Abstract
Historically, native populations in the Republic of Djibouti have experienced only low and unstable malaria transmission and intermittent epidemics. In recent years, efforts at malaria control have been aggressively pursued. This study was performed to inform revised malaria prevention recommendations for military service members and international travelers to the country. Laboratory-confirmed cases of malaria documented at large medical facilities and within military and civilian health care systems in the Republic of Djibouti from 1998 to 2009 were reviewed. In recent years, fewer than 5% of febrile cases among the three largest passive surveillance systems were laboratory-confirmed as malaria, and incidence of confirmed malaria was well below 1/1,000 persons/year. As efforts in the Republic of Djibouti progress toward elimination, and in conjunction with continued efforts at surveillance, emphasizing mosquito-avoidance measures and standby emergency treatment will become reasonable recommendations for malaria prevention.
Introduction
The Republic of Djibouti is located in the Horn of Africa where the Rift Valley meets the Gulf of Aden and the Red Sea. It is a small, hot, dry, and sparsely populated country marked by rugged volcanic hypersaline geology. With irregular rainfall totaling < 130 mm annually,1 the country presents a harsh ecology traditionally inhospitable to all but the pastoralist Issa and Afar peoples. Recently, it has emerged as an important regional crossroad for trade and a growing hub for adventure tourism and international military activity.2
Historically, native populations have experienced only low and unstable malaria transmission and intermittent epidemics,3 with the majority of cases attributable to importation from neighboring countries.4 Early surveys by the French physician Bouffard in 1901 identified malaria foci along the main river valley (or wadi) near Djibouti City.3 In 1973 autochthonous malaria cases began appearing principally along routes of overland immigration of refugees from neighboring countries,4 associated with an increase in Anopheles gambiae, a vector first confirmed in 1901.1,5
As human settlement increased and irrigation expanded during the 1970s and 1980s, hypo-endemic6 chloroquine-sensitive Plasmodium falciparum spread throughout the country.3 These foci proved amenable to small-scale larvacidal campaigns.7,8 Despite these efforts, serologic evidence of continued heavy exposure was noted in populations near Djibouti City.9 From 1988 to 1989 an epidemic of P. falciparum malaria, principally attributed to the Anopheles arabiensis vector,3 affected over 3,000 inhabitants of Djibouti City and the southwestern town of As-Eyla.10 During this time, the Ethiopia-Djibouti railroad was also confirmed as a source of continued importation.10
Efforts at larval control progressively decreased throughout the 1990s.11 As the city of Djibouti grew to encompass the large Ambouli wadi, cases attributable to these foci dominated malaria epidemiology. In 1991, a record 7,338 microscopy confirmed cases of malaria were reported, declining to 4,770 cases in 1993.3 The last major outbreak, marked by the emergence of chloroquine resistance, occurred in and around Djibouti City between March and June of 1999 after a major rainfall.11
Despite autochthonous cases being confirmed throughout the country, malaria has remained hypoendemic with transmission only weakly related to rainfall and temperature.11 Genotypic review of isolates collected over the last decade suggest high parasite diversity,11,12 consistent with importation and localized microepidemics.12 Although malaria cases observed in Djibouti are overwhelmingly caused by P. falciparum,3 isolated cases of Plasmodium vivax have been identified.13 In addition to widespread chloroquine resistance,14,15 low levels of proguanil,11 pyrimethamine,11,15 and cycloguanil resistance have also been reported.15
The Republic of Djibouti hosts a large French expatriate presence, and French forces are based at various locations throughout the country. Beginning in 2003, a large United States (U.S.) military presence was also established next to Ambouli International Airport at Camp Lemonnier on the outskirts of Djibouti City. German and Spanish military forces have also been present within the Republic of Djibouti for several years, housed mainly in downtown Djibouti City. Recently, other military forces, including those of Japan, have deployed small contingents to Camp Lemonnier. Because of the intermittent epidemic nature of malaria in the Republic of Djibouti, these military populations have been considered at potential risk for malaria, and many have been subject to either mandatory seasonal or year-round malaria chemoprophylaxis, according to varying national policies and recommendations (Figure 1).16,17
Figure 1.
