Abstract
Worldwide, invasive meningococcal disease affects about 500,000 people annually. Case fatality in developed countries averages 10%, and higher rates are reported in less prosperous regions. According to the World Health Organization, the most important pathogenic serogroups are A, B, C, W-135, X, and Y. Clinical features of invasive meningococcal disease make diagnosis and management difficult. Antibiotic measures are recommended for prophylaxis after exposure and for treatment of invasive meningococcal disease cases; however, resistant strains may be emerging. Vaccines are generally regarded as the best preventative measure for invasive meningococcal disease. Polysaccharide vaccines against serogroups A, C, W-135, and Y using protein conjugation technology have clear advantages over older plain polysaccharide formulations without a protein component. The first quadrivalent meningococcal conjugate vaccine (MenACWY-D) was licensed in the US in 2005. More recently, MenACWY-CRM (Menveo®) was licensed in Europe, the US, the Middle East, and Latin America. MenACWY-CRM uses cross-reactive material 197, a nontoxic mutant of diphtheria toxin, as the carrier protein. MenACWY-CRM offers robust immunogenicity in all age groups, with a tolerability profile similar to that of a plain polysaccharide vaccine. Given its potential for protecting persons from infancy to old age, MenACWY-CRM offers the opportunity to protect broad populations against invasive meningococcal disease. The most optimal strategy for use of the vaccine has to be assessed country by country on the basis of local epidemiology, individual health care systems, and need.
Keywords: invasive meningococcal disease, quadrivalent meningococcal conjugate vaccine, Neisseria meningitidis
Introduction
Neisseria meningitidis is a Gram-negative diplococcus, and humans are its only known reservoir. Despite advances in therapy and vaccine technologies during the late twentieth century, invasive meningococcal disease (IMD) remains a global public health challenge. Sudden-onset epidemic and sporadic septicemia and bacterial meningitis are the most common manifestations of IMD, although other presentations occur. The meningococcal polysaccharide capsule is a major virulence factor for IMD and has been used to distinguish approximately a dozen serogroups.1–3
Vaccines against the various meningococcal serogroups were developed during the twentieth century, and in the last decade novel quadrivalent conjugate vaccines against serogroups A, C, W-135, and Y became available. These vaccines can protect against four of the five serogroups that cause the vast majority of IMD. We discuss the newest of these quadrivalent conjugate vaccines, ie, Menveo® (MenACWY-CRM) in the context of the clinical importance and management of IMD to provide a fuller picture of the potential contribution of this novel vaccine to public health. This review updates previous reviews by Cooper et al, Pace, Pace et al, and Deeks,4–7 providing detailed information about the clinical use of antibiotics for IMD and recent clinical data.
Importance and clinical characteristics of meningococcal disease
Worldwide, an estimated 500,000 cases of IMD occur annually, with an average mortality rate of 5%–10% in developed nations.1,8–10 In the European Union, case fatality ratios are lowest (<5%) in children 5–9 years and highest (≥20%) in the elderly. The vast majority of IMD, nearly 90% of typable cases, is caused by serogroups A, B, C, W-135, and Y. Another serogroup, X, has caused epidemics in Africa, and is considered by the World Health Organization to be of concern.1–3,9–12
The meningococcus spreads from person to person by respiratory droplets, which requires close contact. In most individuals, acquisition of meningococci leads to asymptomatic colonization in the nasopharynx, after which the bacteria are naturally cleared. Meningococcal carriage has a population prevalence of about 10%, which varies with age and in different settings. Carriage is rather low in young children, increases through childhood to a peak in 19-year-olds, who have a carriage rate >20%, and declines in adulthood.13,14
A minority of nasopharyngeally carried meningococci, even among virulent strains, will cause IMD. Some individuals are rendered susceptible through anatomic or functional asplenia, inborn or acquired deficiencies of complement proteins, or properdin deficiency. Disease may develop quickly and without warning, most commonly within the first week of colonization.2,11,15,16 Goldschneider et al demonstrated the importance of humoral immunity when they showed an inverse correlation between the incidence of IMD and age-related acquisition of serum bactericidal antibodies.17
Meningitis occurs in approximately half of IMD cases. The remaining cases are mainly bacteremia, although meningococcal pneumonia, pericarditis, septic arthritis, and other localized infections may occur.11,16,18–20 Less usual variations in clinical presentation include occult bacteremia in patients having mild transient illness, and chronic meningococcemia with fever, rash, and arthritis similar to the arthritis-dermatitis syndrome of gonococcemia.18,21–24
Early IMD symptoms, such as fever and headache, are nonspecific and similar to those of any mild viral illness. Late-stage symptoms are more distinctive, and include stiff neck, high fever, photophobia, and confusion. In septicemia, coagulopathy and a nonblanching petechial rash may occur. Shock and circulatory collapse may occur rapidly. In spite of prompt initiation of antibiotic therapy and intensive care measures, mortality rates remain consistent in many areas, and up to 20% of survivors suffer from permanent long-term sequelae, including deafness, cognitive deficits, seizures, amputation, endocrinopathy, and neuropsychiatric disorders.2,11,18–20,25
Early suspicion and prompt initiation of antimicrobial therapy provides the best hope for IMD patients.20 Diagnosis of IMD involves clinical suspicion and laboratory confirmation. Definitive diagnosis requires isolation of the organism from blood, cerebrospinal fluid, or tissue.24 The sensitivity of bacterial culture methods may be low, and can affect the accuracy of clinical diagnosis as well as epidemiologic surveillance.2,16 Polymerase chain reaction is a more sensitive method for detection, particularly in persons who receive an antibiotic before samples are obtained for culture.11 Given the difficulties of early recognition, the rapid course of the disease, and persistent rates of disability and mortality even with appropriate treatment, prevention by vaccination is the most viable means of controlling IMD.1,4–6,18
Epidemiology of meningococcal disease
The incidence of IMD usually ranges from 0.2 to 5 per 100,000 persons, although this may increase to nearly 1% during major epidemics. About half of all cases occur in infants and young children. In industrialized countries, most IMD is caused by serogroups B and C.9,10 Specific strains, clonal complexes, and serogroups are associated with case fatality and other unwanted outcomes.23,26
In the US, the incidence of IMD varied cyclically during the late twentieth century, and decreased to a rate of 0.33/100,000 population by 2007. Most cases are caused by serogroups B, C, or Y. Of interest, serogroup Y accounted for approximately 2% of cases in 1991, but more than one-third of cases by 1998,26,27 a pattern of increase also evident in Columbia and Argentina, where it was accompanied by an increase in W-135 disease.28,29 Global travel, which brings carriers of various serogroups into close contact, and capsular switching, a mechanism involving horizontal gene transfer, are thought to contribute to dynamic epidemiology.1,9,30
The greatest burden of meningococcal disease occurs during serogroup A epidemics in the “meningitis belt” of Sub-Saharan Africa during the dry season.1,11 In 2009, nearly 79,000 cases and more than 4000 deaths were reported. Serogroups W-135 and X have become increasingly prominent in this region, the latter serogroup being of concern in part because the incidence during outbreaks has exceeded 25 cases per 100,000. Serogroup W-135 emerged as an important cause of IMD following outbreaks during and after the 2000 and 2001 Hajj pilgrimages. Subsequent cases caused by the same W-135 strain have occurred around the world, and W-135 disease caused by various strains has become endemic in some areas.9,10,30
Clinical management and prevention of IMD
Prompt use of appropriate antibiotics reduces the expected case fatality of IMD from 70%–90% to 5%–10%. Treatment recommendations include prophylaxis after exposure to a confirmed case but before manifestation of clinical signs. The timing of prophylaxis and treatment is crucial, given the rapid progression and course of IMD.3,11,24,31,32
Chemoprophylaxis should be administered to all close contacts, including those previously vaccinated, within 24 hours of an index case of IMD being diagnosed, because the rate of secondary cases is highest immediately following onset of the illness.11,18,33 The US Centers for Disease Control and Prevention defines a contact as an individual exposed to a household member, day care, or nursery school contact within 7 days before the onset of illness in the index case, and anyone directly exposed to oral secretions.32,33 Antibiotics are intended to eliminate meningococci from the nasopharyngeal tract and can overcome the time lag (≥10 days) between vaccination or re-exposure and the development of protective antibodies.33 By 10–14 days after diagnosis of the index case, chemoprophylaxis is not recommended. Chemoprophylaxis should be considered for those travelling on an airplane for ≥8 hours close to an index case.34 Hajj pilgrims from the African meningitis belt countries receive meningococcal chemoprophylaxis at the port of entry to reduce carrier rates.35 Health care workers need prophylaxis if they have been directly exposed to nasopharyngeal secretions of an active case.
