Abstract
The formation of the dorsoventral axis of the Drosophila embryo depends on cell–cell interactions that take place in the female ovary and involve the activation of transmembrane receptors by secreted ligands. The gene windbeutel functions in the somatic follicle cells of the ovary and is required for the generation of a signal that will determine the ventral side of the embryo. This signal originates in the follicle cells during oogenesis, but its actions are only manifested after fertilization, when the egg has already been laid. We have performed a molecular analysis of windbeutel. We have found that windbeutel encodes a putative resident protein of the endoplasmic reticulum, and has homologs in rats and humans. The gene is expressed for a brief period of time in the follicle cells of the ovary, at around the time when the dorsoventral axis of the egg chamber is first established. We propose that Windbeutel is responsible for the folding and/or modification of a specific factor that is secreted from the follicle cells and participates in the activation of the ventralizing signal.
Keywords: Drosophila, pattern formation, oogenesis, endoplasmic reticulum, secretion
In Drosophila, the establishment of dorsoventral polarity occurs during oogenesis and requires communication between the germ-line-derived oocyte and the somatically derived follicle cells of the ovary. Initially, the oocyte sends a dorsalizing signal to the follicle cells. This signal is received by the follicle cells via Egfr, the Drosophila homolog of the human epidermal growth factor receptor (Wadsworth et al. 1985; Price et al. 1989; Schejter and Shilo 1989). Egfr, which is expressed in the follicle cells, is believed to be locally activated by Gurken (Grk), a TGFα type molecule, produced by the oocyte (Neuman-Silberberg and Schüpbach 1993). Egfr activation by Grk takes place on the dorsal side of the follicular epithelium and regulates a second process of cell communication, relating dorsoventral information from the follicle cells back to the egg. Ultimately, the signal from the follicle cells leads to the specification of the dorsoventral axis of the embryo.
This second signaling process from the follicle cells to the embryo requires the action of 11 genes that belong to the dorsal group of maternal-effect genes. Loss-of-function mutations in these genes lead to a dorsalization of the embryo. According to current models, the signal from the follicle cells results in the activation of the Toll receptor only on the ventral side of the egg, and ultimately leads to the formation of the nuclear gradient of the transcription factor Dorsal (for review, see Morisato and Anderson 1995). The activation of the Toll receptor is mediated by the product of the gene spätzle, whose cleaved, active form is thought to be present only on the ventral side of the embryo (Morisato and Anderson 1994; Schneider et al. 1994). Spätzle is itself activated by processing through a proteolytic cascade of serine proteases encoded by the genes gastrulation defective, snake, and easter.
Three of the dorsal group genes, windbeutel (wind), nudel, and pipe, have been shown by genetic mosaic experiments to be required in the somatic follicle cells rather than in the germ line (Schüpbach et al. 1990; Stein et al. 1991). These genes are therefore candidates for encoding proteins that may directly produce the ventral signal in the follicle cells. The gene nudel has been cloned and shown to encode a modular protein with an extracellular matrix domain and a serine protease domain (Hong and Hashimoto 1995). It has been suggested that Nudel is secreted by the follicle cells and may possibly be incorporated in the vitelline membrane, thus specifying the site of generation of the active Spätzle ligand, after fertilization of the oocyte (Hong and Hashimoto 1995). This idea of a localized protease would fit well with the model of proteolytic activation of the ligand for the Toll receptor by sequential processing of a series of serine protease pro-enzymes (for review, see Morisato and Anderson 1995).
The gene Egfr is also required in the somatic follicle cells, and when mutant, causes the generation of ventralized phenotypes in eggs and embryos. Epistasis experiments have shown that the Grk–Egfr pathway negatively regulates the production of the ventralizing signal in the follicle cells, although the exact nature of this interaction remains to be shown (Schüpbach 1987; Roth and Schüpbach 1994). In particular, double mutants between Egfr and wind give rise to dorsalized progeny (Schüpbach et al. 1990). Therefore, it appears that wind acts downstream of Egfr, and that the Grk–Egfr signaling may directly, or indirectly, be responsible for the restriction of Wind activity to the ventral side of the follicular epithelium.
The follicle cells form a single-cell epithelial layer that surrounds the nurse cells and the developing oocyte. Their role during oogenesis is multifunctional as they are responsible for the secretion of both structural components of the egg, like the chorion, vitelline membrane, and yolk proteins (for review, see Spradling 1993), and regulatory molecules like Nudel (Hong and Hashimoto 1995) or Torso-like (Stevens et al. 1990; Savant-Bhonsale and Montell 1993), that participate in the establishment of embryonic polarity (for review, see Ray and Schüpbach 1996). Thus, the follicle cells form a population of cells highly secretory in nature and are, in addition, the recipients and transmitters of extracellular signals.
To elucidate the mechanism by which the follicle cells provide ventral information, we have undertaken the molecular analysis of the gene wind. We show that wind encodes a protein that is a putative resident of the endoplasmic reticulum (ER). It is well documented that the ER plays a role in the translocation, folding, and degradation of membrane bound and secreted proteins (for review, see Rapoport et al. 1996). Our findings lead us to propose that the product of the gene wind is specifically involved in the modification and/or folding of a factor secreted by the follicle cells over the ventral side of the oocyte. This factor is then responsible for the activation of the proteolytic cascade, and possibly for its restriction to the ventral side of the egg. The cascade will release the active Spätzle ligand that will subsequently bind and activate the Toll receptor. The proposed model is based on the temporal expression pattern of wind as well as on the structural motifs present on the putative Wind protein.
Results
Phenotypic analysis of the gene wind
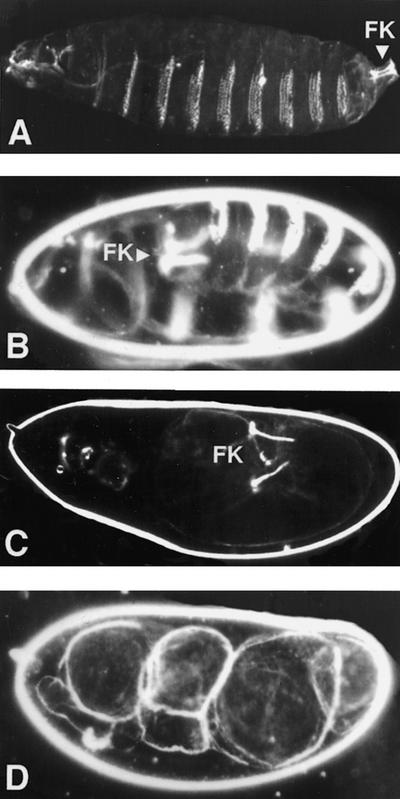
The gene wind was identified in a screen for female sterile mutations (Schüpbach and Wieschaus 1989). Females homozygous for wind mutations produce eggs that have normal morphology but give rise to embryos that are dorsalized. At the end of embryogenesis, such embryos secrete a dorsalized cuticle, which is characterized by the absence of ventral structures such as ventral denticle bands, and the expansion of dorsal epidermal structures. The severity of the cuticle phenotype varies depending on the allelic combination, ranging from weakly dorsalized (D2), to strongly dorsalized (D0) (Fig. 1; for a definition of classes of dorsalization, see Anderson et al. 1985). The maternal defect, caused by wind mutations, cannot be rescued zygotically.
Figure 1.
Embryonic cuticles. (A) Wild type embryo with normal head structures, three thoracic segments, eight abdominal segments carrying ventral denticle belts and telson [Filzkörper (FK)]. (B) Weakly dorsalized embryo (D2), in which development has arrested at the extended germ-band stage, causing the production of an elongated, u-shaped embryo with Filzkörper (FK) on the dorsal side. (C) More dorsalized embryo (D1), still exhibiting Filzkörper structures. (D) Extremely dorsalized embryo (D0). All embryos are shown inside the vitelline membrane.
We have characterized the existing six EMS induced wind alleles in trans to deficiencies that remove the gene and also in all possible trans-heterozygous combinations (Table 1). Five of these alleles show reduced viability in adults, surviving at a rate ranging from 8%-62% of the expected progeny. Females homozygous for these alleles give rise to strongly dorsalized progeny, 100% of which show the mutant phenotype. The sixth allele (windAR51) appears weaker because it shows better viability in trans to the rest of the alleles and to the deficiency, and gives rise to milder dorsalized phenotypes (Table 1).