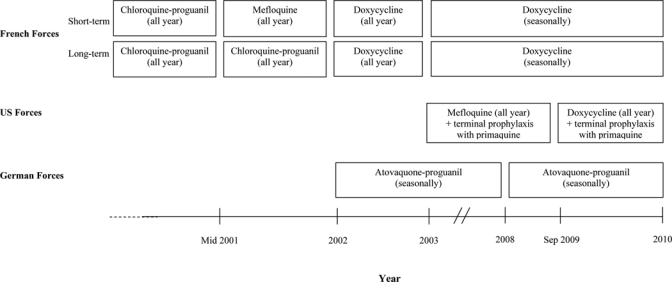
Evolution of malaria chemoprophylaxis among French, U.S., and German military personnel deployed to the Republic of Djibouti.
In this study, recent surveillance data on laboratory-confirmed cases of malaria attributable to the Republic of Djibouti among a subset of the civilian and foreign population are summarized, to inform future recommendations for malaria prevention among international visitors and military personnel.
Methods
Large medical facilities and military and civilian health care systems in the Republic of Djibouti perform passive surveillance for malaria within their patient populations. For this study, records from 1998 to 2009 of laboratory-confirmed malaria from five such sources were reviewed and summarized. Record sources included the Hôpital Général Peltier (Peltier General Hospital), the Caisse National de Sécurité Sociale (Djibouti National Healthcare Insurance Program), the Hôpital Médico-Chirurgical Bouffard (Bouffard French Military Hospital), and the French military health surveillance system. From 2003 to 2009 record sources also included the U.S. military health surveillance system. Cases of malaria reported were tabulated, and, where available, the number of laboratory tests for malaria ordered across systems. Annual malaria incidence rates were also estimated across systems using approximate beneficiary populations. Characteristics of each surveillance system and the method of laboratory confirmation used in each are described below.
Peltier General Hospital.
Peltier General hospital is the leading civilian tertiary and referral hospital within the Republic of Djibouti. From its location in downtown Djibouti City, the hospital serves a diverse civilian and refugee population of uncertain and variable size. From 2006 to 2008, more than 5,500 patients were hospitalized there each year. The number of patients seen annually at its emergency department has increased from 7,081 in 2007 to 13,506 in 2008. Microscopic blood slide examinations for malaria are ordered as clinically indicated for cases of fever, and conducted by trained laboratory staff. Records of laboratory-confirmed cases of malaria, defined as any pathological event or symptom associated with positive blood slides, are retained for surveillance purposes and reported to the Djiboutian Ministry of Health (MOH).
Djiboutian National Healthcare Insurance Program.
The Djiboutian National Healthcare Insurance Program provides free health care to all registered Djiboutian salaried employees and their families at a small network of private health clinics and hospitals throughout the country's six regions. The program's beneficiary population comprises ~73,000 people including 32,000 salaried employees, 30,000 relatives, and 11,000 retired persons. As with Peltier General Hospital, microscopic blood slide examinations for malaria are ordered as clinically indicated for cases of fever, and conducted by trained laboratory staff. Records of laboratory-confirmed cases of malaria are similarly retained for surveillance purposes and reported to the Djiboutian MOH.
Bouffard French Military Hospital.
Bouffard serves as a tertiary and referral hospital providing care primarily to French military personnel and their families as well as to civilian expatriates. The hospital also provides referral and tertiary care to Djiboutian military personnel and their families and to military personnel of other nations stationed in the Republic of Djibouti. Excluding French military personnel, the hospital's patient population comprises ~25,000 people. Bouffard performs laboratory examination for malaria as clinically indicated for cases of fever primarily using microscopic blood slide examination and the Quantitative Buffy Coat (QBC) malaria diagnosis system.18 Cases of malaria for surveillance are defined as any pathological event or symptom associated with a positive laboratory examination. Records of laboratory-confirmed cases occurring among civilians, non-French military, and French military family members are retained for surveillance purposes and reported to the Djiboutian MOH. Cases occurring among French military personnel, however, are reported as described below.
French military.
Approximately 2,900 French military personnel are stationed at various locations in the Republic of Djibouti, and all are subject to surveillance through a passive surveillance system at a network of French military clinics and at the Bouffard General Hospital. Clinics perform laboratory examination for malaria as clinically indicated for cases of fever using either a rapid antigen test, or by forwarding a blood specimen to Bouffard for microscopic blood slide examination or QBC test. Cases of malaria are defined as any pathological event or symptom associated with confirmed laboratory evidence of infection. Among French military personnel, cases of malaria are attributed to a stay in the Republic of Djibouti unless there is evidence of antecedent presence in another malaria endemic area. Cases of malaria are reported to French forces health surveillance officials, who retain records of relevant sociodemographic characteristics, referring clinic, diagnosis, and where available, Plasmodium species.
