Abstract
Schizosaccharomyces pombe divides by means of a centrally placed division septum. The initiation of septation must be tightly coordinated with events in mitosis, as premature formation of the septum can lethally cut the undivided nucleus. The Spg1p GTPase and the Cdc7p kinase, with which it interacts, play a central role in signaling the initiation of septum formation. Loss-of-function mutations in either gene prevent septation, whereas inappropriate activation of Spg1p can induce septum formation from G1 or G2 interphase cells. Increased expression of either gene leads to multiple rounds of septation without cell cleavage, emphasizing the need for precise cell cycle regulation of their activity. To understand the mechanisms underlying this regulation, we have investigated whether these key initiators of septum formation are controlled by changes in their activity and/or location during mitosis and cytokinesis. We demonstrate that Spg1p localizes to the spindle pole body in interphase and to both spindle poles during mitosis. In contrast, Cdc7p shows no discrete localization during interphase, but early in mitosis it associates with both spindle pole bodies and, as the spindle extends, is seen on only one pole of the spindle during anaphase B. Spg1p activity is required for localization of Cdc7p in vivo but not for its kinase activity in vitro. Staining with an antiserum that recognizes preferentially GDP–Spg1p indicates that activated GTP–Spg1p predominates during mitosis when Cdc7p is associated with the spindle pole body. Furthermore, staining with this antibody shows that asymmetric distribution of Cdc7p may be mediated by inactivation of Spg1p on one spindle pole. Deregulated septation in mutant cells correlates with segregation of Cdc7p to both spindle poles.
Keywords: Mitosis, spindle pole body, cytokinesis, kinase, GTPase
Schizosaccharomyces pombe cells grow mainly by elongation at their tips, then divide by formation of a centrally placed septum to produce daughter cells of approximately equal size. The position of the division septum is defined early in mitosis, but its synthesis is not initiated until the end of mitosis, when the daughter nuclei are well separated from each other and the spindle begins to break down. The onset of septum formation must be properly coordinated with mitosis. If septum formation occurs before mitosis is completed, the nucleus may be “cut” by the developing septum (see, e.g., Hirano et al. 1986).
The main morphological events of septum formation and cytokinesis in fission yeast have been reviewed (Robinow and Hyams 1989). As in higher eukaryotes, the fission yeast cytoskeleton undergoes a characteristic series of rearrangements during mitosis and cytokinesis. In interphase cells, a basket of microtubules runs from end to end in the cell (Hagan and Hyams 1988). At the onset of mitosis, the cytoplasmic microtubule array is replaced by a short intranuclear spindle, which elongates as mitosis proceeds. As in higher eukaryotes, spindle formation in S. pombe occurs by interdigitation of two microtubule arrays extending from the duplicated spindle pole bodies (the functional equivalent of the mammalian centrosome), which span the nuclear membrane. The spindle breaks down at the end of mitosis, to be replaced by a new interphase array that is seeded from microtubule-organizing centres (MTOCs) at the cell equator (Hagan and Hyams 1988; for review, see Hagan et al. 1997).
The distribution of F-actin in S. pombe cells is intimately linked with sites of growth or septum formation. During interphase, F-actin is observed mainly as cortical patches at the growing ends of the cell, though actin cables can also be seen (Marks and Hyams 1985; Balasubramanian et al. 1996). At the onset of mitosis, a medial F-actin-containing ring is formed at the site where the septum will be synthesized later (for review, see Gould and Simanis 1997). F-actin patches are subsequently polarized to this ring, and the cell is thus primed for septation. At the end of anaphase, the primary septum grows inward from the cell cortex. Secondary septa are formed on either side of the primary septum, which is dissolved to effect cell separation. F-actin patches are then relocated to the old (pre-existing) end of the cell, where growth resumes.
Because the position of the division site is determined early in mitosis (Chang et al. 1996), the temporal coordination of mitotic events with septum formation is likely to be mediated through the proteins that trigger septum biosynthesis. The products of the cdc11 (Nurse et al. 1976), cdc14 (Fankhauser and Simanis 1993), cdc7 (Fankhauser and Simanis 1994), plo1 (Ohkura et al. 1995), and spg1 genes (Schmidt et al. 1997) are all essential for initiating septation, whereas loss of either cdc16 or byr4 activity deregulates septation, resulting in multiple rounds of septum formation without cell cleavage (Minet et al. 1979; Fankhauser et al. 1993; Song et al. 1996). Increased expression of Plo1p or Spg1p will induce septation in either G1- or G2-arrested interphase cells (Ohkura et al. 1995; Schmidt et al. 1997). Moderate-level expression of Cdc7p prevents septum formation from being turned off but does not induce septation from G2-arrested cells (Fankhauser and Simanis 1994). The cdc7 gene encodes a protein kinase, whereas Spg1p is a GTPase of the Ras superfamily. The two proteins have been shown to interact, and the induction of septum formation by Spg1p requires functional Cdc7p (Schmidt et al. 1997). In this study we have investigated the biological role of the interaction of Spg1p and Cdc7p. We show that Spg1p is associated with the spindle pole body throughout the cell cycle. Activation of Spg1p is required for Cdc7p to associate with the spindle pole body early in mitosis, from where it signals the onset of septum formation at the end of mitosis. In contrast to all other spindle pole body markers examined to date, Cdc7p is distributed asymmetrically to one of the two poles in late anaphase. The implications of these unexpected results for regulation of septum formation are discussed.
Results
Cdc7p kinase activity does not require Spg1p function
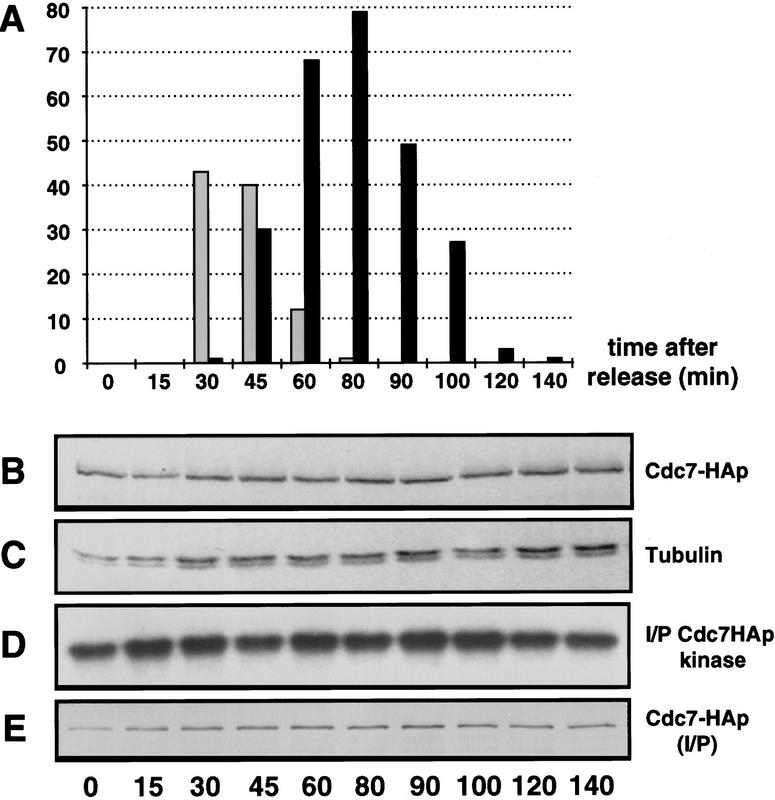
Because Cdc7p kinase activity is required for the onset of septum formation (Fankhauser and Simanis 1994), we assayed its activity in a synchronized population of cells generated by arrest–release of a cdc25-22 mutant (Figure 1A). This demonstrated that the steady-state level of Cdc7p (Fig. 1B,C), and its kinase activity (Fig. 1D,E) were similar in extracts prepared from cells in G2, mitosis, or at the time of septum formation. We conclude that Cdc7p has the potential to be active at all stages of the cell cycle.
Figure 1.
The steady-state level and in vitro kinase activity of Cdc7p do not change significantly as cells proceed from interphase through mitosis, septum formation, and cytokinesis. Cells were synchronized by arrest–release of cdc25-22 cdc7–HA as described in Materials and Methods. Protein samples were prepared at intervals. The number of cells that were in mitosis or forming a division septum at each time point was determined. (A) The percentage of cells in anaphase (shaded bar), and forming a division septum (solid bar) at each time point; (B) Western blot of total protein extracts, probed with mAb 12CA5; (C) the same samples as in B, probed with TAT-1 as loading control; (D) kinase activity of Cdc7p, assayed using myelin basic protein as substrate; (E) Western blot of Cdc7–HAp in the immunoprecipitates used in D, using mAb 12CA5.
