Abstract
In Drosophila, pattern formation at multiple stages of embryonic and imaginal development depends on the same intercellular signaling pathways. We have identified a novel gene, eyelid (eld), which is required for embryonic segmentation, development of the notum and wing margin, and photoreceptor differentiation. In these tissues, eld mutations have effects opposite to those caused by wingless (wg) mutations. eld encodes a widely expressed nuclear protein with a region homologous to a novel family of DNA-binding domains. Based on this homology and on the phenotypic analysis, we suggest that Eld could act as a transcription factor antagonistic to the Wg pathway.
Keywords: wingless, Drosophila, Bright, DNA binding, eye development, segmentation
Development of a multicellular organism from a fertilized egg requires cells to sense their position relative to other cells and to use this information to differentiate appropriately. This process of pattern formation has been shown to involve the operation of a number of intercellular signaling molecules. In Drosophila, one such molecule is the secreted product of the wingless (wg) gene, a Wnt family member (Nusse and Varmus 1992). wg has multiple functions in development (for review, see Klingensmith and Nusse 1994; Siegfried and Perrimon 1994). In the embryo, the pattern of each segment is determined by the interaction between a stripe of cells expressing wg and an adjacent stripe expressing another secreted molecule encoded by hedgehog (hh) (for review, see DiNardo et al. 1994). In the absence of either wg or hh, expression of the other gene is not maintained; this leads to a segment polarity phenotype in which part of each segment is lost and the remaining region is duplicated with inverted polarity. wg subsequently acts locally on the row of cells posterior to it (Dougan and DiNardo 1992) and over a longer range on anterior cells (Baker 1988; Bejsovec and Martinez-Arias 1991), determining their cell fate. wg has additional functions later in embryogenesis, which include directing the development of a region of the gut (Immergluck et al. 1990).
In the imaginal discs, signaling pathways are used to establish compartment boundaries, defined as barriers to the movement of clonally-related cells (Garcia-Bellido et al. 1973). In the larval leg disc, wg is expressed along the ventral portion of the anterior–posterior compartment boundary (Cohen et al. 1993), where it determines ventral cell fates and distal outgrowth of the leg (Campbell et al. 1993; Struhl and Basler 1993; Wilder and Perrimon 1995). During wing development, wg functions at several stages, reflecting the sequential compartmentalization of the wing disc. wg is first required for the establishment of the wing as distinct from the body wall or notum (Couso et al. 1993; Ng et al. 1996), then for development of the ventral compartment (Williams et al. 1993), and finally, to determine the wing margin (Couso et al. 1994), which forms at the dorsal–ventral compartment boundary and has a characteristic pattern of bristles. wg also promotes cell proliferation in the wing hinge region (Neumann and Cohen 1996).
The eye disc, unlike the other imaginal discs, does not have distinct anterior and posterior compartments, but develops progressively, as a wave of differentiation moves from posterior to anterior; the transient boundary between differentiated and undifferentiated cells is known as the morphogenetic furrow (MF) (Ready et al. 1976). Progression of the furrow depends on the expression of hh by differentiating photoreceptors located posterior to the MF (Heberlein et al. 1993; Ma et al. 1993; Heberlein et al. 1995). Hh protein induces more anteriorly-located cells to differentiate and themselves express hh, thereby creating a cycle of induction that moves the furrow across the eye disc. The initiation of differentiation requires decapentaplegic (dpp), a transforming growth-factor-β (TGF-β) homolog expressed at the margins of the disc (Wiersdorff et al. 1996; Chanut and Heberlein 1997; Pignoni and Zipursky 1997). wg is expressed at the dorsal and ventral margins of the eye disc, where it specifies regions of the head cuticle (Royet and Finkelstein 1996) and prevents dpp from inappropriately initiating photoreceptor differentiation, thereby restricting initiation to the posterior margin (Ma and Moses 1995; Treisman and Rubin 1995). Ectopic wg expression can block both initiation and progression of differentiation (Treisman and Rubin 1995).
Although many gaps remain in our understanding of the intracellular response to Wg signaling, some downstream elements have been identified. A frizzled-like molecule, Dfz2, has been proposed recently to act as the Wg receptor (Bhanot et al. 1996). Transmission of the Wg signal also requires a novel conserved protein encoded by dishevelled (dsh) (Klingensmith et al. 1994; Theisen et al. 1994) and the β-catenin homolog encoded by armadillo (arm) (Peifer and Wieschaus 1990). The protein kinase encoded by shaggy/zeste-white3 (sgg) acts downstream of Dsh and upstream of Arm to antagonize Wg signaling (Bourouis et al. 1990; Siegfried et al. 1990, 1992, 1994; Noordemeer et al. 1994). In vertebrates, adenomatous polyposis coli (APC), a large protein with multiple sequence repeats present also in Arm, and the high mobility group (HMG) box proteins, Tcf-1 and LEF-1, have been proposed to act in Wnt signaling because of their molecular or functional interactions with the Arm homolog β-catenin or the sgg homolog GSK3 (Rubinfeld et al. 1993, 1996; Su et al. 1993; Behrens et al. 1996; Huber et al. 1996; Molenaar et al. 1996). A Drosophila Tcf-1 homolog, pangolin (pan), has been shown recently to be an essential component of the Wg pathway and to bind to the Arm protein (Brunner et al. 1997; Riese et al. 1997; van de Wetering et al. 1997). However, the Drosophila APC homolog is not expressed at the time when wg acts on embryonic segmentation (Hayashi et al. 1997). The Arm–Pan complex may activate the transcription of Wg target genes (Brunner et al. 1997; van de Wetering et al. 1997) such as engrailed (en) in the embryonic epidermis (DiNardo et al. 1988), Ultrabithorax in the midgut (Riese et al. 1997), achaete and cut at the wing margin (Couso et al. 1994), and optomotor-blind, nubbin, vestigial, and distalless in the wing pouch (Diaz-Benjumea and Cohen 1995; Grimm and Pflugfelder 1996; Ng et al. 1996; Zecca et al. 1996). All of these genes encode transcription factors that mediate the subsequent effects of Wg.
wg appears to interact with other known pathways; in particular, it has many phenotypes in common with Notch (N) (Couso and Martinez-Arias 1994), which encodes a cell-surface receptor mediating cell fate choices triggered by local contact (for review, see Artavanis-Tsakonas et al. 1995). At the wing margin, an interaction between N and its ligands, Delta (Dl) in the ventral compartment and Serrate (Ser) in the dorsal compartment (Couso et al. 1995; Diaz-Benjumea and Cohen 1995; Kim et al. 1995; de Celis et al. 1996; Doherty et al. 1996), is required for wg expression along the margin (Diaz-Benjumea and Cohen 1995; Rulifson and Blair 1995; de Celis et al. 1996), explaining the loss of wing margin in both N and wg mutants. There also appears to be a negative interaction between wg and N signaling at the wing margin; Dsh, which interacts physically with the intracellular domain of N, inhibits N function, thereby limiting the domain of wg expression (Axelrod et al. 1996; Rulifson et al. 1996). In addition, the same gene, sgg/zw3, was isolated independently as an inhibitory element of the Wg pathway and a positive element of the N pathway (Bourouis et al. 1989; Perrimon and Smouse 1989).
We have isolated mutations in a novel gene, eyelid (eld), that have many similarities to sgg/zw3 mutations, suggesting that eld may be involved in signaling through the Wg or N pathways. In the eye, wing, and embryo, eld appears to antagonize the function of wg. The eld gene has been cloned and encodes a highly proline-rich protein with a region homologous to the newly defined DNA-binding domains of the Drosophila dead ringer and mouse Bright proteins (Herrscher et al. 1995; Gregory et al. 1996). Eld protein is expressed ubiquitously in the early embryo and in imaginal discs and is localized to the nucleus, where we suggest that it counteracts the effects of wg on its target genes.
