ABSTRACT
Central to Q fever pathogenesis is replication of the causative agent, Coxiella burnetii, within a phagolysosome-like parasitophorous vacuole (PV) in mononuclear phagocytes. C. burnetii modulates PV biogenesis and other host cell functions, such as apoptotic signaling, presumably via the activity of proteins delivered to the host cytosol by a Dot/Icm type IVB secretion system (T4BSS). In this study, we utilized a C. burnetii strain carrying IcmD inactivated by the Himar1 transposon to investigate the requirements for Dot/Icm function in C. burnetii parasitism of human THP-1 macrophage-like cells. The icmD::Tn mutant failed to secrete characterized T4BSS substrates, a defect that correlated with deficient replication, PV development, and apoptosis protection. Restoration of type IVB secretion and intracellular growth of the icmD::Tn mutant required complementation with icmD, -J, and -B, indicating a polar effect of the transposon insertion on downstream dot/icm genes. Induction of icmDJB expression at 1 day postinfection resulted in C. burnetii replication and PV generation. Collectively, these data prove that T4BSS function is required for productive infection of human macrophages by C. burnetii. However, illustrating the metabolic flexibility of C. burnetti, the icmD::Tn mutant could replicate intracellularly when sequestered in a PV generated by wild-type bacteria, where Dot/Icm function is provided in trans, and within a phenotypically similar PV generated by the protozoan parasite Leishmania amazonensis, where host cells are devoid of Dot/Icm T4BSS effector proteins.
IMPORTANCE
Coxiella burnetii, the cause of human Q fever, is the only bacterial pathogen known to replicate in a vacuole resembling a phagolysosome. The organism manipulates host macrophages to promote the biogenesis of a vacuolar compartment permissive for growth. By analogy to the well-established cellular microbiology of Legionella pneumophila, the Dot/Icm type IVB secretion system of C. burnetii is implicated as a critical virulence factor in host cell modification that delivers proteins with effector functions directly into the host cell cytosol. Using new genetic tools, we verify that Dot/Icm function is essential for productive infection of human macrophages by C. burnetii. Interestingly, despite the production of homologous secretion systems, L. pneumophila and C. burnetii have strikingly different temporal requirements for Dot/Icm function during their respective infectious cycles.
Introduction
The zoonotic disease agent Coxiella burnetii is a highly infectious Gram-negative bacterium that causes human Q fever (1). Aerosol-transmitted C. burnetii has a tropism for mononuclear phagocytes such as alveolar macrophages (2, 3). Consequently, infection of cultured primary or immortalized human monocytes/macrophages is considered the most physiologically relevant in vitro model of C. burnetii-host cell interactions (4). Regardless of the host cell type, C. burnetii replicates within a membrane-bound compartment or “parasitophorous vacuole” (PV). The C. burnetii PV is unique among vacuoles occupied by bacterial pathogens in maturing through the endolysosomal pathway to ultimately acquire characteristics of a phagolysosome (4, 5). Here, the pathogen resists lysosomal hydrolases while exploiting the acidic pH for metabolic activation (6).
The notion of the C. burnetii PV as a large phagolysosome is oversimplified. Indeed, the PV also has interactions with autophagic and secretory pathways that promote C. burnetii replication (7–9). A mature PV filled with C. burnetii can occupy nearly the entire host cell cytoplasm (10); thus, extensive recruitment of membrane from other host vesicular compartments must occur during the PV expansion phase. Cumulative evidence indicates that C. burnetii actively mediates PV fusogencity/maintenance (11) and other host cell processes, such as autophagy (9) and apoptosis (12–15), that benefit pathogen growth. Inhibition of apoptosis is considered a pathogenic strategy that accommodates the slow growth rate of C. burnetii (15), while fusion with autophagosomes may provide critical nutrients (9).
Type IVA and IVB secretion systems are critical bacterial virulence factors that deliver effector proteins directly to the host cytosol (16). Circumstantial evidence suggests that proteins translocated by a Dot/Icm type IVB secretion system (T4BSS) are critical for successful parasitism of macrophages by C. burnetii (17). This model is based on the well-established role of the homologous Dot/Icm T4BSS of Legionella pneumophila in subverting host cell processes, such as organelle trafficking, to generate a permissive intracellular growth environment (18). The C. burnetii genome contains 23 homologues of the 26 Legionella dot/icm genes (19), and 62 C. burnetii Dot/Icm substrates have been identified by using L. pneumophila as a surrogate host (12, 20–23, 28). Many C. burnetii T4BSS substrates have features of eucaryotic proteins, such as ankyrin repeat domains, that may functionally mimic or antagonize the activity of host proteins (12, 21–23). However, only AnkG has a defined function in inhibiting apopotosis via binding of the proapoptotic protein gC1qR (p32) (12).
The historic obligate intracellular nature of C. burnetii has severely impeded the development of genetic tools to study type IVB secretion and other putative virulence factors. Fortunately, significant advances in the genetic manipulation of C. burnetii have recently been made (24). Using host cell-based propagation, Beare et al. (25) genetically transformed C. burnetii to chloramphenicol resistance and mCherry red fluorescent protein expression using the mariner-based Himar1 transposon (Tn). Limitations of the procedure include the 2 to 3 months required for the isolation of individual transposon mutants and a technically challenging micromanipulation cloning method. These problems were solved with the development of an axenic (host cell-free) method of C. burnetii propagation (26, 27). Under microaerobic conditions, a medium called acidified citrate cysteine medium 2 (ACCM-2) supports approximately 4 logs (log10) of C. burnetii growth in liquid medium and the formation of small (~0.5 mm in diameter) colonies in solid medium (26). A completely axenic C. burnetii genetic transformation system is now available that allows the isolation of genetic transformants in about 2.5 weeks. ACCM-2 recently facilitated the development of RSF1010 ori-based shuttle vectors that autonomously replicate in C. burnetii (22, 28). These shuttle vectors were used to confirm cytosolic delivery by C. burnetii of 15 Dot/Icm substrates originally identified utilizing the heterologous L. pneumophila system (20, 22, 28).
Axenic growth also allows the isolation of C. burnetii mutants incapable of growth in macrophages. Screening of colonies composed of clonal C. burnetii Himar1 transformants revealed a clone with a transposon insertion in icmD (dotP). In L. pneumophila, IcmD is an inner membrane component of the Dot/Icm T4BSS (29) that is required for productive host cell infection (30). In the present study, we utilized the C. burnetii icmD::Tn mutant, along with new systems for genetic complementation and inducible gene expression, to probe the requirements of Dot/Icm function in C. burnetii parasitism of human macrophages.
