Abstract
Pemphigus vulgaris (PV) is a severe autoimmune disease involving blistering of the skin and mucous membranes. It is caused by autoantibodies against desmoglein 3 (Dsg3), an adhesion molecule critical for maintaining epithelial integrity in the skin, oral mucosa, and esophagus. Knowing the antigen targeted by the autoantibodies renders PV a valuable model of autoimmunity. Recently, a role for Dsg3-specific CD4+ T helper cells in autoantibody production was demonstrated in a mouse model of PV, but whether these cells exert cytotoxicity in the tissues is unclear. Here, we analyzed 3 Dsg3-specific TCRs using transgenic mice and retrovirus induction. Dsg3-specific transgenic (Dsg3H1) T cells underwent deletion in the presence of Dsg3 in vivo. Dsg3H1 T cells that developed in the absence of Dsg3 elicited a severe pemphigus-like phenotype when cotransferred into immunodeficient mice with B cells from Dsg3–/– mice. Strikingly, in addition to humoral responses, T cell infiltration of Dsg3-expressing tissues led to interface dermatitis, a distinct form of T cell–mediated autoimmunity that causes keratinocyte apoptosis and is seen in various inflammatory/autoimmune skin diseases, including paraneoplastic pemphigus. The use of retrovirally generated Dsg3-specific T cells revealed that interface dermatitis occurred in an IFN-γ– and TCR avidity–dependent manner. This model of autoimmunity demonstrates that T cells specific for a physiological skin-associated autoantigen are capable of inducing interface dermatitis and should provide a valuable tool for further exploring the immunopathophysiology of T cell–mediated skin diseases.
Introduction
Desmoglein 3 (Dsg3) is a cadherin-type glycoprotein expressed in stratified squamous epithelium, including the skin, oral mucosa, and esophagus, and it plays a critical role in cell-cell adhesion. Dsg3 is the IgG-targeted autoantigen in pemphigus vulgaris (PV) (1). Binding of anti-Dsg3 autoantibodies to epithelial cell surfaces in PV patients and PV model mice inhibits Dsg3 function and leads to the loss of cell-cell adhesion, which manifests clinically as skin blisters and erosions, and histologically as suprabasilar acantholysis (2–4). The antigen-specific autoimmunity in PV makes this disease a valuable model for studying not only autoreactive B cells, but also T cells. Given the importance of the helper function of CD4+ T cells in Ab production (5, 6), autoreactive CD4+ T cells are believed to play critical roles in the pathogenesis of PV. Recently, Dsg3-reactive CD4+ T cell clones were established from Dsg3–/– mice (7, 8). Some clones showed helper activity for anti-Dsg3 IgG production and induced the PV phenotype when adoptively transferred with Dsg3–/– B cells into Rag2–/– mice. Analysis of these clones demonstrated that IL-4 production was critical for Dsg3-reactive T cells in inducing anti-Dsg3 IgG production and the PV phenotype in vivo (7).
T cells are also involved in a rare subset of pemphigus called paraneoplastic pemphigus (PNP). PNP occurs in association with hematopoietic neoplasms and exhibits more complex features than PV (9–11). Anti-Dsg3 and -Dsg1 autoantibodies are detected in PNP, as are autoantibodies against the plakin family, including plectin, desmoplakin I and II, BP230, envoplakin, and periplakin. In addition to acantholysis caused by anti-Dsg3 and -Dsg1 autoantibodies (12), the coexistence of cellular infiltration into lesional epithelial tissue, known as interface dermatitis, is a characteristic histopathological feature in PNP (13). Interface dermatitis is a distinct histological condition in which epidermal basal cell damage occurs subsequent to inflammation at the dermal-epidermal junction. The inflammatory infiltrates consist predominantly of T lymphocytes, and the presence of apoptotic keratinocytes suggests T cell immunity against putative antigens displayed on the surfaces of keratinocytes. Interface dermatitis is observed not only in PNP, but also commonly in lichen planus (LP), lichen sclerosis (LS), toxic epidermal necrolysis/Stevens-Johnson syndrome (TEN/SJS), graft-versus-host disease (GVHD), lupus erythematosus, and other diseases (9, 14–17).
Despite the notion that these skin-infiltrating T cells are autoreactive in nature, there have been few studies of the target antigens, which remain largely unknown, because, unlike Abs, which recognize native proteins, T cell receptors recognize protein peptides in the context of class I or II MHCs. MHC polymorphism and the difficulty in preparing a screening library for peptide-MHC complexes have hampered progress in defining antigen specificity in T cells that infiltrate in inflammatory skin diseases. Consequently, it is unclear whether autoreactive T cells contribute to the pathogenesis of inflammatory skin diseases.
Here, we report the generation of transgenic mice in which T cells express Dsg3-specific TCRs. Dsg3-specific CD4+ T cells that developed in the absence of Dsg3 caused severe mouse pemphigus when cotransferred with Dsg3–/– B cells into Rag2–/– mice. Of interest, histological examination of these mice revealed not only epidermal acantholysis (loss of keratinocyte adhesion), but also T cell infiltration of skin that exhibited interface dermatitis. Studies using WT T cells that were retrovirally transduced with Dsg3-specific TCRs revealed that T cells expressing different Dsg3-specific TCRs could induce interface dermatitis and that IFN-γ was a critical factor in inducing this phenotype.
Results
Cloning of 3 different TCR genes from Dsg3-reactive T cell clones.
Several Dsg3-reactive T cell clones were previously established from lymph node cells of Dsg3–/– mice that were immunized with the extracellular domain of recombinant Dsg3 (7). cDNAs encoding the TCR-α and -β chains were obtained from 3 different T cell clones: 140#27 (AV8S13-J21 and BV6S1-XDX-Jβ1.3), 162#24 (AV20S1-J39 and BV8S1-XDX-Jβ2.7), and 164#2 (AV15S1-J45 and BV6S1-XDX-Jβ2.3). These were subcloned into cassette vectors to enable the expression of both the TCR-α and -β chains (Supplemental Table 1; supplemental material available online with this article; doi: 10.1172/JCI57379DS1). The mRNA and amino acid sequences, including the CDR3 regions from these TCR-α and -β chains, are shown in Supplemental Figures 1–6.
Dsg3-specific TCRs recognize Dsg3 peptides with different avidities.
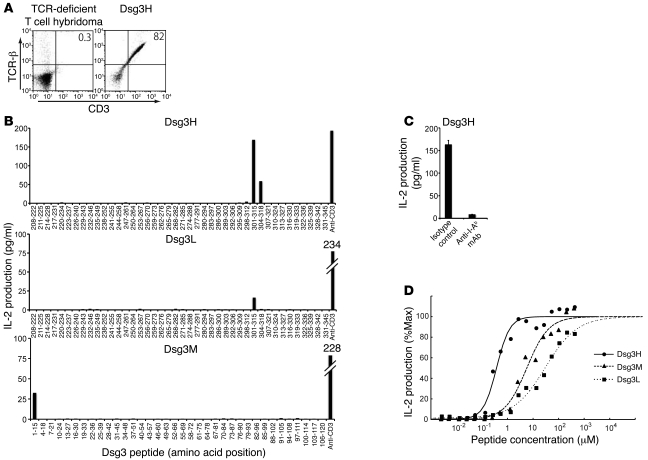
Vectors constructed to express TCR from 140#27, 162#24, and 164#2 were introduced into TCR-deficient hybridoma cell lines by electroporation to generate Dsg3-specific T cell hybridoma cell lines, referred to as Dsg3H, Dsg3M, and Dsg3L, respectively. TCR-β chain and CD3 molecules were detected on the cell surface of the stable transfectants, indicating that the transduced TCR-α and -β chains were properly assembled on the cell surfaces as a complex with CD3 molecules (Figure 1A and other data not shown). Because the parental T cell clones 140#27 and 164#2 showed proliferative responses on stimulation with the recombinant Dsg3 extracellular domain (aa 210–345), and 162#24 to the Dsg3 extracellular domain (aa 1–119) (7), overlapping Dsg3 peptides covering aa 208–345 and aa 1–120 of Dsg3 were prepared to determine the epitopes for Dsg3H, Dsg3M, and Dsg3L TCR. The Dsg3H hybridoma cell line vigorously produced IL-2 when cocultured with irradiated splenocytes and the peptide Dsg3(aa 301–315), which has the sequence RNKAEFHQSVISQYR (Figure 1B). Interestingly, the Dsg3L hybridoma cell line also produced IL-2 on stimulation with Dsg3(aa 301–315), demonstrating that Dsg3H and Dsg3L recognize identical Dsg3 epitopes. The Dsg3M hybridoma cell line produced IL-2 on stimulation with Dsg3(aa 1–15), which has the sequence EWVKFAKPCREREDN, the endmost peptide of the N-terminus of the mature Dsg3 protein. The reactivity of Dsg3H, Dsg3M, and Dsg3L with the corresponding peptides was inhibited when cultured in the presence of anti–MHC class II blocking Ab (Figure 1C and other data not shown), demonstrating that these TCRs recognized Dsg3 peptides in an I-Ab–restricted manner. The synthetic peptides tested here may not necessarily be identical to the ones processed from the native Dsg3 protein. Nevertheless, these TCRs recognized Dsg3 peptides processed in vivo, suggesting that the synthetic peptide Dsg3(aa 301–315) is at least similar, if not identical, to a naturally processed peptide in vivo (Figure 2D). Although appropriate expression of the TCR-α chains of these 3 TCRs could not be confirmed using commercial anti-Vα Abs, this functional assay indicated that both the TCR-α and -β chains were properly expressed.