Generally recommended antibiotics for chemoprophylaxis include rifampin, ciprofloxacin, and ceftriaxone. Rifampin is preferred in industrial countries, but is contraindicated in pregnant women and those with liver disease or alcoholism. Ciprofloxacin, the drug of choice in South Africa, eradicates carriage, but is also contraindicated in pregnancy. Azithromycin generally eradicates carriage and can be used in areas with ciprofloxacin-resistant strains.35 Ceftriaxone can be used in pregnant women and those with contraindications to other agents.7–35 Penicillin, tetracycline, and erythromycin should not be used for chemoprophylaxis.31 Research is needed to evaluate prophylaxis with other antibiotics, including third-generation cephalosporins.
Although mass antibiotic prophylaxis in outbreak situations suppresses meningococcal carriage, it generally rebounds, increasing the risk of emergence of resistant strains. Re-emergence of meningococcal colonization may occur within 8–12 weeks,37 although early recolonization has been observed in crowded living conditions (eg, military communities).
Treatment recommendations in Europe and the US31 include various antibiotics (see Table 1). One US guideline recommends separate empirical antimicrobial treatment, ie, ampicillin and aminoglycoside or cefotaxime, in suspected cases of bacterial meningitis in children under 1 month of age. A further US guideline differentiates treatment depending on the N. meningitidis penicillin minimal inhibitory concentration, recommending penicillin G or ampicillin for minimal inhibitory concentrations <0.1–1.0 μg/mL. Chloramphenicol is recommended in cases of penicillin allergy.31 Tetracycline and erythromycin are contraindicated for IMD.
Table 1.
Antibiotic recommendations for treating and preventing invasive meningococcal disease31
| Region | Therapy recommended | Duration (days) | Alternative therapies |
|---|---|---|---|
| US | Penicillin G, ampicillin, third-generation cephalosporin | 5–7 | Chloramphenicol,* meropenem, fluoroquinolone |
| Canada | Penicillin G | 5–7 | Not specified |
| Europe | Benzyl penicillin, third-generation cephalosporin | 5–7 | Chloramphenicol,* meropenem, moxifloxacin |
Note:
In cases of penicillin allergy.
As an adjuvant treatment, dexamethasone can attenuate inflammatory responses and may decrease cerebral edema, increased intracranial pressure, cerebral vasculitis, and neural injury. However, dexamethasone treatment also reduces blood–brain barrier permeability, and might reduce penetration of antibiotics and sterilization of the cerebrospinal fluid, suggesting a need for further study.31
Possibility of antibiotic-resistant meningococcal strains
Meningococci are not particularly efficient in developing resistance to antibiotics, apart from the sulfonamides (>25% of isolates are resistant).38 Intermediate penicillin resistance is widespread, being 4% in the US, 23% in Sweden, and 38% in Portugal. In Romania, 38.7% of 2007–2008 isolates had intermediate resistance to penicillin and 3.3% were resistant.39 Since the 1980s, penicillin-resistant meningococci have been detected in the UK, Spain, Italy, Greece, and other Mediterranean countries.40 Resistance has not been reported to extended-spectrum cephalosporins, eg, cefotaxime and ceftriaxone. Chloramphenicol resistance is rarely reported.
Meningococcal strains isolated during 1999–2006 in Dhaka, Bangladesh, were susceptible to penicillin, ampicillin, chloramphenicol, ciprofloxacin, and ceftriaxone. Cotrimoxazole resistance increased from 50% to 100% during 2002–2006. Resistance to azithromycin emerged in 2002, and increased to 31% in 2004, but isolates collected in 2005–2006 were susceptible to this antibiotic.41
Among more than 75 meningococcal strains isolated in 2000–2008 in Singapore,42 all were susceptible to rifampicin, but 17% had intermediate resistance and 3% were resistant to penicillin. Two percent were resistant to nalidixic acid, a quinolone. Of note, 74% of Neisseria gonorrhae isolates were resistant to quinolones, and ciprofloxacin use is widespread in Singapore.
During a 2005–2006 outbreak of IMD around New Delhi, India, 15.4% of isolated strains were penicillin-resistant and 15.4% had intermediate resistance. All isolates were susceptible to the third-generation cephalosporins, azithromycin and rifampicin. Resistance to quinolones was very high at 100% for levofloxacin, 84.6% for ofloxacin, and 65.4% for ciprofloxacin.40,43
In 2009, the emergence of ciprofloxacin-resistant meningococci in the US was reported. Resistant strains isolated in North Dakota and Minnesota had an indistinguishable pattern of pulse-field gel electrophoresis, belonged to the same clonal complex, and had the same multilocus sequence type ST-162 and porA, porB, and fetA types. By active surveillance, additional ciprofloxacin-resistant isolates were identified in California in 2007.44
In a study of isolates collected in the African meningitis belt, a large number were resistant to tetracycline and erythromycin. Six percent of meningococcal isolates collected during 2001–2005 in South Africa had intermediate resistance to penicillin.45
Emerging resistance to antibiotics, especially penicillin and fluoroquinolones, raises concerns about treatment and chemoprophylaxis for IMD. Although resistant strains may go undetected, widespread resistance seems unlikely because cases remain rare in the presence of routine antibiotic use (eg, ciprofloxacin).38
Drug-resistant N. meningitidis can potentially cause a global public health threat, especially in this age of global travel. A recent case of drug-resistant IMD in Italy was diagnosed in a man following a 10-day business trip in India with a stopover at Frankfurt airport, thus presenting multiple opportunities for transmission.46 The meningococcal strain affecting this patient was resistant to ciprofloxacin, levofloxacin, and trimethoprim + sulfamethoxazole. Although chemoprophylaxis was initiated in a timely fashion, 15 adult contacts received ciprofloxacin and had to be offered rifampin after susceptibility testing was completed.
