Abstract
Background:
The purpose of this study was to clarify whether it is possible to extrapolate results from studies of the hydrolyzing activity of disaccharidases from rats to humans.
Materials and methods:
We measured disaccharidase activity in humans and rats using identical preparation and assay methods, and investigated the similarity in hydrolyzing activity. Small intestinal samples without malignancy were donated by five patients who had undergone bladder tumor surgery, and homogenates were prepared to measure disaccharidase activity. Adult rat homogenates were prepared using small intestine.
Results:
Maltase activity was the highest among the five disaccharidases, followed by sucrase and then palatinase in humans and rats. Trehalase activity was slightly lower than that of palatinase in humans and was similar to that of sucrase in rats. Lactase activity was the lowest in humans, but was similar to that of palatinase in rats. Thus, the hydrolyzing activity of five disaccharidases was generally similar in humans and rats. The relative activity of sucrose and palatinase versus maltase was generally similar between humans and rats. The ratio of rat to human hydrolyzing activity of maltase, sucrase, and palatinase was 1.9–3.1, but this was not a significant difference. Leaf extract from Morus alba strongly inhibited the activity of maltase, sucrase, and palatinase, but not trehalase and lactase, and the degree of inhibition was similar in humans and rats. L-arabinose mildly inhibited sucrase activity, but hardly inhibited the activity of maltase, palatinase, trehalase and lactase in humans and rats. The digestibility of 1-kestose, galactosylsucrose, and panose by small intestinal enzymes was very similar between humans and rats.
Conclusion:
These results demonstrate that the digestibility of newly developed saccharide materials evaluated by rat small intestinal enzymes can substitute for evaluation using human enzymes.
Keywords: disaccharidase, maltase, sucrase, trehalase, palatinase, digestibility
Introduction
Nondigestible sugar substitutes, such as oligosaccharides and sugar alcohols, have some beneficial health effects and are used in functional foods with health claims.1,2 The digestibility of newly developed oligosaccharide materials is important when evaluating their health benefits, which can be carried out using the digestive enzymes from the small intestine of animals, such as rats and pigs. It is difficult to obtain human intestinal tissues to examine this type of digestibility.3–6
Disaccharidases, such as maltase, sucrase, isomaltase, trehalase, and lactase, localize in the brush border membrane of small intestinal mucosa, but not in the digestive fluid. The hydrolyzing activity is highest at the proximal jejunum and decreases toward the distal ileum.7 The hydrolyzing activity of disaccharidases in the small intestine has been measured extensively in humans and experimental animals.3,4,8–14 Recently, some oligosaccharides that are resistant to digestion and α-glucosidase inhibitors have been developed and used in processed foods with health benefits, such as prebiotics, inhibitors of blood glucose elevation, and stimulators of intestinal mineral absorption.
However, it has not been clarified whether results on the digestibility of these oligosaccharides and their inhibitory effect on digestion, which have been evaluated using rat small intestinal enzymes, can be extrapolated directly to humans. Furthermore, there is no evidence of any similarity in hydrolyzing activity of intestinal disaccharidases between humans and rats, even when using identical preparation and analytical methods.
The objective of the present study was to investigate whether there is any similarity between humans and rats with regard to hydrolyzing activity of small intestinal disaccharidases, and to establish whether the results obtained in rats can be extrapolated to humans. Accordingly, the hydrolyzing activity of maltase, sucrase, palatinase, trehalase, and lactase was simultaneously measured using identical preparation and assay methods in the human and rat small intestine. Furthermore, to compare the inhibitory effect of disaccharidases in the human and rat small intestine, the inhibitory effect for disaccharidases was measured using the leaf extract of Morus alba, which is a competitive inhibitor for disaccharidase15 and L-arabinose, which is an noncompetitive inhibitor.16 Finally, the digestibility of some oligosaccharides, such as 1-kestose, galactosylsucrose, and panose, was simultaneously tested by identical assay methods, using human and rat intestinal homogenates. The evidence that hydrolyzing activity of disaccharidase was generally similar between humans and rats, and that the digestibility of saccharides by rats can be extrapolated to humans, should contribute to the future development of functional foods that use the beneficial health effects of newly developed saccharide materials.