Results
eyelid affects patterning of the eye imaginal disc
A dominant mutation in the rough (ro) gene, roDOM, has been shown to arrest movement of the MF in the eye disc, resulting in a reduced adult eye that specifically lacks the anterior portion of the retina (Heberlein et al. 1993) (Fig. 1B). In wild-type eye discs, a smooth gradient of ommatidial maturation is observed behind the MF, reflecting the stepwise recruitment of photoreceptors (Tomlinson and Ready 1987) (Fig. 1E,I). Differentiating photoreceptors are recognized by the expression of the neuronal protein elav (Robinow and White 1991), and the MF is visualized by the expression of a dpp reporter (dpp–lacZ) (Blackman et al. 1991). Eye discs from roDOM third-instar larvae display a so-called “furrow-stop” phenotype, the landmarks of which are the presence of mature ommatidia that contain a full complement of photoreceptor cells in the anterior-most ommatidial row, and the loss of dpp expression in the furrow (Fig. 1F,J). To identify additional genes involved in furrow movement, a genetic screen for dominant enhancers and suppressors of the roDOM eye phenotype was carried out (A. Luk and U. Heberlein, unpubl.; see Materials and Methods). Several ethylmethane sulfonate (EMS)-induced suppressors (Fig. 1C; see Materials and Methods) failed to complement each other’s recessive lethality and that of P-element insertions located at cytological position 90C1-2. Surprisingly, an analysis of eye discs from larvae heterozygous for both roDOM and any of the 90C1-2 mutants revealed that the observed increase in eye size was not attributable to relief of the roDOM-induced block to furrow progression in the central region of the disc. Rather, photoreceptor differentiation reinitiated at the dorsal and ventral edges of these discs (Fig. 1, arrowheads in G,K,L). This produced two ommatidial fields, each preceded by dpp–lacZ-expressing furrows, which moved anteriorly, presumably fusing later along the midline. The presence of a scar in the center of suppressed adult eyes (arrow in Fig. 1C) is consistent with this proposal.
Figure 1.
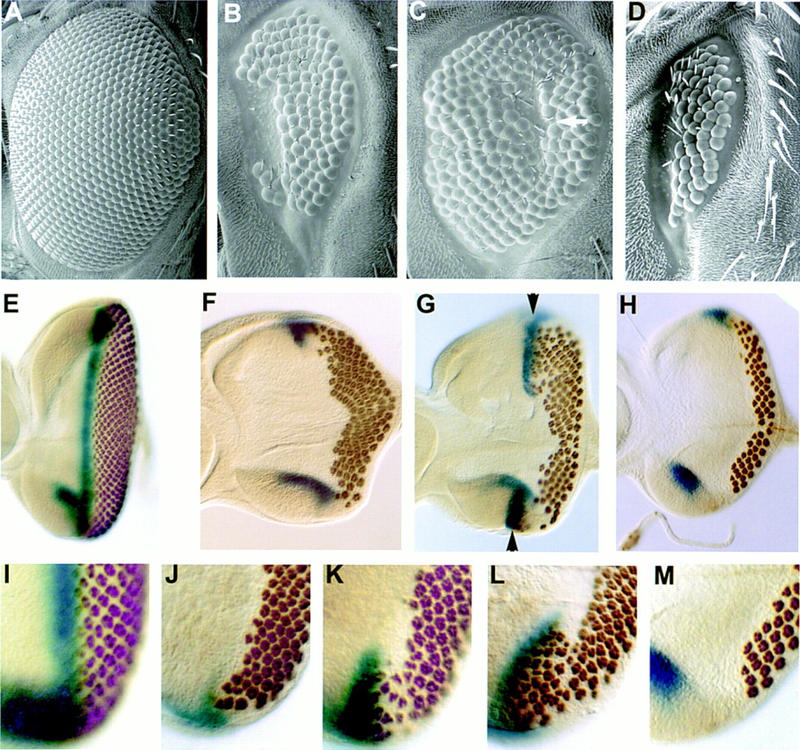
Effect of Eld and Wg on MF movement. (A–D) Scanning electron micrographs of adult eyes. (A) Wild type; (B) roDOM/+; (C) roDOM/eld308 (arrow indicates a scar in the eye); (D) wgCX4/+; roDOM/+. eld suppresses the roDOM phenotype, whereas wg enhances it. E–M) Eye discs stained with antiElav antibody and X-gal to visualize a dpp–lacZ reporter. (E,I) Wild type; (F,J) roDOM/+; (G,K,L) roDOM/eld308; (H,M) wgCX4/+; roDOM/+. (I–M) Enlargements of the ventral posterior region of eye discs. eld restores furrow movement to the lateral edges of roDOM discs (arrowheads in G), whereas wg reduces the amount of furrow movement observed there.
The mechanism of roDOM suppression by the 90C1-2 mutants suggests that the dosage of this suppressor is most critical at the lateral edges of the disc, regions in which wg has been shown to inhibit precocious differentiation (Ma and Moses 1995; Treisman and Rubin 1995). A reduction of wg function had the opposite effect on roDOM, reducing the eye size (Fig. 1D) and the extent of photoreceptor differentiation along the disc margins (Fig. 1H,M). We do not understand why reducing the function of an inhibitor of differentiation, such as wg, enhances rather than suppresses the roDOM phenotype, as would be expected. Curiously, genetic interactions between roDOM and mutations in genes involved in furrow progression are also reversed. For example, mutations in hh, a gene necessary for furrow movement, suppress roDOM, whereas mutations in patched, an inhibitor of furrow movement, enhance roDOM (U. Heberlein, unpubl.). The 90C1-2 mutants also displayed a dominant genetic interaction with the blink allele of dpp (dppd-blk). They acted as enhancers of the small-eye phenotype displayed by dppd-blk, whereas wg is known to act as a suppressor (Treisman and Rubin 1995). The enhancement of blink led us to name the affected gene eld. In both of these situations eld mutations showed effects opposite to those of wg mutations, suggesting that eld may normally function as an antagonist of wg signaling.
Because eld mutants die as embryos, we investigated the function of eld in eye development in mosaic retinae. Clones of cells homozygous mutant for eld were generated by the FLP–FRT method, consisting of the yeast FLP recombinase and its target FRT site (Golic 1991; Xu and Rubin 1993) (see Materials and Methods). Very few eld mutant cells were found when clones mutant for strong eld alleles were analyzed in adult eyes (Fig. 2A,B). However, such clones were associated with scars, suggesting that eld mutant cells were present at one stage and interfered with normal development. Clones analyzed in the eye disc did contain eld mutant cells, although in general the eld mutant clones were small in comparison to the wild-type twin spot clones (Fig. 2C), suggesting that eld is required for cell proliferation and/or survival. Exceptions were clones that included the posterior margin of the disc, which were frequently much larger than internal clones (Fig. 2D). The reason for this differential effect is unknown; however, it has been shown that initiation of development at the posterior margin is driven by dpp (Wiersdorff et al. 1996; Chanut and Heberlein 1997; Pignoni and Zipursky 1997), whereas its internal propagation depends on hh (Heberlein et al. 1993, 1995; Ma et al. 1993). Moreover, clones of cells mutant for the Dpp receptor thick veins proliferate significantly only when they include the posterior margin (Burke and Basler 1996).
Figure 2.
