995a). Thus, prominent activation of the EGF receptor leads to the production of Argos, which diffuses to neighboring cells and reduces the level of signaling in these cells, maintaining the EGF receptor activation gradient (Golembo et al. 1996b; Gabay et al. 1997).
This work describes a paradigm for successive utilization of two ligands triggering the EGF receptor. Activation of DER is capable of providing not only a negative feedback loop through Argos, but also a positive one. We show that in the context of the embryonic ventral ectoderm, Spitz and Vein act sequentially. vein expression is triggered by Spitz-induced DER activation. Diffusion of Vein to lateral cells induces intermediate levels of DER activation. This assures continued receptor activation in lateral cells in situations in which Spitz levels emanating from the midline may be compromised.
Results and Discussion
vein expression in the ventral ectoderm
The embryonic expression pattern of vein is extremely dynamic. We will refer here only to the patterns that pertain to the embryonic ventral ectoderm. Prior to gastrulation, vein is expressed in the future neuroectoderm. This early phase of expression appears to cooperate with Spitz in the induction of medial neuroblast cell fates (Skeath 1998). Following gastrulation at stage 9, vein expression in the neuroectoderm becomes confined to three to four cell rows on each side of the midline. This pattern gradually restricts, such that by stage 11 only one cell row on each side of the midline expresses vein (Fig. 1A, B).
Figure 1.
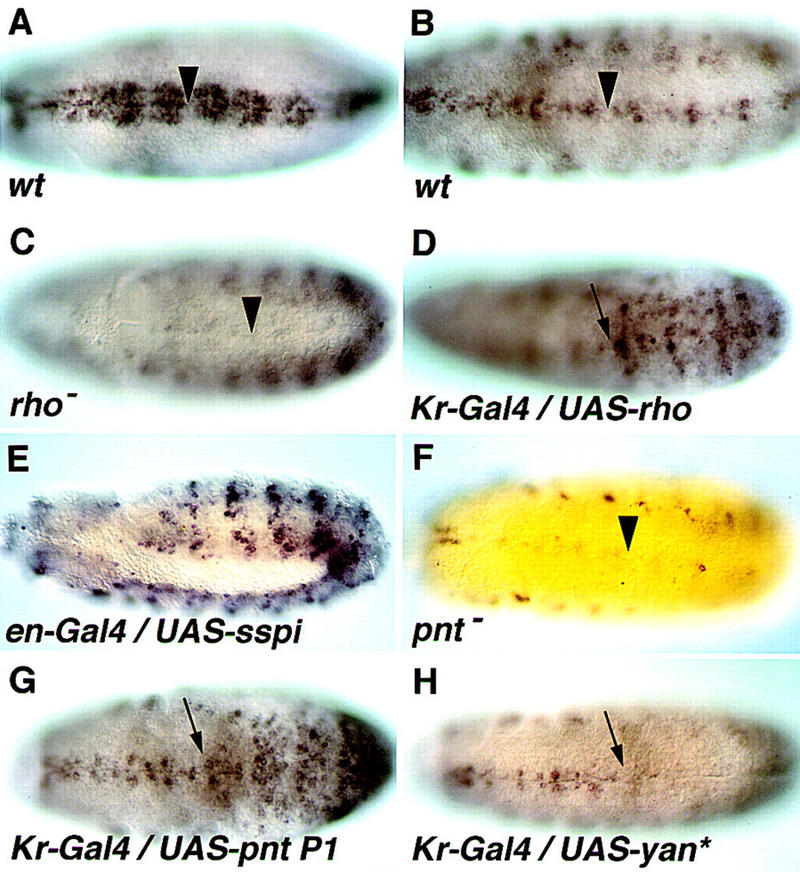
Expression of vein in the ventral ectoderm is induced by DER through Pointed P1. (A) In wild-type embryos at stage 9, vein is expressed in three to four cell rows on each side of the ventral midline. (B) By stage 11, expression of vein is confined to one cell row. (C) In rhoΔ38 mutant embryos, expression of vein in the ventral ectoderm is eliminated at stage 10/11, whereas all other aspects of vein expression are normal. (D) Induction of rho expression by Kr–Gal4 gives rise to ectopic expression of vein in the same domain. (E) Induction of secreted Spitz expression by en–Gal4 gives rise to ectopic expression of vein in a stage 11 embryo. (F) Expression of vein in the ventral ectoderm is eliminated in a pointedΔ88 stage 11 mutant embryo. (G) Induction of Pointed P1 expression by Kr–Gal4 gives rise to ectopic vein expression in the same domain. (H) Induction of activated Yan expression by Kr–Gal4 eliminates vein expression in the same domain. Arrowheads mark the ventral midline; arrows show anterior border of Kr domain in the T1/T2 segment boundary.
