Abstract
T cell Ig domain and mucin domain protein 1 (TIM-1) is a costimulatory molecule that regulates immune responses by modulating CD4+ T cell effector differentiation. However, the function of TIM-1 on other immune cell populations is unknown. Here, we show that in vivo in mice, TIM-1 is predominantly expressed on B rather than T cells. Importantly, TIM-1 was expressed by a large majority of IL-10–expressing regulatory B cells in all major B cell subpopulations, including transitional, marginal zone, and follicular B cells, as well as the B cell population characterized as CD1dhiCD5+. A low-affinity TIM-1–specific antibody that normally promotes tolerance in mice, actually accelerated (T cell–mediated) immune responsiveness in the absence of B cells. TIM-1+ B cells were highly enriched for IL-4 and IL-10 expression, promoted Th2 responses, and could directly transfer allograft tolerance. Both cytokine expression and number of TIM-1+ regulatory B cells (Bregs) were induced by TIM-1–specific antibody, and this was dependent on IL-4 signaling. Thus, TIM-1 is an inclusive marker for IL-10+ Bregs that can be induced by TIM-1 ligation. These findings suggest that TIM-1 may be a novel therapeutic target for modulating the immune response and provide insight into the signals involved in the generation and induction of Bregs.
Introduction
T cell Ig domain and mucin domain (TIM) proteins constitute a family of costimulatory molecules that play an important role in effector differentiation of CD4+ cells (1). The 8 murine and 3 human genes encoding the TIM family are clustered in a chromosomal region (5q32.2 in humans and 11B1.1 in mice) closely associated with autoimmune disease. For example, TIM domain protein 1 (TIM-1) polymorphisms are associated with susceptibility to human asthma, eczema, and rheumatoid arthritis (2, 3). TIM-1 is expressed on activated CD4+ cells and Th2 cells after polarization in vitro (4). TIM-4 is a putative TIM-1 ligand; however, these phosphatidylserine receptors may interact indirectly through an exosome bridge (5, 6). The role of TIM-1 has previously been studied using anti–TIM-1 mAbs. For example, TIM-1 ligation with a high-affinity mAb, 3B3, promotes expansion of antigen-specific T cells expressing Th1 and Th17 cytokines while inhibiting Tregs (4, 7, 8). Concordantly, 3B3 treatment exacerbates EAE (7) and prevents allograft tolerance mediated by anti-CD154 (8). In contrast, a lower-affinity anti–TIM-1 mAb, RMT1-10, inhibits EAE (7) and, when combined with rapamycin, induces long-term allograft acceptance in mice (9). Prolonged engraftment by RMT1-10 is dependent on Th2-cytokine skewing and Treg activity (9). Thus, TIM-1 is a potent regulator of T cell effector responses in both auto- and alloimmunity.
In addition to humoral immunity, B cells play an increasingly recognized role in shaping T effector cell responses through antigen presentation, costimulation, and cytokine production (10). For example, in both humans and mice, B cell deficiency or depletion can ameliorate autoimmune diseases primarily mediated by T cells, including type 1 diabetes and rheumatoid and collagen-induced arthritis (11–13). However, in various other murine models, such as EAE, inflammatory bowel disease, and contact hypersensitivity, B cell deficiency or depletion worsens disease (14–18), which suggests that B cells can also exhibit immunomodulatory function. Indeed, subpopulations of splenic B cells from naive or autoimmune mice can inhibit inflammation in an IL-10–dependent manner (10, 19–21). However, definitive identification has been challenging because such regulatory B cells (Bregs) are rare, lack a specific marker, and express detectable IL-10 only upon ex vivo stimulation.
Various Breg phenotypes have been described. For example, Bregs have been detected within splenic marginal zone (MZ) populations (22–24) or less-mature transitional 2–MZ precursor (T2-MZ) populations (18, 25). Some studies suggest that Bregs may also reside within the much larger follicular (FO) B cell subset (23, 25, 26). Recently, Yanaba et al. identified a small subset (~2%) of splenic B cells expressing a CD1dhiCD5+ phenotype that partially overlaps with that of MZ, T2-MZ, and B1 B cells (15). CD1dhiCD5+ B cells are more enriched for IL-10–producing cells (9%–15%) than other B cell subsets, and it was suggested that they might account for most Breg activity observed in spleen. However, most IL-10+ B cells fall outside of the CD1dhiCD5+ population. A marker that can identify the majority of IL-10+ B cells is critical for improved understanding of Breg biology, including the relationships among Bregs exhibiting different phenotypes.
In the present study we showed that in vivo, TIM-1 was predominantly expressed on B cells, both constitutively and after activation. Surprisingly, the tolerogenic effects of RMT1-10 were completely dependent on TIM-1+ B cells. TIM-1 identified a large majority of B cells capable of IL-4 and IL-10 expression regardless of other markers. Finally, TIM-1+ Bregs were induced by TIM-1 ligation and could transfer long-term acceptance of islet allografts to otherwise-untreated recipients in an IL-10–dependent fashion and could also inhibit allergic airway disease.
Results
B lymphocytes express high levels of TIM-1 compared with T cells.
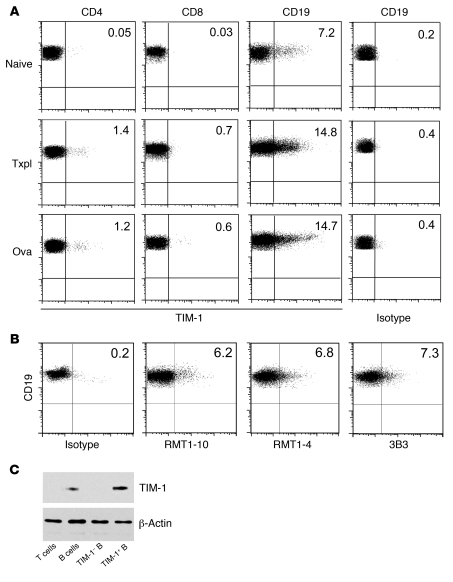
Previous reports have demonstrated that TIM-1 is expressed by activated T cells, in particular by polarized Th2 cells, in vitro (4). However, in vivo, we found that less than 2% of splenic CD4+ or CD8+ T cells expressed TIM-1, even after immunization with an islet allograft or OVA (Figure 1A). This was not increased by gating on Th1 (IFN-γ+) or Th2 (IL-4+ or IL-10+) CD4+ T cells induced by these stimuli (Supplemental Figure 1A; supplemental material available online with this article; doi: 10.1172/JCI46274DS1). Similarly, TIM-1 expression on Foxp3+ Tregs and on CD11c+ or CD11b+ leukocytes was minimal (Supplemental Figure 1, A and B). In contrast, we found that in naive mice, 5%–8% of splenic B cells constitutively expressed TIM-1, and this increased to 10%–15% after immunization with an allograft or OVA (Figure 1A). The same level of TIM-1 expression on B cells was observed using 3 different previously described (4, 7, 9, 27) anti–TIM-1 mAbs (Figure 1B). Finally, levels of TIM-1 surface expression, as detected by flow cytometry, correlated with protein expression, determined by Western blotting of sort-purified T cells, B cells, and TIM-1+ and TIM-1– B cells (Figure 1C).
Figure 1. B lymphocytes express relatively high levels of TIM-1.
(A) Representative flow cytometry plots showing TIM-1 expression on splenic CD4+ and CD8+ T cells and CD19+ B cells in naive BALB/c mice or at 14 days after immunization with either allogeneic (B6) islet transplantation (Txpl) or OVA (20 μg with 4 mg alum i.p. on days 0 and 7). Cell staining was performed in the presence of anti-CD16/CD32 to block FcR binding, and isotype- and fluorochrome-matched negative controls were used to set the cursors. Isotype control staining for CD19+ cells is shown at right. n ≥ 3 per group. (B) Representative flow cytometry plots showing TIM-1 expression on CD19+ B cells from naive mice assessed by indirect staining with anti–TIM-1 mAbs RMT1-10, RMT1-4, and 3B3 followed by PE-conjugated anti-rat Ig secondary mAb. n = 3 per group. (C) Anti–TIM-1 immunoblot of cell lysates from sort-purified T cells, B cells, TIM-1+ B cells, and TIM-1– B cells. Representative of 2 independent experiments. Numbers denote percent TIM-1+ cells within each population.
