Abstract
Learning is associated with structural changes in the human brain that can be seen and studied by MRI. These changes are observed in gray matter and surprisingly also in white matter tissue. Learning a wide range of skills, from sports, computer games, music, and reading, to abstract intellectual learning, including classroom study, is associated with structural changes in appropriate cortical regions or fiber tracts. The cellular changes underlying modifications of brain tissue during learning include changes in neuronal and glial morphology as well as vascular changes. Both alterations in axon morphology and myelination are thought to contribute to white matter plasticity during learning but to varying degrees depending on age. Structural changes in white matter could promote learning by improving the speed or synchrony of impulse transmission between cortical regions mediating the behavior. Action potentials can stimulate oligodendrocyte development and myelination by at least three known mechanisms that involve signaling molecules between axons and oligodendrocytes, which do not require neurotransmitter release from synapses. Integrating information from cellular/molecular and systems-level research on normal cognitive function, development, and learning is providing new insights into the biological mechanisms of learning and the structural changes produced in the brain.
Keywords: white matter, gray matter, learning and memory, MRI, DTI, brain imaging, myelin, NG2, activity-dependent plasticity, neuron-glia interactions, oligodendrocytes, enriched environment
Search for the Engram: A Brief History
How learning engraves its indelible trace in the brain has intrigued philosophers and scientists for ages. Karl Lashley’s strategy to tease out where in the brain the memory trace, or “engram,” was hidden was to train animals and then ablate parts of the cerebral cortex. After decades of research, he conceded defeat, concluding in 1950 that it was not possible to localize a memory trace anywhere in the brain (Lashley 1950).
The anatomical approach to finding changes in the brain during learning was supplanted by a search for electrophysiological changes made possible by technological developments. The evidence was slim at first. “Reverberating circuits” of sustained activation proposed by Eccles (1953) could conceivably store information in the brain for short periods, but this could not explain long-term memories and learned skills that persist for decades or survive extreme disruptions of brain activity following seizure or anesthesia.
In the 1950s and 1960s, the idea that the engram was instead a biochemical change in brain tissue became a leading hypothesis, rising in parallel with stunning breakthroughs in molecular biology and biochemistry. Swiss biochemist Holger Hydén extracted RNA and protein from single neuronal and glial cells that he meticulously dissected by hand from rats and rabbits after training (Hydén 1959). His technically demanding single-cell biochemical analysis revealed changes in the base ratios of RNA and in the abundance of specific proteins in brain cells after training animals (Hydén 1967; Hydén and Edyházi 1962).
Such findings led to conjecture that memory was in some way recorded in the base sequences of DNA, RNA, and protein, just as hereditary information is coded in genes. Spectacular reports in high-profile journals of success in transferring memory between animals by injections of RNA or protein extracted from the brains of trained animals into naive animals buoyed the belief in a molecular memory trace (Babich and others 1965; Rosenblatt and others 1966; Ungar and Oceguera-Navarro 1965; Reiniš and Koloušek 1968). These studies were subsequently discredited (Luttges and others 1966; Byrne and others 1966), but the dispute continued into the 1970s with positive results reported in leading journals (Golub and others 1970).
With the benefit of hindsight, it is now clear that Hyden’s fundamental observations were true, but his experimental findings preceded by decades a detailed understanding of intracellular signaling networks, transcription factors, and posttranscriptional regulation, activated in neurons by functional activity or environmental experience. Interestingly, one protein that Hyden found had changed in abundance in brain cells after learning was S100, a protein in neurons but predominantly found in glia (Hydén and Lang 1970). The possibility that glia could be involved in learning generated little interest, and it would not do so for another 40 to 50 years.
By serendipity, the approach taken by Hydén, of training animals and analyzing brain tissue for biochemical changes, unexpectedly resurrected the unfashionable view that structural changes in the brain might underlie the learning process. The findings also pressed to the fore once again the unconventional view that cells other than neurons might be involved in learning. Rosenzweig and colleagues raised rats in an enriched environment that provided the young animals with increased social interaction and environmental stimulation under the theory that learning during early life experience would produce biochemical changes that could be measured in the cerebral cortex. The researchers predicted that a rise in the enzyme acetyl cholinesterase (AChE) would be found in the brains of rats exposed to environmental complexity and training.
The results, reported in 1960, did yield biochemical differences in AChE activity, but they were exactly opposite to what had been hypothesized (Krech and others 1960). Animals raised in enriched environments had significantly less AChE activity per unit weight of cerebral cortical tissue. The rest of the brain, presumed to be less relevant to learning, had more AChE activity per unit tissue weight.
The results were replicated two years later by the same researchers and explained by a remarkable unanticipated finding—the cerebral cortex had grown larger in the animals after experiencing the enriched environment (Rosenzweig and others 1962). The apparent decrease in AChE activity was explained simply by the fact that the cortical weight increased faster in the animals raised in the enriched environment than the increase in total AChE activity.
The finding that cortical weight increases as a consequence of environmental complexity was not anticipated. … The unexpected finding of a morphological change as a consequence of environmental complexity and training is as intriguing as our predicted finding of a biochemical change. … Possibilities include increases in the volume of neural cell bodies, or neural cell processes or of glial cells, or increases in myelin or in the vascularization of tissue. (Rosenzweig and others 1962, pp. 435–6)
This unexpected turn redirected their future research, which yielded evidence for all of these anatomical responses during learning. Uncovering the causal basis for these correlations between tissue changes during learning and separating cellular cause from cellular effects remain difficult problems today.
Better microscopy methods in the 1980s to 1990s enabled searching at a subcellular level for anatomical substrates for learning. The prevailing concept was that synapses were structurally static, but quantitative electron microscopic analysis in combination with electrophysiological recording proved that synapses were structurally dynamic in association with functional changes (Fields and Ellisman 1985). Development of confocal and two-photon microscopy allowed structural dynamics in dendritic spines to be observed in living tissue. A vigorous area of investigation today is to determine the mechanisms and relevance of these morphological changes in dendrites and synapses to learning (for reviews, see Segal and others 2010; Barnes and Finnerty 2010) and in glia that are closely associated with synapses (for review, see Haber and Murai 2006).