Figure 1. Identification of epitope peptides for 3 Dsg3-specific TCRs and a comparison of the differences in their avidities.
(A) TCR-deficient T cell hybridoma was stably transfected by Dsg3H TCR-α and -β chain genes. Successful coexpression of TCR-β chain and CD3 molecules was subsequently detected by flow cytometry. (B) After the Dsg3H, Dsg3L, or Dsg3M hybridoma cell line was cultured with each Dsg3 peptide and irradiated splenocytes or stimulated by anti-CD3 Ab, IL-2 in the supernatant was quantified by ELISA. (C) The Dsg3H-transfectant was stimulated with peptide Dsg3(aa 301–315) in the presence or absence of anti-I-Ab Ab. Then, IL-2 was quantified by ELISA. (D) Dsg3H (line), Dsg3M (thick dotted line), and Dsg3L (fine dotted line) hybridoma cell lines were cultured with various concentrations of the corresponding peptide and irradiated splenocytes and the supernatant was subjected to IL-2 ELISA. Similar results were obtained in 2 separate experiments.
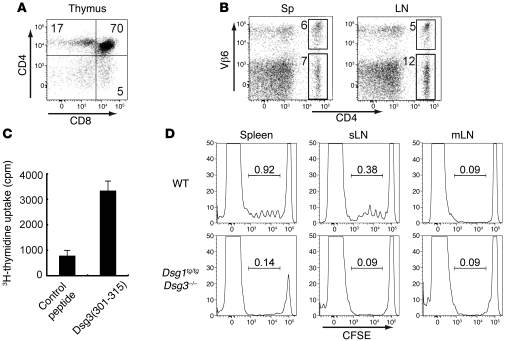
Figure 2. Generation of the Dsg3-specific TCR-transgenic mouse, Dsg3H1 mouse, and Dsg3 reactivity of transgenic T cells.
(A) Thymocytes were stained with anti-CD4 and -CD8 Abs and analyzed by flow cytometry. (B) Single-cell suspensions from the spleen and LNs were stained with anti-CD4 and -Vβ6 Abs and analyzed. (C) Splenocytes from Dsg3H1 mice were cultured with the peptide Dsg3(aa 301–315) or a control peptide. 3H-thymidine incorporation by these splenocytes is shown as the in vitro reactivity against Dsg3 peptide. (D) CFSE-labeled CD4+ T cells from Dsg3H1 mice were transferred into B6 WT mice and Dsg1tg/tgDsg3–/– mice. 3 days later, CFSE dilution was analyzed by flow cytometry after gating CD4+Vβ6+ cells of the spleen, skin-draining LN (sLN), and mesentery LN (mLN) from both recipients. Proportions of dividing cells were shown in each histogram. Similar results were obtained in 2 separate experiments. Data represent mean ± SEM.
The avidity of each TCR complex was further evaluated by stimulation with various concentrations of antigenic peptides. All of the Dsg3-specific T cell hybridoma cell lines responded to the corresponding Dsg3 peptides in a dose-dependent manner (Figure 1D). Interestingly, Dsg3H TCR recognized the MHC peptide complex with greater avidity than Dsg3L TCR (ED50 0.37 μM for Dsg3H and 29.3 μM for Dsg3L). Dsg3M TCR showed medium avidity, with an ED50 of 5.68 μM, and together with Dsg3H and Dsg3L, provided an interesting set of Dsg3-specific TCRs with differing levels of avidity, at least in the I-Ab background.
Generation of Dsg3-specific TCR-transgenic mice.
The development of transgenic mice that express Dsg3-specific TCRs should enable a better understanding of the roles of autoreactive T cells in the pathogenesis of pemphigus in vivo. T cell clone 140#27 induced anti-Dsg3 Ab production and the PV phenotype in vivo when cotransferred with Dsg3–/– B cells into Rag2–/– mice (7). Thus, the TCR from this clone was used to generate TCR transgenic mice, Dsg3H mice. Ultimately, to be able to study the development of autoreactive T cells in detail, it was necessary to select a line of transgenic mice in which T cell development occurred in a manner comparable to that in WT mice. We chose the transgenic line Dsg3-specific transgenic (Dsg3H1) from among several lines because transgenic T cells in Dsg3H1 started to express the TCR-β chain at the DN4 stage in the thymus, as do WT αβ T cells under physiological conditions (Supplemental Figure 7). CD4 single-positive T cells developed in the thymuses of Dsg3H1 mice (Figure 2A). Of the CD4+ population in the spleen and lymph nodes of the Dsg3H1 mouse, 30%–40% were Vβ6+ cells (Figure 2B).
Transgenic CD4+Vβ6+ T cells from Dsg3H1 mice react to Dsg3.
First, we examined the in vitro reactivity of CD4+Vβ6+ Dsg3H1 T cells to Dsg3. Splenocytes from Dsg3H1 mice were cultured with irradiated splenocytes in the presence of the peptide Dsg3(aa 301–315), and their proliferation was analyzed using a 3H-thymidine uptake assay. Addition of Dsg3 peptide induced proliferative responses, demonstrating that splenocytes from Dsg3H1 mice contained T cells that were functionally reactive to Dsg3(aa 301–315) ex vivo (Figure 2C).
We confirmed the antigen specificity of Dsg3H1 T cells in vivo. Because APCs present various antigenic peptides, including self antigens in the context of MHC class II molecules (18, 19), we evaluated whether APCs from WT mice were able to stimulate the proliferation of CD4+ Dsg3H1 T cells in vivo. CD4+ Dsg3H1 T cells were labeled with CFSE and then adoptively transferred into C57BL/6 WT mice or Dsg1tg/tgDsg3–/– C57BL/6 mice. Dsg1tg/tgDsg3–/– mice express Dsg1, driven by the keratin 5 promoter, which functionally compensates for the loss of Dsg3, leading to better survival in various experimental settings (20). Three days after the transfer, CFSE dilution of CD4+Vβ6+ cells was detected in both the spleen and skin-draining LNs, but not in the mesenteric LNs of C57BL/6 WT mice. Importantly, no CFSE dilution was detected in any of the Dsg1tg/tgDsg3–/– mice, indicating that CD4+ Dsg3H1 T cells proliferated only in the presence of Dsg3, further ensuring the antigen reactivity of these cells in vivo (Figure 2D).
Transgenic Dsg3H1 T cells undergo Dsg3-dependent T cell selection.
Despite the forced expression of Dsg3-specific TCR, only 30%–40% of the CD4+ T cells were Vβ6+ in Dsg3H1 mice. Conceivably, the relatively low number of Vβ6+ cells is due to the presence of tolerance mechanisms in these Dsg3-expressing animals. To determine the effect of the presence or absence of Dsg3 during T cell development, BM cells from Dsg3H1 mice (Ly9.2, I-Ab) were transferred into sublethally irradiated 129/Sv WT and 129/Sv Dsg3–/– mice (Ly9.1, I-Ab) to generate BM chimeric mice, referred to as Dsg3H1→WT mice and Dsg3H1→Dsg3–/– mice, respectively (Supplemental Figure 8). Because of the sublethal irradiation dose, efficient adaptation of the donor BM occurs in the presence of recipient hematopoietic cells that are not eradicated by irradiation, allowing the evaluation of Dsg3H1 T cell development as a subpopulation among a diverse repertoire of T cells. The donor Ly9.2+ T cells can be distinguished from recipient Ly9.1+ T cells using an anti-Ly9.1 Ab. Strikingly, in Dsg3H1→Dsg3–/– mice, in which Dsg3H1 T cells developed in the absence of Dsg3, Ly9.1–CD4+ Vβ6+ cells constituted over 95% of the donor-derived CD4+ T cells. Under the Dsg3+/+ condition in Dsg3H1→WT mice, the proportion of Ly9.1–CD4+ Vβ6+ T cells was much decreased, to proportions similar to those of Dsg3H1 mice (Supplemental Figure 9A). These results demonstrated that T cell tolerance mechanisms against Dsg3 exist in WT mice and that Dsg3H1 T cells undergo Dsg3-dependent negative selection in Dsg3H1 mice. However, the incomplete deletion may reflect Dsg3H1 T cells that had “escaped” from the deletion process.
Tolerance mechanisms inhibit the induction of PV, but not interface dermatitis, by Dsg3H1 T cells.
We questioned whether the Dsg3-reactive CD4+Vβ6+ T cells that were present in the periphery of Dsg3H1 mice, which presumably had escaped the above-described tolerance mechanisms, still had the capacity to induce PV. To determine this, Dsg3H1 T cells were isolated from Dsg3H1 mice directly and cotransferred with Dsg3–/– B cells into Rag2–/– mice. Fine scales and mild erythema developed on the ears, neck, tail, and soles within 1 week of adoptive transfer; these progressed to erosions and swelling of the arms, perioral region, and trunk (Figure 3A). This phenotype was not observed in the recipients that were cotransferred with simultaneously isolated WT CD4+ T cells and Dsg3–/– B cells.