B lymphocytes are required for prolonged allograft survival induced by anti–TIM-1.
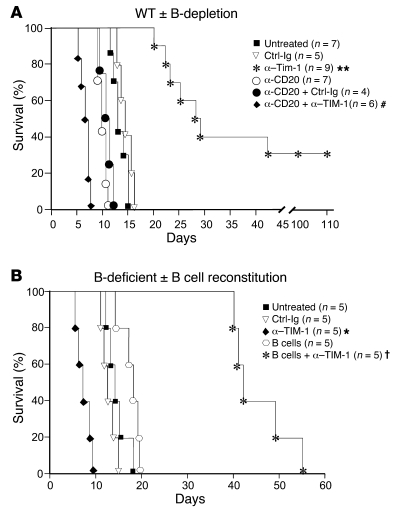
Based on high levels of expression, we addressed the role of TIM-1 on B cells. Chemically diabetic BALB/c recipients of C57BL/6 (B6) islet allografts were untreated or treated with anti–TIM-1 mAb RMT1-10, which we previously showed to inhibit EAE and prolong cardiac allograft survival (7, 9). In untreated WT mice or those treated with isotype control mAb, islet rejection occurred with a median survival time (MST) of 13 days (Figure 2A). Treatment with 3 doses of anti–TIM-1 significantly prolonged islet allograft survival (MST, 28 days), with approximately 30% of mice achieving long-term engraftment (>100 days). Depletion of B cells in recipients prior to transplantation using anti-CD20 slightly shortened allograft survival compared with B cell–intact mice (MST, 10 days). Surprisingly, rather than prolonging allograft survival, anti–TIM-1 treatment significantly accelerated allograft rejection in B cell–depleted recipients (MST, 6 days). This was confirmed in chemically diabetic B cell–deficient JHD (BALB/c) recipients of B6 islets. Untreated JHD recipients, or those treated with isotype control mAb, rejected islet allografts with a MST of 12–14 days (Figure 2B). As in B cell–depleted WT recipients, JHD mice treated with anti–TIM-1 exhibit a 2-fold acceleration in rate of acute rejection (MST, 7 days). Reconstitution of JHD mice with 107 WT B cells, followed by anti–TIM-1 treatment, significantly enhanced allograft survival (MST, 42 days). Thus, not only were B cells required for enhanced allograft survival by anti–TIM-1, but in their absence, this mAb paradoxically shortened survival. This reveals a key role for B cells in TIM-1–mediated tolerance.
Figure 2. B lymphocytes are required for anti–TIM-1–mediated prolongation of allograft survival.
(A) Chemically diabetic BALB/c mice were untreated or subjected to B cell depletion with anti-CD20 (250 μg i.v. on days –14 and –1) followed by transplantation with B6 islets. Allograft recipients were either untreated or treated with RMT1-10 or control Ig at 0.5 mg (day –1) and 0.3 mg (days 0 and 5). (B) JHD mice were unmanipulated or received 107 WT syngeneic B cells followed by transplantation with B6 islets (day 0). Allograft recipients were treated as in A. Shown are Kaplan-Meir plots of graft survival. *P < 0.05, **P < 0.01 vs. untreated or control Ig; #P < 0.05 vs. other anti-CD20 groups; †P < 0.01 vs. B cells.
B cells are required for Th2 cytokine expression induced by anti–TIM-1.
Previously, we showed that RMT1-10 augmented Th2 responses and that this was required for prolonged cardiac allograft survival (9). Similarly, in islet allograft recipients, anti–TIM-1 inhibited IFN-γ expression and augmented IL-4 and IL-10 expression by CD4+ T cells (Figure 3, A and C). B cell depletion alone (anti-CD20) modestly increased IFN-γ expression in allograft recipients. However, treatment of B cell–depleted recipients with anti–TIM-1 significantly enhanced IFN-γ and completely prevented the normally observed increase in Th2 cytokines. Thus, B cells are required for the Th2 shift observed after anti–TIM-1 treatment. Previous findings also suggest that anti–TIM-1 can enhance Treg function (9). This may be explained in part by the enhanced IL-10 expression by Foxp3+ Tregs we observed after anti–TIM-1 treatment, averaging 1.6-fold (Figure 3B and data not shown). However, in the setting of B cell depletion, anti–TIM-1 reduced IL-10 expression by Tregs by an average of 40%, which suggests that B cells may also support Treg function after TIM-1 ligation.
Figure 3. B lymphocytes are required for Th2-deviation induced by anti–TIM-1.
IL-4 EGFP reporter mice (BALB/c) were untreated or subjected to B cell depletion with anti-CD20 followed by transplantation with B6 islets. Allograft recipients were either untreated or treated with anti–TIM-1 or control Ig as in Figure 2. On day 14 after transplantation, cytokine expression on splenic CD4+ T cells was examined by flow cytometry after in vitro stimulation (see Methods). IL-4 was detected by EGFP expression, and IFN-γ, IL-10, and Foxp3 were detected by intracellular staining. (A) Representative IL-4, IFN-γ, and IL-10 expression on CD4+ T cells, determined by flow cytometry. n ≥ 5 mice/group. (B) Representative Foxp3 and IL-10 expression by CD4+ T cells, determined by flow cytometry. n = 3 mice/group. (C) Frequency (mean + SD) gated on CD4+ T cells expressing IFN-γ, IL-4, or IL-10 in naive mice or allograft recipients treated as indicated. n ≥ 5 mice/group. *P < 0.05 vs. untreated or control Ig–treated allograft recipients; †P < 0.05 vs. other anti-CD20–treated allograft recipients; #P < 0.05 vs. anti–TIM-1–treated allograft recipients. Numbers denote percent of gated cells within the respective quadrants.
B cell IL-4 and IL-10 are primarily expressed by the TIM-1+ subset and are induced by TIM-1 ligation.
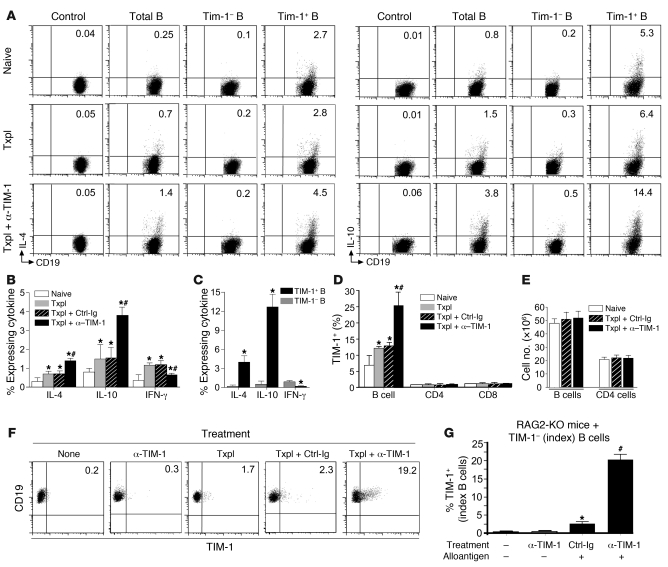
Anti–TIM-1 enhances Th2 cytokine expression in the presence of B cells, yet promotes expression of Th1 cytokines in the absence of B cells. Cytokines produced by B cells can influence T cell polarization (28, 29). This raised the possibility that TIM-1 ligation augments IL-4 production by B cells, which could then promote a Th2 response. Using IL-4 EGFP reporter mice (BALB/c) and WT littermates as negative controls, we detected very low but reproducible IL-4 expression by splenic B cells from naive mice (Figure 4, A and B). Immunization by islet transplantation increased IL-4 expression from 0.25% to 0.7% of B cells, and this doubled to 1.4% when recipients received anti–TIM-1. Compared with the overall B cell population, TIM-1+ B cells were highly enriched for IL-4 expression, which was localized almost entirely within this subset (Figure 4, A and C). Moreover, the percentage of TIM-1+ B cells expressing IL-4 increased when allograft recipients were treated with anti–TIM-1, reaching 4.5% (Figure 4, A and C).
