Abstract
Some protozoan infections such as Toxoplasma, Cryptosporidium, and Plasmodium can be transmitted through stem cell transplantations. To our knowledge, so far, there is no study about transmission of Leishmania parasites in stem cell transplantation and interactions between parasites and stem cells in vitro. Therefore, the aim of this study was to investigate the interaction between different species of Leishmania parasites and adipose tissue-derived mesenchymal stem cells (ADMSCs). ADMSCs have been isolated, cultured, characterized, and infected with different species of Leishmania parasites (L. donovani, L. major, L. tropica, and L. infantum). Infectivity was examined by Giemsa staining, microculture, and polymerase chain reaction methods. As a result, infectivity of ADMSCs by Leishmania parasites has been determined for the first time in this study. According to our findings, it is very important that donors are screened for Leishmania parasites before stem cell transplantations in regions where leishmaniasis is endemic.
Introduction
Although parasitic diseases affect millions of people worldwide, only 5% of more than 340 known infectious diseases have been reported to be detected during transplantation.1 The fact that several protozoa (Toxoplasma, Cryptosporidium, and Plasmodium types) could also create a risk factor in stem cells transplantations has been reported in several studies.2–4 The most important reason that these protozoa could be risk factors is the relapse of the latent infection in the recipient.5 Therefore, the detection of these protozoan diseases in donors and recipients within endemic regions before transplantation is crucial. However, in latent infections, it is not always possible to diagnose the existence of a parasite causing the disease with known diagnostic procedures.6
Leishmaniasis is one of the most important protozoan diseases that cause latent infections.7 In one of our previous studies, it was shown that Leishmania infection could be transmitted during pediatric organ transplantations.8 However, there is no report that has been published on the existence of Leishmania parasites in stem cell transplantations. Distinct from other infections, the lack of clinical evidence related to Leishmania infections in stem cell transplantations could be because of the facts that leishmaniasis is difficult to diagnose and that it sometimes leads to a misdiagnosis.9 Also, leishmaniasis is often neglected or underestimated from the clinical point of view.10
After infecting the host, Leishmania promastigotes are engulfed with receptor-dependent phagocytosis by skin macrophages. Phagocytosed promastigotes turn into amastigote forms, proliferate, and are released by bursting the host cell. These amastigotes again infect other macrophages and settle in reticuloendothelial system organs such as the spleen, liver, and bone marrow.11
It has been reported that parasites infect not only professional phagocytic cells such as macrophages but also numerous other cell types such as amniotic epithelial cells, human epithelial cells, and fibroblast cells.12
Stem cells have the ability of self-renewal and differentiate into a variety of other cell types in a given tissue.13 Bone marrow-derived mesenchymal stem cells have become a major stem cell source in both research and therapeutic studies for years. Mesenchymal stem cells from other sources such as umbilical cord and adipose tissue also gained interest because of several advantages over bone marrow-derived counterparts.13 Especially the adipose tissue, mainly because of the ease of the procedure, low morbidity, and increased aspirated volume, has become one of the primary mesenchymal stem cell sources. Recent reports also show that adipose tissue-derived mesenchymal stem cells (ADMSCs), when combined with autologous fat or various biomaterials, can be a novel and effective therapeutic source in stem cell-based therapies.14 Although increased numbers of reports have been published on the effectiveness of ADMSCs in animal models and several phase III clinical trials, similar disease transmission risks can also be applicable in stem cell transplantations using adipose tissue and its cellular derivatives.15
Although the phagocytic property of ADMSCs has been reported in one study,16 to our knowledge, there have not been any studies that report the interaction between ADMSCs and Leishmania parasites. For this reason, in this study, the interaction between ADMSCs and the different types of Leishmania parasites have been investigated in vitro. The infectivity status of mesenchymal stem cells by Leishmania parasites has also been tested with different methods.
Materials and Methods
Parasite culture.
L. tropica (MHOM/TR/99/EP39),17 L. donovani (HOM/IN/83/AG83), L. major (HOM/IL/81/Friedlin), and L. infantum (MCAN/TR/2005/EP126) promastigotes were kindly provided by K. P. Chang (Chicago Medical School, Chicago, IL). Promastigotes were cultured in Roswell Park Memorial Institute-1640 (RPMI-1640) medium with l-glutamine (Sigma Chemical Co., St. Louis, MO), 10% fetal calf serum (FCS) (Sigma Chemical Co.), and gentamicin (80 μg/mL) (Sigma Chemical Co.) at 27°C. Cultures were passaged after 4 days incubation.18 The growth of promastigotes was monitored daily using an inverted microscope (Olympus CKX 41).