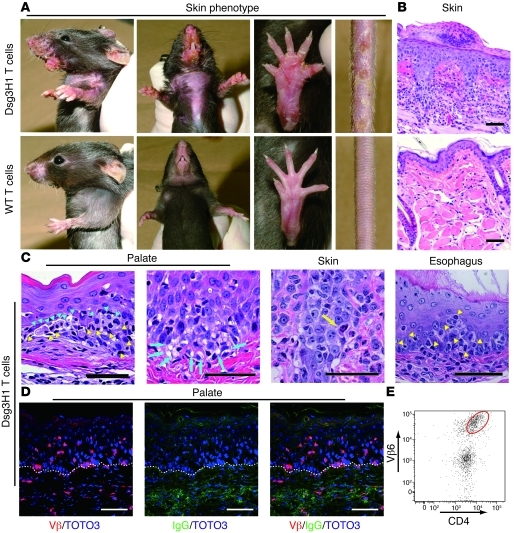
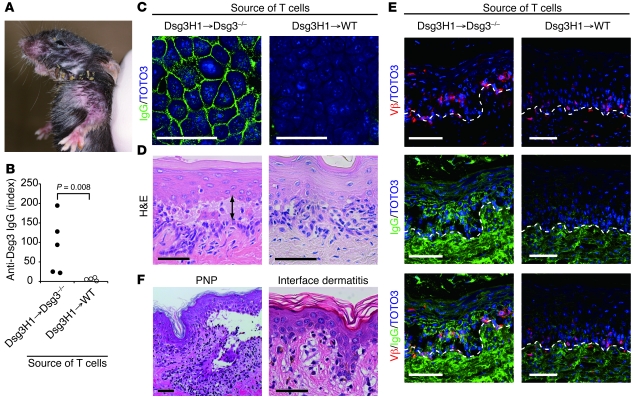
Figure 3. Tolerized Dsg3H1 T cells induced interface dermatitis, but not PV.
(A) The skin phenotype of Rag2–/– mice (n = 3 per group) given CD4+Vβ6+ cells from Dsg3H1 mice or CD4+ T cells from C57BL/6 mice in combination with Dsg3–/– B cells. Erosive and crusted lesion in the perioral region, upper limbs, paws, and tail and hair loss on the chest were evident (upper panels). (B) Pathology of the skin in the recipients given CD4+Vβ6+ cells from Dsg3H1 mice or WT CD4+ T cells. (C) Various Dsg3-expressing tissues in the recipients of CD4+Vβ6+ cells from Dsg3H1 mice were stained with H&E. Blue arrowheads indicate degenerated cells in the epithelium. Yellow arrowheads indicate intraepithelial inflammatory cells. Blue arrows indicate liquefaction degeneration in the basal layer of the lesional epithelium. Yellow arrow indicates a degenerated cell well stained with eosin, a so-called Civatte body. (D) The palate was stained with anti–TCR-β chain (red) and anti-IgG (green) Abs and TOTO3 (blue). Dotted lines indicate the basement membrane zone. (E) A single-cell suspension was prepared from the lesional skin of the recipients given CD4+Vβ6+ cells from Dsg3H1 mice and analyzed by flow cytometry after gating into the CD45+7-AAD– population. Scale bars: 50 μm.
Strikingly, however, no anti-Dsg3 IgG Abs were found in serum from these mice (data not shown). Histopathological studies revealed massive inflammatory cell infiltrates not only in skin, but also in the palate and esophagus, the epithelia of which express Dsg3 (Figure 3, B and C, and Supplemental Figure 10). Lesional epithelia exhibited acanthosis and hyperkeratosis, and keratinocytes undergoing apoptosis and degeneration were observed among the infiltrating lymphocytes. Liquefaction degeneration was observed in the basal cell layers (Figure 3C), where apoptotic keratinocytes resembled Civatte bodies (Figure 3C), as seen in human diseases that involve interface dermatitis (21, 22). These features indeed fulfilled the criteria of human interface dermatitis (Table 1). Immunofluorescence microscopy revealed the infiltration of TCR-β+ T cells in the epidermis (Figure 3D), and a major population of these was identified as CD4+Vβ6+ T cells by flow cytometry (Figure 3E). In contrast with the lymphocyte infiltration, neither suprabasilar acantholysis nor IgG deposition on the surface of keratinocytes was detected (Figure 3, C and D), consistent with the absence of anti-Dsg3 IgG Abs in serum from these mice. Thus, Dsg3H1 T cells that had developed in the presence of Dsg3 did not exert helper activity for B cell autoantibody production to cause PV, but were capable of inducing interface dermatitis, a distinct T cell–mediated inflammation at the dermal-epidermal junction seen in LP, LS, lupus, GVHD, SJS/TEN, PNP, and other diseases.
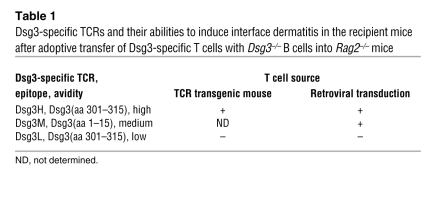
Table 1 .
Dsg3-specific TCRs and their abilities to induce interface dermatitis in the recipient mice after adoptive transfer of Dsg3-specific T cells with Dsg3–/– B cells into Rag2–/– mice
Nontolerized Dsg3H1 T cells induce both anti-Dsg3 IgG production and interface dermatitis.
Paradoxically, whereas the parental T cell clone of Dsg3H1 TCR, 140#27, helped Dsg3–/– B cells to produce anti-Dsg3 autoantibodies, Dsg3H1 T cells could not. We next asked whether the presence or absence of Dsg3 during T cell development influenced the fate of T cell development in Dsg3H1 mice. 140#27 was originally established from Dsg3–/– mice (7). Thus, to mimic this developmental setting, the pathogenic activity of transgenic Dsg3H1 T cells was evaluated using BM chimeric Dsg3H1→Dsg3–/– mice. Ly9.1–CD4+ T cells in Dsg3H1→Dsg3–/– mice, referred to as Dsg3H1→Dsg3–/– CD4+ T cells, showed the CD44lo naive T cell phenotype and expressed the Vβ6 chain (Supplemental Figure 9, A and B). These naive Dsg3H1→Dsg3–/– CD4+ T cells were isolated and cotransferred with Dsg3–/– B cells into Rag2–/– mice (Supplemental Figure 8) and were analyzed 1 week after adoptive transfer. Ly9.1–CD4+ T cells in Dsg3H1→WT mice, referred to as Dsg3H1→WT CD4+ T cells, were used as a control. Because Dsg3H1→WT CD4+ T cells contained approximately 30% of the CD44hi population (memory/activated phenotype; Supplemental Figure 9B), naive T cells were isolated by depleting CD44hi memory cells from the Ly9.1–CD4+ cell population of Dsg3H1→WT mice (Supplemental Figure 9B) and transferred with Dsg3–/– B cells into Rag2–/– mice (Supplemental Figure 8). The recipient mice that received naive Dsg3H1→Dsg3–/– CD4+ T cells and Dsg3–/– B cells developed erythma and crusted lesion on the trunk, neck, limbs, face, and paws (Figure 4A). ELISA showed that anti-Dsg3 IgG was produced in these recipient mice (Figure 4B). Living cell staining also demonstrated that IgG from these mice bound to native antigens expressed on the surfaces of the mouse epidermal cell line (Pam cells) (Figure 4C). Consistently, histopathological analysis revealed suprabasilar acantholysis in the palate (Figure 4D). In addition to suprabasilar acantholysis, however, CD4+ T cell infiltration of the epithelium and lamina propria mucosae was observed in the palate. Immunofluorescence analysis demonstrated the coexistence of IgG deposition on the epithelial cell surfaces and Vβ+ T cell infiltration (Figure 4E and Table 2). There were no detectable circulating IgG Abs against desmoplakin, envoplakin, and periplakin in the recipient mice (data not shown). The combination of suprabasilar acantholysis and interface dermatitis observed in this mouse model is similar to the pathological changes observed in human PNP (Figure 4F).
Figure 4. Nontolerized Dsg3H1 T cells induce both anti-Dsg3 IgG production and interface dermatitis.
BM cells from Dsg3H1 mice (Ly9.2) were transferred into sublethally irradiated 129/Sv WT and Dsg3–/– mice (Ly9.1) to generate BM chimeric mice, referred to as Dsg3H1→WT and Dsg3H1→Dsg3–/– mice, respectively. Donor-derived naive CD4+Vβ6+CD44–Ly9.1– cells were enriched from Dsg3H1→WT and Dsg3H1→Dsg3–/– mice and were cotransferred with Dsg3–/– B cells into Rag2–/– mice. (A) Skin phenotype in the recipient mice that received donor-derived CD4+ T cells from Dsg3H1→Dsg3–/– mice and Dsg3–/– B cells. Erythema and crusted lesions were observed in face, neck, and limbs. (B) Anti-Dsg3 IgG Ab titers in sera from the recipients were analyzed by ELISA. Statistical analysis was performed using the Mann-Whitney U test. (C) Sera from the recipients were added to the culture medium of Pam cells. IgG deposition on the cell surfaces was subsequently detected by anti-IgG Ab Alexa Fluor 488. Nuclei were stained with TOTO3 (blue). (D) Palates of the recipients were analyzed by H&E staining. The arrow indicates suprabasilar acantholysis. (E) Intraepidermal T cells (red) and IgG deposition (green) in the recipient’s palate were detected using anti–TCR-β Ab and anti-mouse IgG Ab, respectively. Dotted lines show the basement membrane zone. Similar results were obtained in 2 separate experiments. (F) The skin histopathology of PNP and interface dermatitis (GVHD) are shown. Scale bars: 50 μm.