RESULTS
A genetic transformant of C. burnetii contains a Himar1 transposon insertion in icmD.
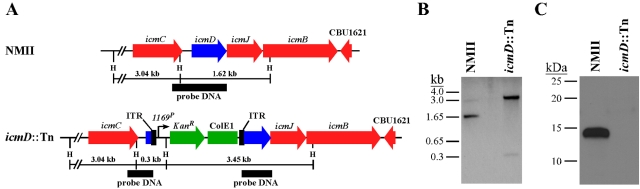
We recently published an axenic protocol for the genetic transformation and clonal isolation of C. burnetii using ACCM-2 (26). An unpublished component of this study confirmed the clonality of randomly picked ACCM-2 colonies by mapping the transposon insertion sites of C. burnetii Nine Mile phase II (NMII) transformed with Himar1. One clone contained a transposon insertion at base 75 of the 414-bp icmD gene (Fig. 1A). Southern blotting confirmed a single transposon insertion in this transformant and, as predicted by the genome sequence, disruption of the icmD-containing 1.62-kb HindIII fragment (Fig. 1B). IcmD-specific antiserum did not detect the IcmD protein (14.3 kDa) in lysates of the icmD::Tn mutant by immunoblotting (Fig. 1C).
FIG 1 .
A Himar1 transposon insertion inactivates C. burnetii icmD. (A) Schematic showing the location of a Himar1 transposon insertion in icmD. The transposon inserted at base 75 of the 414-bp gene. ITR, inverted terminal repeat; H, HindIII site. (B) Southern blot assay of HindIII-digested genomic DNA from NMII and the icmD::Tn mutant hybridized with a probe specific to icmD (black bar in panel A). A 1.62-kb icmD-containing HindIII fragment was disrupted in the icmD mutant. (C) Immunoblotting of lysates from NMII and the icmD::Tn mutant grown in ACCM-2 for 6 days. IcmD-specific antiserum detected the 14.3-kDa IcmD protein in lysates from NMII but not in those of the icmD::Tn mutant.
icmD is dispensable for uptake by macrophages but not productive infection.
To ascertain whether IcmD is required for the productive infection of macrophages, one-step growth curves of C. burnetii in THP-1 macrophages were determined and immunofluorescence microscopy was performed. The axenic replication of NMII and that of the icmD::Tn mutant were indistinguishable, with both strains attaining roughly 10,000-fold increases in genome equivalents (GE) over 6 days of incubation (Fig. 2A). Conversely, the icmD::Tn mutant showed an only 7.9-fold increase in GE at 9 days postinfection (p.i.) of THP-1 macrophages, whereas NMII GE increased by over 10,000-fold. At 6 days p.i., the icmD::Tn mutant did not form the single large, perinuclearly localized PV typical of C. burnetii. Instead, the organism was generally lodged in tight-fitting vacuoles randomly dispersed throughout the cytoplasm (Fig. 2B). The growth deficiency of the icmD mutant was not due to defective uptake by THP-1 macrophages, as entry assays showed cells with equal numbers, i.e., 1.06 ± 0.097 and 1.18 ± 0.107, of internalized NMII and icmD::Tn mutant bacteria, respectively.
FIG 2 .
icmD is required for intracellular growth of C. burnetii. (A) One-step growth curves of NMII and the icmD::Tn mutant grown in ACCM-2 (left panel) or THP-1 macrophages (right panel). Growth was measured by enumerating GE. ACCM-2 results are expressed as the means of three biological replicates from one experiment and are representative of two independent experiments. Error bars indicate the standard deviations of the means. THP-1 macrophage results are expressed as the means of two biological replicates from one experiment and are representative of three independent experiments. Error bars indicate the standard deviations of the means. (B) Fluorescence micrographs of THP-1 macrophages infected for 6 days with NMII or the icmD::Tn mutant. C. burnetii (red) is stained by indirect immunofluorescence, and DNA (blue) is stained with DAPI. Cells contained multiplying NMII harbored within 1 or 2 large and spacious PVs per cell, while the icmD::Tn mutant was typically observed as a single organism in dispersed, tight-fitting PVs. Bar, 20 µm.
icmD is required for cytosolic delivery of T4BSS substrates.
To confirm that the deficiency in intracellular replication of the icmD::Tn mutant correlated with defective cytosolic delivery of T4BSS substrates, the CyaA translocation assay was conducted using THP-1 macrophages infected with C. burnetii transformants expressing CyaA fusions to C. burnetii proteins CpeD and CpeE. Both proteins have been shown to be secreted into the host cytosol by C. burnetii using CyaA and β-lactamase translocation assays and by L. pneumophila in a DotA-dependent fashion using the CyaA assay (22). NMII and the icmD::Tn mutant were transformed with Tn7 constructs encoding CyaA alone or a CyaA fusion protein under the control of the constitutive CBU1169 promoter (22, 24). Tn7 was chosen over RSF1010 ori plasmids for transformations to circumvent potential gene dosage effects associated with plasmid copy number (~3 to 6) (24). We have shown that, as in other Gram-negative bacteria, Tn7 is inserted as a single copy into the C. burnetii chromosome in an intergenic region downstream of glmS (CBU1787), which encodes glucosamine-6-phosphate synthetase (24, 31).
CyaA translocation assays were conducted at 2 days p.i. CpeD and CpeE fusion proteins were secreted by NMII as indicated by a greater-than-100-fold increase in cyclic AMP (cAMP) levels relative to cells harboring organisms expressing CyaA alone (Fig. 3). Conversely, secretion by the icmD::Tn mutant was not detected. Negative secretion was not due to a lack of CyaA fusion protein, as immunoblotting revealed the production of equal amounts of fusion protein by NMII and the icmD::Tn mutant (Fig. 3). Thus, IcmD is required for the secretion of type IVB effectors into the host cell cytosol.
FIG 3 .
icmD is required for cytosolic delivery of T4BSS substrates. Cytosolic levels of cAMP following infection of THP-1 macrophages for 2 days with NMII or the icmD::Tn mutant expressing CyaA alone or CyaA fusions to the previously defined Dot/Icm substrates CpeD and CpeE. Elevated levels of cAMP, indicating secretion, were observed only with NMII expressing CyaA-CpeD or -CpeE fusion protein. Results shown are from one experiment conducted in duplicate and are representative of two independent experiments. Error bars indicate the standard errors of the means. Immunoblotting signals of CyaA and CyaA-effector fusion proteins, depicted above respective histogram bars, show comparable levels of protein expression by NMII and the icmD::Tn mutant after 6 days of growth in ACCM-2. Blots were probed with anti-CyaA antibody.
icmD is required for inhibition of apoptosis.