Materials and methods
Human small intestinal samples and homogenate preparation
Small samples of resected small intestinal tissue without malignancy were donated by patients who had undergone bladder tumor surgery. The samples were mainly from the ileum and from older patients (Table 1). Tissue samples were washed with 0.9% NaCl, and the normal mucosa around the pathological portion was gently scraped with two slide glasses onto an ice-chilled glass plate. The mucosa was homogenized in 19 volumes of 0.9% NaCl (5% homogenate) with a homogenizer (Polytron®; Kinematica Inc, Lucerne, Switzerland) and stored at −80°C until assay. Before the assay of disaccharidase activity, the homogenate was adequately diluted with 0.9% NaCl.
Table 1.
Characteristics of human intestinal samples and comparison of disaccharidase activities in the small intestinal region of individual subject
| N | Sex | Age | Maltase | Sucrase | Palatinase (Isomaltase) | Trehalase | Lactase |
|---|---|---|---|---|---|---|---|
| 1 | M | 51 | 67.8 | 14.0 | 3.4 | 2.5 | 0.5 |
| 2 | F | 55 | 99.4 | 25.4 | 6.5 | 8.2 | 1.2 |
| 3 | M | 74 | 87.1 | 15.7 | 4.2 | 0.9 | 1.0 |
| 4 | F | 68 | 47.5 | 9.0 | 2.5 | 0.9 | 0.2 |
| 5 | F | 63 | 84.5 | 13.3 | 4.0 | 1.0 | 0.2 |
| Mean ± SD | 77.3 (20.1) | 15.5 (6.1) | 4.1 (1.5) | 2.7 (3.1) | 0.6 (0.5) |
Notes: Values are mean ± SD from 5 humans and expressed μ moles of substrate hydrolyzed/mg protein/h at 37°C, pH 6.0.
Abbreviations: M, male; F, female; SD, standard deviation.
Rat feeding and preparation of small intestinal homogenate
Six male Wistar rats (about 300 g) were raised on a synthetic diet with AIN93 composition17 for two weeks ad libitum under a 12-hour light/dark cycle at 23°C. Before decapitation, rats were fasted for 14 hours and given only water. Immediately after decapitation, all regions of the small intestine were removed and soaked in cold 0.9% NaCl. The mucosa was scraped with two glass slides onto an ice-chilled glass plate and homogenized in nine volumes of 0.9% NaCl with a homogenizer (Kinematica Inc). After adequate dilution with 0.9% NaCl, the homogenate was used for the assay of disaccharidase activity and protein content.
Assay of disaccharidase activity
The assay of disaccharidase activity in the homogenates of human and rat small intestinal mucosa was carried out according to the method of Oku et al18 which was a modification of the method of Dahlqvist et al.19 The substrates for the assay of disaccharidase activity were maltose, sucrose, palatinose, trehalose, and lactose, and those for the digestibility of oligosaccharides were 1-kestose, galactosylsucrose, and panose.
We pipetted 0.1 mL of adequately diluted homogenate into a small test tube, and 0.1 mL of 112 mM substrate in 0.1 M maleate-Na buffer (pH 6.0) was added to the test tube after preincubation for 2–3 minutes at 37°C. After incubation for 10–30 minutes at 37°C, 2.4 mL of TGO reagent, which contained 10 U/mL glucose oxidase, 5 U/mL peroxidase, 0.05 mM 4-aminoantipyrine, and 0.4 mM p-phenolsulfonic acid sodium salt in 0.5 M Tris-HCl (pH 7.0), was added to the test tube to stop the disaccharidase reaction, and to start the reactions of glucose oxidase and peroxidase, and the mixture was further incubated for 10 minutes at 37°C. Two drops of 4 N NaOH was added to the test tube to stop the reactions, and we read the optical absorbance at 500 nm using a spectrophotometer (UV1240; Shimadzu, Kyoto, Japan). The leaf extracts from M. alba or L-arabinose were added to the assay system after addition of enzyme solution, to observe the inhibitory effects on disaccharidases.