Vaccination strategies against meningococcal disease
The extracellular polysaccharide coat of meningococcus (except serogroup B) has long been known to induce protective antibodies. Purified polysaccharide preparations, both unconjugated and protein-conjugate vaccines, are serogroup-specific.1 Protein-conjugate vaccines based on capsular polysaccharides induce a T cell-dependent immune response, therefore immunological memory, bringing about elevated or booster responses to subsequent vaccine doses. An important advantage of many polysaccharide-protein conjugate vaccine formulations is their high immunogenicity in young children and infants; serogroup C vaccines have successfully been implemented into the routine infant immunization calendar in many countries. These vaccines have reduced nasopharyngeal carriage and thereby contribute to herd protection.1,18,47,48
MenACWY-CRM for serogroups A, C, W-135, and Y
Following an extensive clinical development program in persons of various ages, MenACWY-CRM is currently approved by the US Food and Drug Administration for use in persons aged 2–55 years and was granted marketing authorization by the European Union for all 27 member states in March 2010 for people aged 11 years and older.4,49 Clinical data have been previously reviewed by Cooper et al, Pace, Pace et al, and Deeks.4–7
Protein production and conjugation
MenACWY-CRM uses cross-reactive material 197 (CRM197), a nontoxic mutant of the diphtheria toxin, as the conjugation protein. This nontoxic version of diphtheria toxin does not need to be inactivated by formaldehyde or glutaraldehyde, as routinely required for use of diphtheria toxin in vaccine applications. Treatment of diphtheria toxin with aldehydes results in intramolecular and intermolecular cross-links of the molecule and significant epitope modifications, which may decrease the accessibility of amino acid residues for coupling of the carrier protein with polysaccharide chains. In CRM197, all 39 ɛ-amino groups of lysine residues remain accessible for the conjugation process.50
The preparation of the polysaccharides in MenACWY-CRM includes fractioning of the individual oligosaccharide chains, which results in a population of antigens of restricted and well-defined medium length and eliminates very short chains, which may be less immunogenic. The final pool of oligosaccharides is activated via reductive animation followed by an adipic linker bearing a terminal active ester to the reducing ends of the sugar chains, ensuring that the saccharides are orientated radially to the carrier protein. The conjugation process also results in a consistent degree of glycosylation (saccharide to protein ratio). A 0.5 mL dose of MenACWY-CRM contains 10 μg Men A, 5 μg each of Men C, Men W-135, and MenY antigens, and approximately 47 μg of CRM197. No adjuvant or preservative is used.32,50
Handling and storage
MenACWY-CRM is packaged as a lyophilized serogroup A component and a liquid component, containing serogroup C, W-135, and Y antigens, for reconstitution immediately before intramuscular administration, preferably in the upper arm. Misapplication, such as subcutaneous injection, may result in reduced immune response. The vaccine should be stored in the original packaging at 2°C–8°C and not frozen. The unreconstituted product remained within specifications when stored at temperatures of up to 25°C for 24 months and up to 40°C for 6 months in stability studies. Clinical experience indicates that reconstitution may be facilitated if the vaccine is brought to room temperature and/or shaken vigorously during reconstitution. Although reconstituted MenACWY-CRM should be used immediately, it may be held at or below 25°C for up to 8 hours.4
Immunogenicity and tolerability
As observed in previous reviews,4–6 the MenACWY-CRM clinical research and development program included studies in persons aged from 2 months to 65 years. In these studies, clinical endpoints used accepted correlates of protection for meningococcal disease in a serum bactericidal assay using human complement (hSBA), with titers ≥4 being considered protective in persons who were seronegative prior to vaccination and titers ≥8 being used in some studies as a more conservative standard.4–6,17,51
Clinical trials in infants and children
Published data describing the immunogenicity and safety profile of MenACWY-CRM in infants supports its suitability for clinical use. As noted by a number of investigators, MenACWY-CRM is the first quadrivalent conjugate vaccine to provide substantial supportive evidence of immunogenicity in young infants.4–6,52 Pace indicates that the possibility to protect persons under 2 years of age against serogroups W-135 and Y disease is of particular importance in meningococcal vaccination.5,6
Preliminary data presented by Vesikari et al supported the tolerability and safety profile of MenACWY-CRM in infants as well as further development, as did published results describing a trial of an earlier MenACWY formulation using an adjuvant.4–6,51,52 One month following study vaccination in an open-label trial in 175 infants, approximately 80%–100% of those receiving MenACWY-CRM at 6 and 12 months of age had an hSBA titer ≥8 to serogroups A, C, W-135, and Y. In contrast, about 60%–95% of those who received MenACWY-CRM at 12 months of age and 50%–100% of those who received the vaccine at 18 months of age had similar titers.4–6,53 Boosting for serogroup C was evident in toddlers who received the monovalent serogroup C conjugate vaccine at 12 months of age, followed by MenACWY-CRM at 18 months of age.53