Table 1.
Complementation analysis of wind mutations
| Alleles
|
Percent viability
|
Percent hatch
|
Phenotype of unhatched progeny (%)
|
||
|---|---|---|---|---|---|
| D0
|
D1
|
D2
|
|||
| RP54/M46 | 8 | 0 | 100 | ||
| RP54/M88 | 13 | 0 | 100 | ||
| RP54/T6 | 27 | 0 | 100 | ||
| RP54/E4 | 16 | 0 | 100 | ||
| RP54/AR51 | 32 | 0 | 60 | 40 | |
| M46/M88 | 14 | 0 | 100 | ||
| M46/T6 | 60 | 0 | 100 | ||
| M46/E4 | 43 | 0 | 100 | ||
| M46/AR51 | 49 | 0 | 100 | ||
| M88/T6 | 24 | 0 | 100 | ||
| M88/E4 | 40 | 0 | 100 | ||
| M88/AR51 | 62 | ∼99 | N.A. | N.A. | N.A. |
| T6/E4 | 19 | 0 | 100 | ||
| T6/AR51 | 32 | 0 | 50 | 50 | |
| E4/AR51 | 33 | 0 | 100 | ||
| AR51/AR51 | 100 | 0 | 100 | ||
| RP54/DfP34 | 47 | 0 | 100 | ||
| M46/DfP34 | 31 | 0 | 100 | ||
| M88/DfP34 | 30 | 0 | 100 | ||
| T6/DfP34 | 47 | 0 | 100 | ||
| E4/DfP34 | 18 | 0 | 100 | ||
| AR51/DfP34 | 58 | 95 | 100 | ||
(N.A.) Not applicable.
The lethal phase for three of the stronger alleles (windRP54, windM88, and windT6) was determined to be between the second and third instar larval stages. This suggests that wind is also required zygotically, and functions in at least one other stage of development.
Molecular analysis of the wind locus
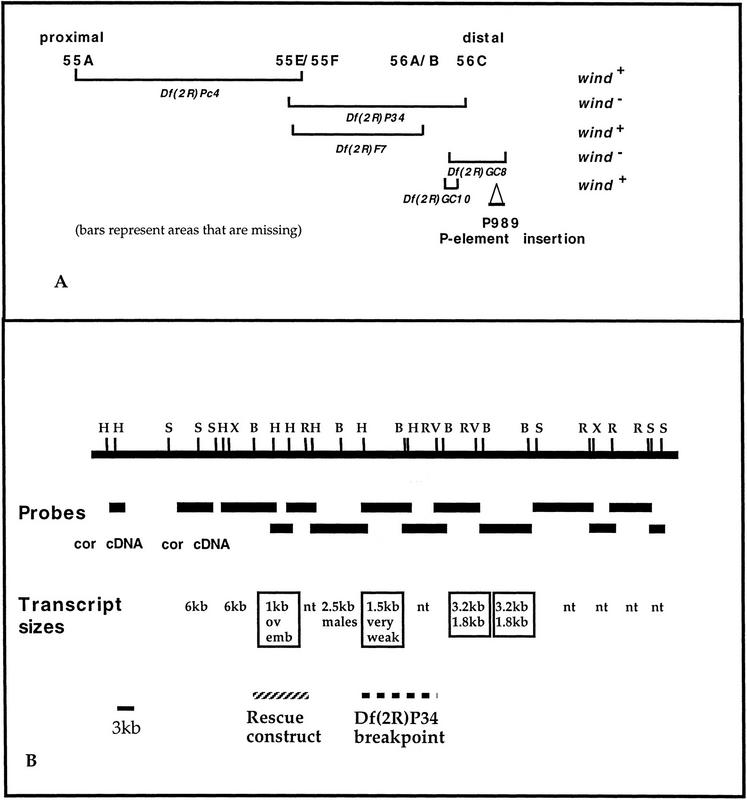
By meiotic mapping, wind was positioned at 2–86 on the second chromosome (Schüpbach and Wieschaus 1989). Complementation tests, using various chromosomal deficiencies, showed that wind is uncovered by the Df(2R)P34, which spans the region 55E–56C and by the Df(2R)GC8, which maps in the proximal 56C region and uncovers the coracle (cor) locus (Fehon et al. 1994). The results of the deficiency analysis are shown in Figure 2A.
Figure 2.
Analysis of the genomic region containing wind. (A) Deficiency mapping of the 55A-56C area and complementation analysis of wind. The P-element insertion P989 is also indicated. (B) Schematic representation of the genomic walk in the 56C area. The total size of the area depicted is ∼90 kb. Black horizontal bars below the solid line represent the fragments that were used as probes in the reverse Northern and Northern analyses. Beneath the probes, the sizes of transcripts that were detected are indicated. (nt) No transcript. The 6-kb transcript corresponds to the cor transcription unit and the 2.5-kb transcript was present only in RNA from male flies. Boxed are the transcripts that were expressed in ovaries. Also shown are the fragments used in the rescue construct (hatched line), and the area where the distal breakpoint of deficiency Df(2R)P34 maps (thick broken line).
Using the P-element insertion P989 (Karpen and Spradling 1992), we were able to determine that Df(2R)GC8 extends more distally than Df(2R)P34 (Fig. 2A). In addition, we created wild-type chromosomes by recombination between cor1 and windRP54, and with the help of flanking markers, showed that wind maps distal to cor. We tested a total of 60,000 chromosomes and found that three independent recombination events gave rise to wild-type chromosomes. The distance between the two genes was calculated by the frequency of the single recombinations and was estimated to be 0.01 cM (Ashburner 1989). On the basis of these calculations, we concluded that the wind gene should lie very close to coracle.
We generated a chromosomal walk on a λ phage genomic library, using as probes a cDNA fragment from the cor gene for the proximal end and a genomic fragment adjacent to the P-element insertion P989 for the distal end (see Materials and Methods). Part of the genomic area represented in the chromosomal walk is covered by overlapping fragments from a P1 phage (DM02733) that we mapped in the relevant area. The chromosomal walk covers a total of 90 kb of genomic DNA (Fig. 2B). The distal breakpoint of Df(2R)P34 was also mapped by Southern analysis (Fig. 2B).
To identify the transcription units that are present in the candidate genomic area, we performed reverse Northern and Northern analyses, by use of poly(A)+ RNA from ovaries. Three candidate transcription units were detected, shown boxed in Figure 2B. All of these transcripts were expressed in ovaries, as determined by Northern analysis. In situ RNA hybridization analysis on ovaries, by use of genomic DNA fragments corresponding to these transcripts as probes, was also performed. The 3.2-/1.8-kb set of alternatively spliced transcripts was found to hybridize predominantly to RNA in the nurse cells. The 1.5-kb transcript gave no detectable signal on ovaries. Given that the Northern hybridization signal from this particular transcript was extremely low, it is possible that in situ hybridization was not sensitive enough to detect it. Finally, the 1-kb transcript was expressed mainly in the follicle cells, thus it was selected as the most promising candidate for the wind gene.
Subsequently, a rescue construct was built, containing ∼6 kb of DNA (hatched bar in Fig. 2B), surrounding this 1-kb transcript. After standard P-element-mediated transformation (Spradling 1986), we obtained six independent transformed lines. Three of the lines, which had integrated the P-element in the first or third chromosome, were crossed into a wind mutant background and all three insertions were found to rescue the female sterility conferred by the wind mutation. wind mutant females carrying the transgene were also shown to be 100% viable, as compared with their siblings without the transgene. Thus, one copy of the the 1-kb transcript fully rescues the mutant phenotype, which shows that this transcript corresponds to the wind gene.