Protein determination
The protein concentration was determined by the Bradford method using bovine serum albumin as the standard.20
Chemicals
Maltose (>99% purity), trehalose (>99.9% purity), and panose (>99% purity) were donated by Hayashibara Biochemical Laboratory Inc (Okayama, Japan), palatinose (>98% purity) was donated by Mitsui Sugar Co (Tokyo, Japan), 1-kestose (>99% purity) was donated by Meiji Seika Kaisha Co Ltd (Tokyo, Japan), galactosylsucrose (>97% purity) was donated by Ensuiko Sugar Refining Co Ltd (Tokyo, Japan), and sucrose, lactose and L-arabinose were purchased from Wako Pure Chemical Industries Ltd (Osaka, Japan). The extract from leaves of M. alba was kindly donated by Toyotama Healthy Food Co (Tokyo, Japan).
Calculations and statistical analysis
Hydrolyzing activity was calculated with specific activity shown as μmol substrate hydrolyzed/mg protein/hour at 37°C (pH 6.0). Disaccharidase activity in six rats and five human samples were calculated as the means and standard deviations and compared with the levels by Student’s t-test for normally distributed data and Mann–Whitney U test for nonparametric data. A P value < 0.05 was considered to be significant using SPSS for Japan version 16.0 (SPSS Inc, Tokyo, Japan).
Ethics
The study protocol was approved by the respective committees of University of Nagasaki and Juzenkai Hospitals in Nagasaki, Japan. Each patient who donated small intestine tissue gave his or her written informed consent to participate in this study before their surgery. The rat experiments were performed under the guidelines on the care and use of laboratory animals of University of Nagasaki, Siebold. All of the experiments were carried out in the Laboratory of Public Health Nutrition, University of Nagasaki, Siebold.
Results
Disaccharidase activity in human small intestine
The average specific activity (μmol substrate hydrolyzed/mg protein/hour) of disaccharidases from five human samples was 77.3 ± 20.1 for maltase, 15.5 ± 6.1 for sucrase, 4.1 ± 1.5 for palatinase, 2.7 ± 3.1 for trehalase, and 0.6 ± 0.5 for lactase, respectively (Table 1). Maltase activity was significantly higher than that of sucrase (P < 0.05), and the activity of palatinase, trehalase, and lactase was very low in comparison with that of maltase (P < 0.05). These results agreed with previously reported data,9–13 although the standard deviation for each disaccharidase activity was very large among the five human samples.
Comparative activity of disaccharidases in human and rat small intestine
Comparison of average disaccharidase activity in the small intestine from five human subjects and six rats is shown in Table 2. The maltase activity was the highest, followed by sucrase and then palatinase in humans and rats. Trehalase activity was slightly lower than palatinase, and lactase activity was the lowest among the human disaccharidases. In rats, trehalase activity was higher than that of palatinase and was similar to that of sucrase. Lactase activity in rats was the lowest, as in humans, but it was similar to that of palatinase. These results support the previously published data.8–11,15,16,21 The activity of maltase, sucrase, and palatinase in humans was slightly higher than that in rats. To compare the relative activity of disaccharides in humans and rats, we calculated the ratio of activity of sucrase, palatinase, lactase, and trehalase versus maltase activity (Table 2). In humans, the sucrase activity ratio was 20, and that of palatinase, trehalase, and lactase was 5, 3, and 1, respectively, when maltase activity was considered to be 100. In rats, sucrase activity ratio was 12 and that of palatinase, trehalase and lactase was 4, 13 and 3, respectively.
Table 2.
Comparison of disaccharidase activities and ratio of disaccharidase versus maltase activity between humans and rats
|
Human (n = 5) |
Rats (n = 6) |
Relative activity (human/rat) | |||
|---|---|---|---|---|---|
| Specific activity ± SD | Ratio to maltase ± SD | Specific activity ± SD | Ratio to maltase ± SD | ||
| Maltase | 77.3 ± 20.1a | 100 | 40.4 ± 5.1a | 100 | 1.9 |
| Sucrase | 15.5 ± 6.1b | 20 ± 4 | 4.9 ± 0.9b | 12 ± 3 | 3.1 |
| Palatinase | 4.1 ± 1.5c | 5 ± 1 | 1.5 ± 0.2c | 4 ± 0.3 | 2.7 |
| Trehalase | 2.7 ± 3.1 | 3 ± 3 | 5.1 ± 0.9 | 13 ± 2 | 0.53 |
| Lactase | 1.0 ± 0.5d | 1 ± 0.5 | 1.2 ± 0.5d | 3 ± 0.5 | 0.83 |
Notes:
Significant difference between same letters (P < 0.05). The specific activities of disaccharidases are expressed as μ moles of substrate hydrolyzed/mg protein/h, at 37°C, pH 6.0. Values are mean ± SD in 5 humans and 6 rats. Whole region of the small intestine of rat was used to determine the activity.