A preliminary report of data from the US cohort of a large Phase III trial conducted in infants in Latin America and the US indicated that MenACWY-CRM administered at 2, 4, and 6 months of age induced robust immune responses considered consistent with protection. MenACWY-CRM was generally well tolerated in these infants.54,55
In a randomized Phase II study of 619 healthy children aged 2–10 years in the US, MenACWY-CRM and MPSV4 (unconjugated quadrivalent meningococcal polysaccharide vaccine; Menomune®, Sanofi Pasteur Inc, Swiftwater, PA) were generally well tolerated and demonstrated robust immunogenicity. One and 12 months following vaccination, significantly more MenACWY-CRM recipients had hSBA titers ≥4 (the primary endpoint) compared with MPSV4 recipients. Compared with values 1 month following vaccination, hSBA geometric mean titers declined against each serogroup by 12 months following vaccination in both vaccine groups. MenACWY-CRM was generally well tolerated, as assessed by adverse events and solicited injection site and systemic reactions.56
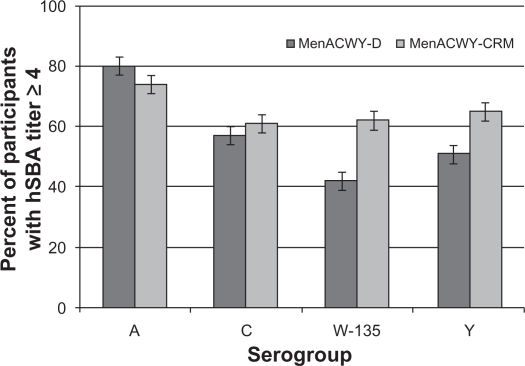
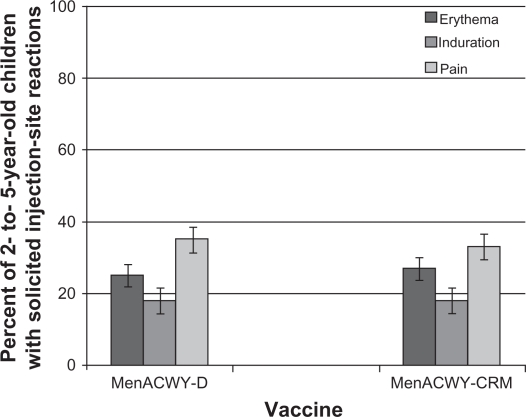
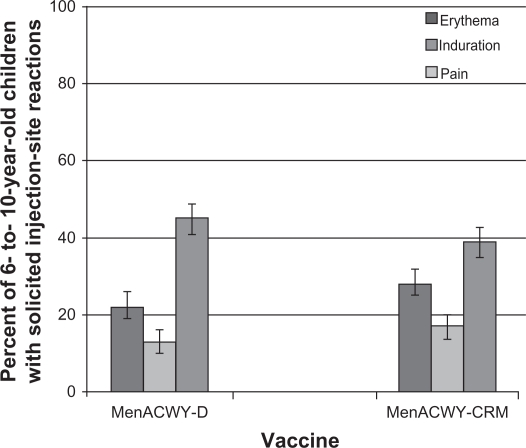
In a Phase III study conducted in 2907 children aged 2–10 years in the US and Canada, 61%–74% of vaccinees had evidence of seroresponse (a four-fold rise for those with hSBA titers ≥4 at baseline or a titer ≥8 for those with titers <4 at baseline) following a single dose of MenACWY-CRM (see Figure 1). These values were significantly higher compared with the MenACWY-D comparator for serogroups C, W-135, and Y. In cohorts of children identified by age strata (2–5 years and 6–10 years), a higher proportion of MenACWY-D recipients had a seroresponse to sero-group A compared with MenACWY-CRM, while responses to serogroups C, W-135, and Y met predefined criteria for noninferiority. Injection site reactions were reported in 17%–39% of children receiving MenACWY-CRM, as were systemic reactions in 2%–14%. The safety profile of MenACWY-CRM was generally similar to that observed with MenACWY-D (see Figures 2 and 3).57
Figure 1.
Percent of children aged 2–10 years with seroresponse 30 days following a single dose of MenACWY-D or MenACWY-CRM.
Figure 2.
Percent of children aged 2–5 years reporting solicited injection site reactions within the 7 days following a single dose of MenACWY-D (n = 684) or MenACWY-CRM (n = 693).
Figure 3.
Percent of children 6–10 years reporting solicited injection site reactions within the 7 days following a single dose of MenACWY-D (n = 571) or MenACWY-CRM (n = 582).
Clinical data in adolescents and adults
In all published studies in adolescents, MenACWY-CRM induced robust immune responses to serogroups A, C, W-135, and Y, and was generally safe and well tolerated.4–7,58–62 Persistence of immunogenicity has been observed for periods of up to 5 years in clinical trials.63
In a study in 524 adolescents, more MenACWY-CRM recipients had hSBA titers ≥4 against serogroups A, C, and Y compared with recipients of MPSV4, and more of those in the conjugate vaccine group also had evidence of an enduring immune response 12 months following vaccination when compared with the unconjugated polysaccharide vaccine group.58
In a randomized study of 2180 adolescents who received MenACWY-CRM or MenACWY-D, robust immune responses were seen in both vaccine groups as assessed by the percentage of participants with hSBA titers ≥8 to each of the four serogroups. In addition, hSBA geometric mean titers to all serogroups were significantly higher among MenACWY-CRM recipients than among MenACWY-D recipients.59 An average of 22 months after vaccination, persistent immune effects for all serogroups were observed among adolescents who had received MenACWY-CRM.60,61
A study in 1620 healthy adolescents assessed concomitant use of MenACWY-CRM and Tdap (tetanus, reduced diphtheria, and three-component acellular pertussis vaccine; Boostrix™, GlaxoSmithKline, Rixensart, Belgium) and human papilloma virus vaccine (Gardasil™, Merck and Co, Whitehouse Station, NJ).62 Immune responses to MenACWY-CRM, human papilloma virus, tetanus, and pertussis toxoids were similar in cohorts who received Tdap and the human papilloma virus vaccine concomitantly with MenACWY-CRM197 or in a sequential dosing regimen. As expected, given the relationship between diphtheria toxoid and CRM197, antidiphtheria responses were somewhat augmented with concomitant administration of MenACWY-CRM and Tdap. Concomitant administration of Tdap with MenACWY-CRM and human papilloma virus vaccine resulted in slightly reduced antibody responses to filamentous hemagglutinin and pertactin, acellular pertussis antigens with no established correlate of protection.
Each of the quadrivalent meningococcal vaccines administered in the studies in adolescents was found to be generally well tolerated in the study population, as were the comparator vaccines in the Arguedas et al study.58–62 In each of these studies, the most commonly reported injection site reaction was pain and the most commonly reported systemic reaction was headache.