C. burnetii protein synthesis is required to protect infected cells against apoptotic stimuli (13–15). Moreover, the C. burnetii T4BSS substrate AnkG has defined antiapoptotic activity (12). To determine if defective type IVB secretion by the icmD::Tn mutant correlated with reduced protection from apoptotic cell death, THP-1 macrophages infected for 2 days were treated for 4 h with staurosporine, an intrinsic apoptosis inducer. Following treatment, cells were scored for the presence of nuclear cleaved poly(ADP-ribose) polymerase (PARP), a marker of the terminal stages of apoptosis (32). Cultures infected with the icmD::Tn mutant had approximately the same percentage (~18.5%) of cleaved PARP-positive nuclei as uninfected cell cultures (Fig. 4). This percentage was significantly higher than that in cultures infected with NMII (~3.7%), thereby confirming that C. burnetii type IVB secretion is required to block apoptosis.
FIG 4 .
icmD is required for inhibition of apoptosis. THP-1 macrophages were infected with NMII or the icmD::Tn mutant for 2 days and then treated with staurosporine for 4 h to induce apoptosis. Uninfected cells were used as a control. (A) Detection of cleaved PARP-positive nuclei. C. burnetii (red) and cleaved PARP (green) were labeled by indirect immunofluorescence. DNA (blue) was stained with DAPI. Bar, 20 µm. (B) Enumeration of cleaved, PARP-positive (apoptotic) nuclei. The results shown are from one experiment conducted in triplicate and are representative of two independent experiments. A total of 750 cells were counted for each condition. Error bars indicate the standard deviations of the means, and asterisks indicate a statistically significant difference (P < 0.0005) from cells infected with NMII.
Coinfection with NMII or L. amazonensis rescues intracellular growth of the icmD::Tn mutant.
Alone, the icmD::Tn mutant does not productively infect cultured macrophages. We were curious about whether growth could be rescued upon trafficking of the mutant to PVs harboring isogenic NMII, where Dot/Icm T4BSS functions would be provided in trans. THP-1 macrophages were coinfected with NMII and the icmD::Tn mutant. Immunofluorescence microscopy revealed PVs filled with roughly equal numbers of each strain at 6 days p.i. (Fig. 5), suggesting that the icmD::Tn mutant replicates when coinhabiting a vacuole generated by NMII. GE measurements conducted at 6 days p.i. confirmed the enhanced replication of the icmD::Tn mutant during coinfection, with the mutant yielding 109.2 (±4.7)-fold more GE than bacteria in singly infected cells (P < 0.0001).
FIG 5 .
Coinfection with NMII or L. amazonensis rescues intracellular growth of the icmD::Tn mutant. (Top) THP-1 macrophages were coinfected for 6 days with NMII expressing mCherry red fluorescent protein (24) and the icmD::Tn mutant. Both C. burnetii strains (green) and LAMP-3 (blue) were stained by indirect immunofluorescence. Confocal fluorescence micrographs show coinhabited PVs with replicating NMII (yellow due to red and green overlay) and icmD::Tn mutant bacteria (green only). Bar, 5 µm. (Bottom) Vero cells were infected for 1 day with L. amazonensis promasitgotes expressing GFP and then superinfected for 4 days with the icmD::Tn mutant. The icmD::Tn mutant (red) and LAMP-3 (blue) were stained by indirect immunofluorescence. Confocal fluorescence micrographs showed coinhabited PVs with replicating L. amazonensis and icmD::Tn mutant bacteria. Bar, 5 µm.
The PV of C. burnetii and that of the protozoan parasite L. amazonensis are superficially similar in being fusogenic, moderately acidic (pH 5), and decorated with lysosomal markers (33). Moreover, in Chinese hamster ovary cells, L. amazonensis traffics to preexisting C. burnetii PVs, where it replicates (34). To establish whether the icmD::Tn mutant could traffic to and replicate within the foreign L. amazonensis PV, Vero cells infected with L. amazonensis for 1 day were superinfected with the icmD::Tn mutant. The infection was then allowed to proceed for 4 more days. This coinfection protocol was employed because, in our experiments, it optimized the generation of coinhabited PVs. These vacuoles were still found in less than 10% of the cells, which reflected the low infection efficiency of L. amazonensis. Nonetheless, the icmD::Tn mutant trafficked to L. amazonensis PVs and, without exception, replicated in this compartment, as indicated by vacuoles containing bacteria well in excess of the initial multiplicity of infection (MOI) of 1 (Fig. 5). Enumeration of the icmD::Tn mutant GE was not conducted due to the low number of coinhabited PVs within cell cultures. Nonetheless, these data indicate the icmD::Tn mutant is capable of intracellular growth in the total absence of Dot/Icm T4BSS effector functions if harbored in a suitable acidic compartment.
Complementation of the icmD::Tn mutant requires expression of icmD, icmJ, and icmB.
The C. burnetii icmD gene is the first gene in a predicted operon including icmJ and icmB (35). Therefore, the transposon insertion in the icmD::Tn mutant could have a polar effect that requires complementation of the mutant with icmD, -J, and -B. Consequently, complementation studies were conducted using Tn7 plasmid constructs encoding icmD (pMiniTn7-CAT::icmDP-icmD), icmDJ (pMiniTn7-CAT::icmDP-icmDJ), and icmDJB (pMiniTn7-CAT::icmDP-icmDJB) (see Table S1 and Fig. S1 in the supplemental material). These constructs contain 191 bp upstream of icmD predicted to encode the endogenous icmD promoter.
Functional complementation, as scored by significant GE increases at 6 days p.i. and the production of large PVs harboring replicating bacteria, was observed only with the icmD::Tn mutant transformed with Tn7::icmDJB (Fig. 6A and B). The GE yield of the icmD::Tn mutant transformed with Tn7::icmD or Tn7::icmDJ did not surpass that of the untransformed mutant. QuantiGene transcript analysis of C. burnetii cultivated in ACCM-2 for 4 days revealed negligible levels of icmD, -J, and -B transcripts in the icmD::Tn mutant that were restored to wild-type levels following single-copy, in cis complementation with Tn7::icmDJB (Fig. 6C). Conversely, CBU1169, encoding the small heat shock protein Hsp20, was equally expressed by NMII, the icmD::Tn mutant, and the complemented icmD::Tn mutant (Fig. 6C). Immunoblotting confirmed the production of IcmD by the complemented mutant (Fig. 6D). Collectively, our complementation results support an operon structure for icmD, -J, and -B and confirm that deficient intracellular replication of the icmD::Tn mutant is due to inactivation of the Dot/Icm T4BSS.