New technology has once again driven research on this longstanding problem in new directions. New MRI brain imaging methods are forcing a renewed search for gross structural changes in the brain during learning and shifting the investigation in a new area: into the white matter brain regions underlying the cerebral cortex. These regions of brain were of little interest to memory researchers who were guided by Hebbian theories of synaptic plasticity as the fundamental mechanism of learning. Clinical studies, however, first showed that there were differences in white matter in the human brain in association with normal variation in cognitive function and in association with psychiatric disorders (for review, see Fields 2008). This led to experimental studies using structural MRI to search for anatomical changes in the brain during learning, which is the focus of this review.
Structural Changes in Gray Matter in the Human Brain during Learning
Voxel-based morphometry (VBM) enables semi-quantitative, unbiased analysis of anatomical data from magnetic resonance imaging. Automated segmentation partitions structural features from MRI into tissue categories of gray matter, white matter, skull, and ventricles. Normalization of the MRI data to neuroanatomical features allows pooling data from different individuals, and statistical analysis is then performed on a voxel-by-voxel manner to identify regional differences that vary significantly.
Significant differences in brain structure detected by MRI in individuals with specific highly developed skills provide evidence suggestive of use-dependent increases in gray matter volume in cortical regions known to be important in performing the task. One of the first studies to do so concerned professional taxi drivers in London, who were found to have significantly larger posterior hippocampal volume compared with control subjects. The anterior hippocampal gray matter volume in taxi drivers was significantly smaller than controls (Maguire and others 2000). The posterior hippocampus in humans is preferentially involved with previously learned spatial information, whereas the anterior hippocampal region is more involved in encoding new environmental layouts. The left platum temporale in musicians with perfect pitch is larger than in nonmusicians (Schlaug and others 1995), and professional keyboard musicians have larger gray matter volume in motor, auditory, and visual-spatial brain regions compared with age-matched nonmusicians (Gaser and Schlaug 2003). A recent study reports that professional golfers have enlarged premotor and pariteal cortex, areas involved in sensory-motor control and cognitive processes (Jäncke and others 2009).
Such differences in elite performers could represent use-dependent changes in the brain during the extensive practice required to achieve superiority in these skills, or they could reflect intrinsic differences in the brain that predisposed the individuals to excel as taxi drivers, musicians, or golfers. Retrospective analysis shows that the increase in posterior hippocampal volume in London taxi drivers (and the decrease in anterior hippocampal volume) correlates linearly with the amount of professional driving experience of individual drivers, suggesting that anatomical remodeling of the hippocampus in taxi drivers was generated by their learning experience (Maguire and others 2000). Similar correlations between the hours practiced and the magnitude of changes in gray matter volume are also documented in golfers (Jäncke and others 2009).
A definitive test of this interpretation requires longitudinal studies performed on the same individual during the process of learning. A comparison of subjects before and after learning to juggle found structural changes in gray matter (increased volume) in the left posterior intraparietal sulcus, an area involved in spatial analysis of moving objects (Draganski and colleagues 2004). Three months later, the increase was still evident but was somewhat reduced from that seen soon after learning the skill. In another learned skill, increased gray matter density in the left occipitotemporal region was found in a study of 16 adult subjects examined by MRI before and after learning Morse code (Schmidt-Wilcke and others 2010). A longitudinal study on adolescent girls found that the cerebral cortex had thickened in two areas after three months of practice on a visual-spatial problem-solving computer game called Tetris (Haier and colleagues 2009).
An increase in gray matter volume after perfecting a new skill can occur much more rapidly than the several-month comparisons most experimental designs would suggest. In a longitudinal study of practice in mirror image reading, an increase in gray matter in the right dorsolateral occipital cortex was seen after only 15 minutes of practice/day for two weeks (Ilg and others 2008). Repetitive transcranial magnetic stimulation can induce structural changes in gray matter of the auditory cortex within only 5 days (May and others 2007).
Skill learning to perfect a specific sensory and motor function, such as that associated with elite performance in sports, musicianship, navigation, or computer games, might involve structural changes in the brain as the new process becomes increasingly automated, but whether more abstract learning would be associated with structural remodeling of gray matter is an important question.
This possibility was investigated in medical students studying for a major exam in Germany (Draganski and others 2006). The students’ brains were imaged three months before the examination and on the day of the exam. The results were compared with an age-matched control group that had no exams in the last six months and who were not studying for an exam. Gray matter volume increased significantly over the three-month study period in the posterior and lateral cortex bilaterally, whereas there were no changes seen in students who had not studied for the exam. A third scan performed on 23 of the 38 students three months later revealed that these changes persisted. Interestingly, the posterior hippocampus showed a different pattern over time. Posterior hippocampal gray matter increase during the learning period and the increase became more pronounced at the third time point. The results suggest that the acquisition of highly abstract information is accompanied by structural changes in gray matter in specific brain regions involved in declarative memory and the analysis of visual information.
A more recent study performed by a different group of investigators has attempted to replicate these findings (Ceccarelli and others 2009). In this study, Italian medical students were imaged only two weeks before classes involving anatomy, biology, and physiology and compared with a control group of students who were on vacation from school. No differences in gray matter were found between the experimental and control group on the initial scan, but after two weeks of study for classroom exams, there was a significant increase in gray matter volume in the left posterior medial frontal cortex and left precuneus, as well as in the right orbitofrontal cortex. These are cortical regions associated with reasoning and visualization, but they are not the same cortical regions that were found to increase in volume in the first study. The authors speculate that the differences in time points of analysis (15 days vs. 3 months) account for the different gray matter regions that changed in the two studies. (This concept will be explored later in this review.)
To summarize, the results of MRI studies show that learning a wide range of skills is accompanied by an increase in gray matter volume in appropriate areas of the brain (see also Table 1). Structural changes in the cerebral cortex during learning are not limited to skills of increased sensory-motor coordination, but rather they can extend to abstract learning as in intellectual university study. The structural changes can be seen within two weeks of learning, and these changes can persist for weeks or months.
Table 1.