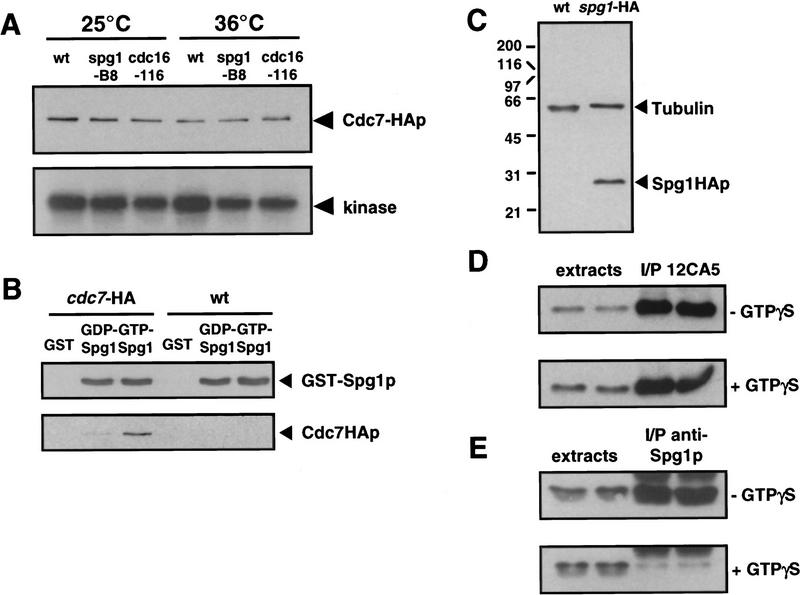
We also examined whether Cdc7p kinase activity is affected by mutation of proteins that are implicated in the regulation of septum formation. Cdc7p kinase assays were performed on protein extracts prepared from the spg1-B8 mutant, which fails to form the division septum, and cdc16-116, which undergoes repeated rounds of septum formation. The activity of Cdc7p in these mutant backgrounds did not vary significantly, whether the extracts were prepared from cells grown at permissive or restrictive temperature (Fig. 2A, cf. assays from each mutant, at 25°C or 36°C). Thus, loss of Spg1p or Cdc16p function does not affect Cdc7p kinase activity in vitro.
Figure 2.
Cdc7p binds to the GTP-bound form of Spg1p, which is not recognized by the anti-Spg1p antiserum (SuSu1). (A) Cells carrying the cdc7–HA allele in a wild-type, spg1-B8, or cdc16-116 background were grown in YE medium at 25°C. Half the culture was shifted to 36°C for 4 hr. Protein extracts were prepared and Cdc7p was immunoprecipitated with the anti-Cdc7p antiserum. The kinase activity associated with the precipitate was assayed using myelin basic protein as in vitro substrate (performed at 25°C or 36°C, respectively). The amount of protein in the immunoprecipitates was analyzed by Western blotting with mAb 12CA5. (B) GST, the GDP-, or the GTP-bound form of GST–Spg1p were mixed with protein extracts prepared from wild-type (972) or cdc7–HA cells. The complexes precipitated with glutathione–agarose were Western blotted and probed with anti-Spg1p antiserum (SuSu1) and mAb 12CA5. (C) Total proteins were prepared from wild-type (972) and spg1–HA cells. The Western blot was probed with a mixture of TAT-1 (loading control) and mAb 12CA5. (D) Proteins were prepared from spg1–HA cells in the absence (top) or presence (bottom) of GTPγS, and Spg1–HAp was immunoprecipitated with mAb 12CA5. The soluble extracts and the immunoprecipitates were analyzed by Western blotting with mAb 12CA5. Note that equal amounts of Spg1–HAp are immunoprecipitated in the presence or absence of GTPγS. (E) Same as in D, except that proteins were prepared from wild-type cells (972), and the anti-Spg1p antibody (SuSu1) was used for immunoprecipitation and Western blotting. Note that much less protein is precipitated by this serum in the presence of GTPγS, suggesting that the antibody recognizes preferentially GDP–Spg1p.
Cdc7p interacts preferentially with GTP–Spg1p
Our previous studies have demonstrated that Spg1p and Cdc7p bind to each other and that mutations in the effector domain of Spg1p interfere with the interaction: It was also proposed that the GTP-bound form of Spg1p was the biologically active form (Schmidt et al. 1997). We therefore tested whether Spg1p binding to GDP or GTP affected its interaction with Cdc7p. A GST–Spg1p fusion protein was expressed in Escherichia coli. The purified protein was stabilized in the GDP- or GTP-bound form and mixed with protein extracts from wild-type S. pombe cells, and proteins binding to Spg1p were recovered by affinity chromatography on glutathione–agarose columns. Western blot analysis showed that Cdc7p was recovered much more efficiently by the GTP-bound form of Spg1p (Fig. 2B).
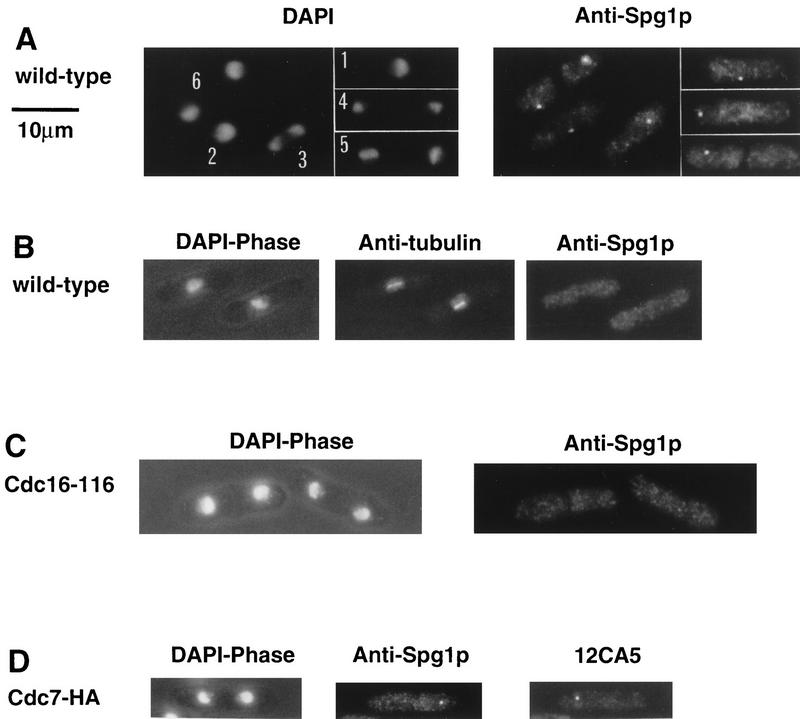
Spg1p is associated with the spindle pole body throughout the mitotic cell cycle
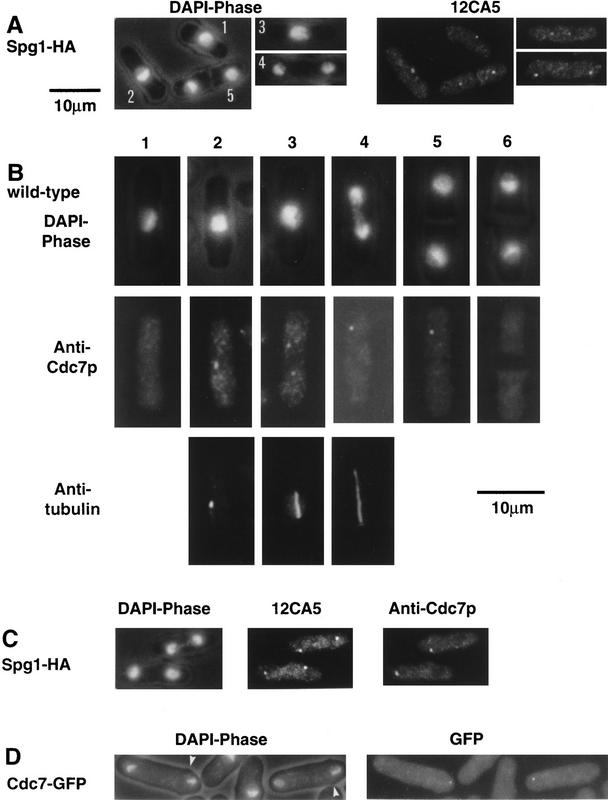
The data described above suggest that regulation of the onset of septation may be mediated by controlling the location of Cdc7p, the availability of substrate, or both. To pursue this idea further, we examined the localization of Cdc7p and Spg1p through the cell cycle. To localize Spg1p, the influenza hemagglutinin (HA) epitope tag, recognized by the 12CA5 monoclonal antibody, was added to the carboxyl terminus of Spg1p (Spg1–HAp) by modifying the chromosomal copy of the spg1 gene (Fig. 2C; see Material and Methods). Indirect immunofluorescence using mAb 12CA5 showed that Spg1–HAp associated with the spindle pole body throughout the cell cycle (Fig. 3A,C). Spg1p staining colocalized with the spindle pole body marker Sad1p during interphase and mitosis (Fig. 4A, Hagan and Yanagida 1995). Staining for Sad1p (data not shown) and microtubules (Schmidt et al. 1997) did not reveal any mitotic defects in the spg1 null allele, indicating that Spg1p is not an essential structural component of the spindle pole body.