FIG 6 .
Intracellular growth of the icmD::Tn mutant requires the expression of icmD, icmJ, and icmB. (A) THP-1 macrophages infected for 6 days with the icmD::Tn mutant transformed with a Tn7 construct containing icmD, icmDJ, or icmDJB under the control of a native C. burnetii promoter. C. burnetii (red) was stained by indirect immunofluorescence, and DNA (blue) was stained with DAPI. Fluorescence micrographs show large PVs harboring replicating C. burnetii only within cells infected with the icmD::Tn mutant transformed with Tn7::icmDJB. Bar, 20 µm. (B) Increases in GE of NMII or the icmD::Tn mutant transformed with Tn7::icmD, Tn7::icmDJ, or Tn7::icmDJB after 6 days of growth in THP-1 macrophages. The asterisk indicates a statistically significant difference (P < 0.05) between the icmD::Tn mutant and the mutant transformed with Tn7::icmDJB. (C) icmD, icmJ, icmB, and CBU1169 transcript levels in NMII, the icmD mutant, and the icmD mutant complemented with Tn7::icmDJB (icmD::Tn comp) after 4 days of growth in ACCM-2. Expression is shown as relative light units (RLU). (D) Immunoblotting of lysates of C. burnetii grown in ACCM-2 for 6 days showing production of IcmD by the complemented mutant and NMII. The experiments depicted in panels B and C were performed in triplicate, and error bars indicate the standard errors of the means.
Induction of icmDJB expression after infection rescues growth of the icmD::Tn mutant.
Successful complementation of the icmD::Tn mutant allowed examination of the temporal requirements of type IVB secretion in productive infection by C. burnetii. These experiments required the development of a system for tightly regulated expression of the icmDJB gene cluster. This was achieved by using a Tn7 plasmid construct containing icmDJB under the control of an anhydrotetracycline (aTc)-inducible promoter (pMiniTn7-CAT::TetRA-icmDJB) (see Fig. S1 in the supplemental material). By immunoblotting, IcmD synthesis by the icmD::Tn mutant transformed with Tn7::TetRA-icmDJB was associated only with induced cultures (Fig. 7A). THP-1 macrophages were infected with the icmD::Tn mutant transformed with Tn7::TetRA-icmDJB, where aTc was added to the culture medium at 0 h or 1 day p.i. These infection conditions were termed concurrent induction (CI) and delayed induction (DI), respectively, and aTc was present in culture medium throughout the infection time course. Infections were also conducted without aTc (no induction, NI). At 6 days postinduction, the CI and DI conditions yielded 50.7- and 9.1-fold increases in transformant GE, respectively (Fig. 7B), and production of large and spacious, LAMP-3-positive PV (Fig. 7C and data not shown). These GE increases were significantly greater than the 4.2-fold GE increase of the transformant under NI conditions, where organisms were found in tight-fitting, LAMP-3-positive phagosomes (Fig. 7B and C).
FIG 7 .

Induction of icmDJB expression after infection rescues the growth of the icmD::Tn mutant. (A) aTc-inducible expression of icmD. The icmD::Tn mutant transformed with a Tn7 construct containing aTc-inducible icmDJB was cultivated in ACCM-2 for 3 days and then induced with aTc for 1 day. Immunoblotting showed aTc-induced synthesis of IcmD. (B and C) aTc induction of icmDJB concurrent with or after infection results in PV production and significant replication by the icmD::Tn mutant at 6 days postinduction. THP-1 macrophages were infected with the icmD::Tn mutant transformed with Tn7::TetRA-icmDJB, where aTc was added to the culture medium at 0 h (CI) or 1 day (DI) p.i. Transformant infections were also conducted without aTc (NI) and with NMII. (B) One asterisk (P < 0.05) and three (P < 0.0005) asterisks indicate significant differences in GE. The experiment was performed three times in duplicate, and error bars indicate the standard deviations of the means. (C) The icmD::Tn mutant grown under CI, but not NI, conditions forms typical large, LAMP-3-positive PVs at 6 days p.i. C. burnetii and LAMP-3 were stained by indirect immunofluorescence and appear red and green, respectively, in the merged image. Bar, 10 µm. (D) Induced expression of icmDJB restores T4BSS function by the icmD::Tn mutant. THP-1 macrophages were infected with the icmD::Tn mutant transformed with a Tn7 construct encoding aTc-inducible icmJBD and a CyaA fusion to CpeD expressed from the constitutive CBU1169 promoter. C. burnetii expressing CyaA alone was used as a control. For induction of icmDJB expression, aTc was added at 1 day p.i. CyaA assays were conducted at 2 days p.i. Elevated levels of cAMP, indicating secretion, were observed only with the complemented mutant expressing CyaA-CpeD under inducing conditions. The results shown are from one experiment conducted in duplicate and are representative of two independent experiments. Error bars indicate the standard errors of the means.
The CyaA translocation assay confirmed that the enhanced replication associated with icmDJB induction correlated with a renewed ability to secrete T4BSS substrates. The icmD::Tn mutant was transformed with a Tn7 construct encoding aTc-inducible icmJBD and a CyaA fusion to CpeD expressed from the constitutive CBU1169 promoter (pMiniTn7-CAT::TetRA-icmDJB-1169P-cyaA-cpeD) (see Fig. S1 in the supplemental material). Assays were conducted using lysates of THP-1 macrophages infected for 2 days with this transformant, with or without aTc induction at 1 day p.i. Cytosolic delivery of CyaA-CpeD by the transformed icmD::Tn mutant occurred only under inducing conditions (Fig. 7D).
Collectively, these data indicate that C. burnetii can initiate significant replication in host cells even when Dot/Icm T4BSS host cell-modulating functions are deployed well after pathogen uptake. Moreover, these data show that the mutant retains viability in a nonpermissive lysosome-like vacuole.