Human Brain Imaging Studies on Effects of Learning on Structure of Gray Matter
| Skill or Learning Activity | Region of Increase in Gray Matter | References |
|---|---|---|
| Navigation (taxi driver) | Posterior hippocampus |
Maguire and others (2000)
Maguire and others (2006) |
| Musical performance | Motor and auditory cortex, left sensorimotor cortex, right cerebellum |
Sluming and others (2002)
Gaser and Schlaug (2003) Bengtsson and others (2005) Han and others (2009) |
| Speech | Parietal |
Golestani and others (2002)
Golestani and Zatorre (2004) Golestani and Pallier (2007) Lee and others (2007) Mechelli and others (2004) |
| Games/sports | ||
| Tetris | Left superior frontal gyrus, left anterior superior temporal |
Haier and others (2009) |
| Golf | Premotor and parietal cortex | Jäncke and others (2009) |
| Juggling | Left parietal sulcus and motor-specific cortical regions, mid-temporal visual cortex, hippocampus, nucleus accumbens |
Draganski and others (2004)
Draganski and others (2006) Boyke and others (2008) Scholz and others (2009) |
| Morse code | Left occipitotemporal and fusiform gyrus | Schmidt-Wilcke and others (2010) |
| Reading | ||
| Learning to read | Left and right angular and middle temporal and dorsal occipital gyri, left supramarginal and posterior superior temporal |
Carreiras and others (2009) |
| Mirror reading | Right occipital | Ilg and others (2008) |
| School learning | Median temporal and posterior parietal, hippocampus, frontal and precuneus |
Draganski and others (2006)
Ceccarelli and others (2009) |
White Matter Changes in the Human Brain during Learning
The investigation on German students studying for a medical exam also found a decrease in gray matter in the occipital parietal lobe between the first and second time points. It is difficult to reconcile a decrease in gray matter volume with learning. The authors suggest that this could be the result of an increase in white matter in the adjacent region, which would reduce the adjacent gray matter volume in the brain scan proportionately (Draganski and others 2006). Although this is a reasonable technical explanation, the biological significance of increased white matter volume in learning is puzzling. The cellular basis of learning is understood to derive from changes in synaptic strength or the number of synaptic connections. White matter regions of the brain were not thought to be involved in learning.
Schlaug and others (1995), however, previously found that the corpus callosum, the large white matter tract connecting the left and right cerebral hemispheres, is significantly larger in musicians. White matter volume differences have also been associated with language abilities. In recent years, interest in exploring the possible involvement of white matter structure in learning has increased with the development of new imaging methods providing greatly improved information on white matter structure and by new experimental information on the effects of functional activity on myelination (see Table 2). In a study of native French speakers who learned to perceive foreign speech sounds (Farsi), Golestani and others (2002, 2006; Golestani and Pallier 2007) report larger white matter volume in the parietal lobe and left Henschl’s gyrus, areas known to be involved in articulation, speech production, and phonological working memory, in the subjects who learned more quickly. The study found no relationships between proficiency in this skill and gray matter volume. The authors interpret the larger white matter volume as an increase in myelination or an increase in the number of axon fibers in white matter tracts, enlargement of axon caliber, or alterations in anatomical arrangement of fibers in white matter tracts adjacent to the cortical gyri and sulci.
Table 2.
Human Brain Imaging Studies on Effects of Learning on Structure of White Matter
| Skill or Learning Activity | White Matter Tract | Reference |
|---|---|---|
| Musical performance | Corpus callosum, corticospinal tract, frontoparietal association tracts, right posterior limb of internal capsule |
Schmithorst and Wilke (2002)
Bengtsson and others (2005) Imfeld and others (2009) Han and others (2009) |
| Speech | Broca’s area, left insula/prefrontal, parieto-occipital sulcus, corpus callosum (nonsignificant trend), left and right inferior parietal |
Golestani and others (2002)
Flöel and others (2009) Golestani and Pallier (2007) |
| Visual/spatial memory and reaction time |
Right optic radiatin, posterior thalamus, medial precuneus, left superior temporal sulcus, left parietal operculum, left and right superior temporal, corpus callosum, left longitudinal fascicle |
Tuch and others (2005)
Begré and others (2007) |
| Games/sports | ||
| Golf | Corticospinal tract and parietal operculum | Jäncke and others (2009) |
| Juggling | Parieto-occipital sulcus | Scholz and others (2009) |
| Go (“Baduk”) | Frontal, cinculum, and striato-thalamic, dorsolateral premotor, parietal |
Lee and others (2010) |
| Reading | Splenium of corpus callosum | Carreiras and others (2009) |
Diffusion tensor imaging (DTI) provides a sensitive measure of changes in white matter microstructure by quantifying the degree of anisotropy of water diffusion in tissue (often expressed mathematically as fractional anisotropy, or FA). Diffusion of water in white matter becomes better oriented along fiber tracts as axons become myelinated, their caliber increases, or fibers become more highly organized (increased packing density, reduced fiber crossing and branching). One of the most unexpected findings of this new technology is wide-ranging evidence suggesting that the mechanisms of learning extend beyond gray matter into the white matter regions of the brain (Fields 2005).
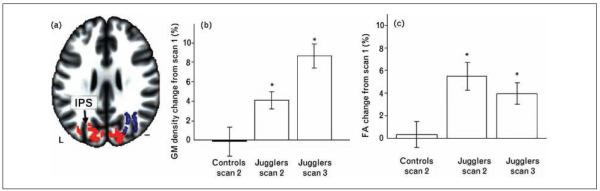
Differences in white matter brain structure are seen in professional musicians compared with nonmusicians (for review, see Ullén 2009); in association with increased proficiency in mathematics, reading, language, and reaction time within the normal range of function; and in a wide range of psychiatric conditions (for review, see Fields 2008). Professional pianists have increased white matter structure in appropriate tracts, and retrospective analysis shows that the increase in FA correlates directly with the number of hours of practice (Bengtsson and colleagues 2005; Han and others 2009). As with structural changes in gray matter during learning, increased FA in white matter is also associated with higher level intellectual ability, including IQ (Schmithorst and others 2005). Greater FA is reported in several white matter tracts in highly skilled players of the Asian chess-like game Go (“Baduk”) relative to controls (Lee and others 2010), principally in frontal brain regions and showing right-side dominance. Learning to read increases white matter volume in the splenium of the corpus callosum, in parallel with increased FA in white matter tracts connecting the left and right angular gyri through the splenium of the corpus callosum, which are areas known to be involved in reading and dyslexia (Carreiras and others 2009). A longitudinal study performed on people learning to juggle recently reported increased FA in white matter tracts underlying the right posterior intraparietal sulcus, which is a cortical region associated with visuomotor function (Scholz and others 2009) (Fig. 1). This study confirmed the increased gray matter volume in people learning to juggle reported previously by Draganski and others (2004) but found that gray and white matter changes developed with different kinetics. The increase in gray matter volume was greater on the third scan than on the second scan performed immediately after the six-week training period, whereas white matter FA did not continue to increase after the second scan. This suggests independent mechanisms may regulate white and gray matter plasticity.