The wind gene is expressed during mid-oogenesis in the follicle cells
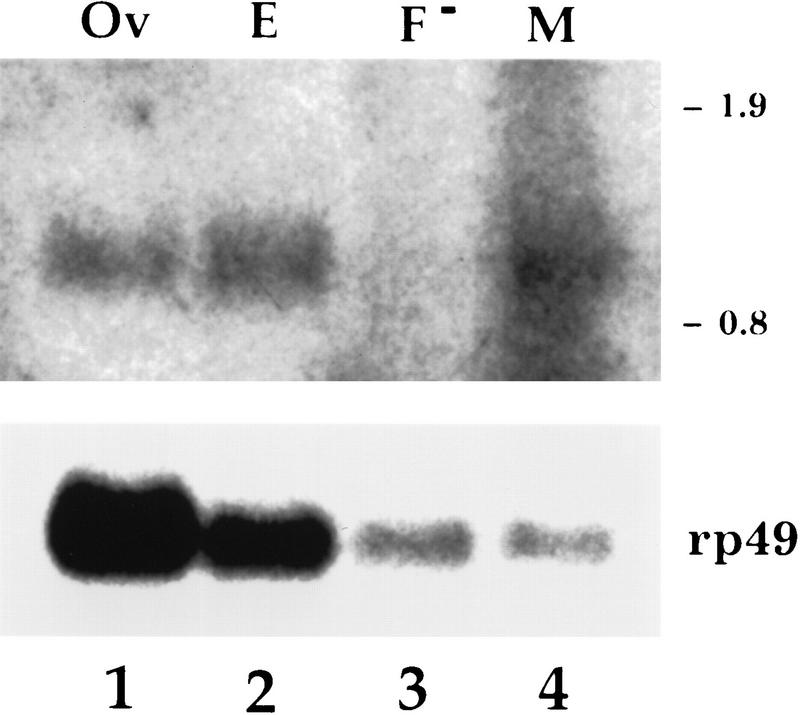
Expression of the wind transcript was tested by Northern blot analysis. The wind transcript is present in ovaries, early embryos (0–4 hr), and adult males but is almost undetectable in female carcasses (Fig. 3).
Figure 3.
Northern analysis of RNA from wild-type tissues. The probe used was the genomic fragment that hybridizes to the 1-kb transcript shown schematically in Fig. 2B. Poly(A)+-selected wild-type RNA was prepaped from ovaries (Ov), 0- to 4-hr embryos (E), females without their ovaries (F−), and males (M). RNA (15–18 μg) was loaded in lanes 1 and 2, 11 μg in lane 3, and 7 μg in lane 4. The same blot was also hybridized to the rp49 probe to provide a loading standard. The numbers at the right correspond to RNA molecular size standards.
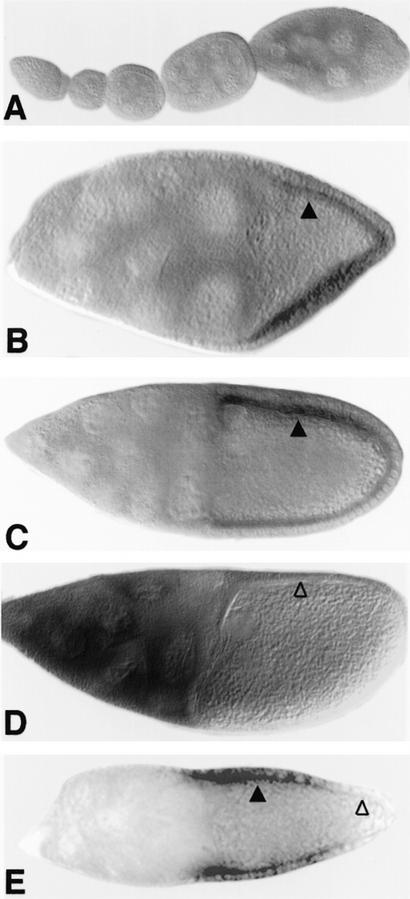
Previously, it has been shown by genetic mosaics that the activity of the gene wind is required in a somatic cell type, most likely in the follicle cells of the ovary (Schüpbach et al. 1990; Stein et al. 1991). To examine the expression pattern of wind in oogenesis, we performed RNA in situ hybridization on ovaries. Expression is not detected in the germarium or early stage egg-chambers (Fig. 4A) and is first detectable in the follicle cells of stage 8 egg-chambers. The peak of expression occurs in the follicle cells of stages 9 and early 10, and the transcript disappears completely before stage 11 (Fig. 4B–D). The germ-line cells (nurse cells and oocyte) do not express any wind RNA, with the possible exception of late stage 10 nurse cells.
Figure 4.
Expression and localization pattern of wind RNA in oogenesis. In situ hybridization on whole-mount ovaries was carried out by use of a wind antisense RNA probe corresponding to the PCR product of the 5′ half of the wind gene. Staining was visualized by an alkaline phosphatase secondary antibody. (A–D) Wild-type egg chambers. (E) Egg chamber mutant for the grk gene. (A) Egg chambers at stages 1–8, wind is not expressed. (B) Egg chamber in stage 9, wind RNA accumulates on the apical side of the follicle cells surrounding the oocyte (▴), it is not expressed in the follicle cells lying over the nurse cells. (C) Egg chamber at stage 10A, wind RNA still accumulates at the apical end of the follicle cells (▴). (D) Egg chamber at late stage 10B, wind RNA is no longer detected in the follicle cells (▵). There is some staining seen in the nurse cells at this stage. It should be noted, however, that nurse cells of that stage have been shown to absorb a great variety of different probes (Spradling 1993); thus, it is difficult to assess whether this staining corresponds to specific expression of wind. (E) In a grk mutant egg chamber, wind RNA is still expressed in the follicle cells that overlie the anterior part of the oocyte (▴). It is not expressed in the follicle cells overlying the posterior end of the oocyte (▵).
Careful examination of the wind expression pattern revealed that only the follicle cells that are located over the oocyte express the wind transcript, in contrast to the follicle cells covering the nurse cells (Fig. 4B). Within the columnar follicle cell epithelium, there does not seem to be any restriction of wind expression relative to the dorsoventral axis. Rather, all the follicle cells over the oocyte are uniformly expressing wind. Interestingly, the transcript appears to be concentrated in the apical cytoplasm of the follicle cells, which in the follicular epithelium is the side facing the oocyte. The functional significance of this localization has not yet been examined but we observed that in situ hybridization by use of two different probes (nudel and cor), which are also expressed in the follicle cells, did not show a similar pattern (data not shown).
We also performed RNA in situ analysis on ovaries from flies hemizygous for the different wind alleles (data not shown). wind RNA was detected in the four of the hemizygous mutant alleles, three of which give a strong dorsalized phenotype (windRP54, windT6, and windE4) and in the weak allele windAR51. In two of the alleles (windM88 and windM46), wind RNA expression was reduced to an almost undetectable level. Given the limitations of the in situ hybridization sensitivity, it is not possible to assess whether RNA expression is completely abolished in these mutants.
Expression of the wind transcript in mutants affecting the dorsoventral pattern
To examine whether wind RNA expression is affected by mutations in other genes involved in the establishment of dorsoventral polarity, we analyzed its expression in different mutant backgrounds. First we examined ovaries mutant for fs(1)K10, a gene that when mutant in females, produces dorsalized eggs and embryos (Wieschaus 1979). fs(1)K10 acts upstream of the dorsoventral Grk–Egfr signaling process (for review, see Ray andSchüpbach 1996). In ovaries mutant for fs(1)K10, the expression of wind RNA is not affected. Similarly, wind RNA expression was unaffected in ovaries mutant for nudel and pipe, the other two genes that act at the same step as wind in the dorsoventral pathway (data not shown).
In ovaries mutant for a strong gurken allele, wind expression was eliminated in all follicle cells around the posterior of the egg chamber (Fig. 4E). This observation can be explained by taking into account the earlier role of the Grk–Egfr signaling process in instructing posterior follicle cells, prior to its role in instructing dorsal follicle cells (Gonzales-Reyes and St Johnson 1995; Roth et al. 1995). The posterior signaling process takes place before stage 8 of oogenesis, and is required for posterior follicle cells to take on the posterior fate, rather than a default anterior fate. In grk mutant egg chambers, the posterior follicle cells behave as anterior follicle cells, and at stages 8 to 10, similar to the anterior follicle cells situated over the nurse cells, they do not express wind (Fig. 4E).