Figure 4. IL-4 and IL-10 are expressed by TIM-1+ B cells and induced by TIM-1 ligation.
Splenic CD19+ B cells from BALB/c mice were naive, transplanted with B6 islets, or transplanted and treated with anti–TIM-1 or control Ig on day 14. (A) IL-4 and IL-10 expression, gated on CD19+ B cells (control and total B cells) or on TIM-1– and TIM-1+ B cells. IL-4 (EGFP) versus control (WT littermates); IL-10 (intracellular staining) versus control (isotype). (B) Frequency (mean + SD) of B cells expressing cytokines from mice in A. *P < 0.05 vs. naive; #P < 0.05 vs. all other groups. (C) Frequency (mean + SD) of cytokine expression on TIM-1– versus TIM-1+ B cells from anti–TIM-1–treated allograft recipients. *P < 0.01 vs. TIM-1–. (D) TIM-1 expression (mean + SD) on B, CD4+ T, and CD8+ T cells. *P < 0.05 vs. naive; #P < 0.05 vs. all other groups. (E) Total number (mean + SD) of splenic B and CD4+ T cells. (A–E) n ≥ 5 mice/group in at least 3 experiments. (F and G) Sorted TIM-1– “index” B cells (see Methods) from naive BALB/c mice were transferred into syngeneic JHD (F) or RAG2-KO (G) mice. Recipients were naive or were exposed to alloantigen (allograft or allogeneic splenocytes) with or without anti–TIM-1 or control Ig. TIM-1 expression on index B cells recovered from spleen (day 14) is shown in representative dot plots (F), or as frequency (G; mean + SD). n = 3 mice/group. *P < 0.05 vs. no allograft; #P < 0.01 vs. all other groups. Numbers denote percent cells in the respective quadrants.
In addition to promoting a Th2 response, B cells might support TIM-1–mediated engraftment through regulatory activity. Breg activity corresponds closely with IL-10 expression (19, 20). B cells expressing IL-10 were infrequent (~1%) among total splenic B cells (Figure 4, A and B). While islet transplantation increased IL-10 expression on B cells from 0.8% to 1.5%, treatment of recipients with anti–TIM-1 increased IL-10 expression on B cells almost 5-fold (3.8%; Figure 4, A and B). As with IL-4, TIM-1+ B cells were highly enriched for IL-10 expression. In naive mice and in untreated allograft recipients, roughly 5% of TIM-1+ B cells expressed IL-10, a 25-fold enrichment compared with TIM-1– B cells and a greater than 5-fold enrichment compared with total splenic B cells (Figure 4A). Treatment of allograft recipients with anti–TIM-1 increased TIM-1+ B cells expressing IL-10 more than 2-fold (~13%; Figure 4, A and C).
In addition to increasing the percentage of TIM-1+ B cells expressing IL-4 and IL-10, anti–TIM-1 treatment also increased the percentage of B cells expressing TIM-1, from 7% to 25% of splenic B cells (Figure 4D). Since neither transplantation nor anti–TIM-1 affected the total number of splenic B cells (Figure 4E), these percentages directly reflect differences in cell number. Thus, RMT1-10 treatment increases both the number of TIM-1+ B cells and the percentage of TIM-1+ B cells expressing cytokines, resulting in a 5-fold increase in the number of B cells expressing IL-4 and IL-10. These increases are likely to promote Th2 cytokine expression and allograft survival. Consistent with this finding, treatment of allograft recipients with anti–TIM-1 mAb 3B3, which promotes rejection and a Th1 response (7, 8), did not augment either TIM-1 or expression of IL-4 or IL-10 by B cells (data not shown). Taken together, these observations suggest that anti–TIM-1 (specifically RMT1-10) induces a subset of TIM-1+ IL-10+ B cells with potential regulatory activity.
To determine whether anti–TIM-1 can induce TIM-1 expression de novo, sort-purified TIM-1– B cells were transferred into naive or transplanted JHD mice with or without anti–TIM-1 treatment. In the absence of an allograft, transferred TIM-1– B cells remained essentially TIM-1–, regardless of whether mice received anti–TIM-1 (Figure 4F). In the presence of an alloantigen (with or without control Ig), approximately 2% of TIM-1– B cells expressed TIM-1. However, treatment of allograft recipients with anti–TIM-1 induced TIM-1 expression on approximately 20% of B cells that were originally TIM-1–. Similar findings were obtained when JHD mice were exposed to alloantigen as i.p. splenocytes (2 × 107; mitomycin C–treated) rather than an allograft (data not shown).
In this setting, anti–TIM-1 could act directly on the small percentage of B cells induced to express TIM-1 by antigen exposure. Alternatively, TIM-1 induction on B cells could occur indirectly through mAb binding to antigen-activated T cells, which expressed similar levels of TIM-1. To address this issue, TIM-1– B cells were transferred into RAG2-KO mice rather than JHD mice (Figure 4G). In naive RAG2-KO mice (with or without anti–TIM-1), TIM-1– B cells remained essentially TIM-1–. When RAG2-KO recipients were exposed to alloantigen plus control Ig, approximately 3% of B cells expressed TIM-1. However, when RAG2-KO mice received alloantigen in combination with anti–TIM-1, TIM-1 was induced on 20% of the B cells. Thus, induction of TIM-1 on B cells by anti–TIM-1 is T cell independent.
Anti–TIM-1–mediated graft survival is associated with B cell TIM-1 expression and with IL-10 and IL-4 signaling.
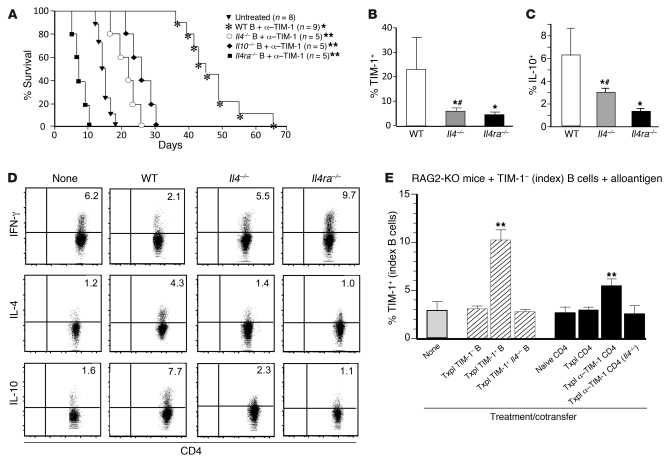
Next we assessed the role of IL-4 and IL-10 expression by B cells on their immunomodulatory function. Anti–TIM-1 shortened allograft survival in B cell–deficient (JHD) allograft recipients, but significantly enhanced allograft survival when recipients were reconstituted with WT B cells (MST, 46 days; Figure 2B and Figure 5A). In contrast, reconstitution of anti–TIM-1–treated JHD recipients with Il4–/– or Il10–/– B cells only modestly prolonged allograft survival (MST, 23 days and 26 days, respectively; Figure 5A). This suggests that IL-10 and IL-4 are both important for the immunomodulatory function of B cells mediated through TIM-1. However, whereas Il10–/– mice had normal B cell TIM-1 expression, Il4–/– mice exhibited a marked reduction in TIM-1+ B cells both constitutively and after transplantation (Supplemental Figure 2A). Indeed, after adoptive transfer into anti–TIM-1–treated JHD allograft recipients, over 23% of WT B cells expressed TIM-1 and greater than 6% expressed IL-10, whereas only 6% of Il4–/– B cells expressed TIM-1 and 3% expressed IL-10 (Figure 5, B and C). The defects in TIM-1 and IL-10 expression were even more severe in B cells from Il4ra–/– mice. After adoptive transfer into treated JHD recipients, only 3% of Il4ra–/– B cells expressed TIM-1, and 1.3% expressed IL-10 (Figure 5, B and C). Concordantly, Il4ra–/– B cells were unable to protect JHD recipients from accelerated rejection after anti–TIM-1 treatment, as seen in mice lacking B cells altogether (Figure 2 and Figure 5A). Thus, prolongation of anti–TIM-1–mediated graft survival by B cells correlates directly with TIM-1 and IL-10 expression. Moreover, normal TIM-1 and IL-10 expression on B cells is critically dependent on IL-4 signaling.