Figure 1.
Increases in gray matter volume and white matter structure after learning to juggle. (a) A six-week period of training to juggle is associated with a significant increase in gray matter density (red) in the intraparietal sulcus (IPS), and the underlying white matter tracts (blue) showed increased fractional anisotropy on the right side. The increases in gray matter and white matter structure showed different kinetics. Subjects were scanned before (scan 1, not shown), after six weeks of training (scan 2), and following a subsequent four-week period without juggling (scan 3). From Scholz and others (2009). Reprinted with permission.
The robust structural changes in white matter and gray matter tissue documented by MRI during learning have broader significance. If brain structure is remodeled by the experience of learning, then physical remodeling of neural circuits may be guided by functional experience to compensate for impairments resulting from disease or injury, developmental delays, dyslexia, psychological and emotional disorders, or cognitive decline in aging.
Effect of Age on Structural Plasticity in the Brain during Learning
One of the important questions in the field of white matter plasticity in learning is whether these changes are the result of activity-dependent effects on development of white matter tracts or whether white matter structure is remodeled during learning. In the human brain, myelination continues postnatally through adolescence and at least into the early adult period in some brain regions, including fiber tracts interconnecting cortical regions. Most studies show that larger structural changes are seen during learning in younger brains, and white matter changes are more evident in tracts that are still undergoing myelination (Bengtsson and others 2005).
Normal aging is associated with gradual brain atrophy, and this includes white matter regions. Widespread reductions in gray matter volume are seen from middle age onward, but earlier reductions in gray matter are detected in the frontal cortex (Schmierer and others 2008). Interestingly, gray matter volume begins to decline in early adulthood, but white matter continues to increase until approximately the fifth decade of life and declines thereafter. There is widespread age-related deterioration in white matter microstructure from young adulthood onward, as shown by decreased FA with age. The decline in FA is primarily due to increased perpendicular diffusivity; diffusivity of water parallel to axons is unchanged with age (Schmierer and others 2008). Increased perpendicular diffusivity is consistent with a reduction in myelin integrity with age occurring in the absence of axonal pathology (Giorgio and others 2010).
More recent research shows that structural changes in the brain during learning persist in adults, although at more restricted locations and to a lesser extent. Only areas of the brain that had not yet completed myelination (frontal lobe regions) showed changes in white matter structure in pianists who began studying the piano at 17 years of age (the arcuate fasciculus), whereas increased white matter integrity was more widespread in pianists who began learning to play the piano at an earlier age (including the isthmus and splenium of the corpus callosum; Bengtsson and others 2005). Studies of skills only learned in adulthood (e.g., driving a taxi) provide strong evidence that changes in specific brain regions involved in the skill are remodeled and enlarged by learning in the adult brain. After retiring from their profession as London taxi drivers, the larger hippocampal volume reduces to that of controls, but taxi drivers of the same age who continue to work in their profession maintain an increased gray matter volume in the posterior hippocampus, showing that experience-dependent structural plasticity in the brain continues even into late age (Maguire and others 2006). In a recent study, illiterate individuals learning to read for the first time as adults had increased white matter in the splenium of the corpus callosum after learning to read compared with matched individuals who had not yet begun the reading program (Carreiras and others 2009). In a longitudinal study on this question, elderly people (mean age of 60 years) were found to be able to learn three-ball juggling but with less proficiency compared with 20-year-olds (Boyke and others 2008). Only 23% of the 60-year-olds were able to sustain juggling for 60 seconds, whereas 100% of the 20-year-olds were able to accomplish this. Nevertheless, similar to the younger group learning to juggle (Draganski and others 2004), gray matter changes in the older brain were seen in the middle temporal area of the visual cortex, left hippocampus, and nucleus accumbens—the same areas of the brain as the 20-year-olds learning to juggle. Together, these data suggest that the strongest effects of learning on white matter structure are seen in tracts that are still undergoing myelination, but learning-dependent changes in white matter do persist in the adult brain, although to a lesser extent.
Controversies in MRI Studies of Structural Changes during Learning
A number of controversies have arisen in the field of MRI research on gray and white matter changes in the brain during learning. The first concerns inconsistencies in replicating findings. Both biological and technical considerations appear to be involved. The second issue is whether volume and FA changes detected by MRI are use dependent or specific to learning. For example, vascular changes associated with increased metabolic demand might produce an increase in tissue volume (or affect FA), but this would be ancillary to the fundamental cellular mechanisms of learning. Both of these questions are being examined by combining fMRI together with structural MRI or DTI in the same subject.
Thomas and others (2009) found no structural changes associated with learning in a longitudinal VBM study of learning a mirror-image, hand-eye coordination task. The investigators conclude that VBM results are inconsistent across minor perturbations in analysis technique that lead to artifactual differences. In this study, 12 adult subjects learned to reverse the direction of controls on a joystick to track moving objects on a computer screen. Although the subjects learned the skill and changes in fMRI were associated with the increased performance, the structural changes that were at first detected by VBM using standard methods were not seen after nonparametric statistics were used or different scans in the sequence of images were used for alignment.
It is difficult or impossible to prove the negative, and several biological confounds could contribute to discrepancies among studies. This includes differences in the age of subjects, the type of skill or learning task, the intensity and duration of the training, and how soon after training the second scan is taken. Most intriguingly, the unknown process by which learning becomes encoded and redistributed to different parts of the brain contributes to uncertainty about where in the brain structural changes are to be expected.
Studies of white matter changes in musicians illustrate difficulties with discrepancies among similar studies (see Ullén 2009 for review). Bengtsson and others (2005) found increased FA in the internal capsule in professional concert pianists, which increased proportionally to the number of hours of practice as determined retrospectively. This finding has been replicated recently by Han and colleagues (2009). In contrast to these findings, Imfeld and others (2009) report the opposite result: lower FA values in this white matter tract, which is consistent with a comparison between musicians and nonmusicians by Schmithorst and Wilke (2002), who observed lower FA in the internal capsule bilaterally but significantly greater FA in the genu of the corpus callosum. Ullén (2009) suggests that a possible explanation for the difference may be that the study by Imfeld and coworkers included musical performers from a large variety of instruments, including keyboard, string, woodwind, and brass instruments, in their musician groups. Professional keyboard artists differ in that they must execute a large number of rapid, complex, and precisely timed finger movements of both hands, leading to increased FA in the pyramidal tract.