Parasite counting.
The parasites were counted using a hemocytometer under standard light microscopy with 200× magnification. Before the parasite count, the parasites were fixed with formalin 2% (v/v) in phosphate-buffered saline (PBS) solution.18
Isolation and cultivation of ADMSCs.
ADMSCs were isolated by enzymatic digestion of adipose tissue after lipoaspiration. Human lipoaspirate samples and corresponding written consent forms were obtained by three donors undergoing cosmetic surgery procedures. This study was approved by the Institutional Review Board (IRB) committee of Yildiz Technical University Bioengineering Department. After transferring the lipoaspirate samples to the laboratory, tissue pieces were washed three to four times with PBS and suspended in Dulbecco-modified Eagle medium (DMEM) (Mediatech Inc., Manassas, VA) supplemented with Glutamax, antibiotics, and 2 mg/mL collagenase type 2 (Sigma Chemical Co.) pre-warmed to 37°C. The tissue was placed in a water bath at 37°C for 60 minutes, placed in hemolysis buffer for 5 minutes, and centrifuged at 1,200 rpm for 10 minutes. The pellet was harvested into DMEM supplemented with Glutamax, antibiotics (100×), and 10% FBS and incubated in the condition of 5% CO2 humidity at 37°C. This initial passage of the primary cell culture was referred to as passage 0 (P0).19
Differentiation and phenotypic characterization of ADMSCs.
For adipogenic differentiation, confluent cultures of primary ADMSCs were induced to undergo adipogenesis by replacing the stromal media with adipocyte induction medium composed of 43.2 mL DMEM, 5 mL FBS, 0.5 mL dexamethasone (Sigma-Aldich, St. Louis, MO), 0.05 mL insulin (Sigma), 2 mL indomethacin (Sigma), 0.05 mL isobutylmethylxanthine (IBMX) (Sigma-Aldich), 0.5 mL penicillin/streptomycin (Sigma), and 0.5 mL l-glutamine (Sigma). Cells were maintained in culture for 2 weeks, and media was replaced every 3 days. After incubation, cultures were washed with PBS three times, fixed in a 10% solution of formaldehyde in PBS for at least 1 hour, washed with 60% isopropanol, stained with Oil red O solution (in 60% isopropanol) for 10 minutes followed by repeated washing with water, and destained in 100% isopropanol for 15 minutes.20
Osteogenic and chondrogenic differentiations have been performed according to previously published protocols.19,21
Phenotypic characterization has been performed by flow cytometric analysis using ADMSC-specific cell surface markers. First, cells were cultured in control medium before analysis. Cells were labeled with the following antihuman antibodies: CD34-PE, CD73-PE, CD90-FITC, and CD105-PE (Becton Dickinson, San Jose, CA). More than 10,000 labeled cells were acquired and analyzed using a CellLab Quanta (Beckman Coulter).22
Infection of ADMSC with Leishmania promastigotes.
ADMSCs (25,000/mL) were harvested in a 12-well plate that contains a coverslip in every well. Leishmania promastigotes, in stationary phases, were washed two times with PBS by centrifugation at 1,500 rpm for 5 minutes; 2.5 × 105 parasites/mL were added into a 12-well plate containing ADMSCs and incubated in 5% CO2 humidity at 37°C. After 4 hours, ADMSCs were washed with PBS to move the unattached parasites away. After washing, the culture was incubated in DMEM with 10% FBS and incubated in 5% CO2 humidity at 37°C.
Giemsa staining.
The coverslips were taken out from the wells for Giemsa staining after 24 hours, 7 days, 14 days, 21 days, and 28 days. They were washed two times with PBS and fixed in methyl alcohol for 3–5 minutes. After fixation, the coverslips were washed with tap water. The coverslips were stained in Giemsa dye for 25–30 minutes. Finally, the coverslips were washed with tap water and dried at room temperature. Dried coverslips were observed under a light microscope with 1,000× magnification using by immersion oil.23 Amastigotes were counted for grading their density according to the method of Chulay and Bryceson.24
Microcapillary culture method.