Figure 3.
Localization of Cdc7p and Spg1p to the spindle pole body. Cells from the indicated strains were grown at 25°C in YE, fixed, and stained, using the indicated antibodies. DAPI shows the DNA, and phase contrast shows the cell outline. (A) spg1–HA cells stained with mAb 12CA5. Cell 1 is in interphase; in cell 2, the two spindle pole bodies have started to separate; cell 3 is in early anaphase; cell 4 is in late anaphase; and cell 5 has formed the division septum. (B) Wild-type cells (972), stained with anti-Cdc7p antibodies and TAT-1. Cell-cycle stages are indicated in the text. (C) Mitotic spg1–HA cells, stained with mAb 12CA5 and anti-Cdc7p antibodies. (D) cdc7–GFP fluorescence in mitotic cells. The arrows indicate the position of the birth scars.
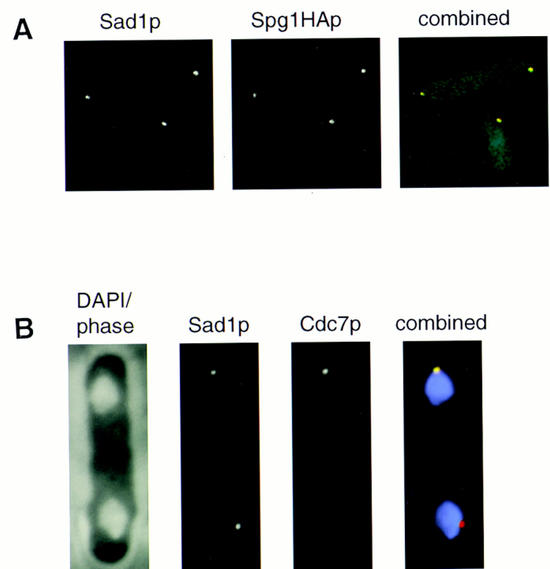
Figure 4.
Cdc7p and Spg1p colocalize with the spindle pole body component Sad1p. (A) spg1–HA cells were stained with anti-Sad1p antibodies and mAb 12CA5. Background fluorescence from secondary antibodies was used to show the cell outline. The lower cell is in interphase; the upper cell is undergoing anaphase. Sad1p was detected with FITC-conjugated secondary antibody, which gives green fluorescence, and Cdc7p was detected with CY3-conjugated secondary antibody, which gives red fluorescence. Colocalization of green and red gives a yellow color, that is seen in the merged image. The individual channels are shown in gray tones, the merged image is in color. (B) Wild-type cells were stained with DAPI, anti-Sad1p (raised in sheep), and anti-Cdc7p antibodies. A mitotic cell is shown. In the combined image, DAPI is shown in violet, Sad1p gives red fluorescence, and Cdc7p gives green fluorescence. Colocalization of Sad1p and Cdc7p on one pole results in yellow (see A) color.
Cdc7p is located on both spindle pole bodies early in mitosis and one spindle pole body late in mitosis
Cdc7p was detected using a polyclonal antibody raised against a region carboxy-terminal to the catalytic domain (Fankhauser and Simanis 1994). In mitotic cells with a short spindle, Cdc7p associated with both spindle pole bodies (Fig. 3B, cells 2 and 3). After initiation of anaphase B, Cdc7p immunofluorescence was observed on only one of the two spindle poles (Fig. 3B, cell 4, and Figs. 3C and 4B), and this asymmetry persisted in cells that were forming a division septum (Fig. 3B, cell 5). Despite the fact that the steady-state level of Cdc7p was constant during interphase, mitosis, and cytokinesis (Fig. 1B), no discrete Cdc7p staining was seen either in cells that had completed the septum (Fig. 3B, cell 6), or in interphase cells (Fig. 3B, cell 1). No discrete Cdc7p staining was detected in cells arrested in late G2 by the cdc25-22 mutation (data not shown), indicating that association of Cdc7p with the spindle pole body depends on entry into mitosis.
To exclude the possibility that the Cdc7p epitopes recognized by the polyclonal serum were masked on one of the two poles, we used strains in which the chromosomal copy of the cdc7 gene had been replaced with a modified version, tagging Cdc7p at its carboxyl terminus with either the HA epitope (cdc7–HA cells; Schmidt et al. 1997) or green fluorescent protein (cdc7–GFP; this study). Indirect immunofluorescence using mAb 12CA5 (Fig. 7D, below) and observation of the Cdc7p–GFP fluorescence (Fig. 3D) confirmed the asymmetric association of Cdc7p with one spindle pole. Using the birth scar as a morphological marker for asymmetry (Mitchison and Nurse 1985), no significant bias in segregation of Cdc7p to the old or new end of the cell was observed (Fig. 3D). We do not know what percentage of the total amount of Cdc7p is associated with the spindle pole body. Therefore, at present, we cannot say whether the asymmetric pattern of Cdc7p localization means that only one of the two daughter cells inherits Cdc7p. The location of Cdc7p during interphase is unknown, but it is possible that the absence of a discrete staining pattern in interphase cells reflects a diffuse cytoplasmic staining that falls below the limit of detection.
Figure 7.
Localization of Spg1p with an antibody recognizing the GDP-bound form of Spg1p (SuSu1). Cells were grown at 25°C in YE and prepared for immunofluorescence, using the indicated antibodies. DAPI shows the DNA; phase contrast shows the cell outline. The antibodies used in each case are indicated. cdc16-116 cells were shifted to 32°C for 1.5 hr before fixation. (A) Wild-type cells (972), stained with the anti-Spg1p antiserum (SuSu1). Cells 1 and 2 are in interphase; cells 3 and 4 are undergoing anaphase B; cell 5 is forming a division septum; cell 6 has completed septum formation. (B) Early mitotic wild-type cells (972) stained with SuSu1 and TAT-1. (C) cdc16-116 cells stained with SuSu1. (D) Late mitotic cdc7–HA cell stained with SuSu1 and mAb 12CA5.
We were concerned about the possibility that Cdc7p staining associated with microtubules, the medial ring, or equatorial MTOCs might fall below the limit of detection. We therefore stained cells expressing a level of Cdc7p ∼20-fold higher than normal from a multicopy plasmid. Although we observed an increase in cytoplasmic staining, no medial ring, microtubule, or equatorial MTOC staining was seen under these conditions, suggesting that Cdc7p does not normally associate with these structures (data not shown). Formally, we cannot exclude the possibility that the number of binding sites for Cdc7p at these locations is limited, precluding its detection with our current reagents; however, we feel that our combined genetic and cytological observations make this interpretation unlikely.