vein expression is induced by Spitz, through Pointed P1
Expression of vein was observed adjacent to the ventral midline, in positions in which activation of the DER pathway by secreted Spitz, emanating from the midline, is maximal. This raised the possibility that vein expression may be triggered by Spitz. In rho mutant embryos in which active Spitz molecules are not produced, no expression of vein was observed at stage 11, demonstrating that Rho/Spitz are indeed necessary for vein expression in the ventral ectoderm (Fig. 1C).
To test if DER activation is sufficient to induce vein expression in the embryo, the pathway was ectopically activated at two different stages. Rho was ectopically expressed at stage 9 in the central domain of the embryo by Kr–Gal4, and gave rise to ectopic vein expression in the same region (Fig. 1D). At stage 11 secreted Spitz was expressed in the engrailed domains, resulting in the induction of vein expression in the same pattern (Fig. 1E). These experiments demonstrate that high levels of DER activation are sufficient for inducing vein expression.
Induction of gene expression by DER in the ventral-most rows of cells was demonstrated previously for the argos, otd, and tartan genes (Gabay et al. 1996). Induction was obtained through inactivation of Yan, an ETS domain transcriptional repressor, and induction of Pointed P1, an ETS domain transcriptional activator. To test if induction of vein by DER is also mediated by Pointed P1, we examined vein expression in pointed mutant embryos. Traces of expression were observed in the midline in stage 10 embryos only, whereas no expression was displayed by the ventral-most and lateral ectodermal cell rows at stage 11 (Fig. 1F). All other aspects of vein expression were normal.
Elimination of Pointed activity can also be obtained in the following manner: An activated form of Yan, in which the inactivating MAP kinase phosphorylation sites have been mutated, has been shown previously to block the activity of Pointed by competing for the same DNA-binding sites (Rebay and Rubin 1995). Indeed, when activated Yan was expressed in the Kr domain, the endogenous expression of vein was abolished in that region (Fig. 1H). To examine if Pointed P1 is sufficient for induction, vein expression was examined in embryos in which Pointed P1 was expressed in the Kr domain. Indeed, expression of vein in the same domain was observed (Fig. 1G). These results demonstrate that under conditions of ectopic expression, Pointed P1 is necessary and sufficient for vein expression.
For other target genes of Pointed P1, elimination of Yan gave rise to an expanded expression pattern (Gabay et al. 1996). This is likely to be caused by the early broad expression pattern of Pointed P1 (that is DER-independent), which is capable of inducing target genes in the absence of the repressive effects of Yan. It is interesting to note that no expansion of vein expression was observed in yan mutant embryos. This suggests that under normal conditions, Pointed P1 may not be sufficient to induce vein expression, as it may cooperate with other factors that have a more restricted distribution or activity.
The role of Vein in the ventral ectoderm
Two different nested cell fates are induced by the DER pathway in the ventral ectoderm, depending upon the distance of the cells from the midline. The cell rows closest to the midline assume the ventral-most fate, as reflected by the expression of target genes such as otd and argos. Intermediate levels of DER activation induce lateral cell fates, reflected by the expression of FasIII that is observed in five rows of cells on each side of the midline. Both ventral-most and lateral markers are eliminated in mutants for DER, as well as in mutants that abolish Spitz or its processing (spitz, rho, or Star). Conversely, ectopic secreted Spitz or Rho are capable of expanding expression of both lateral and ventral-most fates (Golembo et al. 1996a).