A more fundamental issue is raised by these discrepancies, however. How does activity in motor control circuits change during learning? A study of professional golfers found smaller white matter volume and lower FA values in the corticospinal tract at the level of the internal and external capsule and in the parietal operculum compared with less proficient and less experienced golfers (Jäncke and others 2009). How can decreased white matter structure support learning? The authors speculate that skilled golfers may be more proficient in using automated motor programs to execute the golf swing, and these pathways are less taxed than in novices.
A study by Thomas and colleagues (2009) used fMRI and VBM to assess the coincidence of functional and structural changes associated with a simple learning task. Behavioral and fMRI data clearly showed a significant learning effect as subjects learned to reverse the direction of controls on a joystick to track moving objects on the screen. However, during training, activation of the middle frontal gyrus and parietal cortex decreased as the task was learned and activity in the medial frontal cortex increased. fMRI data suggest that learning changes the subject’s cognitive strategy as the task becomes perfected and the relative difficulty of the task declines. Similarly, the study of adolescent girls learning the computer game Tetris found that there was no overlap between the structural changes that were seen by VBM and the changes in neural activity of cortical regions assessed by fMRI as the task was perfected (Haier and others 2009). The authors suggest that practiced subjects developed a more sophisticated approach to the skill that did not invoke brain areas activated when the task was more novel.
An additional complication is that fMRI data measure neuronal “mass activity,” without an ability to distinguish between excitatory and inhibitory activity, both of which may be modified during learning. If, however, structural and functional MRI are performed at the proper time (only two weeks after learning the mirror-reading task investigated by Ilg and coworkers [2008]), gray matter increases in the right dorsolateral occipital cortex correspond to the site of peak fMRI activation during mirror reading.
Thus, structural and functional brain changes after learning do not necessarily occur in the same cortical regions or develop with the same time course. Although this is a serious confound for experimental studies on structural changes in the brain during learning, the combined fMRI and structural MRI data showing the independence of functional and structural changes suggest that the structural changes that are found after learning are not explained simply by the increased level of neuronal activity but rather must be the result of learning remodeling neural tissue.
Cellular Substrates for Structural Changes in the Brain during Learning
The cellular basis for structural changes in the human brain during learning is unknown, as this would require postmortem or biopsy tissue samples, but research on experimental animals indicates that all the cellular changes suggested by Rosenzweig and colleagues in 1962 may contribute to the structural changes during learning determined by MRI. “Possibilities include increases in the volume of neural cell bodies, or neural cell processes or of glial cells, or increases in myelin or in the vascularization of tissue” (Rosenzweig and others 1962, pp. 435–6). In addition to these, neurogenesis (and gliogenesis) could contribute, although neurogenesis provides relatively few cells, and some changes seen using MRI are evident more rapidly than could be accounted for by the generation of new neurons. Histological studies on rats raised in enriched environments, which in contrast to normal cages provide more social interaction and novel objects for exploration, reveal robust cellular changes in gray and white matter. The changes include vascular tissue, glia, neurons, and increased myelination (Markham and Greenough 2004).
Whether the changes in white matter structure in the human brain during learning reflect changes in axonal morophology or arrangement, the number of myelinated axons in the tract, or alterations in the thickness of the myelin sheath are unknown. Histological studies on experimental animals performed after documenting learning-induced changes in the brain by MRI will be required. Large changes in white matter in the cerebellum of Japanese macaques trained to use a rake to retrieve a food reward show that the phenomenon of structural modifications in white matter tracts accompanying learning is not restricted to humans. In this study, the extent of change in white matter structure in individual monkeys correlated with the speed of learning and the proficiency of the animal (Quallo and others 2009). Histological studies on such animals after training and brain imaging will soon provide data on the cellular basis for changes in white matter volume and FA during learning.
Studies of experience-dependent change in white matter regions of rat brain suggest different effects in young and adult animals. Myelination in the rodent brain is increased by environmental enrichment in early life when the fiber tracts are still undergoing active myelination (Markham and Greenough 2004), but in adult rats, the increased volume of white matter tracts in the splenium of the corpus callosum compared with adults housed in standard cages was found to result from an increase in volume of astrocytes and a larger number of unmyelinated axons after environmental enrichment (Markham and others 2009). This suggests axon sprouting and astrocytes responding to experience in white matter tracts that are fully myelinated but that myelin is not affected. Changes in myelin in the adult brain may be more difficult to detect by histology than earlier in development when the tracts are less heavily myelinated, however.
Recent research using transgenic mice with genetically encoded reporters that allow the development of cells forming oligodendrocytes (oligodendrocyte progenitor cells [OPCs]) to be tracked in the adult brain reveals that 5% of the total number of cells in the adult brain are OPCs. These cells can generate neurons or astrocytes under appropriate circumstances, but the large majority remain in the progenitor stage or develop into mature oligodendrocytes (Rivers and others 2008). A significant fraction of the oligodendrocytes in the adult mouse brain (29%) is formed not during development but after sexual maturity. This indicates that there is a continuous source of new oligodendrocytes in the adult brain that is available for repair and possibly to participate in learning (Psachoulia and others 2009). One of the reasons myelination persists for decades in humans, rather than being completed by birth as it is in many other vertebrates, may be that experience directs development of neural circuits in the human brain to optimize brain function for success in the environment experienced in early life.
Cell culture studies have shown that electrical impulses in axons generated by electrical stimulation of axons (Stevens and others 1998; Stevens and others 2002; Ishibashi and others 2006) or drug treatments that inhibit or stimulate sodium channel activity (Demerens and others 1996) can affect myelination. Three molecular mechanisms have been identified for activity-dependent effects on myelination from studies using electrical stimulation of axons during myelination: first, impulse activity regulates expression of a cell adhesion molecule in neurons, L1CAM, that is essential for myelination (Itoh and others 1995). Interestingly, expression of this gene and other cell adhesion molecules is regulated by specific frequencies of action potential firing (Itoh and others 1997). Further research demonstrated frequency-specific effects on myelination as a result of regulating L1 expression (Stevens and others 1998). Second, impulse activity in axons releases the neurotransmitter ATP from axons by a mechanism that is not yet identified, and this can control development of myelinating glia (Stevens and Fields 2000). Myelinating and nonmyelinating glia have membrane receptors for ATP and adenosine, which is generated by hydrolysis of ATP (Fields and Burnstock 2006). ATP released from axons firing action potentials has been shown in cell culture to stimulate astrocytes, causing them to release the cytokine leukemia inhibitory factor (LIF), which stimulates myelination by mature oligodendrocytes (Ishibashi and others 2006). Third, during the progenitor stage, OPCs are directly stimulated by adenosine generated by axon firing, which promotes their development and increases the number of axons that become myelinated (Stevens and others 2002; Fig. 2). Synapses have been detected on some OPCs in white matter (Kukley and others 2007; Ziskin and others 2007), raising a fourth possibility that synapses on OPCs could provide a mechanism for stimulating myelination of electrically active axons, but this has not yet been shown.