In summary, both the spatial and temporal wind RNA expression that we observed in the follicle cells of the egg chamber is consistent with a specific role of wind in dorsoventral patterning during oogenesis.
The wind gene encodes an endoplasmic reticulum protein
Efforts to isolate a wind cDNA from an ovarian library were not successful, although a large number of clones (∼1,200,000) were screened from two different ovarian cDNA libraries (provided by Dr. P. Tolias, Public Health Research Institute, New York, NY, and Dr B. Suter, McGill University, Montreal, Canada). This is likely caused by a low abundance of the particular RNA in the total ovarian RNA population. To overcome this difficulty, we first sequenced the area of the genomic DNA that contains the transcript and made appropriate primers from DNA regions containing open reading frames. These primers were used in two separate PCR reactions (see Materials and Methods) that amplified the 5′ and the 3′ ends of the transcript in two overlapping fragments. Both the genomic and the cDNA fragments were found to contain identical sequences (Fig. 5). The genomic sequence contained a small intron of 193 bp located after amino acid 92 of the putative protein. It appears that the combined PCR products contain all of the cDNA sequence because the size of the transcribed area (956 bp) corresponds very closely to the size of the transcript that was detected on the Northern blot (1 kb). Also, the 3′ end PCR product contained a long poly(A) tail, right after a consensus polyadenylation signal present in the genomic sequence. Sequence analysis showed that the wind transcript encodes a putative protein of 257 amino acids with an estimated molecular mass of 29 kD.
Figure 5.
Nucleotide sequence and deduced protein of the wind gene. The sequence of the cDNA was identical to the genomic sequence (outside of the intron area). The start of transcription was determined by sequencing of the 5′ of the PCR amplified cDNA. A long poly(A) tail was found in the 3′ end PCR amplified product, after a consensus polyadenylation signal present in the genomic sequence. The flanking numbers correspond to nucleotide and amino acid (in parentheses) positions. The 4-amino-acid ER retention signal (KEEL) and thioredoxin-like motif (CTGC) are boxed. Underlined are the translation stop codon (UGA) and consensus polyadenylation signal (AATAAA). Underlined with arrowed lines are the 7-bp deletion found in windM88 and the 14-bp deletion found in windAR51. The amino acid changes in windM46 and windT6 are boxed and shaded and the base change in the intron of windAR51 is boxed.
Database similarity searches revealed that the putative protein encoded by wind is homologous to the rat endoplasmic reticulum protein ERp29 precursor (Demmer et al. 1997). The two proteins share 30% overall identities and 40% homology, taking conserved changes into account (Fig. 6). The identities cluster in specific fragments located in the amino- and carboxy-terminal ends of the putative proteins. There is also a human protein (Hughes et al. 1993) that shows homology to the rat ERp29 protein. The three molecules have very similar sizes (fly, 257 amino acids; rat, 260 amino acids; and human, 261 amino acids). The human and the rat proteins are 90% identical (Fig. 6). The rat protein has been proposed to reside in the ER because it was isolated from the microsomal fraction of the cells (Demmer et al. 1997), and it also contains a variant of the ER retention signal (KEEL, Pelham 1990). Significantly, Wind, like the other two proteins, contains two of those variant ER retention signals, located in comparable positions in the carboxyl terminus of the molecule (Figs. 5 and 6). All three proteins contain putative signal peptides of variable sizes at their amino-terminal end, but no obvious glycosylation sites. The amino-terminal hydrophobic sequences corresponding to the signal peptides in the three homologous molecules are not conserved. Outside of the signal peptide area, the putative proteins are mostly hydrophilic.
Figure 6.
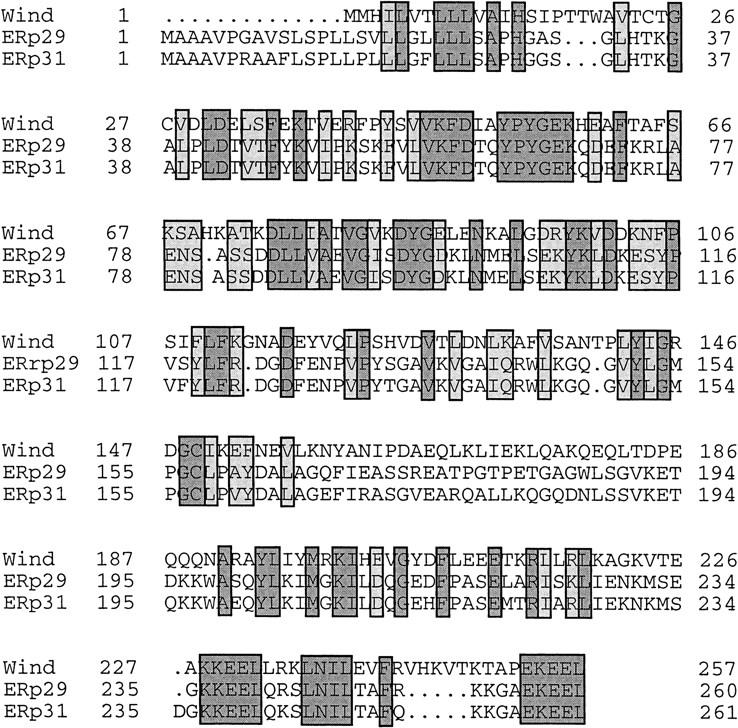
Alignment of the putative amino acid sequences encoded by wind, rat ERp29, and human ERp31. The Drosophila sequence is shorter at the amino-terminal end but outside of this part, the three molecules are almost contiguous. Note that the homologous parts cluster in the amino-terminal half and the carboxy-terminal end of the sequences. Identical amino acids in all three sequences are shown by dark shading; conserved changes are shown in lighter shading.
Secondary structure predictions for the putative Wind protein suggest that the molecule contains two large domains, at the amino and carboxyl termini that form α-helical structures. Similar structures are predicted for the rat ERp29 protein (Demmer et al. 1997), leading to suggestions of the ends of the protein being folded as globular domains. This suggestion is supported by comparisons of the rat and human proteins (90% identical), where the nonconserved amino acids are clustered in the signal peptide and carboxy-terminal parts (Demmer et al. 1997).
Other ER resident proteins like protein disulfide isomerase and related molecules, are involved in folding of nascent polypeptides and the isomerization of disulfide bridges (Noiva and Lennarz 1992). This function is mediated by a thioredoxin-like catalytic domain that contains the motif [Cys-X-X-Cys] (Noiva and Lennarz 1992, Chivers et al. 1996). Wind contains a thioredoxin-like motif between amino acids 24–27 [Cys-Thr-Gly-Cys] as well as a single Cys residue in amino acid position 149. The single Cys is also conserved in the rat sequence, ERp29, which displays significant homology (25% identities over a stretch of 103–122 residues) to two different protein disulfide isomerases, but does not contain any intact thioredoxin-like motif (Demmer et al. 1997). The deduced Wind protein shows 35% identities to the probable protein disulfide isomerase P5 precursor from alfalfa (Shorrosh and Dixon 1992), over a region of 50 amino acids at its carboxyl terminus. Interestingly, this region is part of the conserved areas between the Drosophila and rat homologs. The rat ERp29 protein is, over the same region, 33% identical to the alfalfa P5.
Sequence analysis of wind EMS mutants
To gain additional information regarding the functional domains of the protein, we sequenced some of the wind EMS mutant alleles. Four alleles that were sequenced belong to the group that generates strong dorsalizing phenotypes. They are 100% penetrant, and confer low viability to mutant flies. The fifth allele sequenced is the weakest hypomorphic allele and it is fully viable. PCR-amplified genomic DNA was prepared from flies hemizygous for each of the alleles. Only the transcribed parts of the genomic DNA were sequenced (see Materials and Methods).