To examine the role of Vein in the ventral ectoderm, we tested vein null mutants for the expression of marker genes. No defects in the expression of otd or FasIII were observed (data not shown). These results indicate that at this level of resolution, the function of Vein is redundant. This is also consistent with normal levels of activated MAP kinase that were observed at stage 9 in vein mutant embryos (Skeath 1998).
We wanted to test if a role for Vein may be revealed under conditions in which the level of Spitz is compromised. Flies that are heterozygous for a null allele of Spitz are viable. Normal patterning of the ventral ectoderm takes place in heterozygous spitz embryos, as monitored by the expression pattern of FasIII (Fig. 2A). We now generated embryos that are homozygous for the vein null allele and carry only one functional copy of spitz. In these embryos, patterning of the ventral-most cells is normal, as reflected by the expression of otd (data not shown) or FasIII. However, more lateral cells fail to express FasIII (Fig. 2B). These results indicate that when Spitz levels are compromised in heterozygous embryos, the cell row closest to the midline undergoes normal patterning. However, the levels of Spitz may be too low to pattern the more lateral cells. Under these conditions, induction of Vein appears to be critical to facilitate DER activation in these cells.
Figure 2.
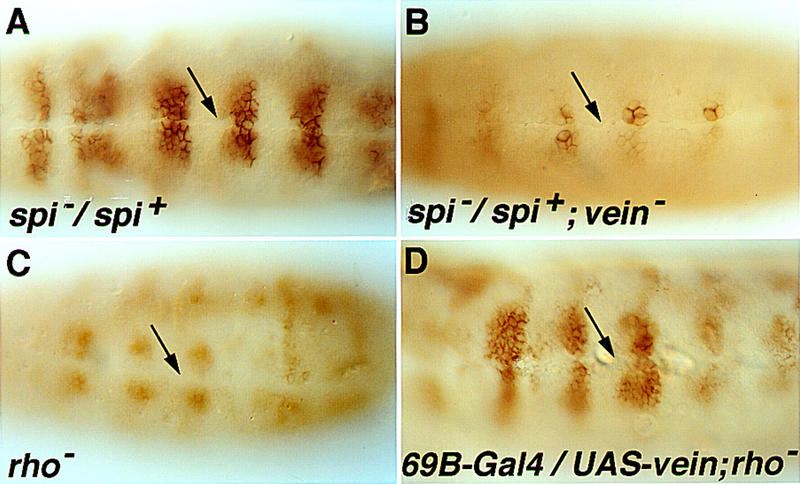
Contribution of Vein to patterning the ventral ectoderm. (A) In embryos heterozygous for the spitzOE92 null mutation, expression of FasIII is identical to the wild-type pattern, namely in four to five cell rows on each side of the midline. (B) In embryos heterozygous for spitz, which are also homozygous for a null heteroallelic combination of vein, expression of FasIII in the cell row adjacent to the midline is normal, but is not observed in more lateral rows. (C) In rhoΔ38 mutant embryos expression of FasIII in the ventral ectoderm is eliminated, and only some expression in the neuroblast layer is retained. (D) Induction of ubiquitous Vein expression in rho mutant embryos by 69B–Gal4 restores the normal expression pattern of FasIII. Arrows mark the ventral midline. Note: In vein homozygous mutant embryos, FasIII expression is normal, as in spitz heterozygous embryos, even when embryos are raised at conditions which may be stressful, such as 29°C.
Upon ectopic expression of Vein, the ventral-most markers are not induced at all or only intermittently, thus reflecting the reduced inherent activity of Vein (Schnepp et al. 1998). To test directly the biological activity of Vein in a controlled setting, it is necessary to eliminate endogenous signaling by Spitz, as well as the presence of Argos. This can be achieved in embryos that are mutated for rho, and thus exhibit no expression of ventral-most markers (such as argos) or lateral markers (Golembo et al. 1996a) (Fig. 2C). Ectopic expression of Vein in rho mutant embryos was capable of restoring FasIII expression (Fig. 2D), but did not induce otd expression (data not shown). Thus, Vein is capable of inducing the lateral cell fates in the absence of secreted Spitz.