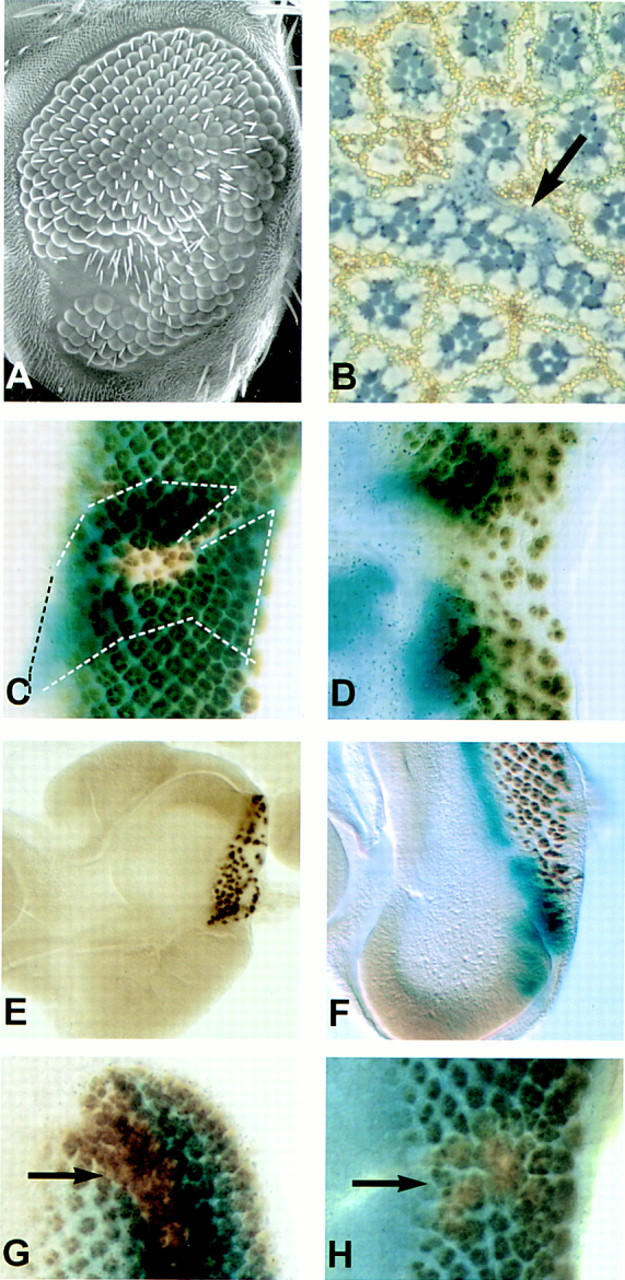
Phenotypes of Eld clones in the eye. (A) Scanning electron micrograph and (B) a tangential section of eld308 clones in the adult eye. The clones form scars with few remaining eld mutant cells, marked by the absence of pigment caused by the white mutation (arrow). (C,D) eld308 clones in eye discs stained with anti-Elav antibody (brown). The clones are marked by absence of X-gal staining (blue). (C) A small internal clone and (D) a large marginal clone. The wild-type twin spot is outlined with dotted lines in C. (E) An eld308, M+ clone induced in a M(3)be background. Although this disc contains a lacZ marker, the clone encompasses all the cells posterior to the furrow that would normally express lacZ; it causes a failure to initiate over the ventral part of the posterior margin. (F) An eld308 clone visible by a loss of anti-Elav stained cells (arrow). The disc is double-labeled with X-gal to visualize a dpp reporter; dpp expression remains at the posterior margin within the eld clone. (G,H) Anti-Elav stained discs containing clones marked by absence of X-gal staining. (G) An E(spl)r16 clone (arrow); (H) An E(spl)r16, eld308 clone (arrow). Both show excess neuronal differentiation.
In addition to its effect on cell proliferation, eld also affects neuronal differentiation. Most photoreceptor clusters that formed within eld clones contained fewer neuronal cells than normal (Fig. 2C,D); therefore, there seems to be a partial, though not absolute, requirement for eld for neuronal differentiation. Larger clones contained a reduced number of clusters as well as a reduced number of cells within each cluster (Fig. 2D). When we increased the size of the eld clones further by using a Minute mutation to slow the growth of the surrounding wild-type tissue (Morata and Ripoll 1975), a complete block of differentiation within the clone was observed frequently, although this usually did not encompass the entire clone (Fig. 2E,F). The lack of differentiation was not simply attributable to poor cell viability, as all the cells within eld clones could be induced to differentiate as neurons by removing the function of the Enhancer of split complex genes (E(spl)) (Fig. 2G,H), a group of helix–loop–helix (HLH) proteins that mediate lateral inhibition of neurogenesis by the N pathway (Delidakis et al. 1991). Therefore, lack of eld blocks neuronal differentiation at a point genetically upstream of E(spl) function and possibly upstream of the lateral inhibitory process.
The block to differentiation caused by loss of eld function in the eye resembles the effect of loss of sgg or ectopic expression of wg (Treisman and Rubin 1995), although it is less extreme unless the eld mutant cells are given a growth advantage. eld is required throughout the eye for differentiation, as is sgg, although eld is additionally required in internal regions of the eye disc for cell proliferation or survival. However, the effects of heterozygosity for eld appear most pronounced at the lateral margins, where wg is expressed, suggesting that wg signaling may be sensitive to the level of eld activity. Ectopic wg expression was not observed in eld mutant clones (data not shown), indicating that the effects of eld are not mediated primarily by activation of wg expression.
eld affects wing patterning
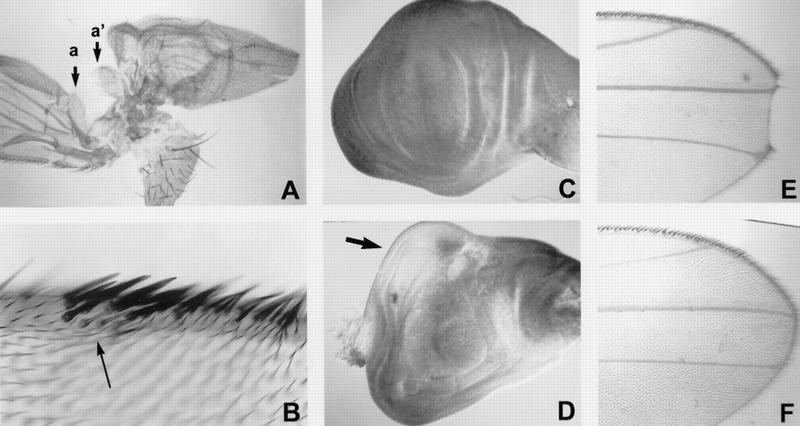
We next determined whether the eld mutant phenotype in other tissues was consistent with an effect on wg signaling. Clones of eld mutant cells induced in the wing disc also produced pattern alterations suggestive of antagonism to wg. One effect of clones produced early in development was the transformation of the posterior notum into a partial second wing (Fig. 3A). These wings had reversed anterior–posterior polarity; their most clearly differentiated structure was an alula produced consistently at their anterior margin. This transformation is the reverse of that produced by the wg1 mutation, which transforms the wing into a duplicated notum (Morata and Lawrence 1977), and is similar to that produced by overexpression of wg, dpp, or optomotor-blind (omb) in the notum (Grimm and Pflugfelder 1996; Ng et al. 1996). The marker we used to identify the eld mutant cells, yellow, marks only those cells that form wing margin bristles; no such mutant cells were detected in these ectopic wings. However, in wing discs containing eld clones and stained with an antibody to the Eld protein (see below), distortion of the disc was associated with regions failing to express Eld (Fig. 3D). These mutant cells must therefore either die at a later stage or fail to contribute to the wing margin, where they can be identified positively.
Figure 3.
Phenotypes of eld in the wing. (A) A second wing formed posterior to the first wing. The alula at the posterior of the normal wing (a) is next to the alula at the anterior of the ectopic wing (a′), indicating reversed A-P polarity. (B) Extra wing margin bristles induced near the wing margin. eld616 mutant bristles are marked with yellow and appear lighter (arrow); they account for only a few of the ectopic bristles. The endogenous margin is out of the focal plane of this photograph. (C) A wing disc stained with an antibody to Eld, showing expression throughout the disc. (D) Distortion of the wing disc in the region of an unstained eld308 clone (arrow). (C) N5419/+ wing; (D) N5419/+; eld308/+ wing. eld suppresses the notching of the distal wing margin caused by N.
Clones induced later in wing development were associated with ectopic wing margin bristles (Fig. 3B). Many or all of these ectopic bristles were not mutant for eld, but they were sometimes seen to form adjacent to eld clones (Fig. 3B; data not shown). Ectopic bristle formation was restricted to the dorsal surface of the wing, within the anterior compartment, and was observed most commonly near the wing margin in tufts of the bristle type appropriate to their position along the anterior–posterior axis. Differentiation of the wing margin is controlled by the wg pathway, and ectopic wing margin bristles are produced in clones mutant for sgg (Simpson et al. 1988; Perrimon and Smouse 1989; Couso et al. 1994). However, sgg clones show neither the non-autonomy nor the positional restrictions observed for eld clones. Overexpression of dsh can also produce ectopic bristles, which are wg-dependent and therefore predominantly found close to the normal wing margin (Axelrod et al. 1996). The restriction of ectopic bristle formation attributable to loss of eld to the anterior dorsal region near the wing margin suggests either that eld represses bristle formation only in this area of the wing, or that eld is required for cell viability in other regions. The second explanation may be correct, as even within the observed clones few eld mutant cells are observed.