Abbreviation: SD, standard deviation.
Inhibitory effect of leaf extract from M. alba and L-arabinose
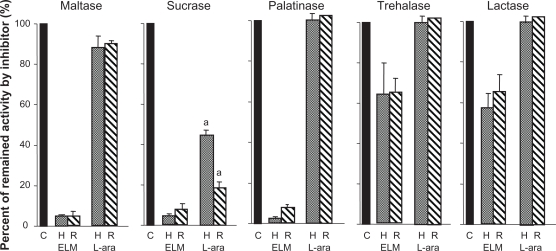
Leaf extract from M. alba competitively inhibits rat small intestinal disaccharidases,15 whereas L-arabinose is a non-competitive disaccharidase inhibitor.16 To compare the effect of these inhibitors on human and rat disaccharidase, we assayed the activity of maltase, sucrase, palatinase, trehalase, and lactase in the presence of M. alba leaf extract or L-arabinose using the small intestinal homogenates of human and rat. The activity of maltase, sucrase, and palatinase was strongly inhibited by M. alba leaf extract, and the level of inhibition was similar in humans and rats. Inhibition of trehalase and lactase was clearly weaker than that of maltase, sucrase, and palatinase, but the inhibitory effect was similar in humans and rats (Figure 1). Inhibition with L-arabinose was generally weaker than that with M. alba leaf extract of five disaccharidases in humans and rats (Figure 1). Inhibition of human sucrase was significantly weaker than that in rats (P < 0.05), but the inhibition of four other enzymes was roughly similar to that in rats. The inhibitory effect of L-arabinose was negligible for palatinase, trehalase, and lactase in humans and rats, and was generally similar, except for sucrase, between humans and rats.
Figure 1.
Comparison of inhibitory effect of M. alba leaf extract and L-arabinose on human and rat disaccharidase activity.
Notes: Data were expressed as the percentage of remaining activity after addition of Morus alba leaf extract and L-arabinose. aSignificant difference between humans and rats (P > 0.05). C, control, no added inhibitor (
 ); H, human (
); H, human (
 ); R, rat (
); R, rat (
 ).
).
Abbreviations: ELM, M. alba leaf extract; L-ara, L-arabinose.
Oligosaccharide digestibility using human and rat small intestinal homogenates
To compare the hydrolyzing activity of human and rat intestinal enzymes for nondigestible or digestible oligosaccharides, the digestibility of 1-kestose, galactosylsucrose, and panose was measured by the same assay method using human and rat small intestinal homogenates. 1-Kestose was not hydrolyzed, galactosylsucrose was barely hydrolyzed, and panose was readily hydrolyzed by human and rat small intestinal enzymes (Table 3). The ratio of hydrolyzing activity of humans versus rats for these three oligosaccharides was between 1 and 1.33, and the hydrolyzing activity towards these oligosaccharides did not differ significantly between human and rat intestinal enzymes.
Table 3.
Digestibility of 1-kestose, galactosylsucrose and panose by human and rat small intestinal homogenates
| Substrate |
Human (n = 5) |
Rats (n = 6) |
|---|---|---|
| Specific activity ± SD | Specific activity ± SD | |
| 1-Kestose | 0.01 ± 0.02 | 0.01 ± 0.04 |
| Galactosylsucrose | 0.28 ± 2.32 | 0.21 ± 0.08 |
| Panose | 3.6 ± 0.4 | 3.1 ± 0.5 |
Notes: The specific activities of disaccharidases are expressed as μ moles of substrate hydrolyzed/mg protein/h, at 37°C, pH 6.0. Values are mean ± SD in 5 humans and 6 rats. There was no significant difference between human and rat. Whole region of the small intestine of rat was used to determine the activity.