Orchestrated induction of EGF receptor ligands in the ventral ectoderm
Patterning of the ventral ectoderm by the EGF receptor pathway relies on a highly coordinated set of events in space and time. The process is initiated when the midline cells begin to express Spitz, Rho, and Star, giving rise to a restricted source of secreted Spitz (Golembo et al. 1996a). Diffusion of Spitz to neighboring ectodermal cells triggers the cascade of DER induction (Gabay et al. 1996). The Spitz gradient should be maintained until the time when the ventral-most cells begin to express and secrete Argos. Upon production of Argos the overall level of DER activation is reduced significantly, so that only higher levels of activating ligands are capable of overcoming Argos inhibition (Golembo et al. 1996b).
The ectodermal cells adjacent to the midline encounter maximal levels of Spitz, and Argos induction assures that maximal signaling will not expand further. But what mechanisms guarantee that sufficient levels of activation are encountered by the lateral cells? This seems to be the task of Vein. Under conditions in which Spitz levels are compromised (e.g., in spitz heterozygous embryos), the availability of Vein is critical for inducing the lateral cell fates.
Vein is constitutively secreted, and is capable of reaching the lateral cells. Because it is a weaker ligand, it can not induce the ventral-most fates but only lateral fates, and thus the level of Vein need not be as carefully regulated as that of secreted Spitz. Immediately after gastrulation, signaling by Vein in the ventral ectoderm may stem from a residual DER-independent expression of Vein. Subsequently, induction of vein expression by Spitz prolongs the capacity to activate the DER pathway.
Two distinct mechanisms have been proposed for patterning by morphogens (Lawrence and Struhl 1996). In one case, a gradient of a single morphogen gives rise to distinct cell fates caused by the induction of different levels of signaling of the same pathway. The complementary scenario involves a relay mechanism: An initial induction by the primary morphogen induces the production of a relay factor triggering another signaling pathway, to pattern the more distant cells. This work describes a combination of the two models. Only the EGF receptor cascade patterns the ventral ectoderm. However, the primary signal, Spitz, induces a relay mechanism by triggering expression of Vein, another ligand of DER. Again, it is important to emphasize that although the restricted spatial distribution of secreted Spitz is critical for correct patterning, Vein and Argos distribution may be more uniform. Argos reduces the overall level of EGF receptor signaling, whereas Vein provides a lower level of activation, capable of inducing only the lateral cell fates. The parallel induction of positive and negative signals by Spitz is schematically presented in Figure 3. vein expression is also induced by the EGF-receptor pathway in follicle cells within the dorsal–anterior corner of the egg chamber (Wasserman and Freeman 1998). It thus appears that Vein may provide a positive feedback loop in several tissues that are patterened by EGF receptor activity.
Figure 3.