A reduction of wg expression at the wing margin caused by heterozygosity for N is associated with nicks in the distal wing margin (Fig. 3E) (Diaz-Benjumea and Cohen 1995; Rulifson and Blair 1995; de Celis et al. 1996). These nicks were suppressed completely by the removal of one copy of eld (Fig. 3F), indicating that reduction of eld expression can compensate for a reduction in either N function or wg expression. Loss of N activity is also associated with extra bristle-forming cells within the proneural region of the wing margin (Rulifson and Blair 1995), suggesting that eld and N might interact in bristle formation. eld clones do not seem to affect another N-dependent process, the formation of notal microchaetae, although the wg-dependent macrochaetae (Phillips and Whittle 1993) are often misplaced, duplicated, or triplicated (data not shown).
eld is required in embryonic segmentation
To further examine the interaction of eld with the wg pathway, we determined its effect on embryonic segmentation. Embryos zygotically mutant for eld appeared to have normal cuticle patterning and normal expression of the segment polarity gene en (data not shown). However, because Eld protein is present at high levels in the early embryo (see Fig. 6, below) and is presumably contributed maternally, these embryos would still contain Eld. To remove both the maternal and zygotic contributions of eld, the FLP–FRT–ovoD system (Chou and Perrimon 1992) was used to produce eld mutant germ-line clones. Embryos derived from these clones showed severe defects in the cuticle pattern (Fig. 4B,C), with many denticle belts either missing, fused, or otherwise abnormal. Interestingly, the provision of a paternal wild-type copy of the eld gene failed to rescue the maternal mutants and made no perceptible difference to the phenotype (data not shown), suggesting either that early expression of eld is critical for its function or that the level of expression of the paternal copy is insufficient. Similarly, embryos maternally mutant for sgg are only partially rescued by a wild-type paternal copy of the gene (Perrimon and Smouse 1989; Siegfried et al. 1992), suggesting that antagonism of the wg pathway may require high levels of gene expression.
Figure 6.
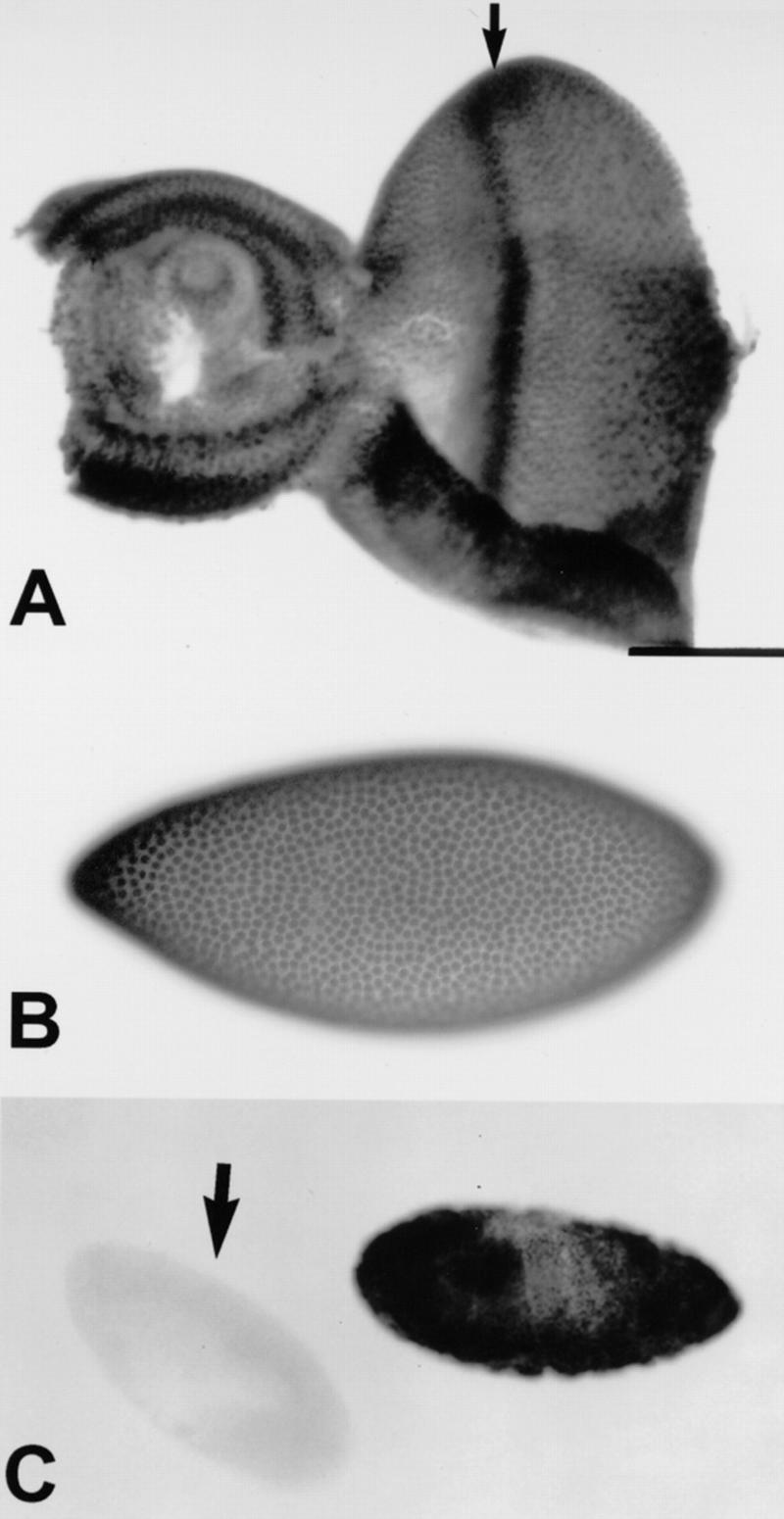
Expression of the Eld protein. All panels show staining with the Eld monoclonal antibody 15A8. (A) An eye disc with ubiquitous nuclear expression, which is enhanced just anterior to the MF (arrow). (B) An embryo at the cellular blastoderm stage, with staining in all nuclei. (C) Two embryos derived from an eld308 germ-line clone. One has a paternal copy of eld and stains with the antibody; the other is maternally and paternally mutant and shows no staining (arrow).
Figure 4.