U.S. military.
Approximately 1,500 U.S. military personnel are stationed at Camp Lemonnier, and all are subject to surveillance through a passive surveillance system. Reports of cases of malaria presenting to the U.S. military health clinic at Camp Lemonnier, or presenting to other U.S. medical facilities following medical evacuation or return from deployment, are electronically reported as Reportable Medical Event (RME) case reports for inclusion in the U.S. Defense Medical Surveillance System (DMSS).19 Such case reports include information on recent countries visited and require formal clinical diagnostic criteria be met. At Camp Lemonnier, this now consists principally of results from the BinaxNOW rapid point of care test, although results of microscopic blood slide examinations, occasionally obtained at Bouffard hospital, may also be used. The DMSS also permits surveillance of diagnostic codes entered in electronic medical records during inpatient and outpatient encounters at U.S. medical facilities. Among cases identified through passive surveillance, electronic records of past deployment, which are also contained in DMSS, may then be reviewed for evidence of deployment to Djibouti before diagnosis. For this analysis, DMSS was queried for all case records of malaria with evidence of travel or deployment to Djibouti, according to previously described methods.20
Results
Numbers of laboratory tests ordered for malaria and numbers of confirmed malaria cases for the three largest Djiboutian surveillance systems are summarized in Table 1 and described below. In addition, the number of cases of malaria identified annually through the French and U.S. military health surveillance systems are displayed in Figure 2 and described below.
Table 1.
Numbers of diagnostic examinations for malaria, positive examinations, and percentage positive examinations at Peltier General Hospital, the Djiboutian National Healthcare Insurance Program, and Bouffard French Military Hospital, by year, 1998–2009
| Peltier General Hospital | Djiboutian National Healthcare Insurance Program | Bouffard French Military Hospital | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Diagnostic tests | Positive exams | % Positive | Diagnostic tests | Positive exams | % Positive | Diagnostic tests | Positive exams | % Positive | |
| 1998 | 2,108 | 144 | 6.8 | 628 | 55 | 8.8 | 2,492 | 100 | 4.0 |
| 1999 | 1,892 | 310 | 16.4 | 2,470 | 255 | 10.3 | 4,257 | 581 | 13.7 |
| 2000 | 775 | 22 | 2.8 | 5,282 | 1400 | 26.5 | 3,237 | 92 | 2.8 |
| 2001 | 1,059 | 116 | 11.0 | 1,629 | 22 | 1.4 | 2,566 | 127 | 4.9 |
| 2002 | 1,360 | 220 | 16.2 | 1,670 | 23 | 1.4 | 2,669 | 136 | 5.1 |
| 2003 | 6,071 | 620 | 10.2 | 2,121 | 106 | 5.0 | 2,857 | 103 | 3.6 |
| 2004 | 6,678 | 289 | 4.3 | 2,391 | 176 | 7.4 | 2,848 | 19 | 0.7 |
| 2005 | 3,486 | 86 | 2.5 | 1,945 | 130 | 6.7 | 1,783 | 10 | 0.6 |
| 2006 | 1,726 | 6 | 0.3 | 1,753 | 26 | 1.5 | 1,460 | 1 | 0.1 |
| 2007 | 1,691 | 39 | 2.3 | 1,129 | 11 | 1.0 | 1,003 | 6 | 0.6 |
| 2008 | 1,545 | 35 | 2.3 | 819 | 4 | 0.5 | 735 | 1 | 0.1 |
| 2009 | 331 | 3 | 0.9 | 672 | 0 | 0.0 | 805 | 18 | 2.2 |
Figure 2.
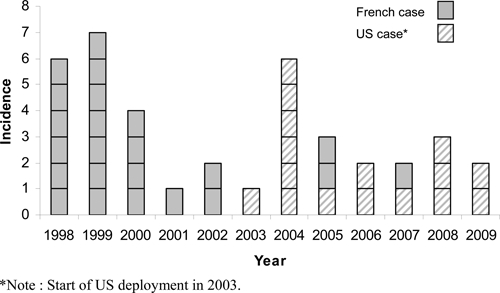
Malaria cases diagnosed among French and U.S. military personnel between 1998 and 2009 in the Republic of Djibouti.