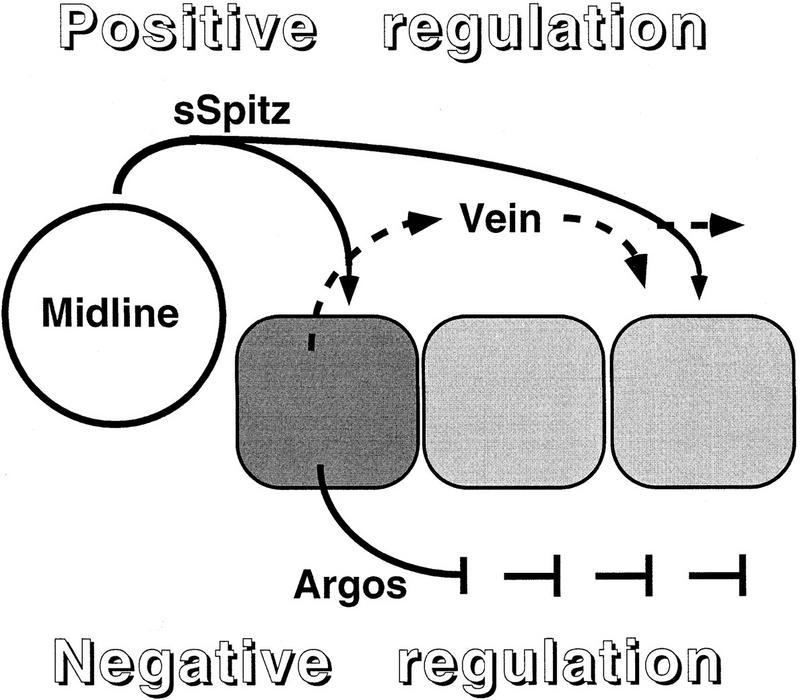
Orchestrated induction of DER ligands in the ventral ectoderm. In the embryonic ventral ectoderm, activation of the DER pathway is triggered by simultaneous expression of the Spitz precursor, Rho and Star in the midline cells, giving rise to a localized source of secreted Spitz. The cells adjacent to the midline encounter maximal levels of secreted Spitz. In these cells, induction of argos expression through Pointed P1 provides a negative-feedback loop, restricting the expansion of ventral-most fates (dark gray). Parallel induction of vein expression through Pointed P1, assures the induction of lateral cell fates (light gray), even under conditions in which the levels of secreted Spitz from the midline may be compromised.
Another facet of the activity of Vein that should be considered is its sensitivity to Argos inhibition. If the lateral cells have not encountered sufficient levels of Spitz prior to the induction of Argos, their capacity to be activated in the presence of Argos is severely compromised, and relies on the continued availability of secreted Spitz from the midline. The induction of Vein in the ventral-most cells, which takes place in parallel to the induction of Argos, may help to overcome this problem. Vein is capable of activating DER in the presence of Argos: In embryos heterozygous for spitz, the activity of Vein induces the lateral fates. This takes place in a situation in which argos is expressed in the ventral-most cells. Vein itself is not capable of inducing expression of argos (Yarnitzky et al. 1998).
In conclusion, this work has revealed a powerful regulatory network, orchestrated by the sequential utilization of Spitz and Vein, two ligands of the EGF receptor with different properties. Induction of Vein takes place once the ventral-most cell fates have been determined already by high Spitz levels. Vein expression prolongs the time window of activation of the DER pathway to ensure that the lateral cell fates will be specified correctly. Vein is suited for this task, as it is a less potent ligand than Spitz, capable of inducing only the lateral cell fates. Vein can activate DER even in the presence of Argos, thus balancing the parallel negative-feedback loop of Argos.
Materials and methods
Probes and antibodies
Probes for RNA in situ hybridization were prepared from a full-length 3.4-kb vein cDNA, or an otd 3.8 kb cDNA fragment (provided by R. Finkelstein). Either random primed DIG-labeled DNA probes or antisense RNA probes (Boehringer Mannheim) were used. FasIII was detected by a mouse monoclonal antibody, using the Vectastain HRP Elite kit.
Fly strains
The following mutant fly strains were used: veindddL6 (Simcox et al. 1996), Df(3L)XAS96 uncovering vein (provided by W.A. Johnson), spiOE92, rhoΔ38, pntΔ88. For ectopic expression the Gal4 drivers Kr (provided by M. Leptin), 69B (recombined with rhoΔ38), or en (provided by A. Brand) were used. The following UAS lines were used: UAS–vein 110, UAS–rho 11-1, UAS–secreted spitz 4b, UAS–pntP1 1.0 (provided by C. Klämbt), UAS–yan activated (provided by I. Rebay). To examine the capacity of ectopic vein to rescue rho mutant embryos, the strains UAS–vein, rhoΔ38/Sb, ftz–lacZ, and 69B–Gal4, rhoΔ38/Sb, ftz–lacZ were used. Homozygous mutant rho embryos were identified by absence of LacZ staining.