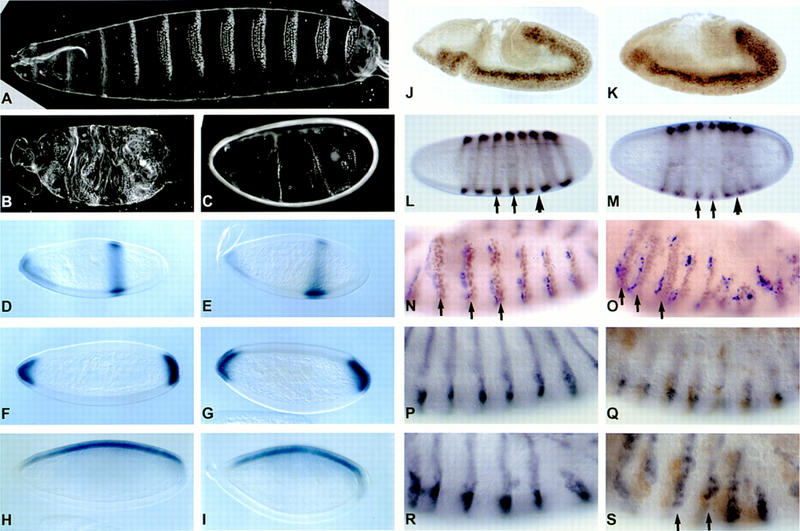
Embryonic phenotype of maternal and zygotic eld mutants. (A,D,F,H,J,L,N) Wild type. (B,C,E,G,I,K,M,O) Embryos derived from eld308 germ-line clones. (A–C) Cuticle preparations. eld embryos show missing and disorganized denticle belts. (D,E) In situ hybridization with a knirps probe. (F,G) In situ hybridization with a huckebein probe. Expression of gap genes is normal. (H,I) In situ hybridization with a zerknullt probe. (J,K) Anti-twist staining. Early patterning along the dorsoventral axis is normal. (L,M) Anti-even-skipped staining. The pattern is slightly altered in eld mutants. Stripes 3 and 4 are weaker (arrows) and the gap between stripes 5 and 6 is smaller (arrowhead). (N,O) Anti-En staining (brown) and in situ hybridization with a wg probe (blue). Wg RNA is present in stripes of approximately the normal width, but several En stripes are expanded (arrows) and others are disorganized. (P–S) Epistasis of eld over wg. (P) Wild type; (Q) wgP; (R) eld616 maternal mutant; (S) wgP; eld616 maternal mutant. The wgP homozyotes, which contain a mutagenic enhancer trap insertion, are identified by staining with anti-β-galactosidase (brown). Embryos are also stained with anti-En (purple). En expression is lost from wgP but is restored in the double mutant (arrows).
Because the cuticle phenotype could be consistent with a number of possible defects in segmentation, we looked at the expression patterns of a variety of genes to determine the stage at which these defects arise. The gap genes knirps (Fig. 4D,E), hunchback, Krüppel (data not shown), huckebein (Fig. 4F,G), and tailless (data not shown) were expressed normally. Slight defects were seen in the expression pattern of the pair-rule gene even-skipped (eve) (Fig. 4L,M)—stripes 3 and 4 often appeared weaker than normal, stripe 2 wider, and stripes 5 and 6 closer together. This pattern is in fact quite similar to that of eve during its early expression in wild-type embryos, suggesting a failure of refinement. The eld mutant embryos also failed to extend their germ bands normally, arresting at a partially extended stage. The dorsal–ventral axis of the embryo is determined by a gradient of Dpp activity (Ferguson and Anderson 1992a), and a similar failure of germ-band extension is seen in ventralized embryos mutant for dpp or for genes in the dpp pathway (Ferguson and Anderson 1992b). However, both patterning along the dorsal–ventral axis, as judged by the expression of the dorsal gene zen (Fig. 4H,I) and the ventral gene twist (Fig. 4J,K), and formation of amnioserosa by the dorsal-most cells (data not shown) appeared normal in the absence of eld. Therefore, eld affects to some degree the expression of pair-rule genes and the morphogenetic movements that occur before the start of wg expression.
wg function is first required in the embryo to maintain the expression of en (DiNardo et al. 1988). Although en expression initiated relatively normally in maternally mutant eld embryos (data not shown), its later expression was abnormal—several stripes appeared broadened, others were partially missing, and their spacing was disrupted. Despite the significant increase in width of the en stripes, however, the wg stripes were not expanded, and ectopic wg was not generally induced posterior to the en stripes (Fig. 4, cf. N and O). These results are consistent with eld acting to counteract Wg signaling posterior to the en stripes, in addition to responding to earlier patterning signals that affect the positioning of pair-rule gene stripes.
We attempted to order eld relative to wg in the segmentation pathway using an epistasis test; we examined embryos maternally mutant for eld and zygotically mutant for wg. en expression is reduced in wg single mutants (Fig. 4P,Q), but is present in both eld single mutants (Fig. 4R) and eld, wg double mutants (Fig. 4S); this would suggest that eld is epistatic to wg. However, we could not use null alleles in this experiment because the doubly heterozygous flies did not survive. Therefore, it is still possible that eld acts upstream of wg and that the absence of eld potentiates the activity of the small amount of remaining wg.
eld encodes a nuclear proline-rich protein with a potential DNA-binding domain
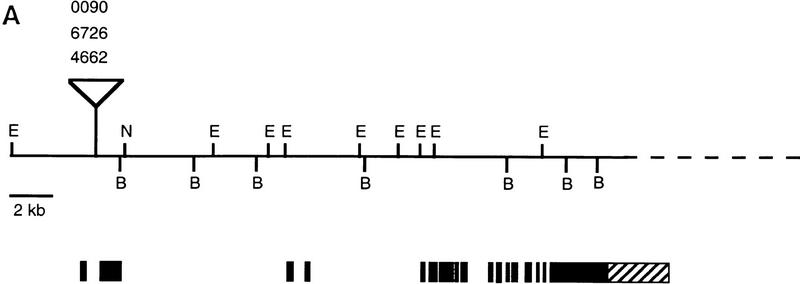
The three P-element alleles of eld allowed us to clone genomic DNA in the region by plasmid rescue. The resulting fragments were used to screen a cosmid genomic library. Probes extending at least 10 kb on each side of the P-element insertion sites were used to screen an eye disc cDNA library, and only a single class of cDNAs was isolated. All of the P elements are located in the first intron of this transcript, downstream of a noncoding exon (Fig. 5A). None of these cDNAs was full-length, and further screening of both eye disc and embryonic cDNA libraries was required to isolate overlapping cDNA clones covering the entire open reading frame (ORF). Eye disc and embryonic cDNAs were identical except for two small exons found only in the eye disc clones (Fig. 5A). The complete ORF deduced from these cDNAs would encode a protein of 2713 amino acids (Fig. 5B). The protein contains a region of homology to the DNA-binding domains of the Drosophila protein dead ringer (Gregory et al. 1996) and the mouse protein Bright (Herrscher et al. 1995; Fig. 5C). There is also a second region of homology to a protein predicted by the Caenorhabditis elegans genome project and to two human expressed sequence tags (ESTs) as well as to ESTs from mouse and rat (Fig. 5D; data not shown). Interestingly, the C. elegans ORF is adjacent to another ORF containing a region homologous to the potential DNA-binding domain of Eld (Fig. 5C), and it is possible that these actually belong to the same protein in C. elegans as well as in Drosophila. Another feature of the Eld sequence is that it is extremely proline-rich; proline comprises 17% of its amino acids. One of the EMS alleles of eld, eld616, was found to contain a stop codon substituted for glutamine 338 (Fig. 5B), and therefore would produce only a small amino-terminal portion of the protein.
Figure 5.