Figure 5. B cell–mediated prolongation of allograft survival requires IL-4–dependent TIM-1 and IL-10 expression.
Diabetic JHD recipients of C57B/6 allografts were reconstituted with 107 naive B cells from WT, Il4–/–, Il4ra–/–, or Il10–/– mice and treated with anti–TIM-1. (A) Kaplan-Meir plots of graft survival. *P < 0.01, **P < 0.05 vs. untreated. TIM-1 (B) and IL-10 (C) expression (mean + SD) on transferred B cells (day 14), detected by flow cytometry. *P < 0.01 vs. WT; #P < 0.05 vs. Il4ra–/–. (D) Representative IL-4, IFN-γ, and IL-10 expression, assessed by flow cytometry, on endogenous CD4+ T cells 14 days after B cell transfer in the JHD recipients. Numbers denote percent CD4+ T cells expressing IL-4, IFN-γ, and IL-10. (E) Sorted index B cells from naive BALB/c mice were adoptively transferred into syngeneic RAG2-KO recipients of B6 islets. Mice received 107 sort-purified congenic TIM-1–, TIM-1+, or Il4–/– TIM-1+ B cells from allostimulated BALB/c mice. Alternatively, mice received 107 sort-purified CD4+ T cells from WT or Il4–/– BALB/c mice (naive, allostimulated, or allostimulated and treated with anti–TIM-1). Shown is frequency (mean + SD) of TIM-1 expression on index B cells in spleen 14 days after transfer. **P < 0.05 vs. other groups. (B–E) n = 3 mice/group.
As shown in Figure 5B, TIM-1 expression remained low on Il4–/– B cells transferred into anti–TIM-1–treated JHD allograft recipients. This indicates that IL-4 produced by B cells themselves is crucial. While this effect could be direct (B cell IL-4 acts in an autocrine fashion to induce TIM-1+ B cells), it could also be indirect. For example, B cell IL-4 (induced by anti–TIM-1; Figure 4) could promote Th2 cytokines (which are B cell dependent; Figure 3), and these could induce TIM-1+ B cells. Indeed, in the absence of B cell IL-4 signaling, treatment of allograft recipients with anti–TIM-1 promoted Th1 rather than Th2 cytokine expression by CD4+ T cells (Figure 5D).
To determine whether IL-4 from B cells or T cells can induce TIM-1 expression, TIM-1– B cells were transferred into alloantigen-exposed RAG2-KO mice. As before (Figure 4G), alloantigen induced TIM-1 expression on approximately 3% of transferred B cells (Figure 5E). However, cotransfer of congenic (CD45.1) TIM-1+ B cells from WT allograft recipients (enriched for IL-4 expression) increased TIM-1 expression in initially TIM-1– B cells to 10%. In contrast, cotransfer of TIM-1– B cells from the same allograft recipients, or TIM-1+ B cells from Il4–/– allograft recipients, had no effect on expression of TIM-1 on initially TIM-1– B cells (3%). This suggests that IL-4 from TIM-1+ B cells can indeed augment TIM-1 expression on TIM-1– B cells.
Next, CD4+ T cells were examined. Cotransfer of CD4+ T cells from anti–TIM-1–treated WT allograft recipients (Th2 enriched) increased TIM-1 expression in initially TIM-1– B cells by almost 2-fold. In contrast, CD4+ T cells from anti–TIM-1–treated Il4–/– allograft recipients or CD4+ T cells from untreated allograft recipients (Th1 enriched) had no effect on TIM-1 expression by B cells. Thus, although IL-4 from CD4+ T cells is not essential, it can augment B cell TIM-1 expression. IL-10 expression on B cells directly paralleled TIM-1 expression (Supplemental Figure 2B).
To further confirm the role of IL-4 signaling on TIM-1 induction, sort-purified TIM-1– B cells from WT, Il4–/–, and Il4ra–/– mice were stimulated in vitro for 48 hours in the presence or absence of exogenous IL-4. TIM-1 expression was readily induced on WT TIM-1– B cells by B cell receptor (BCR) ligation with anti-IgM, increasing from 2%–3% (media alone) to greater than 15% (Supplemental Figure 2, C and D). Addition of exogenous IL-4 to anti-IgM markedly enhanced TIM-1 expression, reaching 35% of WT B cells. In the absence of anti-IgM, IL-4 had only a minor effect compared with media alone (Supplemental Figure 2D). In contrast, TIM-1 induction by anti-IgM was markedly reduced in Il4–/– (TIM-1–) B cells (Supplemental Figure 2C). However, when exogenous IL-4 was added, TIM-1 expression by Il4–/– and WT B cells was comparable. Thus, Il4–/– B cells are capable of TIM-1 induction if IL-4 signaling occurs. In contrast, Il4ra–/– B cells had defective TIM-1 induction after anti-IgM, and, as expected, this was not restored by exogenous IL-4. These results correlated closely with the in vivo effects of B cells from Il4–/– and Il4ra–/– mice (Figure 5); together, these findings indicate that induction of TIM-1 expression requires BCR and IL-4 signaling.
TIM-1+ B cells are regulatory and transfer donor-specific long-term graft survival.
Next, TIM-1+ B cells were directly tested for regulatory activity. BALB/c allograft recipients treated with anti–TIM-1 were sacrificed on day 14. Splenic B cells from these mice were sorted into TIM-1+ and TIM-1– populations and transferred into otherwise untreated JHD recipients of B6 islets. Whereas transfer of TIM-1– B cells had no effect (MST, 15 days), transfer of TIM-1+ B cells markedly prolonged allograft survival, with 50% surviving long-term (Figure 6A). In contrast, TIM-1+ B cells obtained from Il10–/– islet allograft recipients were defective in their regulatory capacity (MST, 22 days) compared with WT TIM-1+ B cells. Similarly, TIM-1+ B cells from Il4ra–/– allograft recipients lacked regulatory activity, consistent with their significantly reduced IL-10 expression.
Figure 6. TIM-1+ B cells prolong allograft survival in a donor-specific and IL-10–dependent manner.