Peltier General Hospital.
From 1998 to 2009, the number of microscopic blood slide examinations ordered annually ranged from 331 (in 2009) to 6,678 (in 2004). The number of laboratory-confirmed cases/year ranged from 620 (10.2% of all examinations, in 2003) to 3 (0.9% of all examinations, in 2009). Since 2002, there has been a monotonic decline in the percentage of microscopic blood slide examinations positive for Plasmodium. In 2009, two of the three cases were attributed to importation from Ethiopia.
Djiboutian National Healthcare Insurance Program.
From 1998 to 2009, the number of examinations ordered annually ranged from 628 (in 1998) to 5,282 (in 2000). The number of laboratory-confirmed cases annually ranged from 1,400 (26.5% of all examinations, in 2000) to 0 (0% of all 672 examinations, in 2009). Since 2004, there has been a monotonic decline in the percentage of examinations positive for Plasmodium.
Bouffard French Military Hospital.
From 1998 to 2009, the number of examinations ordered annually ranged from 735 (in 2008) to 4,257 (in 1999). The number of laboratory-confirmed cases annually ranged from 581 (in 1999) to 1 (in 2006 and 2008). From 2004 to 2008, < 1.0% of malaria examinations was positive. In 2009, an isolated increase was attributed to importation; all but two (16/18) of the cases that year occurred among Djiboutian military personnel returning from training either near the Hamar region of southwest Ethiopia or the Ivory Coast.
French military.
Since 1998, a total of 23 cases of malaria attributable to a stay in the Republic of Djibouti have been identified among French military personnel (Figure 2). The most recent case was diagnosed in 2007. All but four cases were diagnosed during the cold season. Five cases were diagnosed in France. All but one of the cases (22/23) was in males. The average age of cases was 30 years (range 21–42). By military service, cases occurred among the Army (14 cases), the Air Force (5 cases), and the Navy (4 cases). The presentation of malaria was uncomplicated in all but one case. The single case of complicated malaria, in 1998, resulted in death of a male Air Force member. Among French forces, the annual incidence of malaria has decreased from a maximum of 2.1 cases/1,000 person-years to zero currently, with an average incidence over the 12-year period of 0.7 cases/1,000 person-years.
U.S. military.
From 2003 to 2009, 16 cases of malaria were identified among personnel with service at Camp Lemonnier (Figure 2). All cases were in males. Seven cases occurred in those < 25 years of age, and an additional seven in those 25 to 39 years of age, and the remaining two cases in those aged 40 and above. A majority (9/16) of cases occurred among junior enlisted personnel, whereas the remainder of cases (7/16) occurred among senior enlisted and non-commissioned officers. All but six (10/16) cases occurred among infantry personnel. No commissioned officer contracted malaria during the period. The average annual incidence over the 7-year period was ~1.5/1,000 person-years.
Discussion
This 12-year retrospective study of malaria in the Republic of Djibouti reveals a trend of decreasing incidence of laboratory-confirmed malaria. In recent years, significantly fewer than 5% of febrile cases of among the three largest passive surveillance systems were laboratory-confirmed as malaria, and incidence of confirmed malaria based on the population at risk in civilian and military systems was well below 1/1,000/year.