Figure 2.
Oligodendrocyte progenitor cells (OPCs) can respond to neural impulse activity. Dorsal root ganglion neurons in cell culture were stimulated to fire action potentials while monitoring intracellular calcium by confocal microscopy. (a) Before electrical stimulation, intracellular calcium levels are low. (b) The calcium rise in neurons after stimulation indicates action potential firing. (c) OPCs (yellow arrows) respond to action potential firing with an increase in intracellular calcium. The signaling from axons to OPCs involves release of ATP and other neurotransmitters from axons. Adenosine derived from the rapid hydrolysis of ATP activates membrane receptors on OPCs that stimulate development of OPCs into mature oligodendrocytes, resulting in increased myelination of cultures in which axons were stimulated. From Stevens and others (2002). Reprinted with permission.
There is controversy over whether changes in white matter tracts during learning (and in psychological disorders) are initiated by myelinating glia or whether the glial responses are secondary to structural plasticity in axons. Myelination is regulated by axon diameter. Changes in axon diameter during learning could therefore cause oligodendrocytes to alter the thickness of the myelin sheath. Conversely, axons that become demyelinated in diseases, such as multiple sclerosis, can die, and this can cause the death of neurons (for review, see Dutta and Trapp 2007). Thus, a primary event in oligodendrocytes could remodel axons in white matter tracts. Regardless of which cell initiates the response, both axons and glia must maintain a highly coordinated structural relationship that implies constant dialogue between the two cells through adhesive or diffusible cell-cell signaling molecules, some of which may be affected by impulse activity.
White Matter in Information Processing and Learning
The changes in white matter structure and the new molecular mechanisms identified that regulate myelination according to impulse activity in axons expand the traditional view of myelin as being only relevant to pathology, to include participation in a new cellular mechanism for learning. Traditionally, theories of learning focused on the input to neurons, synapses. White matter activity-dependent plasticity expands the scope of learning theory to include consideration of how modifications of the output of neurons, action potential firing in axons, could contribute to learning. Any complex cognitive task in mammals requires transmission of information through a series of distant cortical regions mediating distinct aspects of the cognitive function or skill. Optimizing the speed or synchrony of impulse transmission through cortical circuits could be an important aspect of learning (Fields 2005). Myelin and axon diameter affect the speed of impulse propagation. Changes in white matter, including axonal numbers, diameter, the number of myelinated axons in a tract, the thickness of myelin, or other morphological features such as internodal distance, could affect the speed of impulse propagation and thus contribute to increased functional performance in learning. Similar mechanisms would implicate white matter in psychiatric illnesses, where impaired speed or synchrony of information transmission between distant cortical regions controlling higher level cognitive functions would cause dysfunction.
Conclusions and Challenges for the Future
New methods of human brain imaging, the ability to monitor cellular changes in the living brain by confocal and two-photon microscopy, rapid expansion in understanding the role of glia in supporting neurons and in forming myelin, identification of signaling molecules released by axons in an activity-dependent manner to regulate glia and myelin formation, and an expansion of the cellular concept of learning beyond the synapse, beyond gray matter, and beyond neurons to include myelinating and nonmyelinating glia and the efficiency of impulse transmission through neural circuits now provide a perspective on the long and puzzling search for the memory trace in brain tissue, which shows the predecessors were all on the right tracks. Learning is not limited to gray matter or to synapses, and structural, electrical, and biochemical mechanisms are all necessarily critical to the cellular mechanisms of learning.
The challenges in the future will be to unite new information provided by systems-level studies of cognitive function and learning derived from functional and structural brain imaging with the new cellular and molecular information on neuronal and glial plasticity and how development of OPCs and myelination can be influenced by electrical activity. Activity-dependent communication between axons and oligodendrocytes may involve synapses onto OPCs, but mechanisms of communication beyond the synapse seem likely. How ATP and other neurotransmitters are released from axon segments by electrical activity in the absences of synapses is a major question in the field. Both vesicular and nonvesicular release of signals from axons likely communicate states of activity in neural circuits to other neurons, glia, and vasculature, to stimulate cellular responses that support structural changes in brain tissue, which, using modern brain imaging techniques, enable us to see Lashley’s imagined and elusive engram.
Footnotes
Reprints and permission: http://www.sagepub.com/journalsPermissions.nav
Note Added in Proof A mechanism for non-synaptic release of ATP from axons during action potential firing has been reported recently. Single-photon imaging in combination with imaging intrinsic optical signals in axons shows that ATP is released through volume-regulated anion channels that become activated by microscopic swelling of axons accompanying electrical excitation (Fields and Ni 2010).
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure/Funding The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: supported by the NIH intramural program.
