Abstract
A phenotypic measure commonly used to determine the degree of lactogenic differentiation in mouse mammary epithelial cell cultures is the formation of dome shaped cell structures referred to as mammospheres 1. The HC11 cell line has been employed as a model system for the study of regulation of mammary lactogenic differentiation both in vitro and in vivo 2. The HC11 cells differentiate and synthesize milk proteins in response to treatment with lactogenic hormones. Following the growth of HC11 mouse mammary epithelial cells to confluence, lactogenic differentiation was induced by the addition of a combination of lactogenic hormones including dexamethasone, insulin, and prolactin, referred to as DIP. The HC11 cells induced to differentiate were photographed at times up to 120 hours post induction of differentiation and the number of mammospheres that appeared in each culture was enumerated. The size of the individual mammospheres correlates with the degree of differentiation and this is depicted in the images of the differentiating cells.
Protocol
The HC11 cells were grown to confluence for 6 days in RPMI 1640 medium supplemented with 10% fetal calf serum, 5μg/ml Insulin, 10 mM HEPES, 10ng/ml epidermal growth factor (EGF) to establish competence.
The cells were washed with PBS and maintained in growth media without EGF for 24 hours.
To induce lactogenic differentiation the cells were incubated in differentiation media, i.e. RPMI with dexamethasone (10-6 M), insulin (5 μg /ml) and prolactin (5 μg /ml) referred to as DIP.
At the stated times post-DIP addition, mammospheres were photographed and enumerated by phase contrast microscopy using an Olympus IX71 microscope with digital camera.
Representative Results:
Low power magnification of normal mammary epithelial cell monolayers and multiple mammospheres, as well as high power magnification of a single mammosphere are shown in Figure 1, respectively.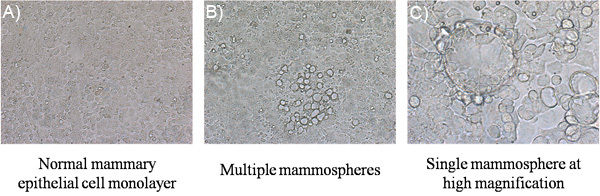 Figure 1. Mammospheres. (a) Normal mammary epithelial cell monolayers should be displayed as a flat layer of confluent cells. (b) After 3-5 days of DIP stimulation multiple mammospheres appear. Photograph taken 5 days post DIP addition. (c) Higher magnification of a single field containing mammospheres at 5 days post DIP addition.
Figure 1. Mammospheres. (a) Normal mammary epithelial cell monolayers should be displayed as a flat layer of confluent cells. (b) After 3-5 days of DIP stimulation multiple mammospheres appear. Photograph taken 5 days post DIP addition. (c) Higher magnification of a single field containing mammospheres at 5 days post DIP addition.
Discussion
In order for this technique to be successful and of the best quality, the epithelial cells must be maintained at confluence prior to being induced with lactogenic hormones. There may be situations where there are numerous mammospheres, which makes the quantification difficult. Thus, for quantitative purposes it is best to photograph the mammospheres at a time point when the number of mammospheres is able to be counted.
While there are various molecular markers used to determine the degree of lactogenic differentiation of mammary epithelial cells, there are few morphological markers used for cells grown in 2D culture. The ability of epithelial cell monolayers to form miniature dome structures where milk proteins can accumulate provides a mode by which the morphological changes that occur during this developmental process can be monitored.
Acknowledgments
Funds from Congressionally Directed Medical Research Fund grant (DAMD17-01-0264) to M.L. Cutler and United States Military Cancer Institute to M.L. Cutler supported the work.
References
- Blatchford DR, Hendry KA, Turner MD, Burgoyne RD, Wilde CJ. Vectorial secretion by constitutive and regulated secretory pathways in mammary epithelial cells. Epithelial Cell Biol. 1995;4:8–16. [PubMed] [Google Scholar]
- Wang W. Glucocorticoid induced expression of connective tissue growth factor contributes to lactogenic differentiation of mouse mammary epithelial cells. J Cell Physiol. 2008;214:38–46. doi: 10.1002/jcp.21159. [DOI] [PubMed] [Google Scholar]