Abbreviation: SD, standard deviation.
Discussion
The practical objective of this study was to clarify whether it is reasonable or not that we use rat intestinal samples instead of human intestinal samples for the evaluation of digestibility or nondigestibility of newly developed oligosaccharides and polysaccharides. This evaluation of digestibility or nondigestibility of oligosaccharides and polysaccharides is essential to validate the health claim of functional foods which contain these saccharides. The results obtained in the present study demonstrate that we can use the rat intestinal tissue instead of human intestinal tissue to evaluate the digestibility of oligosaccharides and polysaccharides.
Many human and animal studies have measured the hydrolyzing activity of intestinal disaccharidases in different laboratories, using various methods, and at different times a long time ago.8–11,15,16,21 However, as far as we are aware, there has been no direct comparison of human and rat intestinal disaccharidase activity measured using identical preparation and assay methods. In the present study, we simultaneously measured the activity of sucrase, maltase, palatinase, trehalase, and lactase in human and rat small intestines using identical preparation and assay methods to compare the hydrolyzing activity between the human and the rat. In addition, inhibition of human and rat disaccharidases by α-glucosidase inhibitors was investigated, along with the digestibility of oligosaccharides with different properties.
The hydrolyzing activity of disaccharidase in human small intestine varied greatly between samples and was generally lower in the present study. One explanation for this variability could be that the region from which the tissue samples were obtained differed considerably between patients. In addition, the distal small intestine region which disaccharidase activity is lower was used for assay. Another explanation for the variability is that the age of the patients was older and widely different, with activity decreasing in older patients.13,22–24
Maltase activity was the highest among the five disaccharidases that we studied in humans, followed by sucrase and palatinase, and the lowest activity was for lactase. The specific activity of maltase, sucrase, and palatinase in humans was between 1.9-fold and 3.1-fold higher than that in rats, and the ratio of specific activity in humans and rats was not as great among these three enzymes (Table 2). The marked variation in enzyme activities between different studies10–14 is greater than the difference between humans and rats in the present study. For example, sucrose activity of samples taken by biopsy from 18 American 60–70-year-olds was 70 ± 18 U/mg protein and the variation was 43–108 U/mg protein, and maltase activity was 260 ± 76 U/mg protein and the variation was 130–474 U/mg protein.24 The variation of hydrolyzing activity is wide among humans with similar age. Therefore, the hydrolyzing activity of disaccharidases appears to be generally similar between humans and rats, although it is slightly higher in humans. The activity ratio of sucrose versus maltase was about 0.20 and 0.12 in humans and rats, respectively. The activity ratio of palatinase versus maltase was only 0.05 and 0.04 in humans and rats, respectively. These activity ratios demonstrate that they are not markedly different between humans and rats, although the activity ratio of sucrase in humans was slightly higher than that in rats. The results also confirm that the hydrolyzing activity of disaccharidases, such as maltase, sucrase, and palatinase, is generally similar between humans and rats. Trehalase activity in rats was much higher than that in humans, but it is not appropriate to compare directly the difference between humans. This is because trehalase activity changes according to region of the small intestine, and is highest in the proximal duodenum and lowest in the distal ileum.10,23,24 Furthermore, it differs considerably between species, such as between humans and rats. Trehalase activity in the present study was very low in most of the human samples of distal ileum, and was similar to sucrase activity throughout the small intestine in rats.
Lactase activity is very low in the distal small intestine,10,22,25 and gradually decreases during aging of the subject.13 Namely, lactase activity is higher in younger than in older subjects. The lactase activity in humans was very low in the present study, because most of the tissue samples used were from the distal ileum and were from elderly persons. Therefore, we cannot draw conclusions about the similarity of lactase activity in humans and rats on the basis of our present data.
Leaf extract from M. alba, which is a disaccharidase inhibitor, showed similar inhibitory effects on human and rat disaccharidases, but the inhibitory effect of L-arabinose on disaccharidases differed slightly between humans and rats. In terms of the mechanism of inhibition of disaccharidase, M. alba leaf extract is competitive, whereas L-arabinose is noncompetitive. Although the law of the inhibition is different, the results obtained appear to suggest that the digestibility of oligosaccharides obtained from rat experiments can be extrapolated to humans.