Although this study represents the most complete published summary of the malaria experience in Djibouti over this period, much of our data is limited to that from a specific subset of the civilian and foreign population under standardized surveillance by passive surveillance systems. As a result of historically limited but rapidly expanding access to health services in some regions and among some peoples, particularly in rural areas of Djibouti and among refugees, this study likely underestimates the true national burden of morbidity caused by malaria. Despite this limitation, we feel that this study nonetheless reflects the changing epidemiology of this disease across the broader population, particularly in densely populated Djibouti City where over 65% of the country's 800,000 residents reside and where government-sponsored care for malaria, including at Peltier General Hospital, has generally been readily accessible to civilian and refugee populations during the period.21
Although not the primary focus of this publication, a recent nationally representative study conducted in 2008 and 2009 by Noor found an overall rate of care-seeking behavior for cases of fever among children < 5 years of age to be in excess of 75%, with over 35% in Djibouti City seeking care at government-run facilities.21 Regions outside of Djibouti City where rates of care-seeking behavior were even higher are in general sparsely inhabited, arid, poorly irrigated, and present few opportune foci for sustained malaria transmission. Reassuringly, of 6,707 individuals in a convenience sample in the outlying regions of Arta, Obock, and Tadjourah recently examined for malaria parasite infection using the rapid diagnostic test (RDT), only 42 (0.6%) were positive for P. falciparum. Individuals found positive in this study lived mostly along main roads or near large seasonal water bodies.21
Together, these findings argue that the epidemiology of malaria in Djibouti is increasingly compatible with World Health Organization (WHO) criteria for implementation of malaria elimination efforts.22 Defining aspects of effective programs in support of elimination include nationwide efforts toward case identification, elimination of onward transmission, management of disease foci, and management of imported cases.22 In recent years, the Djiboutian MOH has made solid gains toward these objectives.21 Toward case identification, in 2009, the Djiboutian MOH began delivery of RDTs to each of its district sanitary centers and established plans for a quality control reference laboratory in the parasitologic unit of the forthcoming Public Health National Laboratory. Efforts at eliminating onward transmission have included, in 2006, a move from chloroquine and sulfadoxine/pyrimethamine to artesunate plus sulfadoxine/pyrimethamine and artemether/lumefantrine as first- and second-line agents for treatment of cases at government-run centers.21 Additionally, since 2006, the MOH has distributed over 146,000 long-lasting insecticide-treated nets, first to pregnant women and children < 5 years of age, and, in recognition of low levels of population immunity, established plans to expand distribution to the remainder of the population. The MOH has also begun regular entomological surveillance at known foci and has established regional vector control teams.21 Recent surveillance by Centers for Disease Control and Prevention (CDC) light-trap has revealed only rare Anopheles species,23 providing some early evidence of the success of such control efforts.
In addition to assessing the incidence of malaria among a subset of the Djiboutian civilian population, this study examined the malaria experience of the French and U.S. militaries stationed in the country. Across both militaries, the average incidence was also well below 1/1,000/year during this period, in consonance with civilian findings. The higher incidence of malaria in the United States as compared with French forces may be explained, in part, by the duties of these personnel. Some U.S. forces, particularly civil affairs and infantry forces, while based in Djibouti, frequently serve a portion of their deployment conducting humanitarian and training activities in other malaria-endemic areas in the Horn of Africa and East Africa region, including Ethiopia, Uganda, Tanzania, and Kenya. The methodology of this study was not able to definitively identify these individuals, and as a result, the risk posed by time in Djibouti may have been overestimated.
This study did not formally assess malaria among members of other militaries stationed in Djibouti, including the Japanese and German forces, although it is believed that no cases of malaria have been diagnosed to date among these forces (Nevin R and Anders D, unpublished data). This study also did not attempt to correlate military cases of malaria with risk factors such as non-compliance with mosquito-avoidance measures. Multiple studies in the Horn of Africa region suggest cases of malaria among international military service members are predominantly associated with non-compliance with both mosquito-avoidance measures and chemoprophylaxis.24–27 Well-designed population studies have also confirmed poor compliance to chemoprophylaxis during prolonged military operations in the region.28
As Djibouti progresses toward malaria elimination, opportunities will emerge for military personnel and international travelers to the country to forgo year-round chemoprophylaxis. Although national travel guidelines specific to Djibouti currently recommend chemoprophylaxis,16,17,29,30 authorities in the United States, France, Germany, and Japan suggest that deferment of chemoprophylaxis is reasonable in settings where cases of malaria occur sporadically and the risk of malaria transmission is very low,29,31 and among certain expatriates and long-term travelers.30–32 Since 2003, official guidance has recommended only seasonal chemoprophylaxis for French forces, and German authorities recommended similar guidance for its forces in 2008. When chemoprophylaxis is deferred, strict adherence to mosquito-avoidance measures, prompt evaluation and treatment of febrile illness, and universal availability of emergency standby treatment of those distant from medical care are strict necessities. When deferred, a decision to resume chemoprophylaxis should be based on evidence from ongoing surveillance of Anopheles vector activity, and in response to focal civilian outbreaks.
Among military service members, the United States experience in Korea, where the risk of malaria has been focal and well characterized in recent years,32 has demonstrated that seasonal chemoprophylaxis targeted to only those populations at risk based on specific duty location is effective.33 Such policies have the advantage of minimizing broader exposure to the risk of adverse events and inappropriate prescribing associated with chemoprophylaxis.31,34 A growing appreciation by military policymakers of the risks associated with the widespread use of certain chemoprophylaxis regimens,34 and a growing acceptance of deferment under well-defined circumstances,35,36 appear ready to inform future discussion in this area.