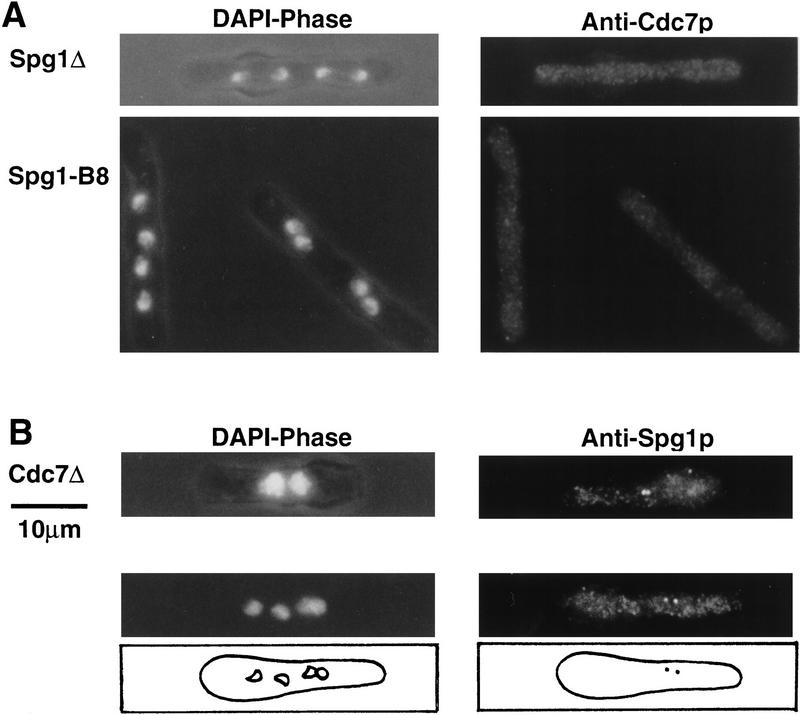
Localization of Cdc7p to the spindle pole body requires Spg1p function, but not vice versa
GTPase-mediated localization of kinases to their sites of action is an emerging theme in the function of signal transduction systems (e.g., Hall 1994; Mochley-Rosen 1995; Peter et al. 1996; Leberer et al. 1997). Because functional Spg1p is not required for Cdc7p kinase activity, we examined the effects of cdc7 and spg1 mutations upon localization of Spg1p and Cdc7p. No discrete Cdc7p staining was detected during mitosis in cells arising from germination of spg1 null allele spores, or in spg1–B8 cells incubated at restrictive temperature (Fig. 5A). Therefore, Spg1p activity is required to localize Cdc7p to the spindle pole body. In cells deleted for the cdc7 gene (Fig. 5B), and the cdc7-24 mutant (data not shown), Spg1p localized to the spindle pole body in both interphase and mitosis [note that the signal is asymmetric in mitotic cells because of the use of a polyclonal antiserum recognizing preferentially GDP–Spg1p (SuSu1; see below)]. Thus, Cdc7p is not required for correct localization of Spg1p. Depolymerization of microtubules by cold shock (Hagan and Yanagida 1995) and staining of the β-tubulin mutant nda3–KM311 (Hiraoka et al. 1984) showed that spindle pole body localization of Cdc7p and Spg1p is independent of microtubule integrity (data not shown).
Figure 5.
Spindle pole body localization of Cdc7p requires Spg1p, but correct localization of Spg1p occurs in the absence of Cdc7p. spg1–B8 cells were grown at 25°C and shifted for 4 hr to 36°C before fixation. Spores deleted for spg1 or cdc7 were germinated in minimal medium containing supplements allowing only spores carrying the null allele to germinate (described in Materials and Methods). (A) spg1 null allele (top) and spg1-B8 (bottom) cells were stained with anti-Cdc7p antibodies (only mitotic cells are shown). (B) cdc7 null cells stained with anti-Spg1p antibodies (SuSu1). The top panels show a binucleate interphase cell; the bottom panels show a cell undergoing the second mitosis. The positions of the nuclei and the spindle pole bodies in the mitotic cell are schematically represented. Note that the right nuclei of each segregating pair almost colocalize. Spindle pole body-mediated clustering of nuclei is often observed in cdc7 mutants (for a detailed description of nuclear positioning in fission yeast, see Hagan and Yanagida 1997).
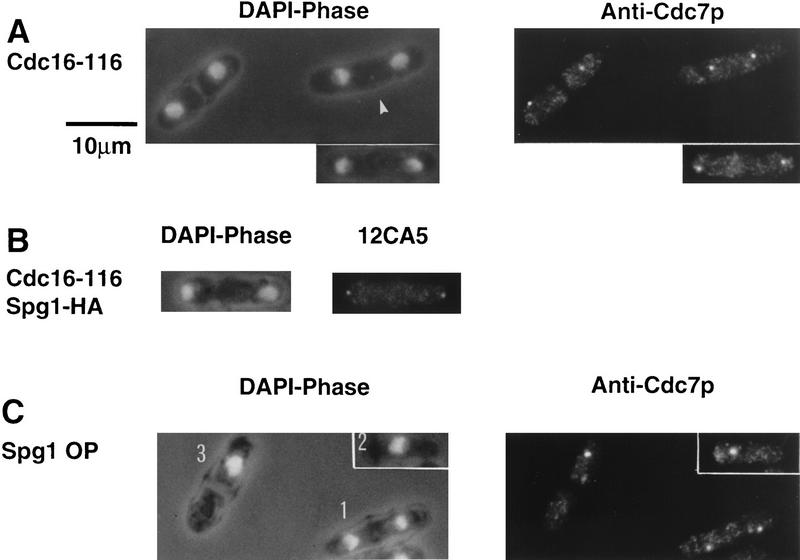
Deregulated septum formation correlates with symmetrical distribution of Cdc7p during mitosis
Because Cdc7p always segregates to only one pole of the spindle in late anaphase in wild-type cells, we examined whether this was the case in cells where septum formation is deregulated. It has been proposed that the cdc16 gene product encodes the GTPase-activating protein (GAP) of Spg1p (Boguski and McCormick 1993; Schmidt et al. 1997). Consistent with this, the multiseptate phenotype resulting from loss of cdc16 function resembles that produced by increased expression of Spg1p and Cdc7p (Mineti et al. 1979; Fankhauser et al. 1993; Fankhauser and Simanis 1994; Schmidt et al. 1997). cdc16-116 cells grown at restrictive temperature were stained with the anti-Cdc7p antiserum. Cdc7p was seen to associate with both spindle pole bodies throughout anaphase and septum formation (Fig. 6A), in contrast to wild-type cells, where Cdc7p was associated with only one pole of late anaphase spindles. Spg1p was also present on both poles (Fig. 6B). The same results were obtained when the experiment was repeated using germinating spores deleted for the cdc16 gene (data not shown). Cells in which septum formation is deregulated by increased expression of Spg1p (Fig. 6C), and cells overexpressing Cdc7p (data not shown), also showed a bipolar distribution of Cdc7p in mitotic cells, confirming the correlation between the symmetric segregation of Cdc7p at mitosis and deregulation of septum formation. Cdc7p was also located on the spindle pole in septated, mononucleate cells induced by increased expression of Spg1p (Fig. 6C, Schmidt et al. 1997). Thus, in wild-type cells Cdc7p is associated with only one spindle pole in late anaphase, whereas deregulation of septum biosynthesis correlates with association of Cdc7p with both poles of the spindle throughout mitosis and septum formation. The potential significance of this is addressed in the Discussion.
Figure 6.
Loss of Cdc16p function results in segregation of Cdc7p to both poles of the mitotic spindle. cdc16-116 cells were shifted from 25°C to 32°C for 1.5 hr before fixation. Cells were prepared for immunofluorescence and stained using the indicated antibodies. DAPI shows the DNA; phase contrast shows the cell outline. (A) cdc16-116 cells stained with anti-Cdc7p antibodies. Note that the cell marked with an arrowhead has completed anaphase and that the spindle pole bodies guide the movement of the nuclei toward the center of the future daughter cells (Hagan and Yanagida 1997). (B) cdc16-116 spg1–HA cell, stained with mAb 12CA5. (C) Cells overexpressing Spg1p (induced for 18 hr at 25°C from the nmt1 promotor SP1805; Schmidt et al. 1997), stained with anti-Cdc7p antibodies. Cell 1 is undergoing anaphase and shows symmetrical Cdc7p staining; cell 2 is in interphase and has Cdc7p associated with the spindle pole body; cell 3 has Cdc7p associated with the spindle pole body and initiated septum formation during interphase.
Inactivation of Spg1p on one of the two spindle poles may mediate the asymmetric distribution of Cdc7p
Two reagents recognizing Spg1p were available: mAb 12CA5 recognizing Spg1–HAp, and an antiserum raised against the carboxy-terminal 19 amino acids of Spg1p (hereafter referred to as SuSu1; Schmidt et al. 1997). We tested whether these antibodies differed in their ability to recognize the GDP- and GTP-bound forms of Spg1p. Protein extracts from wild-type or spg1–HA cells were prepared in the presence or absence of GTPγ-S, a nonhydrolyzable analog of GTP that results in accumulation of the activated GTP-bound Spg1p. This had no effect on the ability of mAb 12CA5 to immunoprecipitate Spg1–HAp (Fig. 2D). However, addition of GTPγ-S reduced significantly the amount of Spg1p precipitated by SuSu1 (Fig. 2E), indicating that the epitopes recognized by this antiserum are masked when Spg1p is in the GTP-bound form. Assuming that the ability of the SuSu1 antiserum to recognize Spg1p epitopes in fixed cells is affected the same way, the SuSu1 antiserum acts as a marker for the GDP-bound form of Spg1p.