Table 2 .
Phenotype of the recipient mice after adoptive transfer of BM chimera-derived Dsg3H1 cells with Dsg3–/– B cells into Rag2–/– mice
In contrast, anti-Dsg3 IgG was not detected in the recipient mice transferred with naive Dsg3H1→WT CD4+ T cells and Dsg3–/– B cells by either ELISA, living cell staining, or immunofluorescence analyses (Figure 4, B, C, and E), and suprabasilar acantholysis by histology was not observed in the palate (Figure 4D). However, some cell infiltration was observed in the palate of the recipient with naive Dsg3H1→WT CD4+ T cells, and immunofluorescence analysis identified the infiltrating cells as Vβ+ cells (Figure 4E and Table 2). Similar infiltration was observed in the skin and esophagus, where Dsg3 was expressed (data not shown). These results demonstrated that nontolerized Dsg3-specific CD4+ T cells that developed in the absence of Dsg3 are able not only to induce suprabasilar acantholysis via the production of anti-Dsg3 autoantibodies, but are also capable of infiltrating skin directly to cause interface dermatitis, which in combination, showed some findings that are seen in PNP. These results suggest that those Dsg3H1 T cells that are capable of inducing PV are highly reactive with Dsg3 and are negatively selected in the Dsg3+/+ environment.
Avidity-dependent induction of interface dermatitis by Dsg3-specific T cells.
Because we had cloned 3 different Dsg3-specific TCRs with differing avidities, this gave us an opportunity to determine whether different TCRs specific for Dsg3 could induce interface dermatitis and to evaluate any relationship between TCR avidity and clinical severity. To determine this, we retrovirally transduced WT CD4+ T cells with Dsg3-specific TCRs Dsg3H, Dsg3M, or Dsg3L, each with differing avidities for Dsg3 (see Figure 1D). By performing transduction on WT CD4+ T cells, it is possible to circumvent TCR avidity–specific events that may occur during thymic selection. WT CD4+ T cells were retrovirally transduced with Dsg3H TCR to generate rvDsg3H T cells, which properly acquired Dsg3 reactivity, shown by the robust IL-2 production on stimulation with Dsg3(aa 301–315) (Supplemental Figure 11). When rvDsg3H T cells were transferred with or without Dsg3–/– B cells into Rag2–/– mice, the recipient mice developed erosive and crusted lesions on the ears, neck, periorbital region, paws, and tail (Figure 5A). Histopathological analysis revealed interface dermatitis in the skin, palate, and esophagus (Figure 5A and other data not shown). Interface dermatitis was not observed in non–Dsg3-expressing tissues, including the liver and small and large intestines (Supplemental Figure 12). Furthermore, recipients to which Dsg3–/– B cells were also transferred showed no anti-Dsg3 IgG production or PV phenotype (Supplemental Figure 13). Control animals that received CD4+ T cells transduced with control retrovirus, rvGFP T cells, did not develop any phenotype (Figure 5A). Overall, the phenotype induced by rvDsg3H T cells was nearly identical to that of Dsg3H1 T cells.
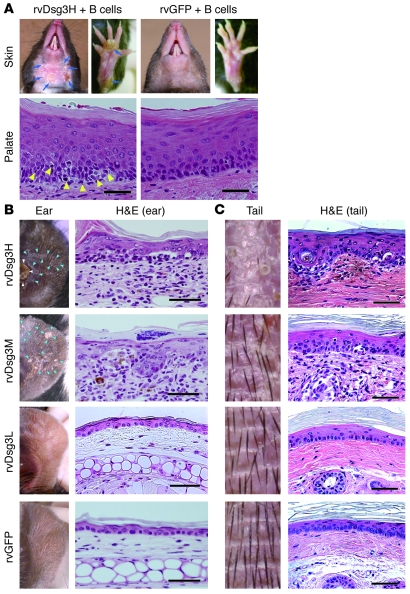
Figure 5. Avidity-dependent induction of interface dermatitis by retrovirally transduced Dsg3-specific T cells.
Dsg3-specific T cells, including rvDsg3H, rvDsg3M, and rvDsg3L T cells, were generated by retroviral transduction of Dsg3H, Dsg3M, and Dsg3L TCRs into WT CD4+ T cells, respectively. Control T cells, rvGFP T cells, were generated using control retrovirus. (A) The skin phenotype and histopathology of the palate from the recipient mice given rvDsg3H T cells and rvGFP T cells in combination with Dsg3–/– B cells. Blue arrows show erosive and crusted lesions. Yellow arrowheads indicate inflammatory cell infiltration. (B and C) rvDsg3H, rvDsg3M, rvDsg3L, and rvGFP T cells were transferred into Rag2–/– mice. 4 weeks later, the skin phenotype and histopathology of the ears (B) and tails (C) were observed. White arrowheads indicate a crusted lesion. Blue arrowheads indicate scaly lesions. Scale bars: 50 μm. Similar results were obtained from 2 independent experiments.
Next, we generated rvDsg3M (medium avidity) and rvDsg3L (low avidity) T cells, and transferred them into Rag2–/– mice with no B cells. Whereas mice that received rvDsg3M subsequently developed scaly erythema, comparable to mice that received rvDsg3H, mice that received rvDsg3L T cells did not develop any skin abnormalities (Figure 5, B and C). The histopathology of the lesional skin induced by rvDsg3H and rvDsg3M revealed interface dermatitis (Table 1). Consistent with earlier experiments, no lesion was seen in non–Dsg3-expressing tissues (Supplemental Figure 12).
To confirm the nonpathogenic nature of Dsg3L TCR in inducing interface dermatitis, a Dsg3L TCR transgenic mouse (i.e., the Dsg3L3 mouse) was evaluated. More than 90% of the CD4+ T cells expressed Vβ6 in the spleen and LNs of Dsg3L3 mice (Supplemental Figure 14A). CD4+Vβ6+ T cells from Dsg3L3 transgenic mice were cotransferred with Dsg3–/– B cells into Rag2–/– mice, but unlike those that received Dsg3H1 T cells, the Dsg3L3 recipient mice did not develop any abnormalities, consistent with the results obtained with the retroviral system (Table 1 and Supplemental Figure 14B).
Thus, T cells expressing Dsg3-specific TCRs with high and medium avidity, but not those with low avidity, were capable of eliciting interface dermatitis. These results collectively demonstrated that interface dermatitis could be induced in an antigen-specific and a TCR avidity–dependent manner by Dsg3-specific CD4+ T cells.
IFN-γ–dependent induction of interface dermatitis by Dsg3-specific T cells.
To further understand the molecular events that influenced Dsg3H1 T cells to elicit different phenotypes depending on their exposure to Dsg3 during developmental processes, cytokine analyses were performed to clarify which T helper subset the Dsg3H1 T cells had differentiated into in vivo. Naive Dsg3H1 T cells that were cotransferred with Dsg3–/– B cells into Rag2–/– mice were isolated ex vivo after disease elicitation and were analyzed for IFN-γ, IL-4, IL-17A, and Foxp3. Dsg3H1→Dsg3–/– CD4+ T cells and Dsg3H1→WT CD4+ T cells were compared (Supplemental Figure 8) because these T cells induced both autoantibody production and interface dermatitis, or only interface dermatitis, respectively (Table 2).
Whereas IFN-γ was the predominant cytokine produced by Dsg3H1 T cells (Ly9.1–CD4+Vβ6+) isolated from the spleens and LNs of both groups, Dsg3H1 T cells isolated from the skin-draining LNs of Dsg3H1→Dsg3–/– mice deviated more toward IL-4 production and expressed relatively lower levels of IFN-γ (Figure 6A). Interestingly, whereas a fraction of Dsg3H1→WT CD4+ T cells had differentiated into IL-17A–expressing T cells, this was not observed in Dsg3H1→Dsg3–/– CD4+ T cells (Figure 6B). Foxp3-expressing Dsg3H1 T cells were found in similar ratios in the spleen and skin-draining LNs from both groups. It seems likely that the presence or absence of an autoantigen influences T cell development and diversifies T cell properties, such as antigen reactivity, leading to striking differences in cytokine deviation and phenotypic outputs.
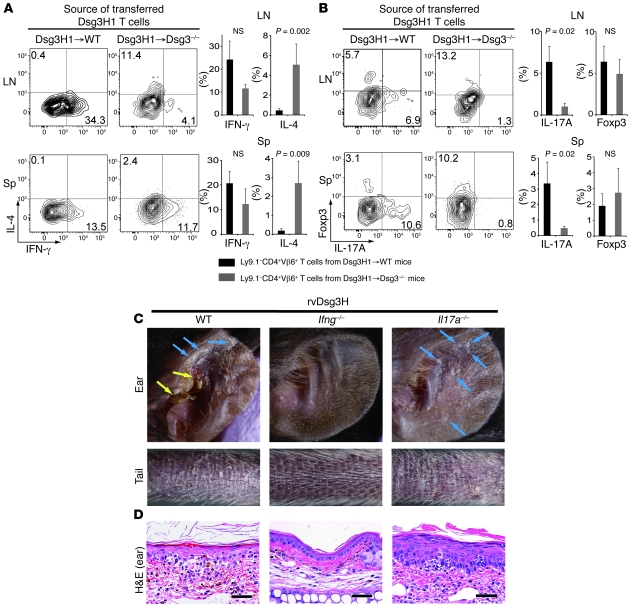
Figure 6. Dsg3-specific T cells induce interface dermatitis in an IFN-γ–dependent manner.