Sequencing of windM88 revealed that the mutant copy of wind contained a 7-bp deletion, between bases 455 and 461 (underlined with arrowed line in Fig. 5). This deletion destroys the reading frame after amino acid 130 and generates a termination codon after another 36 amino acids. Because this deletion occurs in the middle of the coding region, this allele encodes only half of the putative protein, fused to 36 more amino acids of an aberrant sequence. The windM46 allele contains a single base change, resulting in the generation of a stop codon (UAG) after amino acid 187. Thus, this mutant allele also generates a severely truncated molecule. Both the windM88 and windM46 alleles also displayed a common base substitution, relative to the wild-type sequence. A single base change in nucleotide position 594 results in the conversion of an alanine in the wild-type protein, to a valine in both the mutant proteins. Because both of these alleles were generated on the same parental chromosome, it seems likely that this conserved change represents a polymorphism of the parental allele.
The third allele that was sequenced, windT6, also showed a single base change resulting in the conversion of the leucine of amino acid position 239 to a proline (boxed and shaded in Fig. 5). No other change was detected in this allele. Sequencing of the transcribed region of the allele windE4 did not reveal any changes in the nucleotide sequence. Finally, sequencing of the weakest allele windAR51 showed a 14-bp deletion in the intron sequence and a base change of the first nucleotide of the intron (from G to A, boxed in Fig.5). A few other base changes were also detected that only affected the third base of codons.
Discussion
Expression of wind in the follicle cells occurs when the dorsoventral pattern is established
The establishment of polarity in the Drosophila embryo is the result of a network of cellular communication that takes place in the ovary during the process of oogenesis. Both the somatic follicle cells and the germ cells are involved in an intricate exchange of signals, which regulate the polarity of the embryo. Even though the signaling processes are initiated during oogenesis, their activity ultimately leads to the asymetric activation of zygotic genes in the early embryo (Lehmann 1995; Morisato and Anderson 1995; Ray and Schüpbach 1996).
A set of 11 maternal genes participate in a cascade of events that establishes the nuclear gradient of Dorsal protein in the embryo. Loss-of-function mutations in these genes lead to the absence of nuclear Dorsal protein and therefore cause the generation of dorsalized embryos. Together with a twelfth gene, cactus, these genes comprise the dorsal group of genes, and participate in a cascade of events in which dorsoventral information is transmitted from the follicle cells to the embryo. The three genes, wind, nudel, and pipe, are at the top of this cascade, as it has been shown that their action is required in the somatic follicle cells of the ovary (Schüpbach et al. 1990; Stein et al. 1991). The cascade is under the control of the Grk–Egfr pathway, which restricts its action to the ventral side of the egg. This results in the spatially restricted production of the ligand for the Toll receptor, and to the establishment of the Dorsal gradient.
To understand how the Grk–Egfr signaling process is linked to the action of the dorsal group genes, we have undertaken the genetic and molecular characterization of the gene windbeutel. We observed that the gene is expressed during a very brief period in the follicle cells in mid-oogenesis. The timing of wind expression, during stages 9 and early 10, corresponds very well to its expected time of action. The Grk–Egfr signaling process that is required for dorsoventral patterning, is believed to take place during stage 9, after the oocyte nucleus has moved to its dorsal–anterior position during stage 8 (Montell et al. 1991). Early response genes in the follicle cells, such as kek-1 and rhomboid, begin to show a dorsoventral asymmetry in their expression pattern beginning at stage 9 of oogenesis (Ruohola-Baker et al. 1993; Musacchio and Perrimon 1996). The gene nudel has also been shown to be expressed during stage 10 of oogenesis (Hong and Hashimoto 1995).
Even though it is not clear at this point whether the Grk–Egfr signaling process regulates the activity of either wind or nudel directly, the fact that they are expressed at this specific time in oogenesis suggests that their activity is required in the follicle cells close to the time when Egfr activation occurs. Given that this is the stage when the vitelline membrane, the innermost layer of the eggshell, is secreted, this time of expression is consistent with models proposing that asymmetrically distributed dorsoventral factors might be deposited in the vitelline membrane (Manseau and Schüpbach 1989; Stein et al. 1991; Hong and Hashimoto 1995).
Regulation of wind expression
To examine whether wind transcription is regulated by any of the other genes acting in the dorsoventral pathway, we analyzed its expression in mutant backgrounds. This analysis showed that fs(1)K10, which acts upstream of grk in dorsoventral signaling, and is involved in grk RNA localization (Neuman-Silberberg and Schüpbach 1993), does not alter the expression of wind. Expression of wind in a strong grk mutant was abolished in the posteriorly localized follicle cells. It has been shown previously (Gonzales-Reyes and St Johnston 1995; Roth et al. 1995) that Grk–Egfr signaling is required at two times during oogenesis. Grk–Egfr signaling is first required to specify the posterior follicle cells. The second signaling process, at around stage 9, specifies the dorsal follicle cell fate. In strong grk mutants, the first signaling process does not take place and consequently, the posterior follicle cells assume the anterior fate. In the wild-type egg chamber, wind transcript is found only in the follicle cells overlaying the oocyte and not in the follicle cells covering the nurse cells. The absence of wind expression in the posterior follicle cells in grk mutations shows that Grk–Egfr signaling is necessary in posterior follicle cells to express wind. This signaling process, however, is not necessary for lateral follicle cells to express wind, showing that different subpopulations of follicle cells require different inputs to express wind.
Mutations in the gene nudel had no effect on wind RNA expression. Because the Nudel protein seems to be secreted (Hong and Hashimoto 1995) and Wind protein is probably retained in the cell (see below), it is reasonable to suggest that nudel lies downstream of wind. wind expression was also unchanged in pipe mutants, suggesting that this gene may also act downstream or at the same step in the pathway as wind.
Specific role of the endoplasmic reticulum of the follicle cells in dorsoventral patterning
The follicle cells play multifunctional roles during oogenesis. They are responsible for the secretion of yolk proteins that are taken up by the oocyte, as well as the secretion of the vitelline membrane and chorion proteins, which form the protective eggshell around the egg (for review, see Spradling 1993). They are also involved in the signaling processes that help establish the anteroposterior and dorsoventral axes of both the oocyte and embryo (for review, see St Johnston 1995; Ray and Schüpbach 1996).
A common characteristic of these different functions of the follicle cells is the secretion of proteins that serve either a structural or a regulatory role. The follicle cells form an epithelial monolayer around the oocyte–nurse cell complex. The apical side of the follicular epithelium faces the oocyte and is the site of secretion. Our findings that the wind transcript is localized on the apical side and encodes a putative (ER) resident protein suggest that specific parts of the secretory apparatus in the follicle cells are apically localized. Other examples of transcripts that have been found to have a specific subcellular distribution in epithelial cells include the gene crumbs (Tepass et al. 1990) and the gene wingless (Bar et al. 1994) where subcellular localization of the RNA has also been linked to its function.
The sequence homology of Wind to the rat ERp29 protein indicates that Wind is most likely a component of the lumen of the ER. Proteins that contain the 4-residue retention signal (KDEL) are found in the ER lumen and are called reticuloplasmins (Koch 1990). These proteins are transferred into the ER with the help of their signal peptide and are retained there through an interaction of the retention signal with a specific receptor (Pelham 1990, Scheel and Pelham 1996). The major reticuloplasmins described to date (calreticulin, endoplasmin, protein disulfide isomerase, and BiP; Koch 1990; Lucero 1994) are all calcium-binding proteins. They are proposed to function as molecular chaperons during protein assembly and degradation (Nigam et al. 1994) and as calcium buffers (Hubbard 1996). ERp29 was shown to be unresponsive to cellular stresses that induce known heat shock proteins that are related to members of the reticuloplasmin family, and it is not a calcium-binding protein (Demmer et al. 1997). These results suggest that ERp29 and its Drosophila and human homologs form a new group of reticuloplasmins with a conserved, but presently unknown, function.
It is interesting to note that ERp29 protein was isolated from rat enamel cells involved in dentition, and wind is expressed in ovarian follicle cells, both of which are highly secretory cell types. Determining the targets of Wind in the follicle cells should help to elucidate the novel function of these conserved proteins.