Clinical trials of MenACWY-CRM in adults included study participants ≤65 years of age.64,65 Robust immune responses to serogroups A, C, W-135, and Y were observed in the majority of adults who received MenACWY-CRM in these studies. Among the 1359 adults (aged 19–55 years) enrolled in a Phase III study in the US and randomized to receive MenACWY-CRM or MenACWY-D, participants in both vaccine groups had robust immune responses to sero-groups A, C, W-135, and Y. Geometric mean titers were significantly higher among MenACWY-CRM recipients than in MenACWY-D recipients for all four serogroups.64
Similar results were observed in adults enrolled into a Phase III study in Latin America. The majority of healthy adults ≤55 years of age had robust immune responses to Men-ACWY-CRM and MenACWY-D. In a cohort of 326 healthy adults aged 56–65 years, robust immune responses were observed following MenACWY-CRM. In fact, those who received MenACWY-CRM had post vaccination hSBA geometric mean titers that were 1.2–5.4-fold higher for all four serogroups when compared with those who received MPSV4, the only meningococcal vaccine licensed for use in this age group. As observed in adolescents, MenACWY-CRM was generally well tolerated in adults, with a similar safety profile to that of MenACWY-D or MPSV4.65
Taken together, these completed clinical trials in adolescents and adults support the general tolerability and robust immunogenicity of MenACWY-CRM in this population. Robust immunogenicity was observed even in adults aged 56–65 years, a population at increased risk of mortality due to IMD.58–65
Discussion
MenACWY-CRM represents an important advance in the prevention of meningococcal disease. In many regions, including the US, the Middle East, Africa, and Europe, conjugate meningococcal vaccines, including Men ACWY-D, MenACWY-CRM, and a novel serogroup A conjugate vaccine (MenAfriVac®) have replaced or are currently replacing unconjugated polysaccharide vaccines.66 The newest of these vaccines, MenAfriVac is a novel Men-A-TT conjugate vaccine developed specifically for the meningitis belt countries. It received prequalification by the World Health Organization and in December 2010, Burkina Faso became the first country to begin a nationwide campaign to introduce this novel vaccine through the Meningitis Vaccine Project.67 Given the presence of outbreak disease caused by serogroups W-135 and X in the meningitis belt, the epidemiology of disease in this region should be monitored carefully.9 Another recent development in meningococcal vaccines is the recent licensure of MenACWY-D for infants as young as 9 months of age.68
Further study is needed to establish the immunogenicity profile of meningococcal conjugate vaccines following repeated vaccination with unconjugated polysaccharide vaccines. This information is of interest because of the well established observations of hyporesponsiveness following multiple doses of plain (unconjugated) polysaccharide vaccines.69
Based on the published literature, the immunogenicity profile of MenACWY-CRM appears somewhat different from that of MenACWY-D, specifically because no published studies support the use of MenACWY-D in young infants or adults older than 55 years of age. The role of conjugation proteins is of interest in this context, and some studies show differential immunogenicity in Hib vaccines using diphtheria toxoid or CRM197 as carrier proteins.70 Insufficient published data exist to determine whether carrier proteins have a meaningful impact on the clinical efficacy of meningococcal vaccines.49,70,71 A recent study in toddlers who received a booster dose of combined Haemophilus influenzae type B and meningococcal serogroup C vaccine employing a tetanus toxoid protein carrier indicated that boosting was superior among children primed with tetanus toxoid-containing vaccines.72,73 Similar studies have not been performed for CRM conjugate vaccines.
In the UK, the institution of universal vaccination against meningococcal serogroup C met with concerns about the potential for serogroup replacement.1,3,9,18,74 Because the second most prevalent disease-causing serogroup in the UK at that time was B, for which no vaccine was available, these concerns were potentially serious. Carriage studies showed that no serogroup replacement in IMD occurred after the serogroup C vaccine campaigns.75,76 A small but marked increase in serogroup Y disease has been reported in the UK; however, the number of cases remains low at 59 in 2008–2009.77 A recent carriage study among university students indicated the potential for replacement of carriage strains in dormitory housing.78 Of note, increasing rates of serogroup Y disease have been reported in many locations worldwide.1–3,9,10
Meningococcal serogroup C conjugate vaccines reduce asymptomatic carriage, thus leading to indirect herd protection in the UK and the Netherlands after these countries mandated vaccination of infants, young children, and adolescents, who are an important reservoir for meningococcal carriage.14 Given this clinical experience, vaccination programs could include quadrivalent meningococcal vaccines for the very young, who, along with teenagers, have the highest risk of acquiring IMD. However, a reduction in carriage following universal conjugate vaccination policies47 has not been documented for additional serogroups. The first estimate of the effectiveness of MenACWY-D in the US was 80%–85%, but based only on direct protection because coverage rates were too low for herd protection.79 Broader vaccination recommendations following the approval of MenACWY-CRM, including a booster dose for adolescents at 16 years of age, may result in better coverage.49 It is well recognized that additional study is needed to evaluated the possible costs and benefits to be derived from universal adolescent immunization against IMD.
In the future, public health specialists and policymakers will consider available meningococcal vaccines, including MenACWY-CRM in various contexts, such as universal infant vaccination, booster vaccination in various age groups, and as a travel vaccine. These considerations will likely include an evaluation of the benefits and risks of all possible vaccination policies, as well as the costs involved. Of some interest is IMD caused by serogroup B, which is currently not vaccine-preventable in most regions. With the dramatic reduction of IMD cases because of the successful uptake of vaccination and herd protection, the political will to support booster vaccinations to continue a low carriage rate may be lacking in some countries. MenACWY-CRM, like other quadrivalent conjugate vaccines against IMD, promises to provide significant public health benefits, and the advent of vaccines that protect against serogroup B is also necessary to provide protection against the vast majority of cases worldwide. Further research and development efforts80–84 are under way to ameliorate the ongoing public health issue of IMD and to augment the benefits offered by existing vaccines such as MenACWY-CRM.
Table 2.
Overview of published clinical studies of MenACWY-CRM in children and adolescents
| Age group | Immunogenicity among MenACWY-CRM recipients | Safety and tolerability among MenACWY-CRM recipients |
|---|---|---|
| Infants from 2 months of age* | 60%–92% of two-dose and 81%–99% of three-dose recipients had protective hSBA titers (≥4)52 | Most common reactions were injection site redness and irritability52 |
| Infants from 6 months of age | 86%–100% of two-dose recipients had protective hSBA titers (≥4)54 | Most common reactions were injection site redness and irritability54 |
| Toddlers | 86%–100% of two-dose primed and 94%–100% of three-dose primed toddlers had protective hSBA titers (≥4)52 | Most common reactions were injection site redness and irritability52 |
| 65%–100% of unprimed toddlers had protective hSBA titers (≥4) after two doses54 | ||
| Children aged 2–10 years | 83%–95% of two-dose recipients had protective hSBA titers (≥4)56 | Most common reactions were injection site pain, headache, and irritability56 |
| Adolescents | 75%–96% of adolescents had protective hSBA titers (≥4)58,59 | Most common reactions were injection site pain and headache58,59 |
Note:
Used an adjuvant vaccine formulation.
Abbreviation: hSBA, human complement.
Footnotes
Disclosure
The authors are full-time employees of Novartis Vaccines and Diagnostics, a manufacturer of various meningococcal vaccines, including MenACWY-CRM.