ADMSC culture was trypsinized with 1.5 mL 10× trypsin and incubated in 5% CO2 humidity at 37°C for 10 minutes; 5 mL DMEM with 10% FCS were added on to the unattached cells and centrifuged (1,500 rpm for 5 minutes). Supernatant was removed, and the pellet was resuspended; 50- to 60-μL cell suspensions were injected into the microcapillary tubes with 1-mL injectors. The terminal ends were sealed. Microcapillary tubes were incubated at 27°C atmospheric conditions. After 4 days, stem cell–parasite culture was observed under inverted microscope (Olympus CK2) with 200× magnification. Microcapillary culture was prepared every 7 days from ADMSCs culture infected by Leishmania parasites.25–27 Detected motile promastigotes were counted and evaluated according to the method of Chulay and Bryceson24 with slight modifications: Grade 0 = 0 parasites/all fields; Grade 1+ = 1–5 parasites/all fields; Grade 2+ = 6–10 parasites/all fields; Grade 3+ = 11–25 parasites/all fields; Grade 4+ = 26–50 parasites/all fields; Grade 5+ = 51–100 parasites/all fields; Grade 6+ > 100 parasites/all fields.
DNA extraction.
DNA extraction was performed using the phenol-chloroform-isoamyl alcohol method. Cells being infected with Leishmania parasites were cultured for 30 days and cryopreserved. Before DNA extraction, cells were removed from liquid nitrogen and washed with 3 mL PBS; 100 μL lysis buffer (100 mM Tris·HCl, pH 8.0, 5 mM ethylenediaminetetraacetic acid [EDTA], 0.5% sodium dodecyl sulfate [SDS]) and 100 μg/mL proteinase K (Invitrogen Life Technologies, Paisley, UK) were added into the sample and incubated at 56°C for overnight. After incubation, 800 μL phenol-chloroform-isoamyl alcohol (25:24:1) were added, and the mixture was gently vortexed for 30 seconds. The mixture was centrifuged at 12,000 rpm at 4°C for 10 minutes, and the supernatant was collected. Two volumes of chloroform were added to one volume of supernatant, and they were mixed up and down and kept at 4°C for 30 minutes. The mixture was centrifuged at 12,000 rpm at 4°C for 15 minutes. After this centrifugation, supernatant was transferred to a new collection tube and treated with 1 mL ice-cold ethanol. The mixture was gently mixed (5–10 times) and kept at −20°C overnight. The sample was centrifuged at 13,000 rpm for 10 minutes, and the pellet was washed with cold 70% ethanol and then dissolved in 20 μL Tris EDTA (TE) buffer 10/0.1 (10 mM Tris·HCl, 0.1 mM EDTA, pH 8.0).28
Polymerase chain reaction.
Polymerase chain reaction (PCR) was performed for the amplification of the Leishmania-specific gene using DNA extracted from ADMSCs that were previously infected with this parasite.
PCR was set up in a final volume of 25 μL with the 2× Master Mix (Fermentas, St. Leon-Rot, Germany); 100 pM for each primer was used for the detection and identification of Leishmania parasites (primers Fme/Rme: 5¢-TATTGGTATGCGAAACTTCCG-3¢ and 5¢-ACAGAAACTGATACTTATATAGCG-3¢, respectively). This primer pair amplifies a 427-bp DNA fragment specific for L. major, a 389-bp DNA fragment specific for L. donovani, a 403-bp DNA fragment specific for L. tropica, and a 434-bp DNA fragment specific for L. infantum parasites. The reaction mixture consisted of 1 μL template DNA. Forty-five cycles were performed in a thermocycler (Techne). Each cycle consisted of 94°C initial denaturation for 5 minutes, 94°C denaturation (1 minute), 54°C annealing (1 minute), 72°C elongation (1.5 minutes), and 72°C post-elongation (7 minutes). In all assays, positive controls containing Leishmania promastigotes DNA from in vitro culture and a negative control without DNA were included; 10 μL reaction mixture were visualized by 2% agarose gel electrophoresis.29
Results
Primary culture of human ADMSCs.