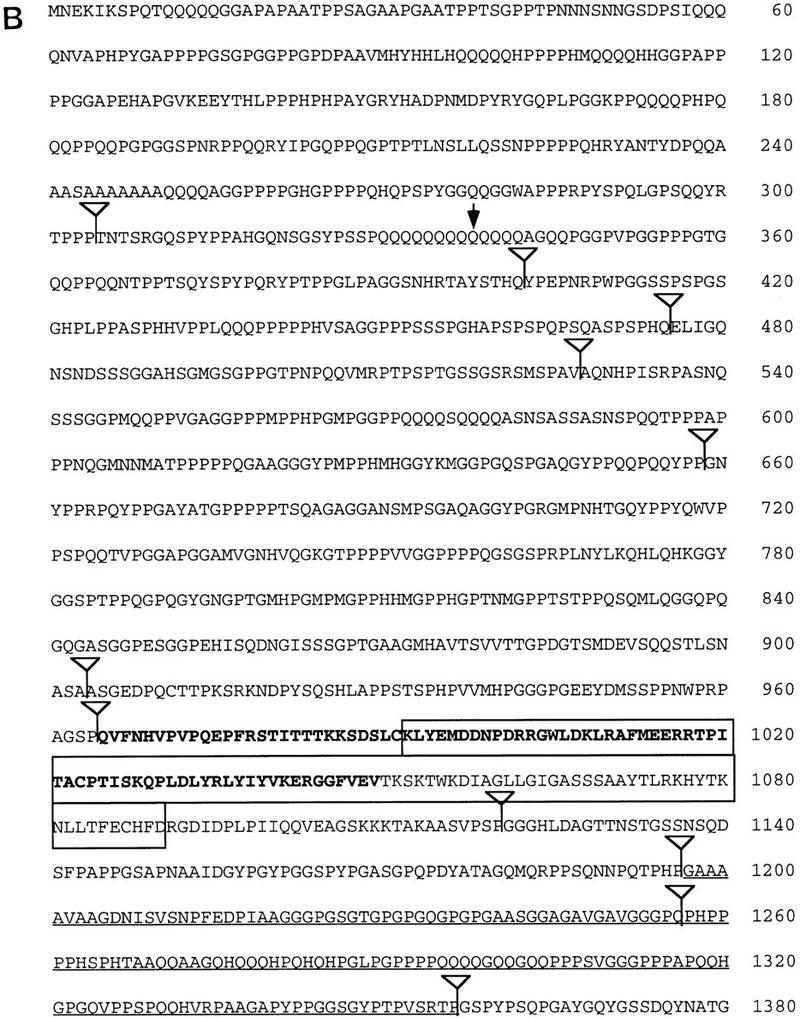
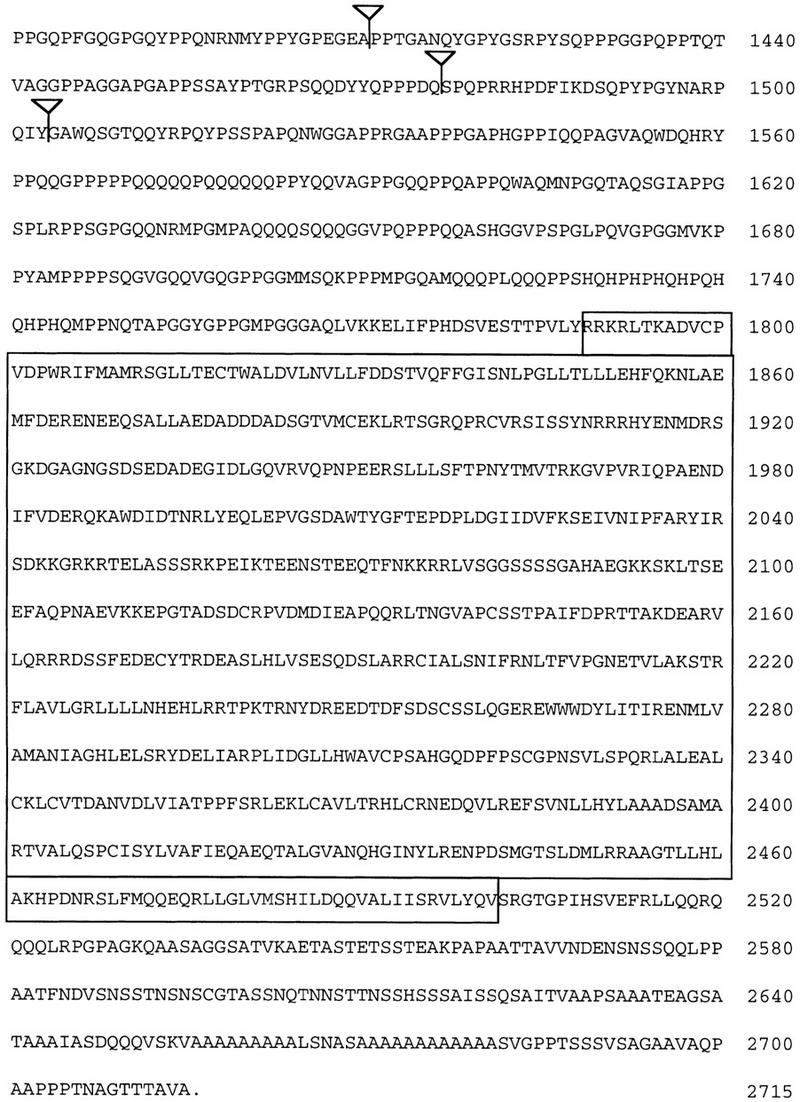
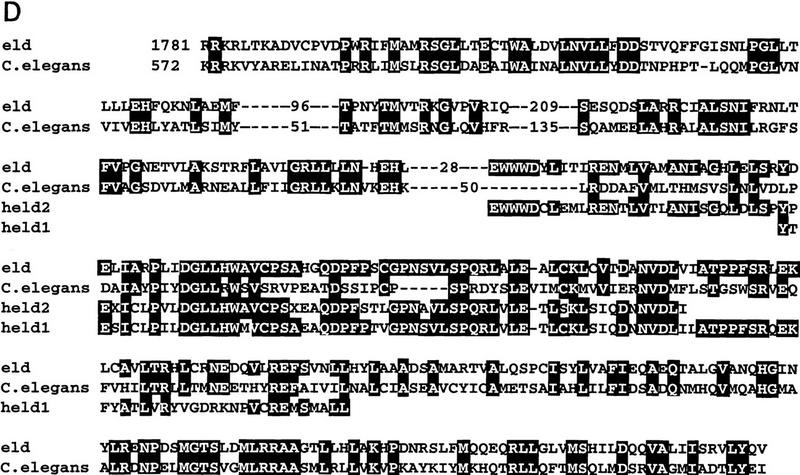
Molecular structure of the eld gene. (A) Genomic map of the eld region. Exons are shown as black boxes; the initiator methionine is in the second exon. The two gray exons are present in eye disc but not in embryonic cDNAs. The genomic structure corresponding to the last 2.3 kb of the eld transcript has not been characterized (hatched box and broken line). The three P-element eld alleles are inserted very close to each other in the first intron. (E) EcoRI; (B) BamHI; (N) NotI. (B) Sequence of the Eld ORF. The positions of identified introns are shown by inverted triangles. The glutamine mutated to a stop codon in eld616 is indicated by an arrow. The region in bold type was used to generate the monoclonal antibody used in Figs. 3 and 6. The regions of homology shown in C and D are boxed. The GenBank accession number for this sequence is 1413215. (C) Homology of Eld to the DNA-binding domains of dead ringer (dri), Bright, and a related predicted protein from C. elegans. Amino acids identical to the Eld sequence appear white on a black background. (D) Homology of Eld to predicted C. elegans and human proteins (held1 and held2). Amino acids identical to the Eld sequence appear white on a black background.
To examine the distribution and subcellular localization of the Eld protein, we raised a monoclonal antibody against a glutathionine S-transferase (GST) fusion protein containing amino acids 965–1049 of Eld. Staining of imaginal discs and embryos revealed that the protein was nuclear (Fig. 6A,B). Its distribution was quite ubiquitous in early embryos, showing no hint of a striped pattern (Fig. 6B), and was also ubiquitous in wing discs (Fig. 3C); although the protein was present everywhere in eye discs, its strongest expression occurred in a band just anterior to the MF, in the position where cells respond to Hh and Dpp signaling (Fig. 6A). To confirm that the antibody was specific for Eld, we stained embryos from eld germ-line clones; although strong staining was seen in those embryos carrying a paternal copy of the gene, the maternally and zygotically mutant embryos were completely unstained (Fig. 6C). This confirmed both that the antibody specifically recognizes the proline-rich protein, and that this protein is the product of the eld gene.
Discussion
eld antagonizes wingless signaling
We have shown for several different tissues in the fly that eld mutations have effects opposite to those caused by the absence of wg signaling. Loss of eld in the eye disc prevents normal photoreceptor differentiation, whereas differentiation is promoted by the loss of wg and inhibited completely by ectopic wg (Ma and Moses 1995; Treisman and Rubin 1995). The regions of the eye where wg is expressed are the most sensitive to a reduction in eld dosage. Loss of eld in the wing disc can cause a transformation of notum to wing, opposite to the wg1 phenotype of wing-to-notum transformation and similar to the effect of ectopically expressed wg (Morata and Lawrence 1977; Ng et al. 1996). Another phenotype associated with loss of eld is ectopic formation of wing margin bristles, similar to the sgg/zw3 mutant phenotype and opposite to the loss of margin seen in wg mutants (Simpson et al. 1988; Blair 1992; Couso et al. 1994). Finally, some of the stripes of en expression in the embryo are expanded in eld maternal mutants, as they all are in sgg maternal mutants (Siegfried et al. 1992); in contrast, en stripes are lost in wg mutants (DiNardo et al. 1988). eld also causes a mild overproduction of notal macrochaetae (data not shown), which are lost in wg mutants (Phillips and Whittle 1993).