The digestibility of 1-kestose, galactosylsucrose and panose, measured by an identical assay method, did not differ significantly between humans and rats, and the ratio of hydrolyzing activity in humans versus rats was 1–1.33 for these oligosaccharides. These results suggest that the digestibility of saccharides measured for rat small intestinal enzymes can be extrapolated to humans. It is difficult to obtain human samples of small intestine to evaluate the digestibility of some food materials with health benefits. Also, we can obtain α-glucosidase prepared from bacteria, but their hydrolyzing activity is very strong and does not reflect digestion in the human gastrointestinal tract.8,9
The evidence that the hydrolyzing activity of disaccharidases is generally similar between humans and rats, and that the digestibility of saccharides measured using rat small intestinal enzymes can be extrapolated to humans, should contribute to the development of functional foods that utilize the beneficial health effects of new saccharide materials.
Conclusion
The activities of maltase, sucrase, and palatinase in the human and rat small intestine were measured using identical preparation and assay methods, and were compared between humans and rats. The activity ratio of sucrose versus maltase in humans and rats was about 0.20 and 0.12, respectively, and that of palatinase was 0.05 and 0.04 in humans and rats, respectively. These activity ratios did not differ markedly between humans and rats. In addition, the relative activity (human/rat) of maltase, sucrose, and palatinase was 1.9–3.1 and not so big, although the region of intestinal samples used for assay was different between the human and the rat. Furthermore, the digestibility of the three different types of oligosaccharides by small intestinal enzymes was also similar between humans and rats. These results suggest that the hydrolyzing activity of disaccharidases is generally similar between humans and rats, and that the digestibility, which is measured using rat small intestinal enzymes, of oligosaccharides and polysaccharides can be used in a practical way to evaluate the health benefits for humans.
Acknowledgments
We wish to thank Hayashibara Biochemical Laboratory Inc for graciously providing maltose, panose, and trehalose, Meiji Seika Kaisha Co Ltd for kindly donating 1-kestose, Ensuiko Sugar Refining Co Ltd for kindly donating galactosylsucrose, and Mitsui Sugar Co Ltd for kindly donating palatinose. This study was supported in part by a Grant-in-Aid for Scientific Research (B)(2) 16390184.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Oku T. Oligosaccharides with beneficial health effects: A Japanese perspective. Nutr Rev. 1996;54:S59–S66. doi: 10.1111/j.1753-4887.1996.tb03820.x. [DOI] [PubMed] [Google Scholar]
- 2.Oku T. Newly developed sugar substitutes and their function in foods. Proceedings of the Second Asian Congress of Dietetics; Seoul, Korea. August 9–12, 1998. [Google Scholar]
- 3.Oku T. Comparison of digestibility and its mechanism of several oligosaccharides and sugar alcohols. Proceedings of the IUFoST 1996 Regional Symposium on Non-digestive Health Factors for Future Food; Seoul, Korea. October 10–11, 1996. [Google Scholar]
- 4.Tsuji Y, Yamada K, Hosoya N, Moriuchi S. Digestion and absorption of sugars and sugar substitutes in rat small intestine. J Nutr Sci Vitaminol. 1986;32:93–100. doi: 10.3177/jnsv.32.93. [DOI] [PubMed] [Google Scholar]
- 5.Fujita K, Hara K, Sakai S, et al. Effect of 4G-β-D-galactosylsucrose (lactosucrose) on intestinal flora and its digestibility in human. Denpun Kagaku. 1991;38:249–255. Japanese. [Google Scholar]
- 6.Karimzadegan E, Clifford AJ, Hill FW. A rat bioassay for measuring the comparative availability of carbohydrates and its application to legume food, pure carbohydrates and polyols. J Nutr. 1979;109:2247–2259. doi: 10.1093/jn/109.12.2247. [DOI] [PubMed] [Google Scholar]