After the enzymatic digestion, cells isolated from the lipoaspirate sample adhered to the tissue culture flask and proliferated. Nonadherent cells, such as red blood cells, were removed by replacing the medium. The initial adherent cells grew into spindle- or satellite-shaped cells, which then developed into visible colonies 2–3 days after the initial plating. The cells began to proliferate quickly and were passaged by trypsinization two times per week. After the second passage, ADMSCs appeared to have a fibroblast-like shape similar to bone marrow stromal cells (Figure 1).
Figure 1.
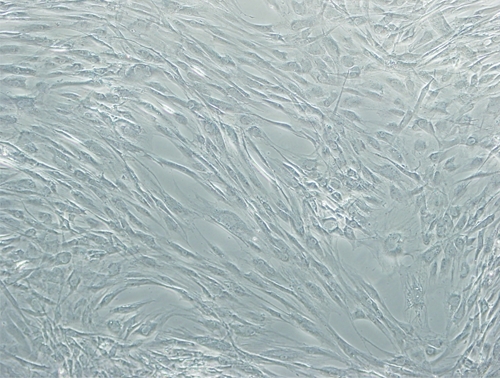
Human ADMSCs isolated from human lipoaspirate tissue and cryopreserved from passage 3 (P3). Original magnification was 40×.
Phenotypic characterization of ADMSCs.
The immunophenotype of undifferentiated human ADMSCs at passage 3 has been examined by flow cytometry. Similarly, we characterized the ADMSC population according to its Cluster of Differentiation (CD) marker profile using flow cytometry. ADMSCs were found to express CD73 (94.2 ± 4.12%), CD90 (95.97 ± 3.67%), and CD105 (70.02 ± 5.58%), but they did not express the expected hematopoietic lineage markers such as CD34 (1.06 ± 0.32%).
Differentiation of ADMSCs in vitro.
For adipogenic differentiation, ADMSCs were cultured in control medium, and then, culture media was replaced by adipogenic medium for 14 days. Cells in adipogenic medium acquired expanded cell morphology, and a time-dependent increase in intracellular lipid vacuoles was observed (Figure 2). These lipid vacuoles became visible after 4 days of adipogenic induction, and the cell size and number of lipid vacuoles increased incessantly afterwards. Osteogenic and chondrogenic differentiation potentials of ADMSCs were detected by Alizarin red S and Alcian blue staining, respectively (data not shown).
Figure 2.
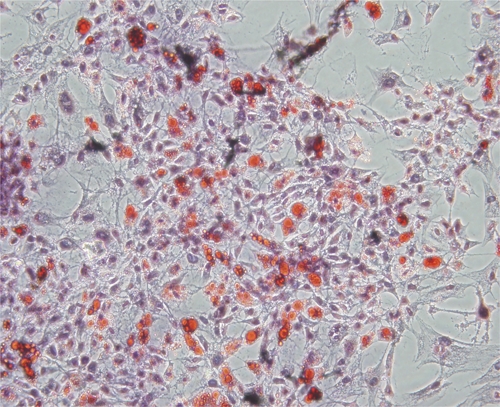
Adipogenic differentiation of ADMSCs in vitro. ADMSCs at P4 were stained for oil red O. Original magnification was 40×.
Detection of intracellular amastigotes by Giemsa method.
After the ADMSC cultures were infected by four different Leishmania species, detection of amastigotes was performed by Giemsa method. According to the results, amastigotes of the four different Leishmania species could be detected microscopically only after 24 hours (Figure 3). The presence of amastigotes in infected ADMSCs cultures could not be detected on days 7, 14, 21, and 28 of the experiment. Moreover, the density of the amastigotes after 24 hours was, in fact, very low, and no parasites could be detected during long-term culture (Table 1).
Figure 3.
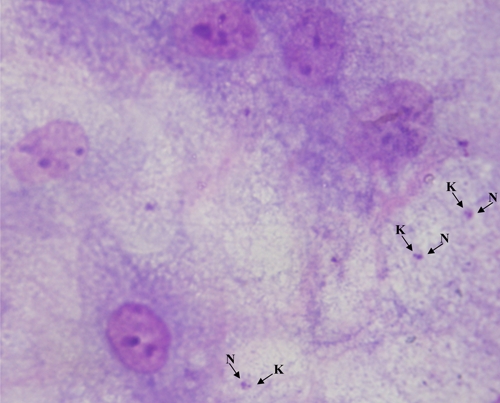
Infected ADMSCs with L. infantum promastigotes at day 1. K = kinetoplast; N = nucleus.