(A) WT, Il4ra–/–, or Il10–/– BALB/c recipients of B6 islets were treated with anti–TIM-1. On day 14, splenic TIM-1+ and TIM-1– B cells were sort-purified and transferred (107) into otherwise-untreated JHD recipients of islet allografts from the same (B6) strain. Shown are Kaplan-Meir plots of graft survival. #P < 0.01 vs. all other groups; *P < 0.05 vs. untreated. (B) TIM-1+ B cells from spleen of naive BALB/c mice or from untreated BALB/c recipients of B6 or C3H islet allografts (day 14) were sort-purified and transferred (107) into JHD recipients of B6 islets without further treatment. Shown are Kaplan-Meir plots of graft survival. #P < 0.01 vs. other groups. (C) JHD IL-4 EGFP (JHD×4get) recipients of B6 islets were untreated or received 107 TIM-1+ or TIM-1– B cells from anti–TIM-1–treated BALB/c allograft recipients as in A. Shown is representative IL-4 (EGFP) or IFN-γ, IL-10, and Foxp3 (intracellular staining) expression by flow cytometry on splenic CD4+ T cells from recipients 14 days after receiving TIM-1+, TIM-1–, or no B cells. Numbers denote percent CD4+ T cells expressing IL-4, IFN-γ, IL-10, or Foxp3. n = 3 mice/group.
Next, we addressed whether TIM-1+ B cells present in the absence of TIM-1 ligation (which augments TIM-1, IL-10, and IL-4 expression) are also regulatory. Adoptive transfer of TIM-1+ B cells from untreated BALB/c recipients of a B6 allograft were able to markedly prolong graft survival in JHD recipients of B6 islets (Figure 6B). In contrast, TIM-1+ B cells obtained from naive (untransplanted) mice were unable to prolong allograft survival when transferred into JHD recipients. These data were consistent with previous reports indicating that Bregs must be activated to be effective (19). Moreover, consistent with other Bregs (15, 16, 30, 31), TIM-1+ Bregs were antigen specific, since TIM-1+ B cells from mice receiving an unrelated (C3H) allograft were unable to prolong graft survival when transferred into JHD recipients of B6 islets (Figure 6B). Likewise, transfer of TIM-1+ B cells from BALB/c mice exposed to B6 alloantigen protected JHD recipients from rejecting B6 islets, but not C3H islets (Supplemental Figure 3A). When JHD mice received both B6 and C3H islets (right and left kidney capsules, respectively), acute rejection did not occur. However, histological assessment of allografts revealed heavy CD3+ infiltration of C3H grafts on day 14 (data not shown) and complete disappearance by day 25, whereas B6 allografts were preserved (Supplemental Figure 3B). This demonstrated that activation of antigen-specific Bregs was insufficient to prevent an immune response against a different antigen in the same host. Thus, TIM-1+ B cells exhibited potent antigen-specific regulatory function and were capable of augmenting IL-4 and IL-10 expression while inhibiting IFN-γ expression by endogenous CD4+ T cells (Figure 6C). Transferred TIM-1+ Bregs also augmented the frequency of Foxp3+ Tregs.
Bregs have previously been implicated in amelioration of allergic airway disease (31–33). Adoptive transfer of TIM-1+, but not TIM-1–, B cells from mice with OVA-induced local inhalational tolerance, markedly reduced OVA-induced allergic airway disease in JHD recipients, as determined by reduced BAL leukocytosis, tissue inflammation, and bronchiolar goblet cell hyperplasia (Supplemental Figure 4), thus confirming our results in an independent model.
TIM-1 identifies the majority of IL-10+ Bregs and encompasses other IL-10–expressing Breg populations.
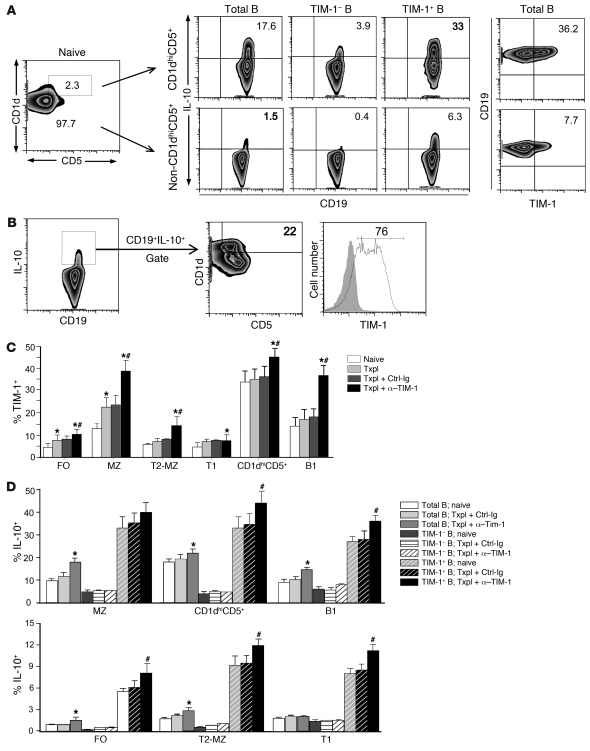
Bregs, defined by their functional capacity and ability to produce IL-10, have been reported as low-frequency cells belonging to various B cell subpopulations. Recently, it was suggested that a novel subset of B cells expressing a CD1dhiCD5+ phenotype might account for most IL-10+ Bregs in spleen (15, 19, 34). Although enriched for IL-10 expression (~18%), this population made up only about 2% of splenic B cells (ref. 15 and Figure 7A). Here, within the predominant (98%) non-CD1dhiCD5+ population, 1.0%–1.5% of B cells also expressed IL-10. When relative number was taken into account, calculations suggested that less that 25% of all IL-10+ B cells should express the CD1dhiCD5+ phenotype. To directly test this, we used multicolor flow cytometry to first gate on CD19+IL-10+ splenic B cells. Only 22% ± 4.5% (SD) of these cells fell within the CD1dhiCD5+ gate (Figure 7B). In marked contrast, 71% ± 5.3% of all IL-10+ B cells were TIM-1+.
Figure 7. TIM-1+ B cells are highly enriched for IL-10 expression across a wide spectrum of phenotypes.
BALB/c splenocytes were assessed for expression of various cell surface markers and IL-10 by multicolor flow cytometry. n = 3–6 mice/group in at least 3 independent experiments. (A) Left: Representative expression of CD5 versus CD1d on CD19+ B cells in naive mice with the CD1dhiCD5+ population (2.3% of total B cells; rectangular gate). Right: IL-10 and TIM-1 expression on CD19+ (total B) and on TIM-1+ and TIM-1– B cells within the CD1dhiCD5+ and non-CD1dhiCD5+ B cell populations. (B) Representative expression of markers on CD19+IL-10+ B cells. Cells within the IL-10+ B cell gate were assessed for CD1d and CD5 or TIM-1 expression. (C) Percent (mean + SD) B cells expressing TIM-1 within FO, MZ, T2-MZ, T1, CD1dhiCD5+, and B1 B cells from naive and transplanted mice with or without anti–TIM-1 or control Ig treatment. *P < 0.05 vs. naive; #P < 0.05 vs. other allograft recipients. (D) IL-10 expression (mean + SD) on B cell subpopulations and mice as in C. *P < 0.05 vs. other total B cell groups; #P < 0.05 vs. other TIM-1+ B cell groups. P < 0.05, TIM-1+ vs. TIM-1– (all groups). Numbers denote percent cells within the designated areas.
Given that most IL-10+ B cells in spleen express TIM-1, we asked whether this marker could further identify IL-10–expressing cells within other B cell subpopulations previously shown to contain Bregs. Within the CD1dhiCD5+ B cell population discussed above, TIM-1+ cells were even more highly enriched for IL-10 (33%). In contrast, TIM-1– cells within the CD1dhiCD5+ subset exhibited an approximate 8-fold decrease in frequency of IL-10 expression (Figure 7, A and D).