ACKNOWLEDGMENTS
We thank Catherine K. Craven, for her assistance in the review of this manuscript and in directing RLN to many useful references; Christopher Rogier, for his valuable assistance and support; Marcus Beckman for his advice and personal communications and information; Thierry Coton for his critical review; numerous departments within the Djiboutian MOH involved in the malaria program; and the French, U.S., and Djiboutian medical teams whose efforts to ensure timely notification of malaria cases serve to inform our understanding of malaria in the Republic of Djibouti.
Disclaimer: This manuscript represents the opinions of the authors alone and does not necessarily reflect the opinions of the French forces, the U.S. Department of Defense, the U.S. Army, or Bayne-Jones Army Community Hospital.
Footnotes
Financial support: This study was principally supported through funds from the French forces Fight against Malaria Master Plan. The French forces reviewed this manuscript before publication and have no objection to its publication.
Authors' addresses: Lénaïck Ollivier, Direction Centrale du Service de Santé des Armées, Fort Neuf de Vincennes, Cours des Maréchaux, Paris Cedex 12, France, E-mail: lenaickollivier@hotmail.com. Remington L. Nevin, Department of Preventive Medicine, Bayne-Jones Army Community Hospital, Fort Polk, LA, E-mail: remington.nevin@us.army.mil. Houssein Y. Darar, Hôpital Général Peltier, Djibouti, Republic of Djibouti, E-mail: yhoussein@yahoo.fr. Jacques Bougère, Christophe Decam, and Alain Todesco, Institut de Recherche Biomédicale des Armées – Antenne de Marseille, Allée du Médecin Colonel Jamot, Parc du Pharo, Marseille cedex 07, France, E-mails: jacquesbougere@yahoo.fr, christophe2km@netcourrier.com, and ens@imtssa.fr. Moustapha Saleh, Caisse National de Sécurité Sociale, Djibouti, Republic of Djibouti, E-mail: djibpharma@intnet.dj. Stéphane Gidenne, Laboratoire Ketterthill, Luxembourg, E-mail: stephane.gidenne@ketterthill.lu. Jérôme Maslin, Hôpital d'Instruction Sainte Anne, Toulon, France, E-mail: maslin_j@yahoo.com. Dietmar Anders, Marinefliegergscwader 3 Bundeswehr, Nordholz, Germany, E-mail: dietmaranders@bundeswehr.org. Bouh A. Khaireh, Service de Santé des Forces Armées Djiboutiennes, Djibouti, Republic of Djibouti, E-mail: bouh.abdi@yahoo.fr. Ammar A. Ahmed, Direction de l'Epidémiologie et de l'Information Sanitaire, Djibouti, Republic of Djibouti, E-mail: ammarmed@yahoo.com.
References
- 1.Rodhain F. Preliminary results of an entomological survey of the potential arbovirus vectors in the French Territory of Afars and Issas. Bull Soc Pathol Exot Filiales. 1976;69:169–174. [PubMed] [Google Scholar]
- 2.Ollivier L, Decam C, Pommier de Santi V, Darar HY, Dia A, Nevin RL, Romand O, Bougère J, Deparis X, Boutin JP. Gastrointestinal illnesses among French forces deployed to Djibouti: French military health surveillance, 2005–2009. Am J Trop Med Hyg. 2010;83:944–950. doi: 10.4269/ajtmh.2010.10-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodier GR, Parra JP, Kamil M, Chakib SO, Cope SE. Recurrence and emergence of infectious diseases in Djibouti city. Bull World Health Organ. 1995;73:755–759. [PMC free article] [PubMed] [Google Scholar]
- 4.Carteron B, Morvan D, Rodhain F. The question of endemic malaria in the Republic of Djibouti. Med Trop (Mars) 1978;38:299–304. [PubMed] [Google Scholar]
- 5.Rodhain F. Mosquitoes of the French Territory of the Afars and Issas. I. The genus Anopheles. Bull Soc Path Exot Filiales. 1977;70:302–330. [PubMed] [Google Scholar]
- 6.Metselaar D, van Thiel PH. Classification of malaria. Trop Geogr Med. 1959;11:157–161. [Google Scholar]
- 7.Carteron B, Morvan D, Rodhain F. The control of culicidae in Djibouti town. An experience of 7 years. Med Trop (Mars) 1979;39:555–558. [PubMed] [Google Scholar]