Table 1.
Average density of different species of Leishmania amastigotes in smears and promastigotes in microculture
| Day | Grading of Leishmania amastigotes in smears* | Grading of Leishmania promastigotes in MCM | ||||||
|---|---|---|---|---|---|---|---|---|
| L. major | L. tropica | L. donovani | L. infantum | L. major | L. tropica | L. donovani | L. infantum | |
| 1st | 1+ | 1+ | 1+ | 1+ | 3+ | 2+ | 3+ | 3+ |
| 7th | 0 | 0 | 0 | 0 | 1+ | 1+ | 1+ | 1+ |
| 14th | 0 | 0 | 0 | 0 | 1+ | 2+ | 2+ | 1+ |
| 21st | 0 | 0 | 0 | 0 | 2+ | 2+ | 2+ | 2+ |
| 28th | 0 | 0 | 0 | 0 | 1+ | 1+ | 2+ | 2+ |
Detection of extracellular promastigotes by microculture method.
The presence of parasites in infected ADMSC culture was also investigated by microculture method (MCM) on days 1, 7, 21, and 28 of the experiment. MCM is based on the facts that intracellular amastigotes can turn into extracellular promastigotes in microaerophylic environments and that these motile promastigotes can be detected under inverted microscope. In our experiment, promatigotes were still detected microscopically by MCM 28 days after infection. Infectivity results of ADMSC with different species of Leishmania parasites are shown in Table 1. According to the results, motile promastigotes were observed at all time points. Likewise, the number of promastigotes has been shown to change with respect to the time points in all Leishmania species (Table 1).
Detection of intracellular amastigotes by PCR.
The presence of parasite-specific DNA amplification in infected ADMSCs was observed at all time points and is shown in Figures 4 and 5.
Figure 4.
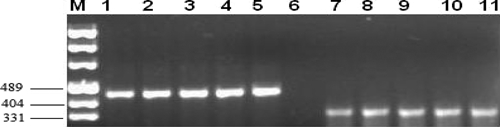
PCR products of infected ADMSC isolates. M = pUC mix marker 8. Lanes 1–5, L. major (lane 1, 7th day; lane 2, 14th day; lane 3, 21st day; lane 4, 28th day; lane 5, positive control). Lane 6, negative control. Lanes 7–11, L. donovani (lane 7, 7th day; lane 8, 14th day; lane 9, 21st day; lane 10, 28th day; lane 11, positive control).
Figure 5.
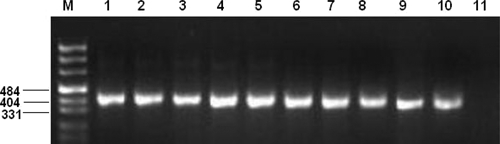
PCR products of infected ADMSC isolates. M = pUC mix marker 8. Lanes 1–5, L. infantum (lane 1, 7th day; lane 2, 14th day; lane 3, 21st day; lane 4, 28th day; lane 5, positive control). Lanes 6–10, L. tropica (lane 6, 7th day; lane 7, 14th day; lane 8, 21st day; lane 9, 28th day; lane 10, positive control). Lane 11, negative control.
Discussion
The presence of leishmaniasis, being one of the important protozoan diseases, has been reported in organ transplantations such as kidneys, pancreas, liver, lungs, and heart tissue.1,8 Although information on other protozoan infections is available, there are no reports where Leishmania infections were investigated in stem cell transplantations. In this study, the interaction between different types of Leishmania parasites and ADMSCs has been investigated in vitro. To our knowledge, this study is the first that documents such a relation between stem cells and leishmaniasis.