References
- Babich FR, Allan L, Jacobson SB, Jacobson A. Transfer of a response to naive rats by injection of ribonucleic acid extracted from trained rats. Science. 1965;149:656–7. doi: 10.1126/science.149.3684.656. [DOI] [PubMed] [Google Scholar]
- Barnes SJ, Finnerty GT. Sensory experience and cortical rewiring. Neuroscientist. 2010;16:186–98. doi: 10.1177/1073858409343961. [DOI] [PubMed] [Google Scholar]
- Begré S, Frommer A, von Känel R, Kiefer C, Federspiel A. Relation of white matter anisotropy to visual memory in 17 healthy subjects. Brain Res. 2007;1168:60–6. doi: 10.1016/j.brainres.2007.06.096. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Forsman L, Forssberg H, Skare S, Ullén F. Extensive piano practicing has regionally specific effects on white matter development. Nature Neurosci. 2005;8:1148–50. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Buchel C, May A. Training-induced brain structure changes in elderly. J Neuroscience. 2008;28:7031–5. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne WL, Samuel D, Bennett EL, Rosenzweig MR, Wasserman E, Wagner AR. Memory transfer. Science. 1966;153:658. doi: 10.1126/science.153.3736.658. others. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Seghier ML, Baquero S, Estévez, Lozano A, Devlin JT. An anatomical signature for literacy. Nature. 2009;461:983–6. doi: 10.1038/nature08461. [DOI] [PubMed] [Google Scholar]
- Ceccarelli A, Rocca MA, Pagani E, Falini A, Comi G, Fillippi M. Cognitive learning is associated with gray matter changes in healthy human individuals: a tensor-based morphometry study. Neuroimage. 2009;48:585–9. doi: 10.1016/j.neuroimage.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci USA. 1996;93:9887–92. doi: 10.1073/pnas.93.18.9887. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Changes in grey matter induced by training. Nature. 2004;427:311–2. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26:6314–7. doi: 10.1523/JNEUROSCI.4628-05.2006. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, Trapp BD. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology. 2007;68:S22–31. doi: 10.1212/01.wnl.0000275229.13012.32. [DOI] [PubMed] [Google Scholar]
- Eccles JC. The neurophysiological basis of mind. Clarendon; Oxford, UK: 1953. [Google Scholar]
- Fields RD. Myelination: An overlooked mechanism of synaptic plasticity? Neuroscientist. 2005;11:528–31. doi: 10.1177/1073858405282304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition, and psychiatric disorders. Trends Neurosci. 2008;31:361–70. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Burnstock G. Purinergic signalling in neuronglia interactions. Nat Rev Neurosci. 2006;7:423–36. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Ellisman MH. Synaptic morphology and differences in sensitivity. Science. 1985;228:197–9. doi: 10.1126/science.3975637. [DOI] [PubMed] [Google Scholar]
- Fields RD, Ni Y. Nonsynaptic communication through ATP release from volume-activated anion channels in axons. Science Signaling. 2010;3:ra73. doi: 10.1126/scisignal.2001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flöel A, de Vries MH, Scholz J, Breitenstein C, Johansen-Berg H. White matter integrity in the vicinity of Broca’s area predicts grammar learning success. Neuroimage. 2009;47:1974–81. doi: 10.1016/j.neuroimage.2009.05.046. [DOI] [PubMed] [Google Scholar]
- Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci. 2003;23:9240–5. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51:943–51. doi: 10.1016/j.neuroimage.2010.03.004. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestani N, Molko N, Dehaene S, Le Bihan D, Pallier C. Brain structure predicts the learning of foreign speech sounds. Cereb Cortex. 2006;17:575–82. doi: 10.1093/cercor/bhk001. [DOI] [PubMed] [Google Scholar]
- Golestani N, Pallier C. Anatomical correlates of foreign speech sound production. Cereb Cortex. 2007;17:929–34. doi: 10.1093/cercor/bhl003. [DOI] [PubMed] [Google Scholar]
- Golestani N, Paus T, Zatorre RJ. Anatomical correlates of learning novel speech sounds. Neuron. 2002;35:997–1010. doi: 10.1016/s0896-6273(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Golestani N, Zatorre RJ. Learning new sounds of speech: reallocation of neural substrates. Neuroimage. 2004;21:494–506. doi: 10.1016/j.neuroimage.2003.09.071. [DOI] [PubMed] [Google Scholar]
- Golub AM, Masiarz FR, Villars T, McConnell JV. Incubation effects in behavior induction in rats. Science. 1970;168:392–5. doi: 10.1126/science.168.3929.392. [DOI] [PubMed] [Google Scholar]
- Haber M, Murai KK. Reshaping neuron-glial communication at hippocampal synapses. Neuron Glia Biol. 2006;2:59–66. doi: 10.1017/S1740925X06000032. [DOI] [PubMed] [Google Scholar]
- Haier RJ, Karama S, Leyba L, Jung RE. MRI assessment of cortical thickness and functional activity changes in adolescent girls following three months of practice on a visual-spatial task. BMC Res Notes. 2009;2:174. doi: 10.1186/1756-0500-2-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Yang H, Lv Y-T, Zhu C-Z, He Y, Tang H-H. Gray matter density and white matter integrity in pianists’ brain: a combined structural and diffusion tensor MRI study. Neurosci Lett. 2009;459:3–6. doi: 10.1016/j.neulet.2008.07.056. others. [DOI] [PubMed] [Google Scholar]
- Hydén H. Quantitative assay of compounds in isolated fresh nerve cells and glial cells from control and stimulated animals. Nature. 1959;184:433. doi: 10.1038/184433a0. [DOI] [PubMed] [Google Scholar]
- Hydén H. Biochemical and molecular aspects of learning and memory. Proc Am Philos Soc. 1967;111:326–42. [Google Scholar]
- Hydén H, Edyházi E. Nuclear RNA changes in nerve cells during learning experiments in rat. Proc Natl Acad Sci U S A. 1962;48:1366–73. doi: 10.1073/pnas.48.8.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hydén H, Lange PW. S100 brain protein: correlation with behavior. Proc Natl Acad Sci U S A. 1970;67:1959–66. doi: 10.1073/pnas.67.4.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg R, Wohlschläger AM, Gaser C, Liebau Y, Dauner R, Wöller A. Gray matter increase induced by practice correlates with task-specific activation: a combined functional and morphometric magnetic resonance imaging study. J Neurosci. 2008;28:4210–5. doi: 10.1523/JNEUROSCI.5722-07.2008. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imfeld A, Oechslin MS, Meyer M, Loenneker T, Jancke L. White matter plasticity in the corticospinal tract of musicians: a diffusion tensor imaging study. Neuroimage. 2009;46:600–7. doi: 10.1016/j.neuroimage.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49:823–32. doi: 10.1016/j.neuron.2006.02.006. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Ozaki M, Stevens B, Fields RD. Activity-dependent regulation of N-cadherin in DRG neurons: differential regulation of N-cadherin, NCAM, and L1 by distinct patterns of action potentials. J Neurobiol. 1997;33:735–48. doi: 10.1002/(sici)1097-4695(19971120)33:6<735::aid-neu3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Itoh K, Stevens B, Schachner M, Fields RD. Regulated expression of the neural cell adhesion molecule L1 by specific patterns of neural impulses. Science. 1995;270:1369–72. doi: 10.1126/science.270.5240.1369. [DOI] [PubMed] [Google Scholar]