- 8.Louis JP, Albert JP. Malaria in the Republic of Djibouti. Strategy for control using a biological antilarval campaign: indigenous larvivorous fishes (Aphanius dispar) and bacterial toxins. Med Trop (Mars) 1988;48:127–131. [PubMed] [Google Scholar]
- 9.Fox E, Abbate EA, Leef M, Mikhail E, Said-Salah Y, Hassan A. Malaria in the Djibouti Republic. Results of a serologic survey in Ambouli. Med Trop (Mars) 1989;49:159–160. [PubMed] [Google Scholar]
- 10.Fox E, Bouloumie J, Olson JG, Tible D, Lluberas M, Shakib SO, Parra JP, Rodier G. Plasmodium falciparum travels by train from Ethiopia to Djibouti. Med Trop (Mars) 1991;51:185–189. [PubMed] [Google Scholar]
- 11.Rogier C, Pradines B, Bogreau H, Koeck JL, Kamil MA, Mercereau-Puijalon O. Malaria epidemic and drug resistance, Djibouti. Emerg Infect Dis. 2005;11:317–321. doi: 10.3201/eid1102.040108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogreau H, Renaud F, Bouchiba H, Durand P, Assi SB, Henry MC, Garnotel E, Pradines B, Fusai T, Wade B, Adehossi E, Parola P, Kamil MA, Puijalon O, Rogier C. Genetic diversity and structure of African Plasmodium falciparum populations in urban and rural areas. Am J Trop Med Hyg. 2006;74:953–959. [PubMed] [Google Scholar]
- 13.de Pécoulas PE, Tahar R, Ouatas T, Mazabraud A, Basco LK. Sequence variations in the Plasmodium vivax dihydrofolate reductase-thymidylate synthase gene and their relationship with pyrimethamine resistance. Mol Biochem Parasitol. 1998;92:265–273. doi: 10.1016/s0166-6851(97)00247-8. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Hameed AA. Antimalarial drug resistance in the Eastern Mediterranean Region. East Mediterr Health J. 2003;9:492–508. [PubMed] [Google Scholar]
- 15.Pradines B, Mamfoumbi MM, Tall A, Sokhna C, Koeck JL, Fusai T, Mosnier J, Czarnecki E, Spiegel A, Trape JF, Kombila M, Rogier C. In vitro activity of tafenoquine against the asexual blood stages of Plasmodium falciparum isolates from Gabon, Senegal, and Djibouti. Antimicrob Agents Chemother. 2006;50:3225–3226. doi: 10.1128/AAC.00777-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Health Information for Djibouti: CDC Travelers' Health. 2011. http://wwwnc.cdc.gov/travel/destinations/djibouti.aspx Available at. Accessed May 20, 2011.
- 17.French Republic, Ministry of Foreign and European Affairs Travel Advice: Djibouti. 2010. http://www.diplomatie.gouv.fr/fr/conseils-aux-voyageurs_909/pays_12191/djibouti_12237/index.html Available at. Accessed May 20, 2011.
- 18.Parija SC, Dhodapkar R, Elangovan S, Chaya DR. A comparative study of blood smear, QBC and antigen detection for diagnosis of malaria. Indian J Pathol Microbiol. 2009;52:200–202. doi: 10.4103/0377-4929.48917. [DOI] [PubMed] [Google Scholar]
- 19.Rubertone MV, Brundage JF. The Defense Medical Surveillance System and the Department of Defense serum repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92:1900–1904. doi: 10.2105/ajph.92.12.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Department of Defense, Armed Forces Health Surveillance Center Update: Malaria, U.S. Armed Forces, 2009. Medical Surveillance Monthly Report. 2010;17:2–5. http://www.afhsc.mil/viewMSMR?file=2010/v17_n01.pdf Available at. Accessed May 20, 2011. [Google Scholar]
- 21.Malaria Public Health and Epidemiology Group, KEMRI/Wellcome Trust Research Program Djibouti National Malaria Indicator Survey 2008–2009. 2009. http://www.map.ox.ac.uk/PDF/Djibouti_MIS_Report.pdf Available at. Accessed May 20, 2011.
- 22.World Health Organization Malaria Elimination: A Field Manual for Low and Moderate Endemic Countries. 2007. http://whqlibdoc.who.int/publications/2007/9789241596084_eng.pdf Available at. Accessed May 20, 2011.