Acknowledgments
We thank A. Brand, R. Finkelstein, W. Johnson, C. Klämbt, M. Leptin, I. Rebay, and A. Simcox for fly strains. This work was supported by grants from the German–Israeli Foundation and the U.S.-Israel Binational Science Foundation to B.-Z.S., and by a grant from the Israel Science Foundation to T.V.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked ‘advertisement’ in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL lvshilo@weizmann.weizmann.ac.il; FAX 972-8-9344108.
References
- Bier E, Jan LY, Jan YN. rhomboid, a gene required for dorsoventral axis establishment and peripheral nervous system development in Drosophila melanogaster. Genes & Dev. 1990;4:190–203. doi: 10.1101/gad.4.2.190. [DOI] [PubMed] [Google Scholar]
- Freeman M. The spitz gene is required for photoreceptor determination in the Drosophila eye where it interacts with the EGF receptor. Mech Dev. 1994;48:25–33. doi: 10.1016/0925-4773(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Freeman M, Klämbt C, Goodman CS, Rubin GM. The argos gene encodes a diffusible factor that regulates cell fate decisions in the Drosophila eye. Cell. 1992;69:963–975. doi: 10.1016/0092-8674(92)90615-j. [DOI] [PubMed] [Google Scholar]
- Gabay L, Scholz H, Golembo M, Klaes A, Shilo B-Z, Klämbt C. EGF rceptor signaling induces pointedP1 transcription and incativates Yan protein in the Drosophila embryonic ventral ectoderm. Development. 1996;22:3355–3362. doi: 10.1242/dev.122.11.3355. [DOI] [PubMed] [Google Scholar]
- Gabay L, Seger R, Shilo B-Z. In situ activation pattern of Drosophila EGF receptor pathway during development. Science. 1997;277:1103–1106. doi: 10.1126/science.277.5329.1103. [DOI] [PubMed] [Google Scholar]
- Golembo M, Raz E, Shilo B-Z. The Drosophila embryonic midline is the site of Spitz processing, and induces activation of the EGF receptor in the ventral ectoderm. Development. 1996a;122:3363–3370. doi: 10.1242/dev.122.11.3363. [DOI] [PubMed] [Google Scholar]
- Golembo M, Schweitzer R, Freeman M, Shilo B-Z. Argos transcription is induced by the Drosophila EGF receptor pathway, to form an inhibitory feedback loop. Development. 1996b;122:223–230. doi: 10.1242/dev.122.1.223. [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, Pickup AT, Lin DM, Goodman CS, Banerjee U. Characterization of Star and its interactions with sevenless and EGF receptor during photoreceptor cell development in Drosophila. Development. 1994;120:1731–1745. doi: 10.1242/dev.120.7.1731. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G. Morphogenes, compartments, and pattern: Lessons from Drosophila? Cell. 1996;85:951–961. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- Mayer U, Nüsslein-Volhard C. A group of genes required for pattern formation in the ventral ectoderm of the Drosophila embryo. Genes & Dev. 1988;2:1496–1511. doi: 10.1101/gad.2.11.1496. [DOI] [PubMed] [Google Scholar]
- Neuman-Silberberg FS, Schüpbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF α-like protein. Cell. 1993;75:165–174. [PubMed] [Google Scholar]
- ————— The Drosophila TGFα-like protein Gurken: Expression and cellular localization during Drosophila oogenesis. Mech Dev. 1996;59:105–113. doi: 10.1016/0925-4773(96)00567-9. [DOI] [PubMed] [Google Scholar]
- Perrimon N, Perkins L. There must be 50 ways to rule the signal: The case of the Drosophila EGF receptor. Cell. 1997;89:13–16. doi: 10.1016/s0092-8674(00)80177-4. [DOI] [PubMed] [Google Scholar]
- Raz E, Shilo B-Z. Establishment of ventral cell fates in the Drosophila embryonic ectoderm requires DER, the EGF receptor homolog. Genes & Dev. 1993;7:1937–1948. doi: 10.1101/gad.7.10.1937. [DOI] [PubMed] [Google Scholar]