- 7.Semenza G, Auricchio S. Small-intestinal disaccharidases. In: Seriver CR, Beauset AL, Sly WS, Valk D, editors. Metabolic Basis of Inherited Disease. 6th ed. New York, NY: McGraw Hill; 1989. [Google Scholar]
- 8.Travel FR, Datema R, Woloszczuk W, Malshall JJ, Whelan WJ. Purification and characterization of a pig intestinal α-dextrinase. Eur J Biochem. 1983;143:575–582. doi: 10.1111/j.1432-1033.1983.tb07129.x. [DOI] [PubMed] [Google Scholar]
- 9.Auricchio S, Rubino A, Tosi R, et al. Disaccharidase activities in human intestinal mucosa. Enzymol Biol Clin (Basel) 1963;3:193–198. doi: 10.1159/000458059. [DOI] [PubMed] [Google Scholar]
- 10.Dahlqvist A. Rat-intestinal dextranase. Localization and relation to the other carbohydrases of the digestive tract. Biochem J. 1963;86:72–76. doi: 10.1042/bj0860072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semenza S, Auricchio S, Rubino A. Multiplicity of human intestinal disaccharidases. 1. Chromatographic separation of maltases and of two lactases. Small-intestinal disaccharidases. Biochim Biophys Acta. 1965;96:487–497. doi: 10.1016/0005-2787(65)90565-4. [DOI] [PubMed] [Google Scholar]
- 12.Alpers DH. The relation of size to the relative rates of degradation of intestinal brush border proteins. J Clin Invest. 1972;51:2621–2630. doi: 10.1172/JCI107080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welsh JD, Poley JR, Bhatia M, Stevenson DE. Intestinal disaccharidase activities in relation to age, race, and mucosal damage. Gastroenterology. 1978;75:847–855. [PubMed] [Google Scholar]
- 14.Howell JN, Mellmann J, Ehlers, Flatz G. Intestinal disaccharidase activities and activity ratios in a group of 60 adult German subjects. Hepatogastroenterology. 1980;27:208–212. [PubMed] [Google Scholar]
- 15.Oku T, Yamada M, Nakamura M, Sadamori N, Nakamura S. Inhibitory effects of extractive from leaves of Morus alba on human and rat small intestinal disaccharidase activity. Br J Nutr. 2006;95:933–938. doi: 10.1079/bjn20061746. [DOI] [PubMed] [Google Scholar]
- 16.Seri K, Sanai K, Matsuo N, Kawakubo K, Xue C, Inoue S. Arabinose selectively inhibits intestinal sucrase in an uncompetitive manner and suppressive glycemic response after sucrose ingestion in animals. Metabolism. 1996;45:1368–1374. doi: 10.1016/s0026-0495(96)90117-1. [DOI] [PubMed] [Google Scholar]
- 17.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 18.Oku T, Konish IF, Hosoya N. Mechanism of inhibitory effect of unavailable carbohydrate on intestinal calcium absorption. J Nutr. 1982;112:410–415. doi: 10.1093/jn/112.3.410. [DOI] [PubMed] [Google Scholar]
- 19.Dahlqvist A. Method of assay of intestinal disaccharidases. Anal Biochem. 1964;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–258. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 21.Dahlqvist A. Specificity of the human intestinal disaccharidases and implication for hereditary disaccharide intolerance. J Clin Invest. 1962;41:463–469. doi: 10.1172/JCI104499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goda T. Dietary regulation of small intestinal disaccharidases. J Japan Soci Nutr Food Sci. 1994;47:81–92. doi: 10.1159/000416401. Japanese. [DOI] [PubMed] [Google Scholar]
- 23.Hietanen E. Interspecific variation in the levels of intestinal alkaline phosphatase, adenosine triphosphatase and disaccharidases. Comp Biochem Physiol. 1973;46:A359–A369. doi: 10.1016/0300-9629(73)90426-x. [DOI] [PubMed] [Google Scholar]
- 24.Triadou N, Bataille J, Schmitz J. Longitudinal study of the human intestinal brush border membrane proteins. Distribution of the main disaccharidases and peptidases. Gastroenterology. 1983;85:1326–1332. [PubMed] [Google Scholar]
- 25.Rubino A, Zimbalatti F, Auricchio S. Intestinal disaccharidase activities in adult and suckling rats. Biochim Biophys Acta. 1964;92:305–311. doi: 10.1016/0926-6569(64)90187-7. [DOI] [PubMed] [Google Scholar]