Using SuSu1, Spg1p was only detected on one of the two spindle pole bodies both in cells undergoing anaphase B, and forming a division septum (Fig. 7A). Moreover, no Spg1p staining was seen in cells with a short spindle (Fig. 7B), or in the nda3–KM311 mutant (data not shown), suggesting that Spg1p is in the GTP-bound form on both poles early in mitosis. In cdc16-116 cells, where Spg1p should be predominantly in the GTP-bound form and Cdc7p segregated to both poles during anaphase (Fig. 6A), GDP–Spg1p could not be detected at any stage of mitosis using SuSu1 (Fig. 7C), though staining with mAb 12CA5 demonstrated that Spg1p was present on both spindle pole bodies in this mutant (Fig. 6B). These data are consistent with the view that SuSu1 preferentially recognizes GDP–Spg1p.
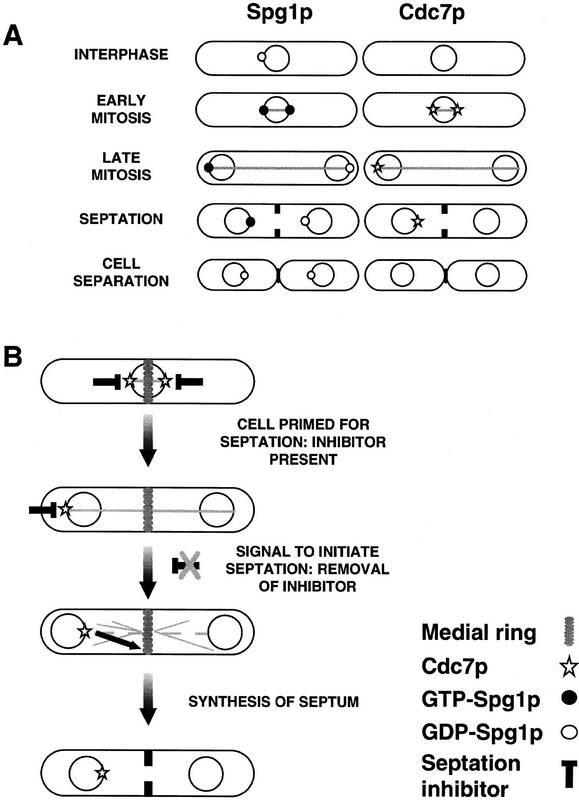
Staining of cdc7–HA cells with both mAb 12CA5 and SuSu1 showed that Cdc7p and inactive Spg1p never colocalized on the same spindle pole in 250 late mitotic cells analyzed (Fig. 7D). This finding is consistent with the observation that Cdc7p interacts preferentially with GTP–Spg1p (see Fig. 2B). Staining of germinating cdc7 null cells with SuSu1 showed asymmetric distribution of GTP-bound Spg1p staining even in the absence of Cdc7p (Fig. 5B). Thus, inactivation of Spg1p on one pole early in anaphase does not depend on the activity of Cdc7p. The localization of Cdc7p and the GTP- and GDP-bound forms of Spg1p are summarized in Figure 8A. Together with the in vitro data shown in Figure 2B, these results suggest that the Cdc7p protein kinase localizes to the spindle pole body by interacting with the activated, GTP-bound form of Spg1p and imply that this is a key step in priming the cell for septum formation.
Figure 8.
(A) Diagram showing the changes in localization of Cdc7p, Spg1p, and GDP–Spg1p during the cell cycle. Note that while Spg1p is always associated with the spindle pole body, it is predominantly in the GTP-bound form during mitosis. Cdc7p is only associated with the spindle pole body during mitosis and is segregated asymmetrically during anaphase B. (B) The signal for the onset of septum formation is delivered from the spindle pole body. The diagram summarizes our working hypothesis. Activation of a Cdc7p-dependent signaling pathway at the end of anaphase sends a signal from the spindle pole body to the medial ring to initiate synthesis of the division septum. Because the cell is primed for septation from the beginning of mitosis, the presence of a putative inhibitor during early mitosis is indicated. The nature of the restraint of Cdc7p signaling in early mitosis is unknown at present. It may be mediated by altering substrate availability or inhibition of the spindle pole body—bound fraction of the enzyme. For further details, see Discussion.
Discussion
Spindle pole body location of inducers of septum formation
In this paper we have demonstrated that the Cdc7p kinase and the Spg1p GTPase, with which it interacts to induce septum formation, are associated with the spindle pole body and that Cdc7p is localized on only one spindle pole late in anaphase. At no stage in mitosis or septum formation have we observed an association of Spg1p or Cdc7p with the medial ring. This result is unexpected, as it positions these key regulatory molecules far from the site where the septum will be synthesized. We believe that this may provide an important insight into how the link between the mitotic apparatus and the septum-forming machinery in fission yeast is established to ensure proper coordination of mitosis and cytokinesis. The Cdc2p/Cdc13p kinase [the S. pombe equivalent of mitosis promoting factor (MPF)] and components of the 20S cyclosome/anaphase promoting complex (APC), localize to the spindle pole body, or its mammalian equivalent, the centrosome (Alfa et al. 1990; Kalt and Schliwa 1993; Tugendreich et al. 1995). Colocalization of regulators of mitotic progression with components of the GTPase switch that is required for the onset of septum formation provides the potential for coordinating their activity during mitosis.
Cdc7p may stand at the head of a GTPase-regulated protein kinase signaling module (Schmidt et al. 1997). In its catalytic domain, Cdc7p is most closely related to Saccharomyces cerevisiae STE11p, which is a member of the mitogen-activated protein (MAP) kinase kinase kinase family (Fankhauser and Simanis 1994). In mammalian cells, activation of a MAP kinase signaling module is accompanied by changes in the subcellular localization of MAP kinase (Lenormand et al. 1993). By analogy with this, we propose that, at the end of mitosis, activation of a Spg1p–Cdc7p-dependent signal transduction pathway sends a signal from the spindle pole body to the medial ring to trigger septum synthesis (shown schematically in Fig. 8B). Transfer of Cdc7p to one spindle pole during mitosis may ensure that the signal for the onset of septum formation will be given only from a single site. It is noteworthy that in all situations where septum formation is deregulated, Cdc7p is seen on both poles during mitosis. Identification and localization of the target(s) and proteins downstream of Cdc7p will be of considerable interest.
Implications of these data for the regulation of septum formation
Kinase assays in vitro suggest that Cdc7p has the potential to be active at all stages of the cell cycle. Assuming that this is an accurate reflection of the in vivo situation, the finding that Spg1p is in the GTP-bound form and Cdc7p is already associated with the spindle poles early in mitosis, suggests that Cdc7p/Spg1p is prevented from signaling the onset of septum formation precociously during the early stages of mitosis. This could be achieved by making the key substrates unavailable, or unresponsive, to Cdc7p kinase activity until the end of mitosis, or by the presence of an inhibitor of Cdc7p during mitosis. Removal of an inhibitor of Cdc7p/Spg1p (or changing substrate availability) at the end of mitosis would provide an excellent means of coordinating mitotic events with the onset of septum formation.
Increased expression of Spg1p in interphase cells promotes medial ring and septum formation (Schmidt et al. 1997). This indicates that the key substrates of Cdc7p/Spg1p are potentially available throughout the cell cycle and therefore suggests that the activity restraining septum formation during mitosis is absent during interphase. Consistent with this, induction of spg1 expression does not induce septum formation in cells arrested in early mitosis by the nda3–KM311 mutation (L. Cerutti and V. Simanis, unpubl.), though it will do so in G1-, S-, or G2-arrested cells (Schmidt et al. 1997). Septation during interphase may therefore be restrained by regulating activation of Spg1p.