DISCUSSION
Since the release of the Nine Mile reference strain genome sequence, the predicted Dot/Icm T4BSS of C. burnetii has been considered essential for pathogen colonization of macrophage host cells without supporting experimental evidence (36–38). In this study, we present data proving that Dot/Icm function is required for productive macrophage infection. Our icmD::Tn mutant has no defect in axenic growth or internalization by THP-1 macrophages. However, intracellularly, the mutant has severe growth defects, fails to generate a large PV, and does not protect cells from apoptosis. These defects correlate with failed cytosolic translocation of the Dot/Icm T4BSS substrates CpeD and CpeE. Our results are consistent with findings recently published by Carey et al. (20) on epithelial cell interactions of an NMII mutant with icmL inactivated by Himar1. The icmL::Tn mutant shows no defect in axenic growth or uptake by HeLa cells but does not replicate in either HeLa or Vero cells. Furthermore, this mutant fails to translocate three C. burnetii Dot/Icm T4BSS substrates (CBU0077, CBU0635, and CBU1524). Thus, two independent studies have confirmed the critical importance of the Dot/Icm T4BSS in C. burnetii infection of disparate host cell types.
In the present study, we used a newly established C. burnetii transformation system based on Tn7 to complement the icmD::Tn mutant. A Tn7 insertion in the glmS-CBU1788 intergenic region has no demonstrable effect on the axenic and in vivo growth of C. burnetii while having the added benefit of allowing single-copy, in cis complementation (24, 31). Complementation required functional expression of icmD, -J, and -B. This result is consistent with an operon prediction by the Database of Prokaryotic Operons (35) and the observation that transposon inactivation of icmD results in dramatically reduced transcription of downstream icmJ and -B. The transcriptional arrangement of C. burnetii icmD differs from that of L. pneumophila where icmD is predicted to lie at the end of an eight-gene operon including lphA and icmMLKEGCD (30, 35).
The icmD::Tn mutant resides in a nonfusogenic and LAMP-3-positive phagosome that does not expand to form the large and spacious PV that supports robust C. burnetii replication (5, 10). However, at 6 days p.i., we consistently observed icmD::Tn mutant GE increases of approximately 5-fold that appear to result from bacterial division within the first few days of infection. We speculate that the mutant is capable of a few rounds of replication in the acidic but nutritionally deficient phagolysosome, with replication ceasing after the depletion of endogenous and/or exogenous nutrient sources. Relative to the uninduced icmD::Tn mutant, induction of icmDJB expression with aTc at 1 day p.i. results in a significant increase in GE, production of a typical PV, and a renewed ability to secrete Dot/Icm T4BSS substrates. However, the GE yields are significantly less than that of the icmD::Tn mutant induced at 0 h p.i., suggesting that the mutant loses some viability over time. This behavior may reflect the acid-activated metabolism of C. burnetii where loss of adenylate energy charge and infectivity is observed when organisms are incubated in an acidic buffer deficient in oxidizable energy sources (6, 39). Consequently, the icmD::Tn mutant may gradually lose viability while maintaining a detectable genome because the nutrient pool is insufficient to maintain ATP levels. The persistence of viable-but-nonreplicating C. burnetii in a phagolysosome was previously observed in NMII-infected Vero cells treated with chloramphenicol (11). The lower GE yield of the icmD::Tn mutant induced at 0 h p.i. relative to NMII may also suggest suboptimal aTc induction of the icmDJB operon.
The icmD::Tn mutant is capable of intracellular replication if sequestered in a PV generated by isogenic NMII. A dotA mutant of L. pneumophila behaves similarly in macrophages, with a strict requirement for synchronous coinfection with wild-type L. pneumophila (40). A more intriguing behavior is replication of the icmD::Tn mutant in the phagolysosome-like PV of L. amazonensis, a situation where there is a complete absence of Dot/Icm T4BSS-modulating functions. To support the growth of the mutant, the biochemical properties of the Leishmania vacuole must closely mimic those of the C. burnetii PV or growth-permissive ACCM-2. In axenic medium, amino acids are preferred carbon and energy sources of C. burnetii and growth declines precipitously when the oxygen level surpasses 7.5% (27). Thus, and as suggested for the C. burnetii PV (41), the L. amazonensis-containing vacuole, in addition to the low pH required for C. burnetii metabolic activation, likely has ample supplies of amino acids and a low oxygen content. Indeed, due to multiple amino acid auxotrophies, Leishmania spp. appear specifically adapted to growth in a hexose-poor but amino acid-rich modified phagolysosome of macrophages (42). As invoked for the C. burnetii PV (9), fusion between the L. amazonensis PV and autophagosomes is predicted to provide important nutrients, including amino acids (8, 43).
There are striking differences between C. burnetii and L. pneumophila in their temporal requirements for Dot/Icm function, a distinction that reflects their unique intracellular replication niches. To promote the development of an endoplasmic reticulum-derived vacuole that supports replication, L. pneumophila must express DotA prior to infection (44). Failure to do so results in rapid fusion (within 15 min) of the nascent phagosome with lysosomes and a lack of pathogen replication. These fates are observed for most L. pneumophila dot/icm null mutants (44, 45). Induction of DotA expression during bacterial uptake does not rescue pathogen replication. Conversely, both NMII and the icmD::Tn mutant traffic by default through the canonical endolysosomal pathway. The icmD::Tn mutant retains viability in a nonfusogenic phagolysosome and can therefore deploy T4BSS effectors that promote PV development and pathogen growth at 1 day p.i. The Dot/Icm system also participates in the uptake of L. pneumophila, with wild-type bacteria phagocytosed 10 to 20 times more efficiently by human macrophages than dot/icm mutants (46). Conversely, NMII and the icmD::Tn mutant are equally internalized, a result consistent with a previous report showing similar rates of uptake of viable and inactivated C. burnetii by murine L929 cells (47). Because C. burnetii is metabolically quiescent at neutral pH (6), secretion of effectors that induce uptake would not be expected.
In summary, this study confirms that successful macrophage parasitism by C. burnetii requires subversion of host cell functions by a repertoire of secreted Dot/Icm effector proteins. The generation of C. burnetii dot/icm mutants allows careful dissection of the specific cellular processes mediated by type IVB secretion, such as apoptotic signaling and PV biogenesis. This work also illustrates the growing amenability of C. burnetii to genetic manipulation.
MATERIALS AND METHODS
C. burnetii, L. amazonensis, Escherichia coli, and mammalian cells.
C. burnetii NMII (clone 4, RSA439) was used in this study. NMII was axenically cultivated in ACCM-2 as previously described (26). L. amazonensis IFLA/BR/67PH8/GFP was cultured as described by Wilson et al. (48). E. coli strains TOP10, PIR1, and BL21-AI (Invitrogen, Carlsbad, CA) were used for recombinant DNA procedures. THP-1 cells, a human acute monocytic leukemia cell line (TIB-202; ATCC), and African green monkey kidney (Vero) cells (CCL-81; ATCC) were maintained in RPMI 1640 medium (Invitrogen) containing 10% fetal calf serum (Invitrogen) at 37°C and 5% CO2.