References
- 1.Tan LK, Carlone GM, Borrow R. Advances in the development of vaccines against Neisseria meningitidis. N Engl J Med. 2010;362(16):1511–1520. doi: 10.1056/NEJMra0906357. [DOI] [PubMed] [Google Scholar]
- 2.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369(9580):2196–2210. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- 3.Stephens DS. Conquering the meningococcus. FEMS Microbiol Rev. 2007;31(1):3–14. doi: 10.1111/j.1574-6976.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 4.Cooper B, DeTora L, Stoddard J. Menveo®: a novel quadrivalent meningococcal CRM197 conjugate vaccine against serogroups A, C, W-135 and Y. Expert Rev Vaccines. 2011;10(1):21–33. doi: 10.1586/erv.10.147. [DOI] [PubMed] [Google Scholar]
- 5.Pace D. Quadrivalent meningococcal ACYW-135 glycoconjugate vaccine for broader protection from infancy. Expert Rev Vaccines. 2009;8(5):529–542. doi: 10.1586/erv.09.18. [DOI] [PubMed] [Google Scholar]
- 6.Pace D, Pollard AJ, Messonier NE. Quadrivalent meningococcal conjugate vaccines. Vaccine. 2009;27(Suppl 2):B30–B41. doi: 10.1016/j.vaccine.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Deeks ED. Meningococcal quadrivalent (serogroups A, C, W135, and Y) conjugate vaccine (Menveo): in adolescents and adults. BioDrugs. 2010;24(5):287–297. doi: 10.2165/11204790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Pollard AJ, Levin M. Vaccines for prevention of meningococcal disease. Pediatr Infect Dis J. 2000;19(4):333–344. doi: 10.1097/00006454-200004000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;27(Suppl 2):B51–B63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 10.Harrison LH. Epidemiological profile of meningococcal disease in the United States. Clin Infect Dis. 2010;50(Suppl 2):S37–S44. doi: 10.1086/648963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344(18):1378–1388. doi: 10.1056/NEJM200105033441807. [DOI] [PubMed] [Google Scholar]
- 12.Olcén P, Fredlund H. Isolation and characterization of Neisseria meningitidis in the vaccine era. Who needs what and when? Scand J Infect Dis. 2010;42(1):4–11. doi: 10.3109/00365540903311177. [DOI] [PubMed] [Google Scholar]
- 13.Yazdankhah SP, Caugant DA. Neisseria meningitidis: an overview of the carriage state. J Med Microbiol. 2004;53(Pt 9):821–832. doi: 10.1099/jmm.0.45529-0. [DOI] [PubMed] [Google Scholar]
- 14.Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(12):853–861. doi: 10.1016/S1473-3099(10)70251-6. [DOI] [PubMed] [Google Scholar]
- 15.Hill DJ, Griffiths NJ, Borodina E, Virji M. Cellular and molecular biology of Neisseria meningitidis colonization and invasive disease. Clin Sci (Lond) 2010;118(9):547–564. doi: 10.1042/CS20090513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson MJ, Ninis N, Perera R, et al. Clinical recognition of meningococcal disease in children and adolescents. Lancet. 2006;367(9508):397–403. doi: 10.1016/S0140-6736(06)67932-4. [DOI] [PubMed] [Google Scholar]
- 17.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner P. Clinical practice. Prevention of meningococcal disease. N Engl J Med. 2006;355(14):1466–1473. doi: 10.1056/NEJMcp063561. [DOI] [PubMed] [Google Scholar]
- 19.Kimmel SR. Prevention of meningococcal disease. Am Fam Physician. 2005;72(10):2049–2056. [PubMed] [Google Scholar]
- 20.Edmond K, Clark A, Korczak VS, et al. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(5):317–328. doi: 10.1016/S1473-3099(10)70048-7. [DOI] [PubMed] [Google Scholar]
- 21.Frank ST, Gomez RM. Chronic meningococcemia. Mil Med. 1968;133(11):918–920. [PubMed] [Google Scholar]
- 22.Sullivan TD, LaScolea LJ., Jr Neisseria meningitidis bacteremia in children: quantitation of bacteremia and spontaneous clinical recovery without antibiotic therapy. Pediatrics. 1987;80(1):63–67. [PubMed] [Google Scholar]
- 23.Levy C, Taha MK, Weil Olivier C, et al. Association of meningococcal phenotypes and genotypes with clinical characteristics and mortality of meningitis in children. Pediatr Infect Dis J. 2010;29(7):618–623. doi: 10.1097/INF.0b013e3181d3ce32. [DOI] [PubMed] [Google Scholar]
- 24.Brigham KS, Sandora TJ. Neisseria meningitidis: epidemiology, treatment and prevention in adolescents. Curr Opin Pediatr. 2009;21(4):437–443. doi: 10.1097/MOP.0b013e32832c9668. [DOI] [PubMed] [Google Scholar]
- 25.Ramakrishnan M, Ulland AJ, Steinhardt LC, et al. Sequelae due to bacterial meningitis among African children: a systematic literature review. BMC Med. 2009;7:47. doi: 10.1186/1741-7015-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohn AC, MacNeil JR, Harrison LH, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: Implications for prevention of meningococcal disease. Clin Infect Dis. 2010;50(2):184–191. doi: 10.1086/649209. [DOI] [PubMed] [Google Scholar]
- 27.Hershey JH, Hitchcock W. Epidemiology and meningococcal serogroup distribution in the United States. Clin Pediatr(Phila) 2010;49(6):519–524. doi: 10.1177/0009922809347797. [DOI] [PubMed] [Google Scholar]
- 28.Ines Agudelo C, Sanabria OM, Ovalle MV. Serogroup Y meningococcal disease, Columbia. Emerg Infect Dis. 2008;14(6):990–991. doi: 10.3201/eid1406.071357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Efron AM, Sorhouet C, Salcedo C, et al. W135 invasive meningococcal strains spreading in South America: significant increase incidence rate in Argentina. J Clin Micro biol. 2009;47(6):1979–1980. doi: 10.1128/JCM.02390-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilic A, Urwin R, Li H, et al. Clonal spread of serogroup W135 meningococcal disease in Turkey. J Clin Micro biol. 2006;44(1):222–224. doi: 10.1128/JCM.44.1.222-224.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Gaudio M, Chiappini E, Galli L, De Martino M. Therapeutic management of bacterial meningitis in children: a systematic review and comparison of published guidelines from a European perspective. J Che mother. 2010;22(4):226–237. doi: 10.1179/joc.2010.22.4.226. [DOI] [PubMed] [Google Scholar]
- 32.Bilukha OO, Rosenstein N, National Center for Infectious Diseases, Centers for Disease Control and Prevention Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2005;54(RR-7):1–21. [PubMed] [Google Scholar]
- 33.Fraser A, Gafter-Gvili A, Paul M, Leibovici L. Antibiotics for preventing meningococcal infections. Cochrane Database Syst Rev. 2006;4:CD004785. doi: 10.1002/14651858.CD004785.pub3. [DOI] [PubMed] [Google Scholar]