- Jäncke L, Koeneke S, Hoppe A, Rominger C, Hänggi J. The architecture of the golfer’s brain. PLOS One. 2009;e4785:1–8. doi: 10.1371/journal.pone.0004785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krech D, Rosenzweig MR, Bennett EL. Effects of environmental complexity and training on brain chemistry. J Comp Physiol Psychol. 1960;53:509–19. doi: 10.1037/h0045402. [DOI] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nature Neurosci. 2007;10:311–20. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- Lashley KS. In search of the engram. Symp Soc Exper Biol. 1950;4:454–82. [Google Scholar]
- Lee B, Park J-Y, Jung WH, Kim HS, Oh JS, Choi CH. White matter neuroplastic changes in long-term trained players of the game of “Baduk” (GO): a voxel-based diffusion-tensor imaging study. Neuroimage. 2010;52:9–19. doi: 10.1016/j.neuroimage.2010.04.014. others. [DOI] [PubMed] [Google Scholar]
- Lee H, Devlin JT, Shakesshaft C, Stewart LH, Brennan A, Glensman J. Anatomical traces of vocabulary acquisition in the adolescent brain. J Neurosci. 2007;27:1184–9. doi: 10.1523/JNEUROSCI.4442-06.2007. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttges M, Johnson T, Buck C, Holland J, McGaugh J. An examination of “transfer of learning” by nucleic acids. Science. 1966;151:834–7. doi: 10.1126/science.151.3712.834. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RSF. Navigation-related structural changes in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97:4398–403. doi: 10.1073/pnas.070039597. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Woollett K, Spiers HJ. London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus. 2006;16:1091–101. doi: 10.1002/hipo.20233. [DOI] [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol. 2004;1:351–63. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Herting MM, Luszpak AE, Juraska JM, Greenough WT. Myelination of the corpus callosum in male and female rats following complex environment housing during adulthood. Brain Res. 2009;1288:9–17. doi: 10.1016/j.brainres.2009.06.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A, Hajak G, Ganssbauer S, Steffens T, Langguth B, Kleinjung T. Structural brain alterations following 5 days of intervention: dynamic aspects of neuroplasticity. Cereb Cortex. 2007;17:205–10. doi: 10.1093/cercor/bhj138. others. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O’Doherty J, Ashburner J, Frackowiak RS. Neurolinguistics: structural plasticity in the bilingual brain. Nature. 2004;431:757. doi: 10.1038/431757a. others. [DOI] [PubMed] [Google Scholar]
- Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009;5:57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quallo MM, Price CJ, Ueno K, Asamizuya T, Cheng K, Lemon RN. Gray and white matter changes associated with tool-use learning in macaque monkeys. Proc Natl Acad Sci U S A. 2009;106:18379–84. doi: 10.1073/pnas.0909751106. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiniš S, Koloušek J. Effect of methionine sulphoximine on memory transfer. Nature. 1968;217:680–1. doi: 10.1038/217680a0. [DOI] [PubMed] [Google Scholar]
- Rivers L, Young K, Rizzi M, Jamen F, Psachoulia K, Wade A. PDGFRa/NG2 glia generate myelinating oligodendrocytes and cortical projection neurons in the adult mouse CNS. Nature Neurosci. 2008;11:1292–401. doi: 10.1038/nn.2220. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt F, Farrow JT, Herblin WF. Transfer of conditioned responses from trained rats to untrained rats by means of a brain extract. Nature. 1966;209:46–8. doi: 10.1038/209046a0. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Krech D, Bennett EL. A search for relations between brain chemistry and behavior. Psychol Bull. 1960;57:476–92. doi: 10.1037/h0044689. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Krech D, Bennett EL, Diamond MC. Effects of environmental complexity and training on brain chemistry and anatomy: a replication and extension. J Comp Physiol Psychol. 1962;55:429–37. doi: 10.1037/h0041137. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Jancke L, Huang Y, Staiger JF, Steinmetz H. Increased corpus callosum size in musicians. Neuropsychologia. 1995;33:1047–55. doi: 10.1016/0028-3932(95)00045-5. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T, Rosengarth K, Luerding R, Bogdahn U, Greenlee MW. Distinct patterns of functional and structural neuroplasticity associated with learning Morse code. Neuroimage. 2010;51:1234–41. doi: 10.1016/j.neuroimage.2010.03.042. [DOI] [PubMed] [Google Scholar]
- Schmierer K, Wheeler-Kingshott CA, Tozer DJ, Boulby PA, Parkes HG, Yousry TA. Quantitative magnetic resonance of postmortem multiple sclerosis brain before and after fixation. Magn Reson Med. 2008;59:268–77. doi: 10.1002/mrm.21487. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M. Differences in white matter architecture between musicians and non-musicians: a diffusion tensor imaging study. Neurosci Lett. 2002;321:57–60. doi: 10.1016/s0304-3940(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp. 2005;26:139–47. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nature Neurosci. 2009;12:1370–1. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M, Vlachos A, Korkotian E. The spina apparatus, synaptopodin and dendritic spine plasticity. Neuroscientist. 2010;16:125–31. doi: 10.1177/1073858409355829. [DOI] [PubMed] [Google Scholar]
- Sluming V, Barrick T, Howard M, Cezayirli E, Mayes A, Roberts N. Voxel based morphometry reveals increased gray matter density in Broca’s area in male symphony orchestra musicians. Neuroimage. 2002;17:1613–22. doi: 10.1006/nimg.2002.1288. [DOI] [PubMed] [Google Scholar]
- Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287:2267–71. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- Stevens B, Porta S, Haak LL, Gallo V, Fields RD. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron. 2002;5:855–68. doi: 10.1016/s0896-6273(02)01067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Tanner S, Fields RD. Control of myelination by specific patterns of neural impulses. J Neurosci. 1998;18:9303–11. doi: 10.1523/JNEUROSCI.18-22-09303.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AG, Marrett S, Saad ZS, Ruff DA, Martin A, Bandettini PA. Functional but not structural changes associated with learning: an exploration of longitudinal voxel-based morphometry (VBM) Neuroimage. 2009;48:117–25. doi: 10.1016/j.neuroimage.2009.05.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proc Natl Acad Sci U S A. 2005;102:12212–7. doi: 10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullén F. Is activity regulation of late myelination a plastic mechanism in the human nervous system? Neuron Glia Biol. 2009;5:29–34. doi: 10.1017/S1740925X09990330. [DOI] [PubMed] [Google Scholar]
- Ungar G, Oceguera-Navarro C. Transfer of habituation by material extracted from brain. Nature. 1965;207:301–2. doi: 10.1038/207301a0. [DOI] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nature Neurosci. 2007;10:321–30. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]