Leishmania parasites can survive in different tissues and organs for decades, even after treatment. In several studies, it has been found that intracellular parasites can persist and keep their viability, especially in fibroblast cells, as latent inactive forms.30 Similarly, in our study, Leishmania parasites stayed viable as inactive forms in ADMSCs in vitro (Figure 3). These results can indicate that, after the treatment, parasites can stay inactive not only in fibroblasts but also in mesenchymal stem cells. Likewise, stem cells in an organism usually stay dormant unless an exogenic stimulus (growth factor expression, physical trauma, etc.) is present. This unique property of stem cells could also imply that these cells could be perfect host systems for Leishmania parasites. Therefore, especially for the stem cell transplantations to be performed within endemic regions, performing a screening that is specific for Leishmania parasites is an important issue. It is simply because of the fact that reactivation of Leishmania parasites after immunosuppressive treatment in organ transplantations has been reported in several studies.1,8
Although several screening methodologies can be performed to detect Leishmania parasites in stem cell transplantations, one of the most important problems is the diagnosis of the leishmaniasis disease. For the diseases that cause latent infections such as Leishmania, the diagnosis become very difficult.9,31 In our study, although we have used several diagnosis approaches such as PCR, Giemsa staining, and MCM, latent infection in ADMSC culture environment could only be detected by microculture approach and PCR. In microscopic Giemsa staining, parasites were only detected after 24 hours of the experiment, albeit in very low density. In our previous studies, it has been shown that MCM is more sensitive than the other classical culture approaches in the diagnosis of visceral and cutaneous leishmaniasis.25–27
It is already known that Leishmania parasites have developed a survival mechanism in which they stay viable in phagolysosome after infecting macrophages by receptor-based phagocytosis.11 In the literature, it has been shown that parasites infect not only professional phagocytotic cells such as macrophages but also amnion epithelial cells, fibroblasts, kidney cells, and dendritic cells in mammalian hosts.11,12 Furthermore, it is documented that parasites enter these cells by binding different cell surface receptors. Although there are many studies that investigate cellular and molecular characteristics of mesenchymal stem cells, the information about the phagocytic properties of these cells is scarce. One study reported that ADMSCs show properties similar to macrophages in wounds.16 In another study, it was shown that pre-adipocytes can develop macrophage-like phagocytic characteristics.32 In our study, although the data are preliminary and it only confers the in vitro results, it has been shown, for the first time, that visceral and cutaneous leishmaniasis-causing parasite types can infect ADMSCs. Additional clinical studies on ADMSCs from infected patients are required to indicate the actual pathogenesis and transmission mechanisms of the parasite. However, these results are important, and infectivity of mesenchymal stem cells by Leishmania parasites can, in fact, be considered as a very important in vitro experimental model system for the analysis of interaction between stem cells and other infectious agents.
In summary, we document, for the first time, the infectivity of ADMSCs by Leishmania parasites in in vitro settings. Our results strongly indicate that screening for Leishmania parasites before stem cell transplantations in endemic regions can be very important and deserves additional clinical investigation.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the Scientific and Technological Research Council of Turkey (TUBITAK) for financial support.
Footnotes
Authors' addresses: Adil M. Allahverdiyev, Melahat Bagirova, Rabia Cakir Koc, Serap Yesilkir Baydar, Necati Findikli, and Olga N. Oztel, Department of Bioengineering, Yildiz Technical University, Istanbul, Turkey, E-mails: adilmoglu@gmail.com, dr.melahatb@gmail.com, rabiacakir@gmail.com, serapoloji@gmail.com, necatif@gmail.com, and onoztel@gmail.com. Serhat Elcicek, Department of Bioengineering, Firat University, Elazig, Turkey, E-mail: serhatelcicek@gmail.com.