Mutations in wind affect coding regions of the protein
Strong mutant alleles of wind cause varying degrees of semi-lethality that is manifested during late larval stages of development. These alleles appear to be strong loss of function, as judged by genetic tests. Because many of the important regulatory genes that act during oogenesis have also equally important functions during the adult development of the fly (Spradling 1993), we considered it possible that the null phenotype of wind might be lethal. The initial allele of the gene wind was identified in female sterile screens (Schüpbach and Wieschaus 1989). Subsequent screens (using as tester a chromosome carrying the original female sterile windRP54 allele), however, did not recover any lethal alleles of wind. In situ RNA analysis on wind mutant ovaries revealed that two of the strongest EMS alleles, windM88 and windM46, had undetectable levels of wind RNA, leading us to suggest that they might represent null alleles. Because the transheterozygous combination of these two mutants is only partially lethal, it appears that even with extremely low, to undetectable, levels of wind RNA, the flies can survive to adulthood.
Two of the strongest wind mutant alleles, windM88 and windM46, were found to encode severely truncated proteins. A third wind allele, windT6, contains a single amino acid change of a leucine to a proline, in its carboxyl terminus. This change is significant, because this specific leucine is part of a well-conserved stretch of amino acids at the carboxyl terminus of all three homologous proteins (Drosophila, rat, and human). Also, proline is a cyclic amino acid that is almost never found in α-helical structures, which is the favorable secondary structure prediction for the carboxyl terminus of Wind and its rat and human homologs. Because windT6 shows a better viability over the Df(2R)P34 than the two truncation alleles, it appears that windT6 may retain some of the function of the molecule.
The weakest allele, windAR51, revealed changes only in the intron sequences. It is possible that the single base change of the first nucleotide at the 5′ splice site affects the level of correct splicing of wind. If this defect is not 100% penetrant, it could account for the weak nature of this allele.
A possible role for Wind protein in the specific folding of a secreted factor?
The ER mediates the translocation of proteins to the membrane and is responsible for the correct folding and chemical modification of secreted or membrane-bound proteins. Several components of the ER have been isolated and specific steps in the process have been characterized (Rapoport et al. 1996). More recently, specific developmental functions have been assigned to components of the ER. It has been shown (McKibben and Cartwright, unpubl.), that an alternatively spliced message of the Dtrp1 gene is expressed in the ER in a tissue specific manner, that is, only in the male reproductive system. It has also been proposed that calmegin, a mouse testis-specific ER protein, is responsible for folding one or more sperm-specific proteins that are involved in adhesion of sperm to the zona pellucida (Ikawa et al. 1997). Finally, the ER-associating protein Vera was recently implicated in the localization of Vg1 RNA in Xenopus oocytes (Deshler et al. 1997).
On the basis of these examples, we would speculate that Wind protein is involved in the specific folding of one or more molecules secreted by the follicle cells. These molecules would be secreted only on the ventral side of the follicular epithelium and would represent the critical factors for the generation of the ventralizing signal that translates into the active ligand for the Toll receptor. Several mechanisms could be proposed that might contribute to the generation of the signal only on the ventral side of the follicular epithelium. The protein expression and/or activity of Wind might be restricted in the ventral follicle cells, in response to the Grk–Egfr signaling. Alternatively, the expression of another factor might be restricted in the ventral follicle cells by the Grk–Egfr pathway, and this factor might be subsequently modified by Wind. In a wind mutant female, the ventral factors would not be active, leading to the absence of functional Toll ligand and absence of nuclear Dorsal protein, and thus to a dorsalized embryo.
Candidate molecules for these secreted factors could be the products of the genes nudel and pipe. Our in situ hybridization results are consistent with the possibility that both of these genes act downstream of wind. In addition, Nudel protein has been proposed to be secreted from the follicle cells (Hong and Hashimoto 1995). An interesting observation regarding the Nudel amino acid sequence is the fact that it contains a highly conserved serine protease domain with eight cysteines (Hong and Hashimoto 1995). It is proposed that these cysteines might form four intramolecular disulfide bonds within the catalytic pocket, as in chymotrypsin. The fact that Wind contains a putative thioredoxin-like motif, involved in the isomerization of disulphide bridges in nascent polypeptides, raises the intriguing possibility that Wind might be involved in the folding of Nudel. Further studies will shed light into the novel finding of the involvement of the ER in the specification of embryonic dorsoventral axis in Drosophila.
Materials and methods
Fly stocks
Six wind EMS-induced mutations were used in this study. The mutation windRP54 is described in Schüpbach and Wieschaus (1989). The mutations windM88 and windM46 were induced on a cn bw sp chromosome that was tested against windRP54, according to standard methods (Clifford and Schüpbach 1989). The mutations windT6 and windE4 were generated by U. Mayer, R. Lehmann, and C. Nüsslein-Volhard (unpubl.) and windAR51 was generated by S. Roth and C. Nüsslein-Volhard (unpubl.).
Df(2R)Pc4 and Df(2R)P34 are described in Lindsley and Zimm (1992). Df(2R)GC8 and Df(2R)GC10 are described in Gertler et al. (1995). Df(2R)F7 was induced by x-rays, screening for reversion events in a strain carrying the H2E P-element insertion (provided by J.A. Lepesant, Institut Jacques Monod, Centre National de la Recherche Scientifique, Paris, France) and was mapped on squashes from salivary gland polytene chromosomes. The cor1 allele was generated by I. Dawson (Fehon et al. 1994). The P-element insertion P989 [l (2) 01103] is described in Karpen and Spradling (1992). The rescue construct was injected in y w flies and cn bw was used for the restriction fragment length polymorphism (RFLP) analysis of the deficiencies. Oregon R was used as the wild-type stock.
Other strains used were fs(1)K10 (Wieschaus 1979), grkHK (Schüpbach 1987), ndl111 (Hong and Hashimoto 1996), and pip664 (Anderson and Nüsslein-Volhard 1984). All marker mutations and deficiencies are found in Lindsley and Zimm (1992).
Genetic crosses
For the complementation analysis, crosses were performed at room temperature (∼22°C) and were put at 25°C at late larval stages to enhance the Cy phenotype. For the determination of the lethal phase of wind EMS alleles, 300–500 embryos were collected from wind/+ × wind/CyO crosses and counted through all stages of development. The recessive markers c and px were used for the isolation of wild-type recombinants between b pr c cor1 px sp, and b pr windRP54bw.
Chromosomal walks
The λ phages were isolated from a λDashII genomic library (constructed by R. Padgett, Waksman Institute, Rutgers University, Piscataway, NJ), by use of a cor cDNA (provided by R. Fehon, Duke University, Durham, NC) as probes, for the proximal end, and genomic DNA adjacent to P989 insertion that was cloned taking advantage of the PZ vector (Mlodzik and Hiromi 1991), for the distal end. The middle part of this genomic area could not be cloned from the λ library because of the presence of repetitive DNA and was eventually isolated in small fragments from the P1 phage DM02733.
The ends of P1 phages mapping in the 56C area were cloned by PCR amplification by use of the T7 and SP6 promoters (Nurminsky and Hartl 1993). The P1 phage DM02733 was chosen because it overlapped with parts of the λ genomic walk. The entire insert of the DM02733 P1 phage was cloned into the Bluescript vector (Stratagene) by generating mini-plasmid libraries with six different restriction enzymes. The minilibraries were kept on grid plates and were used for the isolation of suitable overlapping fragments. The genomic insert of DM02733 was 60-kb long.
RNA in situ hybridization on whole mount ovaries
Ovaries dissected and teased apart were fixed and hybridized as described in Tautz and Pfeifle (1989) and modified by Suter and Steward (1991). Digoxigenin-labeled antisense RNA probes were synthesized by use of the RNA genius kit (Boehringer Mannheim). Ovaries were mounted in Aqua-polymount (Polysciences, Inc.).
Molecular techniques
Genomic DNA was prepared as described in Delidakis and Kafatos (1987), and RNA was isolated as described in Brown and Kafatos (1988). Southern blot, Northern blot, and reverse Northern blot analyses were performed on Zetaprobe nylon membranes (Bio-Rad). For the reverse Northern blot, ∼5 μg of poly(A)+ ovarian RNA was labeled by reverse transcription. All other molecular techniques were done according to Maniatis et al. (1989).
The wind cDNA was isolated in two overlapping fragments by 5′ RACE and 3′ RACE reactions, according to the manufacturer’s instructions (GIBCO BRL). In each reaction, 1 μg of poly(A)+ ovarian RNA was used.