Cell culture infection and aTc induction.
With the exception of C. burnetii-L. amazonensis coinfections, all experiments utilized phorbol 12-myristate 13-acetate (PMA)-differentiated THP-1 cells, which accurately mimic the properties of human primary macrophages (49). To induce differentiation into macrophage-like cells, THP-1 were cells treated with 200 nM PMA (EMD Biosciences, San Diego, CA) for 2 days. Cells were washed twice with phosphate-buffered saline (PBS; 1 mM KH2PO4, 155 mM NaCl, 3 mM Na2HPO4, pH 7.4) to remove PMA prior to infection. For entry assays, cells were incubated for an additional 3 days in fresh RPMI medium following PMA treatment. This procedure induces a flattened cell morphology (49) that assists in the microscopic enumeration of internalized C. burnetii bacteria. Coinfection and growth assays used cells infected at an MOI of 1; cleaved PARP assays used cells infected at an MOI of 25; and CyaA, QuantiGene, immunoblotting, and entry assays used cells infected at an MOI of 200. L. amazonensis infection of Vero cells was conducted with stationary-phase promastigotes at an MOI of 50. With the exception of apoptosis assays, all inocula were removed after a 2-h incubation, cells were washed twice, and then fresh medium was added to infected cell cultures. This time point was considered 0 h p.i. With the exception of entry assays, all experiments used 24-well tissue culture plates. For entry assays, ibiTreat tissue culture-treated channeled µ-Slides VI0.4 (ibidi, LLC, Verona, WI) were employed. Evaluation of C. burnetii replication in ACCM-2 or THP-1 macrophages was conducted by quantitative PCR (Q-PCR) of GE using a primer-probe set specific to the dotA gene as previously described (5, 27). Replication of mutant C. burnetii during coinfection with wild-type bacteria was specifically quantified by Q-PCR using a primer-probe set specific to the kanamycin resistance gene (nptII). Where indicated, aTc (Sigma-Aldrich, St Louis, MO) was added to C. burnetii ACCM-2 cultures and infected THP-1 macrophage cultures at final concentrations of 200 and 400 ng/ml, respectively, for induction of C. burnetii transformant gene expression. Where indicated, staurosporine (EMD Biosciences) was added to cell cultures at a final concentration of 500 nM to induce apoptosis.
Plasmid construction.
The plasmids used in this study are listed in Table S1 in the supplemental material. Details of plasmid construction are given in Table S2 in the supplemental material. DNA sequences were amplified by PCR using Accuprime pfx polymerase (Invitrogen) and gene-specific primers (Integrated DNA Technologies, Coralville, IA).
Transformation with Himar1 and Tn7.
C. burnetii cultured in ACCM-2 was transformed by electroporation as previously described (26). For transformation with Himar1, C. burnetii was coelectroporated with two suicide plasmids that individually code for the Himar1 transposon (pITR-Kan) and the Himar1 transposase (pUC19::1169P-Himar1C9) (see Fig. S2 in the supplemental material). For transformation with miniTn7, C. burnetii was coelectroporated with two suicide plasmids that individually code for the Tn7 transposase (pTnS2::1169P-tnsABCD) and modified Tn7 (derivatives of pMiniTn7T-CAT) (24). Maps of the pMiniTn7T-CAT constructs used in this study are shown in Fig. S1 in the supplemental material. The genomic locations of Himar1 insertions were determined using a modified version of a semirandom two-step PCR protocol described by Chun et al. (50). The Tn-specific primers used in the first- and second-round PCR amplifications were ColE1-3′out (5′ AAGGGAGAAAGGCGGACAGG 3′) and ColE1-3′out-nested (5′ CGCCTGGTATCTTTATAGTCCTGTC 3′), respectively. All of the C. burnetii transformants used in this study were cloned by colony picking.
PV and cleaved PARP staining.
Methanol fixation and staining procedures for indirect immunofluorescence assays were conducted as previously described (11). Guinea pig anti-C. burnetii serum, rabbit anti-cleaved PARP serum (Cell Signaling Technology, Danvers, MA), and a mouse monoclonal antibody directed against LAMP-3 (CD63) (clone H5C6; BD Biosciences) were used as primary antibodies. Alexa Fluor 488 and 594 IgG (Invitrogen) were used as secondary antibodies. Coverslips were mounted using ProLong Gold containing 4',6-diamidino-2-phenylindole (DAPI; Invitrogen) to visualize DNA. Epifluorescence microscopy images were acquired with a TE-2000 microscope equipped with a CoolSNAP HQ digital camera (Roper Scientific, Tucson, AZ) or a Ti-U microscope equipped with a DS-Qi1Mc camera (Nikon, Melville, NY). Confocal fluorescence microscopy was performed with a modified Perkin-Elmer UltraView spinning-disc confocal system connected to a Nikon Eclipse Ti-E inverted microscope. Confocal images (0.2-µm sections) were acquired with a 63× oil immersion objective and a Photometrics Cascade II: 512 digital camera (Princeton Instruments, Trenton, NJ) using Metamorph software (Molecular Devices, Inc., Downingtown, PA). All images were processed similarly using ImageJ software (written by W. S. Rasband at the U.S. National Institutes of Health, Bethesda, MD, and available at http://rsb.info.nih.gov/ij/).
Entry assay.
THP-1 macrophages were exposed to C. burnetii for 2 h, fixed with 2.5% paraformaldehyde for 15 min on ice, and then washed three times with PBS. Subsequent steps were done at room temperature. Cells were blocked with 1% bovine serum albumin (BSA) in PBS for 15 min and then incubated with rabbit anti-C. burnetii serum for 15 min. Cells were washed, permeabilized with 0.1% Triton X-100 in 1% BSA-PBS for 15 min, and then incubated with guinea pig anti-C. burnetii serum for 15 min. Cells were washed and then incubated with anti-rabbit Alexa Fluor 594 and anti-guinea pig Alexa Fluor 488 for 15 min. Cells were mounted in ProLong gold containing DAPI. Intracellular bacteria (green only) were enumerated by epifluorescence microscopy. The experiment was conducted three times in triplicate, and 100 host cells were counted per replicate.