We suggest that eld acts downstream rather than upstream of wg for the following reasons. First, wg expression is present in stripes of approximately normal width in embryos containing no Eld protein, showing that Eld is not required to repress wg transcription in the embryo. Second, ectopic wg expression is not found in clones of eld mutant cells in the eye disc, indicating again that eld does not repress wg expression. Third, En stripes are present in the eld, wg double mutant combination, suggesting that eld is not genetically upstream of wg. We have not been able to order eld relative to other known elements of the wg pathway, however, because of the technical difficulty in making clones on two different chromosomes simultaneously and the semi-lethality of heterozygous combinations of eld and wg pathway mutants. Another possibility that cannot be ruled out is that Eld could act by altering the activity of the Wg protein or the efficiency of wg signaling. Several possible models compatible with the data presented are shown in Figure 7. Further studies are necessary to determine whether Eld receives input from Sgg, Arm, or other factors, or belongs to a separate pathway.
Figure 7.
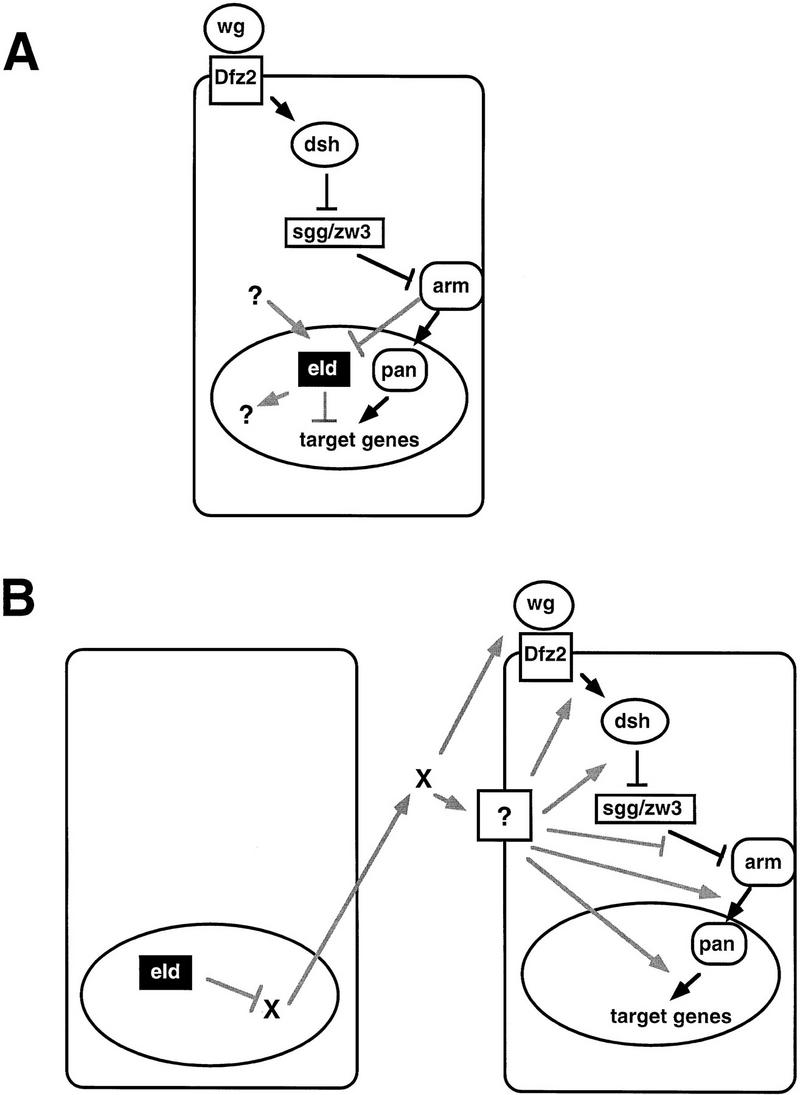
Possible roles of Eld in Wg signaling. (A) In this model, Eld acts as a nuclear repressor of Wg target genes (see Discussion). This model is supported by the finding that eld appears to be epistatic to wg in regulating the width of En stripes in the embryo and by the observation that wg expression is unaffected by loss of eld function. However, the non-autonomy of Eld function during wing development cannot be explained easily by this model. (B) In this model Eld acts as a repressor of a diffusible molecule X that could either augment Wg function directly or enhance the efficiency of Wg signaling at various steps in the signal transduction cascade. This model explains the phenotypes induced by loss of eld function in wing development. However, it fails to explain the epistatic relationship between wg and eld in the embryo. Shaded lines indicate postulated sites of genetic interaction. Arrows indicate positive genetic interactions, and perpendicular intersecting lines indicate negative genetic interactions.
eld could be a nuclear effector of Wg
Little is known about how the Wg signal is transmitted at the molecular level. The Dfz2 molecule is able to act as a Wg receptor in cell culture (Bhanot et al. 1996), although its contribution to Wg signaling in vivo has not been ascertained. The cytoplasmic protein Dsh is phosphorylated by an unknown kinase in response to Wg signaling (Yanagawa et al. 1995). This event appears to lead to the inactivation of the Sgg serine/threonine kinase, which would otherwise phosphorylate the β-catenin homolog Arm. Active, unphosphorylated Arm accumulates in the cytoplasm and nucleus, whereas the phosphorylated form is restricted to adherens junctions (Peifer et al. 1994a,b; Orsulic and Peifer 1996). Pan, a Drosophila homolog of the HMG box protein Lef-1, is a good candidate to act in combination with Arm to activate target genes such as en or Ubx (Brunner et al. 1997; Riese et al. 1997; van de Wetering et al. 1997). These target genes are the only other known nuclear effectors of wg, and because their expression is induced by Wg, they cannot be directly involved in transducing the signal. Eld meets the criteria for a direct nuclear effector; its expression is ubiquitous in the early embryo and imaginal discs, and therefore cannot be dependent on localized Wg signaling. The distribution of Eld protein is not altered in embryos maternally and zygotically mutant for sgg or dsh (data not shown). However, the absence of eld has effects similar to those caused by wg overexpression, suggesting that its activity may normally be inhibited in the wg-expressing regions. Eld appears to function as a repressor of en expression, although this effect need not be direct. Intriguingly, its homolog dead ringer was identified by its ability to bind the consensus target sequence for the En protein (Gregory et al. 1996), which was derived from possible autoregulatory sites within the en genomic region (Desplan et al. 1988; Kassis 1990; Heemskerk et al. 1991). If Eld also binds to these sites, it might compete with En to prevent the establishment of autoregulation. This would imply that eld acts as a repressor; several active repression domains have been shown to have a similarly high proline content (Han and Manley 1993). Further study will be required to determine the DNA-binding specificity and biochemical function of Eld.