- 23.Faulde MK, Ahmed AA. Haematophageous vector monitoring in Djibouti city from 2008 to 2009: first records of Culex pipiens spp. torridus (iglisch), and Anopheles sergentii (theobald) J Egypt Soc Parasitol. 2010;40:281–294. [PubMed] [Google Scholar]
- 24.Wallace MR, Sharp TW, Smoak B, Iriye C, Rozmajzl P, Thornton SA, Batchelor R, Magill AJ, Lobel HO, Longer CF, Burans JP. Malaria among United States troops in Somalia. Am J Med. 1996;100:49–55. doi: 10.1016/s0002-9343(96)90011-x. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez JL, DeFraites RF, Sharp TW, Hanson RK. Mefloquine or doxycycline prophylaxis in US troops in Somalia. Lancet. 1993;341:1021–1022. doi: 10.1016/0140-6736(93)91107-w. [DOI] [PubMed] [Google Scholar]
- 26.Peragallo MS, Sabatinelli G, Majori G, Calí G, Sarnicola G. Prevention of malaria among Italian troops in Somalia and Mozambique (1993–1994) Trans R Soc Trop Med Hyg. 1995;89:302. doi: 10.1016/0035-9203(95)90552-9. [DOI] [PubMed] [Google Scholar]
- 27.Newton JA, Jr, Schnepf GA, Wallace MR, Lobel HO, Kennedy CA, Oldfield EC., 3rd Malaria in US Marines returning from Somalia. JAMA. 1994;272:397–399. [PubMed] [Google Scholar]
- 28.Resseguier N, Machault V, Ollivier L, Orlandi-Pradines E, Texier G, Pradines B, Gaudart J, Buguet A, Tourette-Turgis C, Rogier C. Determinants of compliance with malaria chemoprophylaxis among French soldiers during missions in inter-tropical Africa. Malar J. 2010;9:41. doi: 10.1186/1475-2875-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention . Health Information for International Travel: The Yellow Book. Malaria: 2010. http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-2/malaria.aspx Chapter 2. Available at. Accessed May 20, 2011. [Google Scholar]
- 30.Institute for Public Health Surveillance Health Recommendations for Travelers, 2010. 2010. http://www.invs.sante.fr/beh/2010/21_22/beh_21_22_2010.pdf Weekly Epidemiological Bulletin 21-22, June 1 2010. Available at. Accessed May 20, 2011.
- 31.Chen LH, Wilson ME, Schlagenhauf P. Controversies and misconceptions in malaria chemoprophylaxis for travelers. JAMA. 2007;297:2251–2263. doi: 10.1001/jama.297.20.2251. [DOI] [PubMed] [Google Scholar]
- 32.Kim HC, Pacha LA, Lee WJ, Lee JK, Gaydos JC, Sames WJ, Lee HC, Bradley K, Jeung GG, Tobler SK, Klein TA. Malaria in the Republic of Korea, 1993–2007. Variables related to re-emergence and persistence of Plasmodium vivax among Korean populations and U.S. forces in Korea. Mil Med. 2009;174:762–769. doi: 10.7205/milmed-d-01-6208. [DOI] [PubMed] [Google Scholar]
- 33.Klein TA, Pacha LA, Lee HC, Kim HC, Lee WJ, Lee JK, Jeung GG, Sames WJ, Gaydos JC. Plasmodium vivax malaria among U.S. forces Korea in the Republic of Korea, 1993–2007. Mil Med. 2009;174:412–418. doi: 10.7205/milmed-d-01-4608. 2009. [DOI] [PubMed] [Google Scholar]
- 34.Nevin RL. Mefloquine prescriptions in the presence of contraindications: prevalence among US military personnel deployed to Afghanistan, 2007. Pharmacoepidemiol Drug Saf. 2010;19:206–210. doi: 10.1002/pds.1879. [DOI] [PubMed] [Google Scholar]
- 35.Schlagenhauf P, Petersen E. Malaria chemoprophylaxis: strategies for risk groups. Clin Microbiol Rev. 2008;21:466–472. doi: 10.1128/CMR.00059-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rombo L. Who needs drug prophylaxis against malaria? My personal view. J Travel Med. 2005;12:217–221. doi: 10.2310/7060.2005.12408. [DOI] [PubMed] [Google Scholar]