(A and B) Naive Dsg3H1→WT or Dsg3H1→Dsg3–/– CD4+ T cells were transferred with Dsg3–/– B cells into Rag2–/– mice. More than 6 days later, LN and spleen were collected and used to make a single-cell suspension, which was then treated with PMA, ionomycin, and brefeldin A, and flow cytometric analysis examined the expression of IFN-γ versus IL-4 (A) and IL-17A versus Foxp3 (B). Representative contour plots are shown after gating into the CD4+Vβ6+7-AAD– population. The quadrants were set based on the results of staining with isotype control Abs. Black bars represent the average for Ly9.1–CD4+Vβ6+ T cells from Dsg3H1→WT mice (n = 6). Gray bars represent the average for Ly9.1–CD4+Vβ6+ T cells from Dsg3H1→Dsg3–/– mice (n = 6). Error bars indicate the SEM. Statistical comparison was made using the Mann-Whitney U test. (C and D) CD4+ T cells were isolated from WT, Ifng–/–, or Il17a–/– mice and retrovirally transduced with Dsg3H TCR and transferred into Rag2–/– mice. 3 weeks later, the macroscopic phenotype (C) and histopathology of the skin (D) were observed. Yellow arrows indicate crusted lesions in the ear. Blue arrows indicate scaly lesions. Scale bars: 50 μm. Similar results were obtained from 2 independent experiments.
It caught our strong interest that in settings where interface dermatitis was the predominant phenotype, both IFN-γ– and IL-17A–producing Dsg3H1 T cells were detected, suggesting that these cytokines played important roles in interface dermatitis. To determine this, we took advantage of the retroviral expressing system, and CD4+ T cells isolated from Ifng–/– and Il17a–/– mice were transduced to generate Ifng–/– or Il17a–/– rvDsg3H T cells. Within 3 weeks after the adoptive transfer of WT rvDsg3H T cells and Il17a–/– rvDsg3H T cells, Rag2–/– mice exhibited skin inflammation, showing scale, crusts, and erythema. Strikingly, however, Ifng–/– rvDsg3H T cells failed to elicit any abnormalities (Figure 6C). The histopathology of the skin lesions elicited by WT and Il17a–/– rvDsg3H T cells revealed typical interface dermatitis, while the skin of mice transferred with Ifng–/– rvDsg3H T cells showed no inflammatory infiltrate (Figure 6D). These findings demonstrated that IFN-γ, but not IL-17A, is the key cytokine produced by Dsg3-specific T cells that causes interface dermatitis.
Discussion
We generated Dsg3-specific TCR transgenic mice to analyze the development and roles of autoreactive CD4+ T cells in PV. Bone marrow transplantation experiments demonstrated Dsg3 tolerance mechanisms in vivo. When Dsg3H1 T cells that developed in the absence of Dsg3 were cotransferred with Dsg3–/– B cells into Rag2–/– mice, these mice developed a severe skin phenotype mimicking PNP. Unexpectedly, in addition to Ab production by B cells, direct infiltration of Dsg3H1 T cells was seen in the skin, resulting in histological features that recapitulated interface dermatitis, a phenomenon that is not seen in PV, but in PNP. Retrovirally generated Dsg3H T cells showed that interface dermatitis could be induced independently of B cells, in an IFN-γ– and TCR avidity–dependent manner, indicating that autoimmunity by CD4+ T cells directed against a defined physiological epidermal antigen is sufficient to cause interface dermatitis.
Interface dermatitis exhibits degenerative changes at the dermal-epidermal junction that are accompanied by lymphocytic infiltration (22). Interface dermatitis is divided into 2 subtypes: vacuolar type and lichenoid type (lichenoid dermatitis). Vacuolar types shows small vacuolar spaces at the dermal-epidermal junction, often leaving the junction indistinct. Leukocyte infiltration in this type may not be prominent. On the other hand, lichenoid types show a dense, band-like infiltrate of inflammatory cells, which consists mostly of lymphocytes, in the superficial dermis. Histological findings observed in the skin lesions of our model mice showed interface dermatitis with lymphocytic infiltrations that were not as dense as the band-like infiltration of the lichenoid type and thus more resembled the vacuolar type interface dermatitis, which is found in the original histological description for patients with PNP (9).
Interface dermatitis is observed in various inflammatory conditions of the skin and mucous membranes, including LP, LS, TEN/SJS, GVHD, lupus, and PNP. The infiltrating lymphocytes in LP lesions have been reported to be a mixture of CD4+ and CD8+ T cells (23), which appear, at least histologically, to “attack” the epidermal keratinocytes, causing apoptosis and obscuring the dermal-epidermal junction. It has been described that cytotoxic CD8+ T cells are capable of directly injuring keratinocytes and thus play a central role in interface dermatitis (24, 25). Although there are very few reports that have studied the role of CD4+ T cells in interface dermatitis, one study has clearly demonstrated that CD4+ T cells alone can injure keratinocytes (26). The cytotoxic effector function of CD4+ T cells has been observed in other animal models, including EAE, with results that are also consistent with ours in terms of tissue injury by CD4+ T cells (27). However, more fundamental questions, such as which population of T cells plays the initial role in triggering interface dermatitis and what proportion of infiltrating CD4+ and CD8+ T cells are specific to certain antigens in skin, remain unresolved. Thus, our experimental autoimmune dermatitis (EAD) model described in this study will provide a valuable tool to clarify such unsolved pathophysiological mechanisms of T cell–mediated skin diseases.
Despite the long-standing notion that these lymphocytes might recognize skin-associated autoantigens, strong evidence for autoimmunity and putative autoantigens is lacking. Interestingly, however, in LS and LP, both of which exhibit interface dermatitis, autoantibodies against extracellular matrix protein 1 (28) and type XVII collagen (29) have been reported, suggesting the existence of autoimmunity in these patients, further implying the possibility that skin-infiltrating T cells might also recognize the same autoantigen. CD8+ T cell lines established from the lesional skin of LP patients exerted cytotoxic activity against immortalized autologous keratinocytes, supporting the involvement of skin-targeted autoimmunity in the development of interface dermatitis (30).
Likewise, the autoantibody targets in PNP are well characterized, but the relative importance of T cell immunity in the pathology and nature of the autoantigen were unclear. The dermatitis observed in our EAD model is a CD4+ T cell response. Thus, it does not fully recapitulate the immunity seen in PNP, in which Ab targets are not restricted to Dsg3, and CD8+ T cells also contribute to the pathogenesis. Nevertheless, mice that were cotransferred with Dsg3H1 T cells and Dsg3–/– B cells exhibited PNP-like clinical and pathological features, including both acantholysis and interface dermatitis, resulting in a rapidly progressive course with high mortality. The coexistence of humoral immunity against Dsg3 and interface dermatitis is a phenotypic combination unique to PNP. The results of our murine study imply, for what we believe is the first time, that T cell immunity against Dsg3, among other targets, might exist in human PNP. The finding that direct tissue infiltration of T cells that recognize a specific skin-associated antigen leads to interface dermatitis extends its significance not only to Dsg3 and PV or PNP, but also to other diseases that display interface dermatitis and provides impetus for the further characterization of T cell–mediated inflammatory skin diseases.
Recent research has revealed new subsets of helper T cells and critical cytokines that induce specific responses, including autoimmunity. Th1 and Th17 differentiation and cytokines that support the development of helper T cell subsets, such as IL-12 and IL-23, have been investigated intensively in the context of autoimmune disease models. Th17 cells are important for disease development in collagen-induced arthritis (31–35). In contrast, Th1 cells have been reported to be critical for autoimmune diabetes (36–38). Furthermore, in EAE, conflicting results have been reported and the central role of a particular T helper subset in the induction of EAE is controversial (39–41). This evidence emphasizes that each autoimmune disease may involve differing mechanisms and the pathological mechanisms of each disease must be investigated carefully.
In our EAD model, the importance of IFN-γ, but not IL-17A, was clearly demonstrated in the induction of interface dermatitis caused by Dsg3-specific T cells. This result is consistent with a previous study, in which H2Kb donor cells from Il17a–/– mice, when transferred into H2Kb/d recipients, induced GVHD to an extent similar to Il17a+/+ donor cells (42). However, it is still unclear whether IFN-γ directly acts against keratinocytes to cause tissue destruction or whether IFN-γ acts on T cells themselves to acquire cytotoxic activity. In a model of autoimmune diabetes utilizing NOD mice, IFN-γ was important to induce surface expression of FasL in β cells, which subsequently underwent apoptosis through Fas expressed by activated CD4+ T cells (43). Furthermore, requirement of IFN-γ for induction of interface dermatitis implies that other Th1-associated molecules will be critically involved in this model. In addition to IFN-γ, IL-12 and IL-27 play pivotal roles in Th1 differentiation (44, 45). These cytokines participate in activation of molecules such as Jak1 and -2, and STAT1 and -4, all of which are important components of the signaling cascade that induces T-bet expression as well as IFN-γ expression or amplification. These critical molecules in Th1 differentiation may be potential targets to modulate disease activity, and our EAD model should provide a useful tool for in-depth evaluation.