It is noteworthy that inactivation of Cdc2p kinase in a mitotically arrested cell will bring about cytokinesis or septum formation in both S. cerevisiae and S. pombe (Ghiara et al. 1991; He et al. 1997). Moreover, mutants that arrest late in mitosis with elevated Cdc2p kinase activity do not undergo septum formation and cytokinesis (Moreno et al. 1989; Surana et al. 1993). It is therefore possible that the onset of septum formation is inhibited by high Cdc2p kinase activity during mitosis. Studies of myosin II regulatory light chain phosphorylation in higher eukaryotes provide a precedent for Cdc2p inactivation in triggering cytokinesis. Myosin II regulatory light chain activity appears to be both negatively and positively regulated by changes in its phosphorylation state. During mitosis, it is inhibited through phosphorylation by Cdc2p/cyclin B (Satterwhite et al. 1992). When Cdc2p/cyclin B is inactivated in the normal cell cycle, coincident with the metaphase-anaphase transition, these phosphorylations no longer occur; and coincident with this, phosphorylation of another site by myosin light chain kinase is markedly increased (Yamakita et al. 1994). The changes at these phosphorylation sites are thought to contribute to signaling the initiation of cytokinesis.
The onset of anaphase and inactivation of Cdc2p/cyclin B kinase is brought about by the proteolysis of key substrates targeted by the anaphase-promoting complex (APC). The finding that some cut mutants encode components of the APC (Yamashita et al. 1996; Yamada et al. 1997) argues that the putative inhibitor of septation may not be removed by APC-mediated proteolysis. However, increased expression of the APC component Nuc2p blocks septation but does not interfere with mitosis (Kumada et al. 1995). Clearly, future studies will address how Cdc7p/Spg1p signaling is activated at the end of mitosis and whether the APC plays a role in this signaling.
What mediates the asymmetrical distribution of Cdc7p on the spindle poles during anaphase?
Electron microscopic observation shows that it is highly likely that two apparently identical daughter spindle pole bodies arise by the splitting of a single precursor in S. pombe (Ding et al. 1997). Our data clearly indicate that the two daughter pole bodies produced by this apparently symmetrical structure are not identical.
In S. cerevisiae, a Kar1p–β-galactosidase fusion protein has been shown to localize predominantly to the cytoplasmic face of the new spindle pole body when expressed at high levels (Vallen et al. 1992). However, localization of native Kar1p demonstrated that it is present on the spindle pole body throughout the cell cycle and is a component of the bridge and half-bridge (Spang et al. 1995). The asymmetry observed in the earlier study was attributable to misdirection of the Kar1p–β-galactosidase fusion protein within the spindle pole body. Thus, our data represent the first example of asymmetric distribution of a native molecule on the spindle poles during mitosis. It is interesting to note that an example of nonequivalence in the centrioles of a higher eukaryote has been described recently (Lange and Gull 1995). The antibody marker called Cenexin is acquired by the immature centriole of the pair only at prophase: For the remainder of the cycle, only the mature centriole is Cenexin positive.
The nature of the signal that promotes the switch from bipolar to monopolar Cdc7p localization during anaphase is unknown: It may be attributable to progression past a mitotic checkpoint, or it might be a function of spindle length. The timing of the switch from bipolar to monopolar Cdc7p staining is similar to the timing of the disappearance of Cdc2p/Cdc13p from the poles (Alfa et al. 1990). However, we do not know at present whether APC activity is required for the transition to monopolar Cdc7p localization. Since the disappearance of Cdc7p from one spindle pole correlates with inactivation of Spg1p, and Cdc7p and GDP–Spg1p never colocalize in late anaphase, we favor the view that Cdc7p is delocalized from one pole by asymmetric inactivation of Spg1p. Because Spg1p associates with both spindle pole bodies throughout mitosis, it seems likely that the factors regulating Spg1p activity will be distributed, or regulated, asymmetrically on the spindle poles. Potential negative regulators of Spg1p are Cdc16p (Fankhauser et al. 1993; Schmidt et al. 1997), Byr4p (Song et al. 1996), and Dma1p (Murone and Simanis 1996). Their localization during mitosis will be of considerable interest.
Why does increased expression of cdc7, plo1, or spg1 deregulate septum formation?
Plo1p kinase is essential not only for formation of a bipolar spindle but also for septum formation: Ectopic expression of the protein can also trigger septum formation in interphase cells (Ohkura et al. 1995). Studies of Plk1, the human counterpart of plo1, have demonstrated that microinjection of neutralizing antibodies prevents centrosome maturation (Lane and Nigg 1996). It is tempting to speculate that an untimely increase of Plo1p kinase activity during interphase in S. pombe causes premature spindle pole body maturation and activation of Spg1p. This could be achieved by negative regulation of Spg1p inhibitors, recruitment of Spg1p activators, or both. Because increases in Spg1p activity can induce all stages of septum formation (Schmidt et al. 1997), this would explain the observed phenotypes of plo1 overexpression. It will be interesting to examine the localization of Spg1p and Cdc7p in plo1 mutants, when they become available.
Previous work has shown that cells are very sensitive to increased spg1 expression but much less so to increased cdc7 expression (Fankhauser and Simanis 1994; Schmidt et al. 1997). The finding that spindle pole body association of Cdc7p requires GTP–Spg1p may offer an explanation for this apparent discrepancy. We have shown that Spg1p is mostly GDP bound in interphase cells, so the tendency for Cdc7p to associate with the spindle pole body, where we presume its key substrates are localized, will be low. Thus, increased expression of Cdc7p will not induce septum formation in interphase-arrested cells. In contrast, during mitosis, Spg1p is GTP-bound, and Cdc7p can associate with the spindle pole. It is possible that the excess of Cdc7p titrates away the putative negative regulator from the spindle pole body, giving deregulated signaling and multiple rounds of septum formation at the end of mitosis. Alternatively, it may hinder the inactivation of Spg1p.
The spindle pole body/centrosome as an integrator of cell cycle events
Localization of key cell cycle regulators such as Cdc2p/cyclin B (Bailley et al. 1989; Riabowol et al. 1989; Pockwinse et al. 1997) and polo-like kinases (Lane and Nigg 1996), to the poles of the spindle may explain the observation that isolated centrosomes parthenogenetically activate development in Xenopus, as they may provide a framework on which to build regulatory complexes (Tournier et al. 1989; Klotz et al. 1990). In S. pombe, the mitosis-inducing kinase Cdc2p/Cdc13p is also located on the spindle pole body (Alfa et al. 1990). It is also noteworthy that mutation of Cut12p, a spindle pole body component, is sufficient to circumvent the normal regulation of commitment to mitosis (A.J. Bridge, M. Morphew, R. Bartlett, and I.M. Hagan, in prep.). If the centrosome/spindle pole body acts as a scaffold and communication point through, and upon which, cell cycle pathways are coordinated, association of Cdc7p and Spg1p, the “trigger” for septation, with the spindle pole body provides an excellent means of coordinating mitosis and cytokinesis.
Materials and methods
Yeast techniques
Standard techniques were used for growth, manipulation, and synchronization of fission yeast (Moreno et al. 1991). Cells were grown in yeast extract (YE) or EMM2 minimal medium, supplemented as required. Other techniques have been referred to previously (Fankhauser et al. 1995). To generate synchronous cultures, cdc25-22 cdc7–HA cells were incubated for 4 hr at 36°C and then released from the block by shifting them back to 25°C (Moreno et al. 1989). To examine germinating spores deleted for spg1 or cdc7, the diploid strains heterozygous for either of the null alleles (Fankhauser and Simanis 1994; Schmidt et al. 1997) were induced to sporulate on malt extract plates (Moreno et al. 1991). Spores were prepared and inoculated in minimal medium containing adenine and leucine, so only the spores carrying the null allele could germinate and grow.
Immunofluorescence
Indirect immunofluorescence was performed as described previously (Sohrmann et al. 1996), except that cells were digested for 10 min in 1 mg/ml of Zymolyase 20T. The anti-Sad1p (Hagan and Yanagida 1995), anti-Cdc7p (Fankhauser and Simanis 1994), and anti-Spg1p (SuSu1, Schmidt et al. 1997) antisera were affinity purified before use, according to standard procedures (Harlow and Lane 1988). The monoclonal anti-HA antibody (12CA5) was purchased from BabCO. The α-tubulin mAb TAT-1 (Woods et al. 1989) was a generous gift from K. Gull (University of Manchester). Specificity of the staining was confirmed by competition experiments with the antigen used to raise the antiserum (according to Sohrmann et al. 1996) and by staining of germinating spores deleted for either cdc7 or spg1. The epitopes recognized by the anti-Cdc7p and anti-Spg1p antisera and by mAb 12CA5 are sensitive to glutaraldehyde. Note that fixation in the absence of glutaraldehyde only preserves the mitotic spindle but not the interphase microtubule array. Microtubules were depolymerized by incubating the cells on ice for 25 min before fixation, as described by Hagan and Yanagida (1995). Cells were observed with a Zeiss Axiophot microscope. Production of color images and confocal microscopy has been described (Hagan and Yanagida 1997; Sohrmann et al. 1996).