- 34.Rao D, Hamilton E, Glennie L, et al. Should long-haul flights carry antibiotics on board to treat acute bacterial meningitis and meningococcal septicaemia? Br J Biomed Sci. 2008;65(4):201–202. doi: 10.1080/09674845.2008.11978130. [DOI] [PubMed] [Google Scholar]
- 35.Memish ZA, Goubeaud A, Bröker M, Malerczyk C, Shibl AM. Invasive meningococcal disease and travel. J Infect Public Health. 2010;3(4):143–151. doi: 10.1016/j.jiph.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Stefanelli P. Emerging resistance in Neisseria meningitidis and Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2011;9(2):237–244. doi: 10.1586/eri.10.171. [DOI] [PubMed] [Google Scholar]
- 37.Katz LH, Zelazny A, Scharf S, et al. Mass antibiotic treatment to stop an outbreak of meningococcal disease: a molecular analysis. Clin Micro biol Infect. 2007;13(9):943–946. doi: 10.1111/j.1469-0691.2007.01767.x. [DOI] [PubMed] [Google Scholar]
- 38.Vázquez JA, Enriquez R, Abad R, et al. Antibiotic resistant meningococci in Europe: any need to act? FEMS Microbiol Rev. 2007;31(1):64–70. doi: 10.1111/j.1574-6976.2006.00049.x. [DOI] [PubMed] [Google Scholar]
- 39.Pană M, Ghiţă M, Levenet I, et al. The in vitro susceptibility to 7 antibiotics of Neisseria meningitidis strains isolated last years in Romania. Roum Arch Microbiol Immunol. 2009;68(1):38–43. [PubMed] [Google Scholar]
- 40.Taha MK, Vázquez JA, Hong E, et al. Target gene sequencing to characterize the penicillin G susceptibility of Neisseria meningitidis. Antimicrob Agents Chemother. 2007;51(8):2784–2792. doi: 10.1128/AAC.00412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hossain MA, Ahmed D, Ahmed T, et al. Increasing isolations of Neisseria meningitides serogroup A from blood and cerebrospinal fluid in Dhaka, Bangladesh, 1999–2006. Am J Trop Med Hyg. 2009;80(4):615–618. [PubMed] [Google Scholar]
- 42.Donaldson AD, Tang WY, Tan AL, Barkham T. Neisseria meningitidis with reduced susceptibility to quinolones in Singapore. J Anti microb Che mother. 2010;65(2):362–364. doi: 10.1093/jac/dkp437. [DOI] [PubMed] [Google Scholar]
- 43.Nair D, Dawar R, Deb M, et al. Outbreak of meningococcal disease in and around New Delhi, India, 2005–2006: a report from a tertiary care hospital. Epidemiol Infect. 2009;137(4):570–576. doi: 10.1017/S0950268808001398. [DOI] [PubMed] [Google Scholar]
- 44.Wu HM, Harcourt BH, Hatcher CP, Messonnier NE, Mayer LW, Lynfield R. Emergence of ciprofloxacin-resistant Neisseria meningitidis in North America. N Engl J Med. 2009;360(9):886–892. doi: 10.1056/NEJMoa0806414. [DOI] [PubMed] [Google Scholar]
- 45.Du Plessis M, von Gottberg A, Cohen C, et al. Group for Enteric Respiratory and Meningeal Disease Surveillance in South Africa (GERMS-SA) Neisseria meningitidis intermediately resistant to penicillin and causing invasive disease in South Africa in 2001 to 2005. J Clin Micro biol. 2008;46(10):3208–3214. doi: 10.1128/JCM.00221-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lapadula G, Viganò F, Fortuna P, et al. Imported ciprofloxacin-resistant Neisseria meningitidis. Emerg Infect Dis. 2009;15(11):1852–1854. doi: 10.3201/eid1511.090833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maiden MC, Ibarz-Pavón AB, Urwin R, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis. 2008;197(5):737–743. doi: 10.1086/527401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bettinger JA, Scheifele DW, Le Saux N, et al. The impact of childhood meningococcal serogroup C conjugate vaccine programs in Canada. Pediatr Infect Dis J. 2009;28(3):220–224. doi: 10.1097/INF.0b013e31819040e7. [DOI] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention Licensure of a meningococcal conjugate vaccine (Menveo) and guidance for use – Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(9):273. [PubMed] [Google Scholar]
- 50.Bröker M, Dull PM, Rappuoli R, Costantino P. Chemistry of a new investigational quadrivalent meningococcal conjugate vaccine that is immunogenic at all ages. Vaccine. 2009;27(41):5574–5580. doi: 10.1016/j.vaccine.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 51.Snape MD, Perrett KP, Ford KJ, et al. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA. 2008;299(2):173–184. doi: 10.1001/jama.2007.29-c. [DOI] [PubMed] [Google Scholar]
- 52.Vesikari T, Ceddia F, Karvonen A, et al. Immune response and immunological memory induced by a novel meningococcal ACWY-CRM conjugate vaccine (MenACWY) in toddlers. Presented at the 23rd annual meeting of the European Society for Paediatric Infectious Diseases; Valencia, Spain. May 18–20, 2005. [Google Scholar]
- 53.Halperin SA, Diaz-Mitoma F, Dull P, et al. Safety and immunogenicity of an investigational quadrivalent meningococcal conjugate vaccine after one or two doses given to infants and toddlers. Eur J Clin Microbiol Infect Dis. 2010;29(3):259–267. doi: 10.1007/s10096-009-0848-8. [DOI] [PubMed] [Google Scholar]
- 54.Klein N, Gill CJ, Bedell L, et al. Pivotal safety and immunogenicity of an investigational quadrivalent meningococcal vaccine (MenACWY-CRM; Menveo®) in 4545 infants. Presented atthe annual meeting of the Infectious Diseases Society of America; Vancouver, Canada. October 21–24, 2010. [Google Scholar]
- 55.Klein N, Gill CJ, Bedell L, et al. Pivotal safety and immunogenicity of an investigational quadrivalent meningococcal vaccine (Men ACWY-CRM; Menveo®) ininfants in the United States. Presented at the annual meeting of the Pediatric Academic Societies; Denver, CO. April 30–May 3, 2011. [Google Scholar]
- 56.Black S, Klein NP, Shah J, et al. Immunogenicity and tolerability of a quadrivalent meningococcal glycoconjugate vaccine in children 2–10 years of age. Vaccine. 2010;28(3):657–663. doi: 10.1016/j.vaccine.2009.10.104. [DOI] [PubMed] [Google Scholar]
- 57.Halperin SA, Gupta A, Jeanfreau R, et al. Comparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2–10 years of age. Vaccine. 2010;28(50):7865–7872. doi: 10.1016/j.vaccine.2010.09.092. [DOI] [PubMed] [Google Scholar]
- 58.Jackson LA, Jacobson RM, Reisinger KS, et al. A randomized trial to determine the tolerability and immunogenicity of a quadrivalent meningococcal glycoconjugate vaccine in healthy adolescents. Pediatr Infect Dis J. 2009;28(2):86–91. doi: 10.1097/INF.0b013e31818a0237. [DOI] [PubMed] [Google Scholar]
- 59.Jackson LA, Baxter R, Reisinger K, et al. Phase III comparison of an investigational quadrivalent meningococcal conjugate vaccine with the licensed meningococcal ACWY conjugate vaccine in adolescents. Clin Infect Dis. 2009;49(1):e1–e10. doi: 10.1086/599117. [DOI] [PubMed] [Google Scholar]