Reported mouse models of TEN (46) and GVHD-like disease (47) target neoantigens in which transgenic mice that express membrane-bound ovalbumin, driven by the keratin 5 or 14 promoters, are transferred with OVA-specific CD8+ T cells from OT-I mice. These mice demonstrated a role for CD8+ T cells in inducing the TEN or GVHD-like phenotype and exhibited interface dermatitis on histology. Although CD4+ T cells are likely involved in helping CD8+ T cells, the roles of CD4+ T cells in these models are unclear. Another report showed a local interface dermatitis model in which CD5+CD8– allo-Ia-reactive T cell clones were injected subcutaneously and demonstrated that the immune response against the allo–MHC class II antigen was capable of inducing interface dermatitis (26). The Dsg3-specific TCRs we used are MHC class II–restricted, and these CD4+ T cells were also capable of inducing interface dermatitis. Together, these results demonstrated that CD4+ T cells are sufficient to induce interface dermatitis, at least in the acute phase, and suggest that MHC class II–expressing cells are important cell components in the pathological process.
A major issue in using neoantigen transgenic mice to analyze autoimmune responses is that the level of antigen expression cannot be controlled. Because these are nonfunctional molecules, it is possible that such molecules do not undergo physiological turnover or processing, including degradation and recycling. Studies of the immune reaction against neoantigens may not necessarily reflect the immune reaction against physiological antigens. We believe that the significance of the EAD that we report here lies in the fact that a physiological and functional molecule, which is expressed only in the skin and mucous membranes, is targeted. Dsg3 is also expressed in the thymus by Aire-expressing medullary thymic epithelial cells, and this may influence the output of immune reactions (48). Thus, the use of a physiological antigen as a target antigen of the autoimmune reaction may be important in analyzing autoimmunity. In this regard, Dsg3-specific TCR transgenic mice should provide a useful tool for exploring the development of autoreactive T cells in the thymus, as well as their maintenance or regulation in the periphery. How and where these cells interact with antigen-presenting cells and the processes by which they find their targets are fundamental, important questions in the context of not only PV and PNP, but also in the array of T cell–mediated inflammatory skin diseases that exhibit interface dermatitis.
In conclusion, we generated TCR transgenic mice with autoreactive CD4+ T cells that are specific for Dsg3, a physiological autoantigen expressed by epidermal keratinocytes. The finding that CD4+ T cells specific for a single skin-associated antigen could induce a PNP-like phenotype or interface dermatitis sheds light not only on the pathophysiology of pemphigus, but also on a range of other T cell–mediated inflammatory skin diseases with as-yet-undefined target antigens. This experimental model of autoimmune dermatitis should deepen our understanding of the immunopathophysiology of T cell–mediated skin diseases and provide impetus for investigating targeted autoantigens in human T cell–mediated skin diseases that exhibit interface dermatitis.
Methods
Mice.
C57BL/6 (H-2b, Ly-9.2) and 129/Sv mice (H-2b, Ly-9.1) were purchased from CLEA Japan and Sankyo Labo Service Corporation. C57BL/6 Rag2–/– mice were purchased from the Central Institute for Experimental Animals (Tokyo, Japan). Dsg3–/– mice with a mixed 129/Sv and C57BL/6J genetic background and 129/Sv Dsg3–/– mice were obtained by mating male and female Dsg3–/– mice (Jackson Laboratory) (49). Dsg1tg/tgDsg3–/– mice with a C57BL/6 background were generated as described elsewhere (20). Ifng–/– mice were purchased from Jackson Laboratory. Il17a–/– mice were generated as described previously (42).
The animals were housed under specific pathogen–free conditions. The Keio University Ethics Committee for Animal Experiments approved all of the experiments in this study.
DNA construct for Dsg3-reactive TCR expression.
Rearranged genes including the variable and junctional regions of the TCR-α and -β chains of a Dsg3-reactive T cell clone, 140#27 (Vα8-J21, Vβ6-Dβ1-Jβ1.3) (7), were obtained by RT-PCR using the following primers: Vα-Jα region, forward 5′-TCTCCCGGGTGCACTCAAGGACCAAGTGT-3′ (the XmaI site is underlined), reverse 5′-TTGGCTTCACTGTGAGCACGGTCCCA-3′; Vβ-Dβ-Jβ region, forward 5′-GTCTCGAGTTTCTCTTTTAACTAACTAATGCCC-3′ (XhoI), reverse 5′-CTACAACAATGAGCCGGCTTCCTT-3′. J21 or Jβ1.3 genes with an intron at their 3′ terminus were obtained from genomic DNA by PCR using the following primers: Jα21 intron, forward 5′-GGGGATGGGACCGTGCTCACAGTG-3′, reverse 5′-GCGCGGCCGCCCTGCCAGGAAGTCTAGTCAAAA-3′ (NotI); Jβ1.3 intron, forward 5′-GGAGAAGGAAGCCGGCTCATTGT-3′, reverse 5′-GCCCGCGGCGTAGGACTGTGAACAGTACAT-3′ (SacII). The Vα-Jα and Vβ-Dβ-Jβ genes were linked with the Jα intron and Jβ intron to obtain Vα-Jα intron and Vβ-Dβ-Jβ intron genes, respectively, (Supplemental Figures 1 and 2) by linkage PCR. The genes of the TCR-α and -β chains of a Dsg3-reactive T cell clone, 162#24 (AV20S1-J39 and BV8S1-XDX-Jβ2.7), were obtained similarly by RT-PCR and linkage PCR using the following primers: Vα-Jα region, forward 5′-GCCCGGGGGAGAGATAACTCAAAGCTTCAGAGAAGA-3′ (XmaI), reverse 5′-GAGGTCTGACTCTCAAAATGGTTCCCAAGCCAAA-3′; Vβ-Dβ-Jβ region, forward 5′-GCTCGAGTAGTTCTGAGATGGGCTCCAGACTCTT-3′ (XhoI), reverse 5′-CTAAAACCGTGAGCCTGGTGCCGGGACCGAAGTA-3′. J39 or Jβ2.7 genes with an intron at their 3′ terminus were obtained from genomic DNA by PCR using the following primers: J39 intron, forward 5′-CTATGCAAACAAGATGATCTTTGGCTTGGGAA-3′, reverse 5′-GCCGCGGTCCAAGCTGTGGGCCACACCAGTGAATTT-3′ (SacII); Jβ2.7 intron, forward 5′-ATGAACAGTACTTCGGTCCCGGCACCAGGCTCA-3′, reverse 5′-GCCGCGGCCCACCCAGTGCATGCATACCTCAGAGA-3′ (SacII). Then, Vα-Jα and Vβ-Dβ-Jβ genes were linked with the Jα intron and Jβ intron to obtain Vα-Jα intron and Vβ-Dβ-Jβ intron genes, respectively. The genes of the TCR-α and -β chains of a Dsg3-reactive T cell clone, 164#2 (AV15S1-J45 and BV6S1-XDX-Jβ2.3), were obtained similarly by RT-PCR and linkage PCR using the following primers: Vα-Jα region, forward 5′-GCCCGGGCTTGCATGGCAAGAGATTGCAAGT-3′ (XmaI), reverse 5′-TTGGAGTCACAGTTAAGAGAGTTCCTTT-3′; Vβ-Dβ-Jβ region, forward 5′-GTCTCGAGTTTCTCTTTTAACTAACTAATGCCC-3′ (XhoI), reverse 5′-CGAGAACAGTCAGTCTGGTTCCT-3′. J45 or Jβ2.3 genes with an intron at their 3′ terminus were obtained from genomic DNA by PCR using the following primers: J45 intron, forward 5′-GGAGGCAGCAATTACAAACTGACAT-3′, reverse 5′-GCCGCGGCTGGATCCTGTATTCAATGTGCCTCCCT-3′ (SacII); Jβ2.3 intron, forward 5′-GCAGAAACGCTGTATTTTGGCT-3′, reverse 5′-GCCGCGGCGAGGAGCCGAGTGCCTGGCCCAAA-3′ (SacII).
Genes for the TCR-α and -β chains of 140#27, 162#24, and 164#2 were subcloned into expression cassettes for the TCR-α and -β chains, which were provided by Diane Mathis (Harvard University, Cambridge, Massachusetts, USA) (50), following appropriate treatment with restriction enzymes. The nucleotide sequences of the restriction enzyme sites XhoI and KpnI, which were originally contained in the TCR genes and used for subcloning and linearization before transfection, were altered using a QuikChange Multi Site-Directed Mutagenesis Kit (Stratagene), preserving the amino acid sequence.
Retroviral vector for Dsg3-reactive TCR expression.
Complementary DNAs for the TCR-α and -β chains (Supplemental Figures 1–6) were isolated from the Dsg3-reactive T cell clones 140#27, 162#24, and 164#2 and inserted into the retroviral vector pMXs (51). The retroviral vector pMXs-IG harbors the sequence encoding GFP after the internal ribosomal entry sequence (IRES), and mock retroviral vector pMXs were used as negative controls.
Retroviral transduction.