References
- 1.Barsoum RS. Parasitic infections in organ transplantation. Exp Clin Transplant. 2004;2:258–267. [PubMed] [Google Scholar]
- 2.Nachbaur D, Kropshofer G, Feichtinger H, Allerberger F, Niederwieser D. Cryptosporidiosis after CD34-selected autologous peripheral blood stem cell transplantation (PBSCT). Treatment with paromomycin, azithromycin and recombinant human interleukin-2. Bone Marrow Transplant. 1997;19:1261–1263. doi: 10.1038/sj.bmt.1700826. [DOI] [PubMed] [Google Scholar]
- 3.Raina V, Sharma A, Gujral S, Kumar R. Plasmodium vivax causing pancytopenia after allogeneic blood stem cell transplantation in CML. Bone Marrow Transplant. 1998;22:205–206. doi: 10.1038/sj.bmt.1701299. [DOI] [PubMed] [Google Scholar]
- 4.Straathof CS, Kortbeek LM, Roerdink H, Sillevis Smitt PA, van den Bent MJ. A solitary spinal cord toxoplasma lesion after peripheral stem-cell transplantation. J Neurol. 2001;248:814–815. doi: 10.1007/s004150170101. [DOI] [PubMed] [Google Scholar]
- 5.Munoz P, Valerio M, Puga D, Bouza E. Parasitic infections in solid organ transplant recipients. Infect Dis Clin North Am. 2010;24:461–495. doi: 10.1016/j.idc.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Sra KK, Sracic J, Tyring SK. Treatment of protozoan infections. Dermatol Ther. 2004;17:513–516. doi: 10.1111/j.1396-0296.2004.04054.x. [DOI] [PubMed] [Google Scholar]
- 7.Boelaert M, Aoun K, Liinev J, Goetghebeur E, Van der Stuyft P. The potential of latent class analysis in diagnostic test validation for canine Leishmania infantum infection. Epidemiol Infect. 1999;123:499–506. doi: 10.1017/s0950268899003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozcan D, Seckin D, Allahverdiyev AM, Weina PJ, Aydin H, Ozcay F, Haberal M. Liver transplant recipient with concomitant cutaneous and visceral leishmaniasis. Pediatr Transplant. 2007;11:228–232. doi: 10.1111/j.1399-3046.2006.00660.x. [DOI] [PubMed] [Google Scholar]
- 9.Garcia Menendez L, Santamaria Lopez C, Fernandez Eroles AL, Megido Lahera M, Galende del Canto J, Aguilera Sanz C. Monoclonal component in visceral leishmaniasis: a rare association that can lead to misdiagnosis. Rev Clin Esp. 1998;198:517–520. [PubMed] [Google Scholar]
- 10.Dujardin JC, Campino L, Canavate C, Dedet JP, Gradoni L, Soteriadou K, Mazeris A, Ozbel Y, Boelaert M. Spread of vector-borne diseases and neglect of leishmaniasis, Europe. Emerg Infect Dis. 2008;14:1013–1018. doi: 10.3201/eid1407.071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handman E, Bullen DV. Interaction of Leishmania with the host macrophage. Trends Parasitol. 2002;18:332–334. doi: 10.1016/s1471-4922(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 12.Rittig MG, Bogdan C. Leishmania-host-cell interaction: complexities and alternative views. Parasitol Today. 2000;16:292–297. doi: 10.1016/s0169-4758(00)01692-6. [DOI] [PubMed] [Google Scholar]
- 13.Rebelatto CK, Aguiar AM, Moretao MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, Christofis A, Amaral VF, Brofman PS, Goldenberg S, Nakao LS, Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood) 2008;233:901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- 14.Choi YS, Cha SM, Lee YY, Kwon SW, Park CJ, Kim M. Adipogenic differentiation of adipose tissue derived adult stem cells in nude mouse. Biochem Biophys Res Commun. 2006;345:631–637. doi: 10.1016/j.bbrc.2006.04.128. [DOI] [PubMed] [Google Scholar]
- 15.Shigemura N, Okumura M, Mizuno S, Imanishi Y, Nakamura T, Sawa Y. Autologous transplantation of adipose tissue-derived stromal cells ameliorates pulmonary emphysema. Am J Transplant. 2006;6:2592–2600. doi: 10.1111/j.1600-6143.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- 16.Mazo M, Planat-Benard V, Abizanda G, Pelacho B, Leobon B, Gavira JJ, Penuelas I, Cemborain A, Penicaud L, Laharrague P, Joffre C, Boisson M, Ecay M, Collantes M, Barba J, Casteilla L, Prosper F. Transplantation of adipose derived stromal cells is associated with functional improvement in a rat model of chronic myocardial infarction. Eur J Heart Fail. 2008;10:454–462. doi: 10.1016/j.ejheart.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Allahverdiyev AM, Koc RC, Ates SC, Bagirova M, Elcicek S, Oztel ON. Leishmania tropica: the effect of darkness and light on biological activities in vitro. Exp Parasitol. 2011 doi: 10.1016/j.exppara.2011.04.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Merlen T, Sereno D, Brajon N, Rostand F, Lemesre JL. Leishmania spp.: completely defined medium without serum and macromolecules (CDM/LP) for the continuous in vitro cultivation of infective promastigote forms. Am J Trop Med Hyg. 1999;60:41–50. doi: 10.4269/ajtmh.1999.60.41. [DOI] [PubMed] [Google Scholar]