QuantiGene transcript analysis.
mRNA quantification was performed using the QuantiGene reagent system v.2.0 (Panomics, Santa Clara, CA) as previously described (22) and custom designed probes specific to icmD, icmJ, icmB, and CBU1169 (22). RNA was extracted from C. burnetii after 4 days of growth in ACCM-2.
IcmD antiserum production.
The C. burnetii icmD gene was cloned without its predicted 5′ N-terminal signal anchor sequence-encoding region (bp 1 to 84) into the 6×His expression vector pEXP1 (Invitrogen) to generate pEXP1::icmD-SP (see Table S2 in the supplemental material). E. coli BL21/pEXP1::icmD-SP was induced for 4 h with 0.2% arabinose and 6×His-IcmD-SP purified by Ni2+ chromatography using a His-Bind buffer kit (EMD Biosciences) in the presence of 6 M urea. Urea was removed using an Amicon Ultra centrifugal filter (3-kDa cutoff; Millipore Corp., Billerica, MA). Purified 6×His-IcmD-SP (250 µg) in 25 mM Tris buffer (pH 7.2) with 0.05% Triton X-100 was mixed with the Sigma Adjuvant System and used to immunize a New Zealand White rabbit. Rabbit antiserum was generated according to protocol 2008-32.1, approved by the Rocky Mountain Laboratories Animal Care and Use Committee.
Immunoblotting.
C. burnetii expression of CyaA fusion proteins or IcmD was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting. Membranes were incubated with rabbit anti-IcmD polyclonal antibody or a mouse monoclonal antibody directed against CyaA (clone 3D1; Santa Cruz Biotechnology, Santa Cruz, CA). Reacting proteins were detected using anti-rabbit (IcmD) or anti-mouse (CyaA) IgG secondary antibodies conjugated to horseradish peroxidase (Pierce, Rockford, IL) and chemiluminescence using ECL Pico reagent (Pierce).
CyaA translocation assay.
CyaA translocation assays were performed as previously described, using the cAMP enzyme immunoassay (GE Healthcare, Piscataway, NJ) (23).
Statistical analysis.
Statistical analyses were performed using the unpaired Student t test and Prism software (GraphPad Software, Inc., La Jolla, CA).
SUPPLEMENTAL MATERIAL
Maps of the pMiniTn7T-CAT constructs used in this study. Download Figure S1, PDF file, 0.4 MB.
Maps of the Himar1 transposase and transposon constructs used in this study. Download Figure S2, PDF file, 0.2 MB.
Plasmids used in this study.
Plasmid construction.
ACKNOWLEDGMENTS
We thank Norma Andrews, Mauro Veliz, and Maria Fernandes of the University of Maryland for supplying L. amazonensis and helpful advice.
This work was supported by funding from the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) grant R01AI087669 (D.E.V.), and the Intramural Research Program of the NIH, NIAID (R.A.H.).
Footnotes
Citation Beare PA, Gilk SD, Larson CL, Hill J, Stead CM, et al. 2011. Dot/Icm type IVB secretion system requirements for Coxiella burnetii growth in human macrophages. mBio 2(4):e00175-11. doi:10.1128/mBio.00175-11.
REFERENCES
- 1. Maurin M, Raoult D. 1999. Q fever. Clin. Microbiol. Rev. 12:518–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khavkin T, Tabibzadeh SS. 1988. Histologic, immunofluorescence, and electron microscopic study of infectious process in mouse lung after intranasal challenge with Coxiella burnetii. Infect. Immun. 56:1792–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stein A, et al. 2005. Q fever pneumonia: virulence of Coxiella burnetii pathovars in a murine model of aerosol infection. Infect. Immun. 73:2469–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Voth DE, Heinzen RA. 2007. Lounging in a lysosome: the intracellular lifestyle of Coxiella burnetii. Cell. Microbiol. 9:829–840 [DOI] [PubMed] [Google Scholar]
- 5. Howe D, Shannon JG, Winfree S, Dorward DW, Heinzen RA. 2010. Coxiella burnetii phase I and II variants replicate with similar kinetics in degradative phagolysosome-like compartments of human macrophages. Infect. Immun. 78:3465–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hackstadt T, Williams JC. 1981. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc. Natl. Acad. Sci. U. S. A. 78:3240–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Campoy EM, Zoppino FC, Colombo MI. 2011. The early secretory pathway contributes to the growth of the Coxiella-replicative niche. Infect. Immun. 79:402–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gutierrez MG, et al. 2005. Autophagy induction favours the generation and maturation of the Coxiella-replicative vacuoles. Cell. Microbiol. 7:981–993 [DOI] [PubMed] [Google Scholar]
- 9. Romano PS, Gutierrez MG, Berón W, Rabinovitch M, Colombo MI. 2007. The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell. Microbiol. 9:891–909 [DOI] [PubMed] [Google Scholar]
- 10. Coleman SA, Fischer ER, Howe D, Mead DJ, Heinzen RA. 2004. Temporal analysis of Coxiella burnetii morphological differentiation. J. Bacteriol. 186:7344–7352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howe D, Melnicáková J, Barák I, Heinzen RA. 2003. Maturation of the Coxiella burnetii parasitophorous vacuole requires bacterial protein synthesis but not replication. Cell. Microbiol. 5:469–480 [DOI] [PubMed] [Google Scholar]
- 12. Lührmann A, Nogueira CV, Carey KL, Roy CR. 2010. Inhibition of pathogen-induced apoptosis by a Coxiella burnetii type IV effector protein. Proc. Natl. Acad. Sci. U. S. A. 107:18997–19001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lührmann A, Roy CR. 2007. Coxiella burnetii inhibits activation of host cell apoptosis through a mechanism that involves preventing cytochrome c release from mitochondria. Infect. Immun. 75:5282–5289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Voth DE, Heinzen RA. 2009. Sustained activation of Akt and Erk1/2 is required for Coxiella burnetii antiapoptotic activity. Infect. Immun. 77:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Voth DE, Howe D, Heinzen RA. 2007. Coxiella burnetii inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages. Infect. Immun. 75:4263–4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wallden K, Rivera-Calzada A, Waksman G. 2010. Type IV secretion systems: versatility and diversity in function. Cell. Microbiol. 12:1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Voth DE, Heinzen RA. 2009. Coxiella type IV secretion and cellular microbiology. Curr. Opin. Microbiol. 12:74–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Isberg RR, O’Connor TJ, Heidtman M. 2009. The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat. Rev. Microbiol. 7:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vogel JP. 2004. Turning a tiger into a house cat: using Legionella pneumophila to study Coxiella burnetii. Trends Microbiol. 12:103–105 [DOI] [PubMed] [Google Scholar]