Genomic DNA was isolated from flies hemizygous for the wind EMS alleles over Df(2R)GC8 and was amplified by use of the Advantage cDNA PCR core kit (Clontech).
Sequence analysis
Sequencing of wild-type DNA was done by automated sequencing (Applied Biosystems) and by the method of Sanger et al. (1977). Sequencing of the mutant DNA was done exclusively by automated sequencing. Sequences were assembled and analyzed by use of the AssemblyLign and MacVector DNA analysis programs (IBI sequence analysis software). Database searches were performed by the FASTA program (Lipman and Pearson 1985). The wind GenBank accession number is AF025408.
Acknowledgments
We thank S. Roth, F. Gertler, R. Fehon, I. Dawson, J.-A. Lepesant, R. Padgett, P. Tolias, B. Suter, and C. Hashimoto for their generous supply of fly stocks and reagents. We are greatful to T. Vogt, P. Tolias, L. Nilson, A. Norvel, and A.-M. Queenan for critical reading and comments on the manuscript, and all the members of the Schüpbach and Wieschaus laboratories for support and helpful discussions. We also especially thank F. Farber for technical assistance, and J. Curtis for help in pinpointing the Df(2R)P34 breakpoint. This work was supported by Howard Hughes Medical Institute and by U.S. Public Health Services grant PO1 CA 41086. M. Konsolaki was supported by American Cancer Society fellowship and later by a fellowship from the Office for the Promotion of Mental Retardation and Developmental Disabilities, New Jersey Dept. of Human Services.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL gschupbach@molbiol.princeton.edu; FAX (609) 258-1547.
References
- Anderson KV, Nüsslein-Volhard C. Information for the dorsal-ventral pattern of the Drosophila embryo is stored as maternal mRNA. Nature. 1984;311:223–227. doi: 10.1038/311223a0. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Jürgens G, Nüsslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: Genetic studies on the role of the Toll gene product. Cell. 1985;42:779–789. doi: 10.1016/0092-8674(85)90274-0. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Drosophila: A laboratory handbook. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. Map distance and recombination frequency. [Google Scholar]
- Bar IL, Ledrean BS, Krause H. Subcellular localization of wingless in the Drosophila embryo. Mol Biol Cell. 1994;5:383a. [Google Scholar]
- Brown NH, Kafatos FC. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988;203:424–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- Chivers PT, Laboissiere MCA, Raines RT. The CXXC motif: Imperatives for the formation of native disulfide bonds in the cell. EMBO J. 1996;15:2659–2667. [PMC free article] [PubMed] [Google Scholar]
- Clifford RJ, Schüpbach T. Coordinately and differentially mutable activities of torpedo, the Drosophila melanogaster homolog of the vertebrate EGF receptor gene. Genetics. 1989;123:771–787. doi: 10.1093/genetics/123.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delidakis C, Kafatos FC. Amplification of a chorion gene cluster in Drosophila is subject to multiple cis-regulatory elements and to long-range position effects. J Mol Biol. 1987;197:11–26. doi: 10.1016/0022-2836(87)90605-x. [DOI] [PubMed] [Google Scholar]
- Demmer J, Zhou CM, Hubbard MJ. Molecular cloning of ERp29, a novel and widely expressed resident of the endoplasmic reticulum. FEBS Lett. 1997;402:145–150. doi: 10.1016/s0014-5793(96)01513-x. [DOI] [PubMed] [Google Scholar]
- Deshler JO, Highett MI, Schnapp BJ. Localization of Xenopus Vg1 mRNA by Vera protein and the endoplasmic reticulum. Science. 1997;276:1128–1131. doi: 10.1126/science.276.5315.1128. [DOI] [PubMed] [Google Scholar]
- Fehon RG, Dawson IA, Artavanis-Tsakonas S. A Drosophila homologue of membrane-skeleton protein 4.1 is associated with septate junctions and is encoded by the coracle gene. Development. 1994;120:545–557. doi: 10.1242/dev.120.3.545. [DOI] [PubMed] [Google Scholar]
- Gertler FB, Comer AR, Juang JL, Ahern SM, Clark MJ, Liebl EC, Hoffmann FM. enabled, a dosage-sensitive suppressor of mutations in the Drosophila Abl tyrosine kinase, encodes an Abl substrate with SH3 domain-binding proteins. Genes & Dev. 1995;9:521–533. doi: 10.1101/gad.9.5.521. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes A, Elliott H, St Johnston RD. Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature. 1995;375:654–658. doi: 10.1038/375654a0. [DOI] [PubMed] [Google Scholar]
- Hong CC, Hashimoto C. An unusual mosaic protein with a protease domain, encoded by the nudel gene, is involved in defining embryonic dorsoventral polarity in Drosophila. Cell. 1995;82:785–794. doi: 10.1016/0092-8674(95)90475-1. [DOI] [PubMed] [Google Scholar]
- ————— The maternal nudel protein of Drosophila has two distinct roles important for embryogenesis. Genetics. 1996;143:1653–1661. doi: 10.1093/genetics/143.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard MJ. Abundant calcium homeostasis machinery in rat dental enamel cells. Upregulation of calcium store proteins during enamel mineralization implicates the endoplasmic reticulum in calcium transcytosis. Eur J Biochem. 1996;239:611–623. doi: 10.1111/j.1432-1033.1996.0611u.x. [DOI] [PubMed] [Google Scholar]
- Hughes GJ, Frutiger S, Paquet N, Pasquali C, Sanchez JC, Tissot JD, Bairoch A, Appel RD, Hochstrasser DF. Human liver protein map: Update 1993. Electrophoresis. 1993;14:1216–1222. doi: 10.1002/elps.11501401181. [DOI] [PubMed] [Google Scholar]
- Ikawa M, Wada I, Kominami K, Watanabe D, Toshimori K, Nishimune Y, Okabe M. The putative chaperone calmegin is required for sperm fertility. Nature. 1997;387:607–611. doi: 10.1038/42484. [DOI] [PubMed] [Google Scholar]
- Karpen GH, Spradling AC. Analysis of subtelomeric heterochromatin in a Drosophila minichromosome by single P-element insertional mutagenesis. Genetics. 1992;132:737–753. doi: 10.1093/genetics/132.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch GLE. The endoplasmic reticulum and calcium storage. BioEssays. 1990;12:527–531. doi: 10.1002/bies.950121105. [DOI] [PubMed] [Google Scholar]
- Lehmann R. Establishment of embryonic polarity during Drosophila oogenesis. Semin Dev Biol. 1995;6:25–38. [Google Scholar]
- Lindsley DL, Zimm GG. The genome of Drosophila melanogaster. New York, NY: Academic Press; 1992. [Google Scholar]
- Lipman DJ, Pearson WR. Rapid and sensitive protein similarity searches. Science. 1985;227:1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Lucero HA, Lebeche D, Kaminer B. ER calcistorin/protein disulfide isomerase (PDI). Sequence determination and expression of a cDNA clone encoding a calcium storage protein with PDI activity from endoplasmic reticulum of a sea urchin egg. J Biol Chem. 1994;269:23112–23119. [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular cloning: A laboratory manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Manseau LJ, Schüpbach T. cappuccino and spire: Two unique maternal effect loci required for both the anteroposterior and dorsoventral patterns of the Drosophila embryo. Genes & Dev. 1989;3:1437–1452. doi: 10.1101/gad.3.9.1437. [DOI] [PubMed] [Google Scholar]
- Mlodzik M, Hiromi Y. The enhancer trap method in Drosophila: Its application to neurobiology. In: Cann PM, editor. Methods in neuroscience. Vol. 9. Orlando, FL: Academic press; 1992. pp. 397–414. [Google Scholar]
- Montell DJ, Keshishian H, Spradling AC. Laser ablation studies of the role of the Drosophila oocyte nucleus in pattern formation. Science. 1991;245:290–293. doi: 10.1126/science.254.5029.290. [DOI] [PubMed] [Google Scholar]