Although uncommon, IL-10+ cells within the non-CD1dhiCD5+ population represented a numerical majority of IL-10–expressing B cells in spleen (see above). Unfortunately, to our knowledge there has been no consistent marker allowing identification of rare IL-10+ cells within this vast B cell population. Here, we found that TIM-1 was present on 8% of non-CD1dhiCD5+ B cells, and in this population, TIM-1+ B cells were enriched greater than 15-fold for IL-10 expression compared with their TIM-1– counterparts (Figure 7A). Similar findings were observed using IL-10 reporter mice (B6 background; Supplemental Figure 5A), confirming these findings in a second strain. Moreover, after TIM-1 ligation, TIM-1 expression was increased, reaching 45% of CD1dhiCD5+ B cells, of which approximately 45% expressed IL-10 (Figure 7C and Supplemental Figure 5B). After TIM-1 ligation, TIM-1 expression on non-CD1dhiCD5+ B cells increased to 15%, of which approximately 10% expressed IL-10. Thus, TIM-1 identified most IL-10+ B cells within the CD1dhiCD5+ subset and also identified a large number of IL-10+ B cells excluded by the CD1dhiCD5+ phenotype.
We also examined the distribution of TIM-1 on more typical splenic B cell subsets in naive mice (35). TIM-1 was preferentially expressed on MZ (IgM+IgD–CD21hiCD23–) and B1 (CD5+) B cells (~15%), but was also expressed on approximately 5% of immature transitional 1 (T1; IgM+IgD–CD21–CD23–), T2-MZ (IgM+IgD+CD21+CD23hi), and FO (IgM–IgD+CD21+CD23+) B cells (Figure 7C). Within each subset, TIM-1 identified B cells that were 8- to 20-fold enriched for IL-10 expression compared with their TIM-1– counterparts (Figure 7D). Moreover, TIM-1 ligation in the presence of alloantigen significantly enhanced the percentage of cells expressing TIM-1 (Figure 7C). Thus, in naive mice, 33% of TIM-1+ MZ B cells expressed IL-10. However, anti–TIM-1 treatment of allograft recipients resulted in a 2.8-fold increase in the number of TIM-1+ MZ B cells (Figure 7C) and a 40% increase in the proportion of these TIM-1+ cells expressing IL-10 (Figure 7D), resulting in a 3.5-fold increase in number of TIM-1+ IL-10+ MZ B cells. FO B cells accounted for approximately 40% of splenic B cells, of which less than 1% expressed IL-10 (Figure 7D). However, approximately 6% of TIM-1+ FO B cells expressed IL-10, a more than 20-fold increase over TIM-1– FO B cells. Moreover, TIM-1 ligation in transplant recipients increased the number of TIM-1+ FO B cells 2.2-fold and increased IL-10 expression on TIM-1+ B cells by 45%, resulting in a greater than 3-fold increase in TIM-1+IL-10+ B cells in this large subset (Figure 7, C and D). Taken together, these data suggest that TIM-1 identifies an inducible subset of B cells that are highly enriched for IL-10 production, regardless of other phenotypic markers.
Discussion
TIM-1 has previously been identified as a T cell costimulatory molecule that regulates both auto- and alloimmunity through its effects on CD4+ effector responses (1). We now show, for the first time to our knowledge, that TIM-1 not only broadly identifies Bregs, but plays an important functional role on B cells that is critical for anti–TIM-1–mediated tolerance. TIM-1 ligation with the low-affinity anti–TIM-1 mAb RMT1-10, that normally promotes a Th2 response and prolongs allograft survival, paradoxically induced a Th1 response and accelerated rejection in the absence of B cells. TIM-1 ligation on B cells enhanced their IL-4 and IL-10 expression, and both were required for Th2 responses in the allograft setting (Figure 3A, Figure 5D, and data not shown). Although very few B cells expressed IL-4, we demonstrated that these were greatly enriched in the fraction bearing TIM-1. Of note, overexpression of TIM-1 in T cells augments IL-4 transcription (36), and a similar signaling pathway could operate in B cells. In addition to promoting Th2 deviation, TIM-1 ligation also enhances IL-10 expression on Tregs in a B cell–dependent manner. Both Tregs and Th2 cytokines are required for prolonged allograft survival mediated by anti–TIM-1 (9). However, these data do not preclude the possibility that anti–TIM-1 has important effects on T cells that either are independent or amplify the effects on B cells.
It is now recognized that Bregs promote tolerance in a number of autoimmune models, including EAE, inflammatory bowel disease, collagen-induced arthritis, allergic airway disease, and diabetes mellitus (15, 17–21, 30, 31, 37). Lacking a specific marker, such Breg activity appears to reside within uncommon IL-10–expressing B cells scattered within various B cell subpopulations. Moreover, IL-10 production by B cells capable of regulatory activity does not occur in situ, but can be detected only after brief stimulation ex vivo (10). Even then, only 1%–2% of all B cells in spleen, and even less in LN or bone marrow, are IL-10 competent (10). These factors have complicated the identification and study of Bregs.
The recent identification of CD1dhiCD5+ B cells, which express IL-10 with relatively high frequency, was an important step forward. This subset was thought to identify most IL-10–producing Bregs, including many previously attributed to the MZ or T2-MZ subsets (10, 15). Although strongly enriched for IL-10 expression, the CD1dhiCD5+ population is very small and actually accounts for less than 25% of all IL-10+ B cells in spleen. An approach for identifying infrequent IL-10+ cells scattered within other or larger B cell subpopulations has been lacking. We showed here that within multiple B cell subpopulations,TIM-1+ B cells were enriched 8- to 20-fold for IL-10 expression compared with their TIM-1– counterparts. Indeed, when splenic B cells were first gated on IL-10 expression, TIM-1 encompassed more than 70% of the IL-10+ population. Thus, TIM-1 not only markedly enhanced identification of IL-10–expressing B cells within the CD1dhiCD5+ and MZ populations, but also substantially enriched for IL-10+ B cells within the large FO B cell population (~40% of splenic B cells). Moreover, unlike other markers (19), TIM-1 also identified IL-10+ B cells in LNs and peritoneal cavity. For example, in naive mice, TIM-1 identified 3%–5% of LN B cells, and these were 6-fold enriched for IL-10 expression compared with TIM-1– cells (Supplemental Figure 5C). TIM-1 also identified approximately 15% of peritoneal B1-b and B1-a cells, of which 18%–25% expressed IL-10 (Supplemental Figure 5D). We therefore believe that TIM-1 represents the broadest and most specific marker for Bregs identified thus far.
The finding that TIM-1 ligation on B cells induced TIM-1+ B cells with regulatory activity indicates that TIM-1 signaling is involved in this process. Thus far, anti–TIM-1 appears unique in its ability to induce Bregs across a wide spectrum of phenotypes. In comparison, BAFF and anti-CD40 are reported to induce Bregs specifically within the splenic MZ and T2 populations, respectively (38, 39). Moreover, both of these agents exhibit dual agonist and antagonist functions, raising the concern that they may not uniformly inhibit the immune response in all disease settings.
In addition to increasing the number of TIM-1+ B cells, anti–TIM-1 also augmented their ability to express immunomodulatory cytokines. Thus, TIM-1 ligation in allograft recipients resulted in TIM-1 expression in 35%–40% of CD1dhiCD5+ B cells as well as in the MZ and B1 populations, and IL-10 was expressed by 35%–45% of these cells. Even within the major FO B cell population, anti–TIM-1 treatment increased TIM-1 and IL-10 expression, resulting in a 3-fold increase in number of IL-10+ cells. Since TIM-1 identifies IL-10+ cells within each B cell subset, it may allow the relative regulatory activity and lineal relationship between such cells to be studied.