- Rebay I, Rubin GM. Yan functions as a general inhibitor of differentiation and is negatively regulated by activation of the Ras1/MAPK pathway. Cell. 1995;81:857–866. doi: 10.1016/0092-8674(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Rutledge BJ, Zhang K, Bier E, Jan YN, Perrimon N. The Drosophila spitz gene encodes a putative EGF-like growth factor involved in dorsal-ventral axis formation and neurogenesis. Genes & Dev. 1992;6:1503–1517. doi: 10.1101/gad.6.8.1503. [DOI] [PubMed] [Google Scholar]
- Sapir A, Schweitzer R, Shilo B-Z. Sequential activation of the EGF receptor pathway during Drosophila oogenesis establishes the dorsoventral axis. Development. 1998;125:191–200. doi: 10.1242/dev.125.2.191. [DOI] [PubMed] [Google Scholar]
- Schnepp B, Grumbling G, Donaldson T, Simcox A. Vein is a novel component in the Drosophila epidermal growth factor receptor pathway with similairy to the neuregulins. Genes & Dev. 1996;10:2302–2313. doi: 10.1101/gad.10.18.2302. [DOI] [PubMed] [Google Scholar]
- Schnepp B, Donaldson T, Grumbling G, Ostrowski S, Schweitzer R, Shilo B-Z, Simcox A. EGF domain swap converts a Drosophila EGF receptor activator into an inhibitor. Genes & Dev. 1998;12:908–913. doi: 10.1101/gad.12.7.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer R, Howes R, Smith R, Shilo B-Z, Freeman M. Inhibition of Drosophila EGF receptor activation by the secreted protein Argos. Nature. 1995a;376:699–702. doi: 10.1038/376699a0. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Shaharabany M, Seger R, Shilo B-Z. Secreted Spitz triggers the DER signaling pathway and is a limiting component in embryonic ventral ectoderm determination. Genes & Dev. 1995b;9:1518–1529. doi: 10.1101/gad.9.12.1518. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Shilo B-Z. A thousand and one roles for the Drosophila EGF receptor. Trends Genet. 1997;13:191–196. doi: 10.1016/s0168-9525(97)01091-3. [DOI] [PubMed] [Google Scholar]
- Simcox A. Differential requirement for EGF-like ligands in Drosophila wing development. Mech Dev. 1997;62:41–50. doi: 10.1016/s0925-4773(96)00643-0. [DOI] [PubMed] [Google Scholar]
- Simcox A, Grumbling G, Schnepp B, Bennington-Mathias C, Hersperger E, Shearn A. Molecular, phenotypic, and expression analysis of vein, a gene required for growth of the Drosophila wing disc. Dev Biol. 1996;177:475–489. doi: 10.1006/dbio.1996.0179. [DOI] [PubMed] [Google Scholar]
- Skeath JB. The Drosophila EGF receptor controls the formation and specification of neuroblasts along hte dorso-ventral axis of the Drosophila embryo. Development. 1998;125:3301–3312. doi: 10.1242/dev.125.17.3301. [DOI] [PubMed] [Google Scholar]
- Strumpf D, Volls T. Kakapo, a novel cytoskeletal-associated protein is essential for the restricted localization of the neuregulin-like factor, Vein, at the muscle-tendon junction site. J Cell Biol. 1998;143:1259–1270. doi: 10.1083/jcb.143.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman JD, Freeman M. An autoregulatory cascade of EGF receptor signaling patterns the Drosophila egg. Cell. 1998;95:355–364. doi: 10.1016/s0092-8674(00)81767-5. [DOI] [PubMed] [Google Scholar]
- Yarnitzky T, Min L, Volk T. The Drosophila neuregulin homolog Vein mediates inductive interactions between myotubes and their epidermal attachment cells. Genes & Dev. 1997;11:2691–2700. doi: 10.1101/gad.11.20.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— An interplay between two EGF-receptor ligands, Vein and Spitz, is required for the formation of a subset of muscle precursors in Drosophila. Mech Dev. 1998;79:73–82. doi: 10.1016/s0925-4773(98)00175-0. [DOI] [PubMed] [Google Scholar]