- 60.Gill CJ, Baxter R, Anemona A, Ciavarro GL, Dull PM. Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo®) or Menactra® among healthy adolescents. Hum Vaccin. 2010;6(11):881–887. doi: 10.4161/hv.6.11.12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gill CJ, Anemona A, Ciavarro G, et al. Persistence of bactericidal activity in adolescents 12 and 21 months following receipt of meningococcal vaccines against serogroups A, C, W-135, and Y. Presented at the 28th annual meeting of the European Society for Paediatric Infectious Diseases; Nice, France. May 4–8, 2010. [Google Scholar]
- 62.Arguedas A, Soley C, Loaiza C, et al. Safety and immuno genicity of one dose of Men ACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, when administered to adolescents concomitantly or sequentially with Tdap and HPV vaccines. Vaccine. 2010;28(18):3171–3179. doi: 10.1016/j.vaccine.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 63.Jackson L, Reisinger K, Gill CJ, et al. Persistence of immune responses and boosting in adolescents five years after receiving Men ACWY-CRM or Men ACWY-D. Presented at the annual meeting of the Pediatric Academic Societies; Denver, CO. April 30–May 3, 2011. [Google Scholar]
- 64.Reisinger KS, Baxter R, Block SL, et al. Quadrivalent meningococcal vaccination of adults: phase III comparison of an investigational conjugate vaccine, MenACWY-CRM, with the licensed vaccine, Menactra. Clin Vaccine Immunol. 2009;16(12):1810–1815. doi: 10.1128/CVI.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stamboulian D, Lopardo G, Lopez P, et al. Safety and immunogenicity of an investigational quadrivalent meningococcal CRM197 conjugate vaccine, MenACWY-CRM, compared with licensed vaccines in adults in Latin America. Int J Infect Dis. 2010;14(10):e868–e875. doi: 10.1016/j.ijid.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 66.Memish ZA, Shibl AM. Consensus building and recommendations based on the available epidemiology of meningococcal disease in Gulf Cooperation Council States. Travel Med Infect Dis. 2011;9(2):60–66. doi: 10.1016/j.tmaid.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Kristiansen PA, Diomandé F, Wei SC, et al. Baseline meningococcal carriage in Burkina Faso before the introduction of a meningococcal serogroup A conjugate vaccine. Clin Vaccine Immunol. 2011;18(3):435–443. doi: 10.1128/CVI.00479-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Menactra®.[Package insert] Swift water, PA: Sanofi Pasteur Inc; Apr, 2011. [Google Scholar]
- 69.Poolman J, Borrow R. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Exp Rev Vaccines. 2011;10(3):307–322. doi: 10.1586/erv.11.8. [DOI] [PubMed] [Google Scholar]
- 70.Burrage M, Robinson A, Borrow R, et al. Effect of vaccination with carrier protein on response to meningococcal C conjugate vaccines and value of different immunoassays as predictors of protection. Infect Immun. 2002;70(9):4946–4954. doi: 10.1128/IAI.70.9.4946-4954.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knuf M, Kowalzik F, Kieninger D. Comparative effects of carrier proteins on vaccine-induced immune response. Vaccine. 2011 May 4; doi: 10.1016/j.vaccine.2011.04.053. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 72.Pace D, Snape M, Westcar S, et al. A novel combined Hib-MenC-TT glycoconjugate vaccine as a booster dose for toddlers: a phase 3 open randomised controlled trial. Arch Dis Child. 2008;93(11):963–970. doi: 10.1136/adc.2007.136036. [DOI] [PubMed] [Google Scholar]
- 73.Vesikari T, Karvonen A, Bianco V, Van der Wielen M, Miller J. Tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine is well tolerated and immunogenic when co-administered with measles-mumps-rubella-varicella vaccine during the second year of life: an open, randomized controlled trial. Vaccine. 2011 Apr 4; doi: 10.1016/j.vaccine.2011.03.043. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 74.Campbell H, Andrews N, Borrow R, et al. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol. 2010;17(5):840–847. doi: 10.1128/CVI.00529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maiden MCJ, Stuart JM. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet. 2002;359(9320):1829–1830. doi: 10.1016/S0140-6736(02)08679-8. [DOI] [PubMed] [Google Scholar]
- 76.Balmer P, Borrow R, Miller E. Impact of meningococcal C conjugate vaccine in the UK. J Med Micro biol. 2002;51(9):717–722. doi: 10.1099/0022-1317-51-9-717. [DOI] [PubMed] [Google Scholar]
- 77.Health Protection Agency Health Protection Report. Available at: http://www.hpa.org.uk/hpr/archives/2011/hpr1611.pdf. Accessed May 25, 2011.
- 78.Bidmos FA, Neal KR, Oldfield NJ, Turner DP, Ala’Aldeen DA, Bayliss CD. Persistence, replacement, and rapid clonal expansion of meningococcal carriage isolates in a 2008 university student cohort. J Clin Microbiol. 2011;49(2):506–512. doi: 10.1128/JCM.01322-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.MacNeil JR, Cohn AC, Zell ER, et al. Early estimate of the effectiveness of quadrivalent meningococcal conjugate vaccine. Ped Infect Dis J. 2011;30(6):451–455. doi: 10.1097/INF.0b013e31820a8b3c. [DOI] [PubMed] [Google Scholar]
- 80.Kimura A, Toneatto D, Kleinschmidt A, et al. Immunogenicity and safety of a multicomponent meningococcal serogroup B vaccine and a quadrivalent meningococcal CRM197 conjugate vaccine against serogroups A, C, W-135, and Y in adults who are at increased risk for occupational exposure to meningococcal isolates. Clin Vaccine Immunol. 2011;18(3):483–486. doi: 10.1128/CVI.00304-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Findlow J, Borrow R, Snape MD, et al. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis. 2010;51(10):1127–1137. doi: 10.1086/656741. [DOI] [PubMed] [Google Scholar]
- 82.Snape MD, Dawson T, Oster P, et al. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr Infect Dis J. 2010;29(11):e71–e79. doi: 10.1097/INF.0b013e3181f59f6d. [DOI] [PubMed] [Google Scholar]
- 83.Bambini S, Muzzi A, Olcen P, et al. Distribution and genetic variability of three vaccine components in a panel of strains representative of the diversity of serogroup B meningococcus. Vaccine. 2009;27(21):2794–2803. doi: 10.1016/j.vaccine.2009.02.098. [DOI] [PubMed] [Google Scholar]
- 84.Giuliani MM, Adu-Bobie J, Comanducci M, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A. 2006;103(29):10834–10839. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]