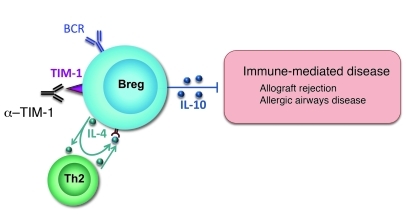
The current study also provides what we believe to be new insight into signals involved in Breg generation (Figure 8). The increase in TIM-1+ B cells induced by anti–TIM-1 is caused, at least in part, by de novo induction of TIM-1 expression on TIM-1– B cells, and this does not require T cells. This apparent paradox may be resolved by the finding that BCR ligation induced TIM-1 expression on a subset of B cells, providing a target for anti–TIM-1 and further enhancing TIM-1 expression. IL-4 signaling in B cells played a key role in both constitutive and induced TIM-1 as well as in IL-10 expression and Breg activity. Thus, both Il4–/– and Il4ra–/– mice were defective in TIM-1, IL-10, and Breg activity. Conversely, TIM-1 ligation augmented B cell IL-4 expression, and this could act in an autocrine fashion to significantly enhance TIM-1 and IL-10 expression. IL-4, provided by either B cells or CD4+ T cells, could also promote TIM-1 (and IL-10) expression by activated B cells. However, as previously noted, in the absence of B cell IL-4 signaling, few T cells expressed IL-4, at least in the allograft setting.
Figure 8. Model of TIM-1+IL-10+ Breg induction.
TIM-1+ B cells account for most IL-4+ and IL-10+ B cells, and both cytokines and TIM-1 itself can be induced by anti–TIM-1 in the face of BCR ligation. B cell IL-4 is required to promote IL-4 expression by Th2 cells in the allograft setting. IL-4 receptor signaling in B cells is essential both for TIM-1 expression and for IL-10 production by B cells. IL-4 can be derived from either B cells themselves or from Th2 cells. IL-10 expression is required for Breg activity, but not for TIM-1 expression.
To our knowledge, this is the first report to identify a requisite role for IL-4 signaling in B cell IL-10 expression and Breg activity, and our findings have a number of implications. First, in contrast to Harris et al. (28), we found that Th2 cells were not required for induction of IL-4 expression by B cells. IL-4 and IL-10 expressed by B cells is known to augment Th2 expression (17, 28, 29, 40). Although B cell IL-10 may act by inhibiting IL-12 expression by DCs (40), IL-4 was thought to directly promote Th2 skewing. However, IL-4 may also enhance CD4+ T cell IL-4 expression indirectly by promoting B cell IL-10, forming another positive regulatory loop. Thus, B cells and T cells engage in reciprocal regulation of IL-4 on several levels, promoting TIM-1, IL-10, and Breg function.
In agreement with previous studies, IL-10 played a critical role in Breg activity. Neither TIM-1+ Il10–/– nor Il4ra–/– B cells (defective in IL-10 expression) could transfer tolerance to otherwise-untreated allograft recipients. However, Il10–/– B cells modestly prolonged allograft survival in anti–TIM-1–treated B cell–deficient recipients, which suggested that in this setting, additional regulatory mechanisms may play a role.
TIM-1 plays an important role in regulating the immune response. We showed here that many of the effects on T cells resulting from TIM-1 ligation with a low-affinity mAb were actually B cell dependent. TIM-1 represented an inclusive marker for Bregs highly enriched for IL-10 expression in spleen and LN of both naive mice and those undergoing active immune responses. Transfer of TIM-1+, but not TIM-1–, B cells after antigen challenge markedly prolonged survival of fully MHC-mismatched allografts in otherwise-untreated recipients. To our knowledge, RMT1-10 is the first agent to selectively induce Bregs in vivo and the first tolerogenic agent to promote allograft tolerance through Bregs. These findings may represent a fresh therapeutic approach and provide insight into the signals involved in the generation and induction of Bregs.
Methods
Mice.
B6 (H-2b) and BALB/c (H-2d) mice (National Cancer Institute) and Il4–/–, Il4ra–/–, Il10–/–, and RAG2-KO BALB/c mice (The Jackson Laboratory) were used. BALB/c IL-4 EGFP reporter mice (also known as 4get) were provided by R. Locksley (UCSF, San Francisco, California, USA). B6 IL-10 GFP reporter (also known as Tiger) and B cell–deficient JHD (BALB/c) mice were provided, respectively, by R. Flavell and M. Shlomchik (Yale School of Medicine, New Haven, Connecticut, USA). All animals were used at 6–10 weeks of age and were housed with food and water ad libitum.
In vivo treatment protocols.
The anti–TIM-1 mAb RMT1-10 (rat IgG2a; BioX Cell) or control rat IgG2a mAb (Biogen-IDEC) was administered i.p. on days –1 (0.5 mg), 0, and 5 (0.3 mg) relative to day of transplantation. As indicated, BALB/c mice were treated with anti–murine CD20 mAb 18B12 (IgG2a; 250 μg i.v.; provided by Biogen-IDEC Pharmaceuticals) on days –14 and –1 relative to transplantation. This mAb depletes greater than 95% of B cells in the circulation, spleen, and LN and 86% of mature and immature B cells in the marrow for 2–3 weeks (41).
Islet isolation and transplantation.
Islets from B6 donors were digested with collagenase V (Sigma-Aldrich), purified by filtration through a 100-μm nylon cell strainer (BD Biosciences), hand-picked under a stereomicroscope, and placed under the left renal capsule of sex-matched BALB/c or JHD recipients with streptozocin-induced diabetes (400 islets/recipient), as we previously described (42, 43). All recipients had glycemia at less than 150 mg/dl by days 2 after transplant. Blood glucose greater than 250 mg/dl after engraftment was defined as rejection.
Flow cytometry.
Biotin- or fluorochrome-conjugated mAbs were purchased from BD Biosciences or eBioscience, except for PE-conjugated anti–TIM-1 (RMT1-4; Biolegend), whose binding did not overlap with that of RMT1-10 (data not shown). The anti–TIM-1 mAb 3B3 was provided by R. DeKruyff (Harvard Medical School, Cambridge, Massachusetts, USA). All staining was performed in the presence of Fc block (anti-CD16/CD32). Flow acquisition was performed on LSRII or FACSCalibur analyzers (BD Biosciences), and data were analyzed using FlowJo software (Tree Star). The numbers in each flow cytometry plot represent the percent of gated cells within the respective quadrants. Background staining was determined with isotype-matched controls. Where IL-4 and IL-10 were detected by EGFP reporter expression, cells from WT littermates were used as negative controls. For detection of intracellular cytokines, T cells were cultured for 4 hours with PMA (50 ng/ml; Sigma-Aldrich), ionomycin (500 ng/ml; Sigma-Aldrich), and GolgiPlug (1 μl/ml; BD Biosciences), and B cells were cultured for 5 hours with LPS (10 μg/ml), PMA (50 ng/ml; Sigma-Aldrich), ionomycin (500 ng/ml; Sigma-Aldrich), and GolgiPlug (1 μl/ml; BD Biosciences), as described previously (15). Intracellular staining was conducted using intracellular staining kits from BD Biosciences or eBioscience.
Western blotting.
Cell lysates were prepared from sort-purified CD3+, CD19+, CD19+TIM-1–, and CD19+TIM-1+ cells using Sigma CelLytic reagent containing leupeptin (10 μg/ml), aprotinin (10 μg/ml), DTT (1 mM), and PMSF (1 mM). 40 μg protein was loaded per lane. After SDS-PAGE, proteins were transferred to a PVDF membrane, incubated with anti–TIM-1 (clone 222414; R&D), and HRP-conjugated anti-rat IgG (Sigma-Aldrich), as described previously (27).
Cell preparation and adoptive transfer.