- Morisato D, Anderson KV. The spätzle gene encodes a component of the extracellular signaling pathway establishing the dorso-ventral pattern of the Drosophila embryo. Cell. 1994;76:677–688. doi: 10.1016/0092-8674(94)90507-x. [DOI] [PubMed] [Google Scholar]
- ————— Signaling pathways that establish the dorsal-ventral pattern of the Drosophila embryo. Annu Rev Genet. 1995;29:371–399. doi: 10.1146/annurev.ge.29.120195.002103. [DOI] [PubMed] [Google Scholar]
- Musacchio M, Perrimon D. The Drosophila kekkon genes: Novel members of both the leucine-rich repeat and immunoglobulin superfamilies expressed in the CNS. Dev Biol. 1996;178:63–76. doi: 10.1006/dbio.1996.0198. [DOI] [PubMed] [Google Scholar]
- Neuman-Silberberg FS, Schüpbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGFα-like protein. Cell. 1993;75:165–174. [PubMed] [Google Scholar]
- Nigam SK, Goldgerg AL, Ho S, Rohde MF, Bush KT, Sherman MY. A set of endoplasmic reticulum proteins possessing properties of molecular chaperons includes Ca(2+)-binding proteins and members of the thioredoxin superfamily. J Biol Chem. 1994;269:1744–1749. [PubMed] [Google Scholar]
- Noiva R, Lennarz WJ. Protein disulfide isomerase: A multifunctional protein resident in the lumen of the endoplasmic reticulum. J Biol Chem. 1992;267:3553–3556. [PubMed] [Google Scholar]
- Nurminsky DI, Hartl DL. Amplification of the ends of DNA fragments cloned in bacteriophage P1. BioTechniques. 1993;15:201–208. [PubMed] [Google Scholar]
- Pelham HRB. The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem Sci. 1990;15:483–486. doi: 10.1016/0968-0004(90)90303-s. [DOI] [PubMed] [Google Scholar]
- Price JV, Clifford RJ, Schüpbach T. The maternal ventralizing locus torpedo is allelic to faint little ball, an embryonic lethal, and encodes the Drosophila EGF receptor homolog. Cell. 1989;56:1085–1092. doi: 10.1016/0092-8674(89)90641-7. [DOI] [PubMed] [Google Scholar]
- Rapoport TA, Jungnickel B, Kutay U. Protein transport across the eucaryotic endoplasmic reticulum and bacterial inner membranes. Annu Rev Biochem. 1996;65:271–303. doi: 10.1146/annurev.bi.65.070196.001415. [DOI] [PubMed] [Google Scholar]
- Ray RP, Schüpbach T. Intercellular signaling and the polarization of body axes during Drosophila oogenesis. Genes & Dev. 1996;10:1711–1723. doi: 10.1101/gad.10.14.1711. [DOI] [PubMed] [Google Scholar]
- Roth S, Schüpbach T. The relationship between ovarian and embryonic dorsoventral patterning in Drosophila. Development. 1994;120:2245–2257. doi: 10.1242/dev.120.8.2245. [DOI] [PubMed] [Google Scholar]
- Roth S, Neuman-Silberberg FS, Barcelo G, Schüpbach T. cornichon and the EGF receptor signalling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 1995;81:967–978. doi: 10.1016/0092-8674(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Ruohola-Baker H, Grell E, Chou T-B, Baker D, Jan LY, Jan YN. Spatially localized rhomboid is required for establishment of dorsal-ventral axis in Drosophila oogenesis. Cell. 1993;73:953–965. doi: 10.1016/0092-8674(93)90273-s. [DOI] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain termination inhibitors. Proc Natl Acad Sci. 1977;72:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savant-Bhonsale S, Montell DJ. torso-like encodes the localized determinant of Drosophila terminal pattern formation. Genes & Dev. 1993;7:2548–2555. doi: 10.1101/gad.7.12b.2548. [DOI] [PubMed] [Google Scholar]
- Schejter ED, Shilo BZ. The Drosophila EGF receptor homolog (DER) gene is allelic to faint little ball, a locus essential for embryonic development. Cell. 1989;56:1093–1104. doi: 10.1016/0092-8674(89)90642-9. [DOI] [PubMed] [Google Scholar]
- Schneider DS, Jin Y, Morisato D, Anderson KV. A processed form of the spatzle protein defines dorsal-ventral polarity in the Drosophila embryo. Development. 1994;120:1243–1250. doi: 10.1242/dev.120.5.1243. [DOI] [PubMed] [Google Scholar]
- Schüpbach T. Germ line and soma cooperate during oogenesis to establish the dorsoventral pattern of egg shell and embryo in Drosophila melanogaster. Cell. 1987;49:699–707. doi: 10.1016/0092-8674(87)90546-0. [DOI] [PubMed] [Google Scholar]
- Schüpbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. I Maternal effect mutations Genetics. 1989;121:101–117. doi: 10.1093/genetics/121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüpbach T, Clifford RJ, Manseau LJ, Price JV. Dorso-ventral signaling processes in Drosophila. In: Gerhart J, editor. Cell-cell interactions in early development. NY: Wiley–Liss Inc.; 1990. pp. 163–174. [Google Scholar]
- Sheel AA, Pelham HRB. Purification and characterization of the human KDEL receptor. Biochemistry. 1996;35:10203–10209. doi: 10.1021/bi960807x. [DOI] [PubMed] [Google Scholar]
- Shorrosh BS, Dixon RA. Molecular characterization and expression of an alfalfa protein with sequence similarity to mammalian ERp72, a glucose regulated endoplasmic reticulum protein containing active site sequences of protein disulfide isomerase. Plant J. 1992;2:51–58. doi: 10.1046/j.1365-313x.1992.t01-50-00999.x. [DOI] [PubMed] [Google Scholar]
- Spradling AC. P element mediated transformation. In: Roberts DB, editor. Drosophila: A practical approach. Oxford, UK: IRL Press; 1986. pp. 175–197. [Google Scholar]
- ————— . Developmental genetics of oogenesis. In: Bate M, Martinez-Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1–70. [Google Scholar]
- Stein D, Roth S, Vogelsang E, Nüsslein-Volhard C. The polarity of the dorsoventral axis in the Drosophila embryo is defined by an extracellular signal. Cell. 1991;65:725–735. doi: 10.1016/0092-8674(91)90381-8. [DOI] [PubMed] [Google Scholar]
- Stevens LM, Fronhoffer HG, Klinger M, Nüsslein-Volhard C. Localized requirement for torso-like expression in follicle cells for development of terminal anlagen of the Drosophila embryo. Nature. 1990;346:660–662. doi: 10.1038/346660a0. [DOI] [PubMed] [Google Scholar]
- Steward R, Zusman SB, Huang LH, Schedl P. The dorsal protein is distributed in a gradient in early Drosophila embryos. Cell. 1989;55:487–495. doi: 10.1016/0092-8674(88)90035-9. [DOI] [PubMed] [Google Scholar]
- St Johnston D. The intracellular localization of messenger RNAs. Cell. 1995;81:161–170. doi: 10.1016/0092-8674(95)90324-0. [DOI] [PubMed] [Google Scholar]
- Suter B, Steward R. Requirement for phosphorylation and localization of the Bicaudal-D protein in Drosophila oocyte differentiation. Cell. 1991;67:917–926. doi: 10.1016/0092-8674(91)90365-6. [DOI] [PubMed] [Google Scholar]
- Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translation control of the segmentation gene hunchback. Chromosoma. 1989;92:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–799. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- Wadsworth SC, Vincent III WS, Bilodeau-Wentworth D. A Drosophila genomic sequence with homology to the human epidermal growth factor receptor. Nature. 1985;314:178–180. doi: 10.1038/314178a0. [DOI] [PubMed] [Google Scholar]
- Wieschaus E. fs(1)K10, a female sterile mutation altering the pattern of both the egg coverings and the resultant embryos in Drosophila. In: le Douarin N, editor. Cell lineage, stem cell and cell differentiation. New York, NY: Elsevier/North-Holland Biomedical Press; 1979. pp. 291–302. [Google Scholar]
- Wieschaus E, Nüsslein-Volhard C. Looking at embryos. In: Roberts D, editor. Drosophila: A practical approach. Oxford, UK: IRL Press; 1986. pp. 199–227. [Google Scholar]