A packaging cell line, PLAT-E, was cultured in DMEM supplemented with 10% FCS, 2 mM l-glutamine, 1 mM pyruvate, 50 U/ml penicillin, 50 μg/ml streptomycin, 0.05 mM 2-ME, 1 μg/ml puromycin, and 10 μg/ml blasticidin. Retroviral vectors were transfected into packaging cell line PLAT-E cells and the supernatant was collected, as described previously (51). CD4+ T cells were enriched from splenocytes of C57BL/6 mice by positive selection using the MACS cell separation system (Miltenyi Biotec) and then cultured in 24-well plates (106 cells/well) in complete medium (RPMI 1640 containing 10% FCS, 2 mM l-glutamine, 1 mM pyruvate, 50 U/ml penicillin, 50 μg/ml streptomycin, and 0.05 mM 2-ME) supplemented with 100 U/ml hIL-2 and 2.5 μg/ml Con A at 37°C for 24 hours. In some experiments, Ifng–/– and Il17a–/– mice were used as the source of CD4+ T cells. The cells remaining after CD4+ T cell enrichment were irradiated (30 Gy) and used for coculture with CD4+ T cells as feeder cells. The activated CD4+ T cells were resuspended in retroviral supernatant and centrifuged (1400 g, 1 hour, 30°C). After incubation at 37°C for 4 hours, the retroviral supernatant was removed, and the cells were cultured in complete medium supplemented with 100 U/ml hIL-2 at 37°C for 44 hours.
Generation of Dsg3-reactive TCR transgenic mice.
Linearized transgenes of the TCR-α and -β chains for the T cell clone 140#27 were injected into fertilized oocytes of C57BL/6J (H-2b) mice to generate TCR transgenic mice, C57BL/6J-Tg(Dsg3TCR140), which were maintained by mating with C57BL/6J mice. The line C57BL/6J-Tg(Dsg3TCR140)1 was referred to as Dsg3H1, and female Dsg3H1 mice were used in this study. Similarly, another TCR transgenic mouse, C57BL/6J-Tg(Dsg3TCR164)3, was generated by using transgenes of the TCR-α and -β chains for T cell clones, 164#2, and referred to as Dsg3L3 mice.
Peptide.
We purchased 111 overlapping 15-mer peptides covering EC1 to EC3 of the Dsg3 extracellular domain (aa 1–394) from Sigma-Aldrich.
In vitro reconstitution of Dsg3-reactive TCR.
The T cell hybridoma TG40-CD4 (52, 53) (provided by Takashi Saito, Riken Research Center for Allergy and Immunology, Yokohama, Japan) loses intrinsic TCR expression and expresses mouse CD4, which was retrovirally introduced. Then, 10 μg of linearized vectors for TCR-α and -β chain expression in addition to pSV2-hph (ATCC) containing the hygromycin-resistance gene were introduced into TG40-CD4 electrically (280 V, 0.975 μF). Stable transfectants were selected by using hygromycin after electroporation. The expression of transduced vectors was confirmed by flow cytometry, detecting coexpression of TCR-β and CD3, which is expressed as a complex with both the TCR-α and -β chains. Stable transfectants were sorted by a MACS cell isolation system using anti-TCRβ6 Ab-PE or anti-TCRβ8 Ab-PE (BD) in combination with anti–PE Ab microbeads (Miltenyi Biotech).
Reactivity of T cells and T cell hybridomas against Dsg3 peptides.
A single-cell suspension of 4 × 104 cells was prepared from the spleens of TCR transgenic mice and cultured with 1 × 105 40 Gy–irradiated splenocytes and 10 μg/ml peptide in a 96-well round-bottom plate, and Dsg3-specific T cell proliferation was measured by 3H-thymidine uptake, as described previously (54). 2 × 104 T cell hybridoma cells were cultured with 1 × 106 40 Gy–irradiated splenocytes and the peptide at the indicated concentration in 96-well flat-bottom plates for 24 hours. Subsequently, the culture supernatant was subjected to IL-2 ELISA (BD). Some of the experiments were performed in combination with anti-MHC class II mAb (M5/114) or isotype-matched rat mAb (BD).
Flow cytometry.
A single-cell suspension of thymus, spleen, or lymph nodes from mice was stained appropriately using CD4-FITC, CD25-FITC, CD44-FITC, IL-17A–FITC, IFN-γ–FITC, TCR-β–PE, TCR-Vβ6–PE, CD4-PerCP-Cy5.5, CD8-PerCP-Cy5.5, 7-AAD, CD25-APC, CD24-APC, IL-4–APC, Foxp3-APC, CD45-APC/Cy7, CD4-PE/Cy7, CD62L-biotin, and CD229.1-biotin in combination with streptavidin-APC. The reagents in the anti-mouse/rat Foxp3 Staining Set (eBioscience) were used for intracellular staining following treatment with PMA, ionomycin, and brefeldin A (Sigma-Aldrich). For intracellular staining, 7-AAD was washed with PBS twice before fixation.
Generation of bone marrow chimeric mice.
CD3-depleted BM cells from Dsg3H1 mice were prepared using CD3 microbeads (Miltenyi Biotech) and transferred intravenously into 7 Gy–irradiated 129/Sv mice or 129/Sv Dsg3–/– mice. Two months later, the recipient mice were used for further experiments.
Adoptive transfer.
Dsg3–/– B cells were prepared from Dsg3–/– mice, as described previously (7). CD4+Vβ6+ T cells were prepared from the spleens and LNs of Dsg3H1 or Dsg3L3 mice by depleting B220+ and CD8+ cells, followed by positive selection of Vβ6+ cells using the MACS cell separation system (Miltenyi Biotech). Then 5 × 106 Dsg3–/– B cells and 2.5 × 106 CD4+Vβ6+ T cells were transferred into Rag2–/– mice intravenously. In some experiments, retrovirally transduced CD4+ T cells were transferred into Rag2–/– mice in combination with or without Dsg3–/– B cells. For CFSE labeling, CD4+ T cells were isolated by depleting B220+, CD8+, Gr-1+, and CD11b+ cells from splenocytes and LN cells of Dsg3H1 mice by MACS and labeled with CFSE (Molecular Probes) before adoptive transfer, as described previously (7). Naive Ly9.1–CD4+ T cells derived from Dsg3H1→WT and Dsg3H1→Dsg3–/– mice, referred to as Dsg3H1→WT and Dsg3H1→Dsg3–/– CD4+ T cells, respectively, were prepared by depleting Ly9.1+, B220+, CD8+, Gr-1+, CD11b+, DX5+, and CD44+ cells from splenocytes and LN cells with magnetic beads, and 3–15 × 105 T cells were transferred with Dsg3–/– B cells into Rag2–/– mice.
Anti-Dsg3 Ab detection.
Anti-Dsg3 IgG Ab was quantified by ELISA and detected by living cell staining, as described previously (49).
Immunoprecipitation-immunoblotting.
Immunoprecipitation-immunoblotting was performed according to the previous report with some modification, in which we used primary mouse keratinocytes as a source of substrate (55).
Histological analysis.
Formalin-fixed tissue was stained with H&E, and observed with an inverted TE2000-U microscope (Nikon). For immunofluorescent staining, 10-μm cryosections of the palate were fixed with acetone, and then stained with anti-mouse IgG Ab Alexa Fluor 488 (Molecular Probes), anti-TCRβ Ab-PE, and TOTO3 (Molecular Probes). Sections were observed under a confocal laser fluorescence FV1000 microscope (Olympus).
Statistics.
Statistical analyses were made using the Mann-Whitney U test. Data represent mean ± SEM. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Diane Mathis (Harvard University) for the gift of cassette vectors for TCR-α and -β chain expression, Takashi Saito (Riken Research Center for Allergy and Immunology, Yokohama, Japan) for the TG40-CD4 T cell hybridoma cell line, Toshio Kitamura (Institute of Medical Science, University of Tokyo) for the pMXs retroviral vector and PLAT-E packaging cell line, H. Itoh for providing excellent animal care, and M. Suzuki for preparing the cryosections. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan; Health and Labor Sciences Research Grants for Research on Measures for Intractable Diseases from the Ministry of Health, Labor and Welfare of Japan; the Uehara Memorial Foundation; and Keio Gijuku Academic Development Funds.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(9):3677–3688. doi:10.1172/JCI57379.