- 19.Ma Y, Li Q, Zhao DQ, Wang SF, Li WZ, Min J, Gu Y. Isolation, cultivation of adipose-derived mesenchymal stem cells and its chondrogenic ability. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2007;23:463–465. [PubMed] [Google Scholar]
- 20.Ogawa R, Mizuno H, Watanabe A, Migita M, Hyakusoku H, Shimada T. Adipogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice-including relationship of sex differences. Biochem Biophys Res Commun. 2004;319:511–517. doi: 10.1016/j.bbrc.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Kroeze RJ, Knippenberg M, Helder MN. Osteogenic differentiation strategies for adipose-derived mesenchymal stem cells. Methods Mol Biol. 2011;702:233–248. doi: 10.1007/978-1-61737-960-4_17. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 23.Campos MB, De Castro Gomes CM, de Souza AA, Lainson R, Corbett CE, Silveira FT. In vitro infectivity of species of Leishmania (Viannia) responsible for American cutaneous leishmaniasis. Parasitol Res. 2008;103:771–776. doi: 10.1007/s00436-008-1039-8. [DOI] [PubMed] [Google Scholar]
- 24.Chulay JD, Bryceson AD. Quantitation of amastigotes of Leishmania donovani in smears of splenic aspirates from patients with visceral leishmaniasis. Am J Trop Med Hyg. 1983;32:475–479. doi: 10.4269/ajtmh.1983.32.475. [DOI] [PubMed] [Google Scholar]
- 25.Allahverdiyev AM, Bagirova M, Uzun S, Alabaz D, Aksaray N, Kocabas E, Koksal F. The value of a new microculture method for diagnosis of visceral leishmaniasis by using bone marrow and peripheral blood. Am J Trop Med Hyg. 2005;73:276–280. [PubMed] [Google Scholar]
- 26.Allahverdiyev AM, Uzun S, Bagirova M, Durdu M, Memisoglu HR. A sensitive new microculture method for diagnosis of cutaneous leishmaniasis. Am J Trop Med Hyg. 2004;70:294–297. [PubMed] [Google Scholar]
- 27.Serin MS, Daglioglu K, Bagirova M, Allahverdiyev A, Uzun S, Vural Z, Kayar B, Tezcan S, Yetkin M, Aslan G, Emekdas G, Koksal F. Rapid diagnosis and genotyping of Leishmania isolates from cutaneous and visceral leishmaniasis by microcapillary cultivation and polymerase chain reaction-restriction fragment length polymorphism of miniexon region. Diagn Microbiol Infect Dis. 2005;53:209–214. doi: 10.1016/j.diagmicrobio.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Marques MJ, Volpini AC, Genaro O, Mayrink W, Romanha AJ. Simple form of clinical sample preservation and Leishmania DNA extraction from human lesions for diagnosis of American cutaneous leishmaniasis via polymerase chain reaction. Am J Trop Med Hyg. 2001;65:902–906. doi: 10.4269/ajtmh.2001.65.902. [DOI] [PubMed] [Google Scholar]
- 29.Marfurt J, Nasereddin A, Niederwieser I, Jaffe CL, Beck HP, Felger I. Identification and differentiation of Leishmania species in clinical samples by PCR amplification of the miniexon sequence and subsequent restriction fragment length polymorphism analysis. J Clin Microbiol. 2003;41:3147–3153. doi: 10.1128/JCM.41.7.3147-3153.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bogdan C, Donhauser N, Doring R, Rollinghoff M, Diefenbach A, Rittig MG. Fibroblasts as host cells in latent leishmaniosis. J Exp Med. 2000;191:2121–2130. doi: 10.1084/jem.191.12.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oren R, Schnur LF, Ben Yehuda D, Mayner V, Okon E, Rachmilewitz EA. Visceral leishmaniasis: a difficult diagnosis and unusual causative agent. J Infect Dis. 1991;164:746–749. doi: 10.1093/infdis/164.4.746. [DOI] [PubMed] [Google Scholar]
- 32.Cousin B, Munoz O, Andre M, Fontanilles AM, Dani C, Cousin JL, Laharrague P, Casteilla L, Penicaud L. A role for preadipocytes as macrophage-like cells. FASEB J. 1999;13:305–312. doi: 10.1096/fasebj.13.2.305. [DOI] [PubMed] [Google Scholar]