CD19+ B cells were enriched by negative selection (EasySep; StemCell Technologies). Purity of the B cell subset was greater than 95%. CD19+TIM-1+ and CD19+TIM-1– B cells were subsequently isolated by FACS using BD FACSAria. For adoptive transfer studies, 1 × 107 sort-purified CD19+, CD19+TIM-1–, and/or CD19+TIM-1+ B cells from spleens of naive BALB/c mice or BALB/c recipients of B6 islets (day 14) were injected i.v. into otherwise untreated JHD allograft recipients. In some experiments, CD19+ cells were obtained from BALB/c mice exposed to alloantigen (2 × 107 mitomycin C–treated B6 spleen cells i.p.). As indicated, 107 sorted CD19+TIM-1– B cells (i.e., index cells) from naive BALB/c mice were adoptively transferred into syngeneic RAG2-KO recipients that were naive or were stimulated for 14 days with alloantigen as described above. RAG2-KO mice were then treated with anti–TIM-1 or control Ig, or received cotransfer of 107 sort-purified congenic TIM-1– or TIM-1+ B cells from allografted BALB/c mice. Alternatively, mice received 107 analogous sort-purified CD4+ T cells. After 14 days, TIM-1 or IL-10 expression on the index cells was determined by flow cytometry.
Statistics.
Statistical analyses used unpaired 2-tailed Student’s t test and log-rank (Mantel-Cox) test. Differences were considered to be significant at P values less than 0.05.
Study approval.
Animal studies were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh (Pittsburgh, Pennsylvania, USA).
Supplementary Material
Acknowledgments
This work was supported by NIH grant AI070820 (to M.H. Sayegh).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(9):3645–3656. doi:10.1172/JCI46274.
References
- 1.Kuchroo VK, Dardalhon V, Xiao S, Anderson AC. New roles for TIM family members in immune regulation. Nat Rev Immunol. 2008;8(8):577–580. doi: 10.1038/nri2366. [DOI] [PubMed] [Google Scholar]
- 2.McIntire JJ, et al. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat Immunol. 2001;2(12):1109–1116. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- 3.Chae SC, Park YR, Song JH, Shim SC, Yoon KS, Chung HT. The polymorphisms of Tim-1 promoter region are associated with rheumatoid arthritis in a Korean population. Immunogenetics. 2005;56(10):696–701. doi: 10.1007/s00251-004-0743-5. [DOI] [PubMed] [Google Scholar]
- 4.Umetsu SE, et al. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat Immunol. 2005;6(5):447–454. doi: 10.1038/ni1186. [DOI] [PubMed] [Google Scholar]
- 5.Meyers JH, et al. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat Immunol. 2005;6(5):455–464. doi: 10.1038/ni1185. [DOI] [PubMed] [Google Scholar]
- 6.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235(1):172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao S, et al. Differential engagement of Tim-1 during activation can positively or negatively costimulate T cell expansion and effector function. J Exp Med. 2007;204(7):1691–1702. doi: 10.1084/jem.20062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Degauque N, et al. Immunostimulatory Tim-1-specific antibody deprograms Tregs and prevents transplant tolerance in mice. J Clin Invest. 2008;118(2):735–741. doi: 10.1172/JCI32562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueno T, et al. The emerging role of T cell Ig mucin 1 in alloimmune responses in an experimental mouse transplant model. J Clin Invest. 2008;118(2):742–751. doi: 10.1172/JCI32451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–299. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 11.Hu CY, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117(12):3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards JC, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350(25):2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 13.Bouaziz JD, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci U S A. 2007;104(52):20878–20883. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe R, et al. CD19 expression in B cells is important for suppression of contact hypersensitivity. Am J Pathol. 2007;171(2):560–570. doi: 10.2353/ajpath.2007.061279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28(5):639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118(10):3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3(10):944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 18.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16(2):219–230. doi: 10.1016/S1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 19.Dilillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 20.Mauri C, Ehrenstein MR. The ‘short’ history of regulatory B cells. Trends Immunol. 2008;29(1):34–40. doi: 10.1016/j.it.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176(2):705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 22.Brummel R, Lenert P. Activation of marginal zone B cells from lupus mice with type A(D) CpG-oligodeoxynucleotides. J Immunol. 2005;174(4):2429–2434. doi: 10.4049/jimmunol.174.4.2429. [DOI] [PubMed] [Google Scholar]
- 23.Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci U S A. 2007;104(35):14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J Clin Immunol. 2005;25(1):29–40. doi: 10.1007/s10875-005-0355-6. [DOI] [PubMed] [Google Scholar]
- 25.Evans JG, et al. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178(12):7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 26.Miyazaki D, Kuo CH, Tominaga T, Inoue Y, Ono SJ. Regulatory function of CpG-activated B cells in late-phase experimental allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2009;50(4):1626–1635. doi: 10.1167/iovs.08-2701. [DOI] [PubMed] [Google Scholar]
- 27.Nakae S, et al. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood. 2007;110(7):2565–2568. doi: 10.1182/blood-2006-11-058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris DP, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1(6):475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 29.Harris DP, Goodrich S, Mohrs K, Mohrs M, Lund FE. Cutting edge: the development of IL-4-producing B cells (B effector 2 cells) is controlled by IL-4, IL-4 receptor alpha, and Th2 cells. J Immunol. 2005;175(11):7103–7107. doi: 10.4049/jimmunol.175.11.7103. [DOI] [PubMed] [Google Scholar]
- 30.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197(4):489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh A, et al. Regulatory role of B cells in a murine model of allergic airway disease. J Immunol. 2008;180(11):7318–7326. doi: 10.4049/jimmunol.180.11.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tedder TF, Matsushita T. Regulatory B cells that produce IL-10: a breath of fresh air in allergic airway disease. J Allergy Clin Immunol. 2010;125(5):1125–1127. doi: 10.1016/j.jaci.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsitoura DC, Yeung VP, DeKruyff RH, Umetsu DT. Critical role of B cells in the development of T cell tolerance to aeroallergens. Int Immunol. 2002;14(6):659–667. doi: 10.1093/intimm/dxf032. [DOI] [PubMed] [Google Scholar]
- 34.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 35.Tung JW, Parks DR, Moore WA, Herzenberg LA. Identification of B-cell subsets: an exposition of 11-color (Hi-D) FACS methods. Methods Mol Biol. 2004;271:37–58. doi: 10.1385/1-59259-796-3:037. [DOI] [PubMed] [Google Scholar]
- 36.de Souza AJ, Oriss TB, O’Malley K J, Ray A, Kane LP. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. Proc Natl Acad Sci U S A. 2005;102(47):17113–17118. doi: 10.1073/pnas.0508643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hussain S, Delovitch TL. Intravenous transfusion of BCR-activated B cells protects NOD mice from type 1 diabetes in an IL-10-dependent manner. . J Immunol. 2007;179(11):7225–7232. doi: 10.4049/jimmunol.179.11.7225. [DOI] [PubMed] [Google Scholar]
- 38.Blair PA, et al. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J Immunol. 2009;182(6):3492–3502. doi: 10.4049/jimmunol.0803052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang M, et al. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J Immunol. 2010;184(7):3321–3325. doi: 10.4049/jimmunol.0902551. [DOI] [PubMed] [Google Scholar]
- 40.Ronet C, et al. Regulatory B cells shape the development of Th2 immune responses in BALB/c mice infected with Leishmania major through IL-10 production. J Immunol. 2010;184(2):886–894. doi: 10.4049/jimmunol.0901114. [DOI] [PubMed] [Google Scholar]
- 41.Hamel K, et al. Suppression of proteoglycan-induced arthritis by anti-CD20 B Cell depletion therapy is mediated by reduction in autoantibodies and CD4+ T cell reactivity. J Immunol. 2008;180(7):4994–5003. doi: 10.4049/jimmunol.180.7.4994. [DOI] [PubMed] [Google Scholar]
- 42.Ariyan C, et al. Cutting edge: transplantation tolerance through enhanced CTLA-4 expression. J Immunol. 2003;171(11):5673–5677. doi: 10.4049/jimmunol.171.11.5673. [DOI] [PubMed] [Google Scholar]
- 43.Salvalaggio PR, et al. Islet filtration: a simple and rapid new purification procedure that avoids ficoll and improves islet mass and function. Transplantation. 2002;74(6):877–879. doi: 10.1097/00007890-200209270-00023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.