Construction of the spg1–HA and the cdc7–GFP strains
To construct spg1–HA, the carboxy-terminal half of the spg1 gene was amplified by PCR using the oligonucleotides VS 135 (ATCAAAGAATGGTACCGTCAAGCTCG) and VS 151 (GAG GATCCTTAATCAGCGGCCGCTGTATTCCAA) and cloned into pDW232 (Weilguny et al. 1991). The triple HA tag (Tyers et al. 1992) was cloned into the NotI site introduced by VS 151 just before the stop codon. The ura4+ gene and 2 kb of genomic sequence 3′ of the spg1 coding sequence were cloned behind the tag. The whole linear insert was transformed into a ura4D18 strain. Stable uracil prototrophs were checked by Southern and Western blotting for correct replacement of the spg1 gene by the tagged version.
The cdc7–GFP strain was constructed as the cdc7–HA strain described previously (Schmidt et al. 1997), except that the HA tag in the transformed linear fragment was replaced by an improved version of the Aequorea victoria GFP (S65T, F64L). All strains in which the tagged gene replaced the wild-type copy were indistinguishable from wild type.
Protein extracts, kinase assays, and immunoprecipitations
Total protein extracts were prepared as described previously (Sohrmann et al. 1996). Extracts of soluble proteins and kinase assays were performed according to Fankhauser and Simanis (1994), except that mAb 12CA5 was used for Western blot analysis, after immunoprecipitation with polyclonal anti-Cdc7p antiserum. To precipitate Spg1p or Spg1–HAp, 5 μl of affinity-purified anti-Spg1p antiserum, or 1 μg of mAb 12CA5, respectively, was added to protein extracts (1.5 mg) prepared in the absence or presence of 0.6 mm GTPγS (Sigma) and incubated for 60 min at 4°C. The complexes were then precipitated using protein A–Sepharose (Sigma) and analyzed by Western blotting using the same antibody as for the immunoprecipitation.
Association of Cdc7p and GST–Spg1p
The spg1 gene was cloned into pGEX-3X (Pharmacia) and expressed in E. coli DH5α. The fusion protein was purified on a glutathione–Sepharose 4B (Sigma) column as described (Jacquet et al. 1994). Glutathione was removed by dialysis. For the GDP/GTP exchange, 2 mm EDTA and 1 mm GTPγS were added to the buffer. To examine the association between Cdc7p and Spg1p, 0.5 μg of GST, GDP-bound Spg1p, or GTP-bound Spg1p was incubated with 3 mg of protein extract for 60 min at 4°C. The GST–Spg1p fusion protein and any bound proteins were collected by affinity chromatography on glutathione–Sepharose and analyzed by Western blotting.
Acknowledgments
We thank Lorenzo Cerutti for the gift of purified GST–Spg1p fusion protein. We are grateful to Bruno Amati, Nicola Beltraminelli, Lorenzo Cerutti, Christian Fankhauser, Xavier LeGoff, Matthias Peter, and Suzan Utzig, for discussions and comments on the paper. We are also grateful to Elena Cano for technical assistance. This work was supported by ISREC, the Swiss National Science Foundation, the Swiss Cancer League, the Cancer Research Campaign (CRC), and a short-term European Molecular Biology Organization (EMBO) fellowship to M.S.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL viesturs.simanis@isrec.unil.ch; FAX +41-21-652-6933.
References
- Alfa CE, Ducommun B, Beach D, Hyams JS. Distinct nuclear and spindle pole body population of cyclin-cdc2 in fission yeast. Nature. 1990;347:680–682. doi: 10.1038/347680a0. [DOI] [PubMed] [Google Scholar]
- Bailly E, Doree M, Nurse P, Bornens M. p34cdc2 is located in both nucleus and cytoplasm; part is centrosomally associated at G2/M and enters vesicles at anaphase. EMBO J. 1989;8:3985–3995. doi: 10.1002/j.1460-2075.1989.tb08581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian MK, Feoktistova A, McCollum D, Gould KL. Fission yeast Sop2p: A novel and evolutionarily conserved protein that interacts with Arp3p and modulates profilin function. EMBO J. 1996;15:6426–6437. [PMC free article] [PubMed] [Google Scholar]
- Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Chang F, Woollard A, Nurse P. Isolation and characterization of fission yeast mutants defective in the assembly and placement of the contractile actin ring. J Cell Sci. 1996;109:131–142. doi: 10.1242/jcs.109.1.131. [DOI] [PubMed] [Google Scholar]
- Ding R, West RR, Morphew M, McIntosh JR. The spindle pole body of Schizosaccharomyces pombe enters and leaves the nuclear envelope as the cell cycle proceeds. Mol Biol Cell. 1997;8:1461–1479. doi: 10.1091/mbc.8.8.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Simanis V. The Schizosaccharomyces pombe cdc14 gene is required for septum formation and can also inhibit nuclear division. Mol Biol Cell. 1993;4:531–539. doi: 10.1091/mbc.4.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— The cdc7 protein kinase is a dosage dependent regulator of septum formation in fission yeast. EMBO J. 1994;13:3011–3019. doi: 10.1002/j.1460-2075.1994.tb06600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Marks J, Reymond A, Simanis V. The S. pombe cdc16 gene is required both for maintenance of p34cdc2 kinase activity and regulation of septum formation: A link between mitosis and cytokinesis? EMBO J. 1993;12:2697–2704. doi: 10.1002/j.1460-2075.1993.tb05931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Reymond A, Cerutti L, Utzig S, Hofmann K, Simanis V. The S. pombe cdc15 gene is a key element in the reorganization of F-actin at mitosis. Cell. 1995;82:435–444. doi: 10.1016/0092-8674(95)90432-8. [DOI] [PubMed] [Google Scholar]
- Ghiara JB, Richardson HE, Sugimoto K, Henze M, Lew DJ, Wittenberg C, Reed SI. A cyclin B homolog in S. cerevisiae; Chronic activation of the Cdc28 protein kinase by cyclin prevents exit from mitosis. Cell. 1991;65:163–174. doi: 10.1016/0092-8674(91)90417-w. [DOI] [PubMed] [Google Scholar]
- Gould KL, Simanis V. The control of septum formation in fission yeast. Genes & Dev. 1997;11:2939–2951. doi: 10.1101/gad.11.22.2939. [DOI] [PubMed] [Google Scholar]
- Hagan IM, Gull K, Glover DM. Poles apart? Spindle pole bodies and centrosomes differ in ultrastructure yet their function and regulation is conserved. In: Endow S, Glover DM, editors. Mechanisms of cell division—Frontiers in cell biology. Oxford, UK: IRL Press; 1997. . (In press). [Google Scholar]
- Hagan IM, Hyams JS. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1988;89:343–357. doi: 10.1242/jcs.89.3.343. [DOI] [PubMed] [Google Scholar]
- Hagen I, Yanagida M. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J Cell Biol. 1995;129:1033–1047. doi: 10.1083/jcb.129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Evidence of a cell cycle specific, spindle pole body mediated, nuclear positioning in fission yeast. J Cell Sci. 1997;110:1851–1866. doi: 10.1242/jcs.110.16.1851. [DOI] [PubMed] [Google Scholar]
- Hall A. A biochemical function for ras—at last. Science. 1994;264:1413–1414. doi: 10.1126/science.8197454. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane E. Antibodies: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- He X, Patterson TE, Sazer S. The S. pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the APC. Proc Natl Acad Sci. 1997;94:7965–7970. doi: 10.1073/pnas.94.15.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Funahashi S, Uemura T, Yanagida M. Isolation and characterization of Schizosaccharomyces pombe cut mutants that block nuclear division but not cytokinesis. EMBO J. 1986;5:2973–2979. doi: 10.1002/j.1460-2075.1986.tb04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y, Toda T, Yanagida M. The NDA3 gene of fission yeast encodes β-tubulin: A cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell. 1984;39:349–358. doi: 10.1016/0092-8674(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Jacquet E, Parrini MC, Bernardi A, Martegani E, Parmeggiani A. Properties of the catalytic domain of CDC25, a Saccharomyces cerevisiae GDP/GTP exchange factor: Comparison of its activity on full-length and C-terminal truncated RAS2 proteins. Biochem Biophys Res Commun. 1994;199:497–503. doi: 10.1006/bbrc.1994.1256. [DOI] [PubMed] [Google Scholar]