References
- 1.Amagai M, Klaus-Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67(5):869–877. doi: 10.1016/0092-8674(91)90360-B. [DOI] [PubMed] [Google Scholar]
- 2.Amagai M, Hashimoto T, Shimizu N, Nishikawa T. Absorption of pathogenic autoantibodies by the extracellular domain of pemphigus vulgaris antigen (Dsg3) produced by baculovirus. J Clin Invest. 1994;94(1):59–67. doi: 10.1172/JCI117349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsunoda K, et al. Induction of pemphigus phenotype by a mouse monoclonal antibody against the amino-terminal adhesive interface of desmoglein 3. J Immunol. 2003;170(4):2170–2178. doi: 10.4049/jimmunol.170.4.2170. [DOI] [PubMed] [Google Scholar]
- 4.Amagai M. Autoimmune and infectious skin diseases that target desmogleins. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(5):524–537. doi: 10.2183/pjab.86.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raff MC. Role of thymus-derived lymphocytes in the secondary humoral immune response in mice. Nature. 1970;226(5252):1257–1258. doi: 10.1038/2261257a0. [DOI] [PubMed] [Google Scholar]
- 6.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281(5373):96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi H, Amagai M, Nishikawa T, Fujii Y, Kawakami Y, Kuwana M. Novel system evaluating in vivo pathogenicity of desmoglein 3-reactive T cell clones using murine pemphigus vulgaris. J Immunol. 2008;181(2):1526–1535. doi: 10.4049/jimmunol.181.2.1526. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi H, Kuwana M, Amagai M. A single helper T cell clone is sufficient to commit polyclonal naive B cells to produce pathogenic IgG in experimental pemphigus vulgaris. J Immunol. 2009;182(3):1740–1745. doi: 10.4049/jimmunol.182.3.1740. [DOI] [PubMed] [Google Scholar]
- 9.Anhalt GJ, et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med. 1990;323(25):1729–1735. doi: 10.1056/NEJM199012203232503. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman MA, Qiao X, Anhalt GJ. CD8+ T lymphocytes in bronchiolitis obliterans, paraneoplastic pemphigus, and solitary Castleman’s disease. N Engl J Med. 2003;349(4):407–408. doi: 10.1056/NEJM200307243490421. [DOI] [PubMed] [Google Scholar]
- 11.Nikolskaia OV, Nousari CH, Anhalt GJ. Paraneoplastic pemphigus in association with Castleman’s disease. Br J Dermatol. 2003;149(6):1143–1151. doi: 10.1111/j.1365-2133.2003.05659.x. [DOI] [PubMed] [Google Scholar]
- 12.Amagai M, Nishikawa T, Nousari HC, Anhalt GJ, Hashimoto T. Antibodies against desmoglein 3 (pemphigus vulgaris antigen) are present in sera from patients with paraneoplastic pemphigus and cause acantholysis in vivo in neonatal mice. J Clin Invest. 1998;102(4):775–782. doi: 10.1172/JCI3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anhalt GJ. Paraneoplastic pemphigus. Adv Dermatol. 1997;12:77–96. [PubMed] [Google Scholar]
- 14.Sontheimer RD. Lichenoid tissue reaction/interface dermatitis: clinical and histological perspectives. J Invest Dermatol. 2009;129(5):1088–1099. doi: 10.1038/jid.2009.42. [DOI] [PubMed] [Google Scholar]
- 15.Matsuoka LY. Graft versus host disease. J Am Acad Dermatol. 1981;5(5):595–599. doi: 10.1016/S0190-9622(81)70123-3. [DOI] [PubMed] [Google Scholar]
- 16.Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007;56(2):181–200. doi: 10.1016/j.jaad.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 17.Oliver GF, Winkelmann RK, Muller SA. Lichenoid dermatitis: a clinicopathologic and immunopathologic review of sixty-two cases. J Am Acad Dermatol. 1989;21(2 pt 1):284–292. doi: 10.1016/S0190-9622(89)70174-2. [DOI] [PubMed] [Google Scholar]
- 18.Rudensky A, Preston-Hurlburt P, Hong SC, Barlow A, Janeway CA., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991;353(6345):622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- 19.Chicz RM, Urban RG, Gorga JC, Vignali DA, Lane WS, Strominger JL. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178(1):27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hata T, et al. Transgenic rescue of desmoglein 3 null mice with desmoglein 1 to develop a syngeneic mouse model for pemphigus vulgaris. J Dermatol Sci. 2011;63(1):33–39. doi: 10.1016/j.jdermsci.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 21. Weedon D.Weedon’s Skin Pathology . Amsterdam, The Netherlands: Churchill Livingstone; 2009. [Google Scholar]
- 22. Bolognia JL, Jorizzo JL, Rapini RP.Dermatology . 2nd ed. Amsterdam, The Netherlands: Elsevier, Mosby, Saunders; 2008. [Google Scholar]
- 23.Akasu R, From L, Kahn HJ. Lymphocyte and macrophage subsets in active and inactive lesions of lichen planus. Am J Dermatopathol. 1993;15(3):217–223. doi: 10.1097/00000372-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 24. Freedberg IM, et al.Fitzpatrick’s Dermatology in General Medicine . New York, New York, USA: McGraw-Hill Publishing; 1999. [Google Scholar]
- 25. Burns T, Breathnach S, Cox N, Griffiths C.Rook’s Textbook of Dermatology . Oxford, United Kingdom: Wiley-Blackwell; 2005. [Google Scholar]
- 26.Shiohara T, Moriya N, Tsuchiya K, Nagashima M, Narimatsu H. Lichenoid tissue reaction induced by local transfer of Ia-reactive T-cell clones. J Invest Dermatol. 1986;87(1):33–38. doi: 10.1111/1523-1747.ep12523539. [DOI] [PubMed] [Google Scholar]
- 27.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78(3):399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 28.Oyama N, et al. Autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Lancet. 2003;362(9378):118–123. doi: 10.1016/S0140-6736(03)13863-9. [DOI] [PubMed] [Google Scholar]
- 29.Baldo M, Bailey A, Bhogal B, Groves RW, Ogg G, Wojnarowska F. T cells reactive with the NC16A domain of BP180 are present in vulval lichen sclerosus and lichen planus. J Eur Acad Dermatol Venereol. 2010;24(2):186–190. doi: 10.1111/j.1468-3083.2009.03375.x. [DOI] [PubMed] [Google Scholar]
- 30.Sugerman PB, Satterwhite K, Bigby M. Autocytotoxic T-cell clones in lichen planus. Br J Dermatol. 2000;142(3):449–456. doi: 10.1046/j.1365-2133.2000.03355.x. [DOI] [PubMed] [Google Scholar]
- 31.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 32.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8(4):345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 33.Lubberts E, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50(2):650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 34.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171(11):6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 35.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198(12):1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bending D, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119(3):565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang B, et al. Interferon-gamma impacts at multiple points during the progression of autoimmune diabetes. Proc Natl Acad Sci U S A. 1997;94(25):13844–13849. doi: 10.1073/pnas.94.25.13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell IL, Kay TW, Oxbrow L, Harrison LC. Essential role for interferon-gamma and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J Clin Invest. 1991;87(2):739–742. doi: 10.1172/JCI115055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Connor RA, et al. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008;181(6):3750–3754. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183(11):7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakae S, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17(3):375–387. doi: 10.1016/S1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 43.Amrani A, Verdaguer J, Thiessen S, Bou S, Santamaria P. IL-1alpha, IL-1beta, and IFN-gamma mark beta cells for Fas-dependent destruction by diabetogenic CD4(+) T lymphocytes. J Clin Invest. 2000;105(4):459–468. doi: 10.1172/JCI8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9(2):91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 45.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19(5):641–644. doi: 10.1016/S1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 46.Azukizawa H, et al. Induction of T-cell-mediated skin disease specific for antigen transgenically expressed in keratinocytes. Eur J Immunol. 2003;33(7):1879–1888. doi: 10.1002/eji.200323630. [DOI] [PubMed] [Google Scholar]
- 47.Shibaki A, Sato A, Vogel JC, Miyagawa F, Katz SI. Induction of GVHD-like skin disease by passively transferred CD8(+) T-cell receptor transgenic T cells into keratin 14-ovalbumin transgenic mice. J Invest Dermatol. 2004;123(1):109–115. doi: 10.1111/j.0022-202X.2004.22701.x. [DOI] [PubMed] [Google Scholar]
- 48.Wada N, et al. Aire-dependent thymic expression of desmoglein 3, the autoantigen in pemphigus vulgaris, and its role in T-cell tolerance. J Invest Dermatol. 2011;131(2):410–417. doi: 10.1038/jid.2010.330. [DOI] [PubMed] [Google Scholar]
- 49.Amagai M, Tsunoda K, Suzuki H, Nishifuji K, Koyasu S, Nishikawa T. Use of autoantigen-knockout mice in developing an active autoimmune disease model for pemphigus. J Clin Invest. 2000;105(5):625–631. doi: 10.1172/JCI8748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kouskoff V, Signorelli K, Benoist C, Mathis D. Cassette vectors directing expression of T cell receptor genes in transgenic mice. J Immunol Methods. 1995;180(2):273–280. doi: 10.1016/0022-1759(95)00002-R. [DOI] [PubMed] [Google Scholar]
- 51.Kitamura T, et al. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp Hematol. 2003;31(11):1007–1014. [PubMed] [Google Scholar]
- 52.Sussman JJ, Saito T, Shevach EM, Germain RN, Ashwell JD. Thy-1- and Ly-6-mediated lymphokine production and growth inhibition of a T cell hybridoma require co-expression of the T cell antigen receptor complex. J Immunol. 1988;140(8):2520–2526. [PubMed] [Google Scholar]
- 53.Zumla A, et al. Co-expression of human T cell receptor chains with mouse CD3 on the cell surface of a mouse T cell hybridoma. J Immunol Methods. 1992;149(1):69–76. doi: 10.1016/s0022-1759(12)80050-0. [DOI] [PubMed] [Google Scholar]
- 54.Kuwana M, Medsger TA, Jr, Wright TM. T cell proliferative response induced by DNA topoisomerase I in patients with systemic sclerosis and healthy donors. J Clin Invest. 1995;96(1):586–596. doi: 10.1172/JCI118071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hashimoto T, et al. Novel non-radioisotope immunoprecipitation studies indicate involvement of pemphigus vulgaris antigen in paraneoplastic pemphigus. J Dermatol Sci. 1998;17(2):132–139. doi: 10.1016/S0923-1811(98)00005-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.