- 20. Carey KL, Newton HJ, Luhrmann A, Roy CR. 2011. The Coxiella burnetii Dot/Icm system delivers a unique repertoire of type IV effectors into host cells and is required for intracellular replication. PLoS Pathog. 7:e1002056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pan X, Lührmann A, Satoh A, Laskowski-Arce MA, Roy CR. 2008. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science 320:1651–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Voth DE, et al. 2011. The Coxiella burnetii cryptic plasmid is enriched in genes encoding type IV secretion system substrates. J. Bacteriol. 193:1493–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Voth DE, et al. 2009. The Coxiella burnetii ankyrin repeat domain-containing protein family is heterogeneous, with C-terminal truncations that influence Dot/Icm-mediated secretion. J. Bacteriol. 191:4232–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beare PA, Sandoz KM, Omsland A, Rockey DD, Heinzen RA. 2011. Advances in genetic manipulation of obligate intracellular bacterial pathogens. Front. Microbiol. 2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beare PA, et al. 2009. Characterization of a Coxiella burnetii ftsZ mutant generated by Himar1 transposon mutagenesis. J. Bacteriol. 191:1369–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Omsland A, et al. 2011. Isolation from animal tissue and genetic transformation of Coxiella burnetii are facilitated by an improved axenic growth medium. Appl. Environ. Microbiol. 77:3720–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Omsland A, et al. 2009. Host cell-free growth of the Q fever bacterium Coxiella burnetii. Proc. Natl. Acad. Sci. U. S. A. 106:4430–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen C, et al. 2010. Large-scale identification and translocation of type IV secretion substrates by Coxiella burnetii. Proc. Natl. Acad. Sci. U. S. A. 107:21755–21760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vincent CD, et al. 2006. Identification of the core transmembrane complex of the Legionella Dot/Icm type IV secretion system. Mol. Microbiol. 62:1278–1291 [DOI] [PubMed] [Google Scholar]
- 30. Purcell M, Shuman HA. 1998. The Legionella pneumophila icmGCDJBF genes are required for killing of human macrophages. Infect. Immun. 66:2245–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Choi KH, et al. 2005. A Tn7-based broad-range bacterial cloning and expression system. Nat. Methods. 2:443–448 [DOI] [PubMed] [Google Scholar]
- 32. Jin Z, El-Deiry WS. 2005. Overview of cell death signaling pathways. Cancer Biol. Ther. 4:139–163 [DOI] [PubMed] [Google Scholar]
- 33. Antoine JC, Prina E, Lang T, Courret N. 1998. The biogenesis and properties of the parasitophorous vacuoles that harbour Leishmania in murine macrophages. Trends Microbiol. 6:392–401 [DOI] [PubMed] [Google Scholar]
- 34. Veras PS, et al. 1995. Entry and survival of Leishmania amazonensis amastigotes within phagolysosome-like vacuoles that shelter Coxiella burnetii in Chinese hamster ovary cells. Infect. Immun. 63:3502–3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mao F, Dam P, Chou J, Olman V, Xu Y. 2009. DOOR: a database for prokaryotic operons. Nucleic Acids Res. 37:D459–D463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Segal G, Feldman M, Zusman T. 2005. The Icm/Dot type-IV secretion systems of Legionella pneumophila and Coxiella burnetii. FEMS Microbiol. Rev. 29:65–81 [DOI] [PubMed] [Google Scholar]
- 37. Segal G, Shuman HA. 1999. Possible origin of the Legionella pneumophila virulence genes and their relation to Coxiella burnetii. Mol. Microbiol. 33:669–670 [DOI] [PubMed] [Google Scholar]
- 38. Seshadri R, et al. 2003. Complete genome sequence of the Q-fever pathogen Coxiella burnetii. Proc. Natl. Acad. Sci. U. S. A. 100:5455–5460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hackstadt T, Williams JC. 1981. Stability of the adenosine 5′-triphosphate pool in Coxiella burnetii: influence of pH and substrate. J. Bacteriol. 148:419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coers J, Monahan JJ, Roy CR. 1999. Modulation of phagosome biogenesis by Legionella pneumophila creates an organelle permissive for intracellular growth. Nat. Cell Biol. 1:451–453 [DOI] [PubMed] [Google Scholar]
- 41. Omsland A, Heinzen RA. Life on the outside: the rescue of Coxiella burnetii from its host cell. Annu. Rev. Microbiol., in press [DOI] [PubMed] [Google Scholar]
- 42. McConville MJ, de Souza D, Saunders E, Likic VA, Naderer T. 2007. Living in a phagolysosome; metabolism of Leishmania amastigotes. Trends Parasitol. 23:368–375 [DOI] [PubMed] [Google Scholar]
- 43. Schaible UE, et al. 1999. Parasitophorous vacuoles of Leishmania mexicana acquire macromolecules from the host cell cytosol via two independent routes. J. Cell Sci. 112:681–693 [DOI] [PubMed] [Google Scholar]
- 44. Roy CR, Berger KH, Isberg RR. 1998. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol. Microbiol. 28:663–674 [DOI] [PubMed] [Google Scholar]
- 45. Wiater LA, Dunn K, Maxfield FR, Shuman HA. 1998. Early events in phagosome establishment are required for intracellular survival of Legionella pneumophila. Infect. Immun. 66:4450–4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hilbi H, Segal G, Shuman HA. 2001. Icm/dot-dependent upregulation of phagocytosis by Legionella pneumophila. Mol. Microbiol. 42:603–617 [DOI] [PubMed] [Google Scholar]
- 47. Baca OG, Klassen DA, Aragon AS. 1993. Entry of Coxiella burnetii into host cells. Acta Virol. 37:143–155 [PubMed] [Google Scholar]
- 48. Wilson J, et al. 2008. Control of parasitophorous vacuole expansion by LYST/Beige restricts the intracellular growth of Leishmania amazonensis. PLoS Pathog. 4:e1000179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. 2010. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One 5:e8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chun KT, Edenberg HJ, Kelley MR, Goebl MG. 1997. Rapid amplification of uncharacterized transposon-tagged DNA sequences from genomic DNA. Yeast 13:233–240 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Maps of the pMiniTn7T-CAT constructs used in this study. Download Figure S1, PDF file, 0.4 MB.
Maps of the Himar1 transposase and transposon constructs used in this study. Download Figure S2, PDF file, 0.2 MB.
Plasmids used in this study.
Plasmid construction.