- Kalt A, Schliwa M. Molecular components of the centrosome. Trends Cell Biol. 1993;3:118–128. doi: 10.1016/0962-8924(93)90174-y. [DOI] [PubMed] [Google Scholar]
- Klotz C, Dabauvalle MC, Paintrand M, Weber T, Bornens M, Karsenti E. Parthenogenesis in Xenopus eggs requires centrosomal integrity. J Cell Biol. 1990;110:405–415. doi: 10.1083/jcb.110.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada K, Su S, Yanagida M, Toda T. Fission yeast TPR-family protein nuc2 is required for G1-arrest upon nitrogen starvation and is an inhibitor of septum formation. J Cell Sci. 1995;108:895–905. doi: 10.1242/jcs.108.3.895. [DOI] [PubMed] [Google Scholar]
- Lane HA, Nigg EA. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J Cell Biol. 1996;135:1701–1713. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange BM, Gull K. A molecular marker for centriole maturation in the mammalian cell cycle. J Cell Biol. 1995;130:919–927. doi: 10.1083/jcb.130.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E, Wu C, Leeuw T, Fourest-Lieuvin A, Segall JE, Thomas DY. Functional characterization of the Cdc42p binding domain of yeast Ste20p protein kinase. EMBO J. 1997;16:83–97. doi: 10.1093/emboj/16.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenormand P, Sardet C, Pages G, L’A Hemain G, Brunet A, Pouyssegur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J, Hyams JS. Localization of F-actin through the cell division cycle of Schizosaccharomyces pombe. Eur J Cell Biol. 1985;39:27–32. [Google Scholar]
- Minet M, Nurse P, Thuriaux P, Mitchison JM. Uncontrolled septation in a cell division cycle mutant of the fission yeast Schizosaccharomyces pombe. J Bacteriol. 1979;137:440–446. doi: 10.1128/jb.137.1.440-446.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison JM, Nurse P. Growth in cell length in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1985;75:357–376. doi: 10.1242/jcs.75.1.357. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D. Localization of protein kinases by anchoring proteins: A theme in signal transduction. Science. 1995;268:247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- Moreno S, Haynes J, Nurse P. Regulation of p34cdc2 protein kinase during mitosis. Cell. 1989;58:361–372. doi: 10.1016/0092-8674(89)90850-7. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Murone M, Simanis V. The fission yeast dma1 gene is a component of the spindle assembly checkpoint, required to prevent septum formation and premature exit from mitosis if spindle function is compromised. EMBO J. 1996;15:6605–6616. [PMC free article] [PubMed] [Google Scholar]
- Nurse P, Thuriaux P, Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol & Gen Genet. 1976;146:167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- Ohkura H, Hagan IM, Glover DM. The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes & Dev. 1995;9:1059–1073. doi: 10.1101/gad.9.9.1059. [DOI] [PubMed] [Google Scholar]
- Peter M, Neiman AM, Park HO, van Lohuizen M, Herskowitz I. Functional analysis of the interaction between the small GTP binding protein Cdc42 and the Ste20 protein kinase in yeast. EMBO J. 1996;15:7046–7059. [PMC free article] [PubMed] [Google Scholar]
- Pockwinse SM, Krockmalnic G, Doxsey SJ, Nickerson J, Lian JB, van Wijnen AJ, Stein JL, Stein GS, Penman S. Cell cycle independent interaction of CDC2 with the centrosome, which is associated with the nuclear matrix-intermediate filament scaffold. Proc Natl Acad Sci. 1997;94:3022–3027. doi: 10.1073/pnas.94.7.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabowol K, Draetta G, Brizuela L, Vandre D, Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989;57:393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- Robinow CF, Hyams JS. General cytology of fission yeast. In: Nasim A, Young PG, Johnson BF, editors. The molecular biology of the fission yeast. New York, NY: Academic Press; 1989. pp. 273–331. [Google Scholar]
- Satterwhite LL, Lohka MJ, Wilson KL, Scherson TY, Cisek LJ, Corden JL, Pollard TD. Phosphorylation of myosin-II regulatory light chain by cyclin-p34cdc2: A mechanism for the timing of cytokinesis. J Cell Biol. 1992;118:595–605. doi: 10.1083/jcb.118.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Sohrmann M, Hofmann K, Woollard A, Simanis V. The Spg1p GTPase is an essential, dosage-dependent inducer of septum formation in Schizosaccharomyces pombe. Genes & Dev. 1997;11:1519–1534. doi: 10.1101/gad.11.12.1519. [DOI] [PubMed] [Google Scholar]
- Sohrmann M, Fankhauser C, Brodbeck C, Simanis V. The dmf1/mid1 gene is essential for correct positioning of the division septum in fission yeast. Genes & Dev. 1996;10:2707–2719. doi: 10.1101/gad.10.21.2707. [DOI] [PubMed] [Google Scholar]
- Song K, Mach KE, Chen CY, Reynolds T, Albright CF. A novel suppressor of ras1 in fission yeast, byr4, is a dosage-dependent inhibitor of cytokinesis. J Cell Biol. 1996;133:1307–1319. doi: 10.1083/jcb.133.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A, Courtney I, Grein K, Matzner M, Schiebel E. The Cdc31p-binding protein Kar1p is a component of the half bridge of the yeast spindle pole body. J Cell Biol. 1995;128:863–877. doi: 10.1083/jcb.128.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surana U, Amon A, Dowzer C, McGrew J, Byers B, Nasmyth K. Destruction of the Cdc28/CLB mitotic kinase is not required for the metaphase to anaphase transition in budding yeast. EMBO J. 1993;12:1969–1978. doi: 10.1002/j.1460-2075.1993.tb05846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier F, Karsenti E, Bornens M. Parthenogenesis in Xenopus eggs injected with centrosomes from synchronized human lymphoid cells. Dev Biol. 1989;136:321–329. doi: 10.1016/0012-1606(89)90259-5. [DOI] [PubMed] [Google Scholar]
- Tugendreich S, Tomkiel J, Earnshaw W, Hieter P. CDC27Hs colocalizes with CDC16Hs to the centrosome and mitotic spindle and is essential for the metaphase to anaphase transition. Cell. 1995;81:261–268. doi: 10.1016/0092-8674(95)90336-4. [DOI] [PubMed] [Google Scholar]
- Tyers M, Tokiwa G, Nash R, Futcher B. The Cln3-Cdc28 kinase complex of S. cerevisiae is regulated by proteolysis and phosphorylation. EMBO J. 1992;11:1773–1784. doi: 10.1002/j.1460-2075.1992.tb05229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallen EA, Scherson TY, Roberts T, van Zee K, Rose MD. Asymmetric mitotic segregation of the yeast spindle pole body. Cell. 1992;69:505–515. doi: 10.1016/0092-8674(92)90451-h. [DOI] [PubMed] [Google Scholar]
- Weilguny D, Praetorius M, Carr A, Egel R, Nielsen O. New vectors in fission yeast: Application for cloning the his2 gene. Gene. 1991;99:47–54. doi: 10.1016/0378-1119(91)90032-7. [DOI] [PubMed] [Google Scholar]
- Woods A, Sherwin T, Sasse R, MacRae TH, Baines AJ, Gull K. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J Cell Sci. 1989;93:491–500. doi: 10.1242/jcs.93.3.491. [DOI] [PubMed] [Google Scholar]
- Yamada H, Kumada K, Yanagida M. Distinct subunit functions and cell cycle regulated phosphorylation of 20S APC/cyclosome required for anaphase in fission yeast. J Cell Sci. 1997;110:1793–1804. doi: 10.1242/jcs.110.15.1793. [DOI] [PubMed] [Google Scholar]
- Yamakita Y, Yamashiro S, Matsumura F. In vivo phosphorylation of regulatory light chain of myosin II during mitosis of cultured cells. J Cell Biol. 1994;124:129–137. doi: 10.1083/jcb.124.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita YM, Nakaseko Y, Samejima I, Kumada K, Yamada H, Michaelson D, Yanagida M. 20S cyclosome complex formation and proteolytic activity inhibited by the cAMP/PKA pathway. Nature. 1996;384:276–279. doi: 10.1038/384276a0. [DOI] [PubMed] [Google Scholar]
- Yanagida M. 20S cyclosome complex formation and proteolytic activity inhibited by the cAMP/PKA pathway. Nature. 1996;384:276–279. doi: 10.1038/384276a0. [DOI] [PubMed] [Google Scholar]