Interactions between signaling pathways
Our analysis indicates that the function of Eld is not restricted to the Wg signaling pathway. Although eld mutant cells behave similarly in many respects to cells that lack sgg or express wg ectopically, significant differences nevertheless exist. In particular, eld clearly functions in embryogenesis before wg is expressed. The effects of eld on individual Eve stripes are reminiscent of those of the JAK/STAT (hopscotch/marelle; Binari and Perrimon 1994; Hou et al. 1996; Yan et al. 1996) signaling pathway, although the particular stripes affected are different. In the eye, eld phenotypes are less extreme than those caused by ectopic wg (Treisman and Rubin 1995) although equally strong phenotypes can be seen if Minute mutations are used to increase the size of clones. However, eld appears to be required for proliferation, whereas ectopic wg stimulates proliferation. eld has been isolated independently as a dominant suppressor of activated Ras1 expressed in the eye (Karim et al. 1996), and its requirement for ras signaling may explain its role in proliferation. The effects of eld on wing margin bristle formation differ from those of sgg in their nonautonomy and their positional restriction; again, the nonautonomy suggests that Eld does not function solely as a nuclear effector of Wg.
eld is not the only gene described with the potential to participate in multiple pathways. The sgg gene also has multiple functions in the embryo; in addition to regulating En maintenance, it is required for normal organization of the nervous system (Perrimon and Smouse 1989). Loss of eld leads to a similar disorganization that is considerably less extreme than the overproduction of neuroblasts seen in N mutants (data not shown). Additional precedents exist for effects on early stages of patterning caused by mutations that also affect neurogenesis; strawberry notch (sno) has defects at the blastoderm stage (Coyle-Thompson and Banerjee 1993), whereas hairy (h), which has neurogenic effects in the notum, wing and eye, acts as a pair-rule gene in the embryo (Rushlow et al. 1989; Brown et al. 1995). The groucho gene appears to function with h in segmentation, with the E(spl) genes in neurogenesis, and with deadpan in sex determination (Paroush et al. 1994), in addition to repressing en expression in the wing disc and embryo (de Celis and Ruiz-Gomez 1995). In the latter case, the phenotypes may be accounted for by a common mechanism, such as co-repression with basic HLH proteins (Paroush et al. 1994), rather than a common genetic pathway. Many questions remain about the mode of Eld action and its regulation by Wg and other signals, which can only be answered by biochemical analysis. Nevertheless, results to date suggest that Eld belongs to the growing class of signal integrators.
Materials and methods
Fly strains
The screen in which eld was identified will be described in detail elsewhere. Briefly, wild-type males were mutagenized with EMS or X-rays and mated to roDOM/TM3, Sb virgin females. The F1 progeny was screened for enhancers or suppressors of the furrow-stop phenotype of roDOM. Two EMS alleles of eld were used for all experiments: eld616, which has a stop codon at position 338, and eld308, which behaved as a stronger allele than eld616, although we were unable to molecularly identify the mutation. Embryos maternally mutant for either allele do not express full-length protein as determined by immunohistochemistry with the monoclonal antibody directed against the predicted DNA-binding domain. The three lethal P-element alleles used were also identified as weak suppressors of roDOM; they failed to complement the lethality of the EMS alleles. l(3)00090, l(3)04662, and l(3)06726 have been described by Spradling et al. (1995). Other alleles used were roDOM (Heberlein et al. 1993), dppd-blk (Masucci et al. 1990), dpp–lacZ BS3.0 (Blackman et al. 1991), wgP (Kassis et al. 1992), wgIL114, wgCX4, E(spl)r16, M(3)be[36e], M(3)w, N317 (Karim et al. 1996), and N5419. Two enhancer trap lines, a generous gift of Kevin Moses (University of Southern California, Los Angeles), were used to mark eld clones in the eye disc; one is at chromosomal position 94F, which expresses only posterior to the furrow (Fig. 2C,E,G), and the other at position 96C, which expresses at lower levels throughout the eye disc (Fig. 2D). An Arm–LacZ line on 3R was also used (Vincent et al. 1994; Fig. 2H).
Genetics
To make eld mutant clones in the adult eye, males of genotype w; FRT82, eld308/TM3, or w; FRT82, eld616/TM3 were crossed to females of genotype w, hsFLP1; FRT82, P(w+)90C. To make eld mutant clones in the eye disc, males of genotype w; FRT82, eld308/TM6B, or w; FRT82, eld616/TM6B were crossed to females of genotype w, hsFLP1; FRT82, P(lacZ, ry+)94F or P(lacZ, ry+)96C. Non-Tb larvae were selected for analysis. To make eld, E(spl) double mutant clones, the males used were w; FRT82, eld308, E(spl)r16/TM6B. To make large eld clones in the eye disc, the females used were w, hsFLP1; FRT82, P(ry+)94F, M(3)be[36e]. To make eld clones in the wing disc, the females were y, w, hsFLP1; FRT82, P(hs-πM)87E, Sb63b, P(y+)96E. To make eld mutant germ-line clones, males of genotype w, hsFLP1/Y; FRT82, ovoD1/TM3 were crossed to females of genotype w; FRT82, eld308/TM3, and non-Sb females resulting from this cross were crossed to eld308/TM3, eve–lacZ males. To make maternal eld, zygotic wg double mutants, males of genotype w, hsFLP1/Y; FRT82, ovoD1/TM3 were crossed to females of genotype w; FRT82, eld616; wgP/SM6.TM6B, and the resulting w, hsFLP1/w; FRT82, ovoD1/FRT82, eld616; wgP/+ females were crossed to w; FRT82, eld616; wgP/SM6.TM6B males. All larvae were heat-shocked for 1 hr at 38.5°C in the first or second instar to induce hsFLP expression.
Histology and immunohistochemistry
Flies were prepared for scanning electron microscopy as described by Kimmel et al. (1990). Adult eyes were fixed, embedded, and sectioned as described by Tomlinson and Ready (1987). Eye imaginal discs were stained with antibodies as described by Xu and Rubin (1993), except that the detergent used was 0.2% Triton. Anti-Elav and anti-Eld were both diluted 1:2. Double labeling with antibody and X-gal was performed as described by Treisman et al. (1995). Embryos were stained with antibodies by blocking in PBS with 0.2% Triton and 5% donkey serum (PBSTS), incubating in primary antibody in PBSTS at 4°C overnight, washing in PBS with 0.2% Triton three times for 20 min at room temperature, incubating in secondary antibody (donkey anti-mouse or anti-rabbit, Jackson Immunoresearch) at a 1:200 dilution in PBSTS for 1 hr at room temperature, washing as above, and developing with a metal-stabilized DAB solution (Pierce). Rabbit anti-Twist was provided by Kathryn Anderson (University of California, Berkeley) and was used at a dilution of 1:5000. Rabbit anti-Eve was provided by Monica Boyle (Rockefeller University, New York, NY) and was used at a dilution of 1:5000. Mouse anti-En was provided by Peter Bokor (Rockefeller University, New York, NY) and was used undiluted. In situ hybridization and in situ/antibody double labeling were performed as described by Ronchi et al. (1993). To examine wings, flies were dehydrated through an ethanol series and the wings were mounted in methyl salicylate/Canada balsam (1:2).
Molecular biology
Standard procedures were used for DNA analysis (Sambrook et al. 1989). DNA surrounding the l(2)00090, l(2)04662, and l(2)06726 P elements was isolated by plasmid rescue (Mlodzik et al. 1990). All these probes were used to screen a cosmid library (Tamkun et al. 1992) and the genomic region was assembled by a cosmid walk. Fourteen cDNAs that cross-hybridized were isolated from a λgt10 third-instar eye-antennal disc cDNA library (constructed by Alan Cowman, Walter and Eliza Hall Research Institute, Victoria, Australia) using probes covering 24 kb of the walk. Further cDNA clones were isolated by a cDNA walk using both this library and a 9- to 12-hr embryonic λgt11 cDNA library (constructed by Kai Zinn, Caltech, Pasadena, CA). Six cDNAs were subcloned into p(Bluescript)SK+ and were sequenced completely on both strands using an Automated Laser Fluorescent DNA sequencer (Pharmacia), and several additional cDNAs were sequenced partially. The exon positions were mapped onto the genomic structure by hybridization and sequencing of junctional regions. Sequencing of the P-element rescue fragments was used to define their precise insertion positions. Sequences were analyzed using Staden and LaserGene software, and homology searches were done using the blastp program.
Antibody production
A fragment encoding amino acids 965–1049 of the Eld protein was generated by PCR, and was subcloned into the BamHI and EcoRI sites of pGEX-1 (Pharmacia) using sites present in the PCR primers. The fusion protein was purified from bacteria on glutathione–agarose beads and injected into mice. Monoclonal antibodies were screened first by ELISA and then by staining embryos.
Acknowledgments
We are grateful to Noah Solomon and Bernard Goldschmidt for DNA sequencing and oligonucleotide synthesis, to Todd Laverty for chromosome in situ hybridization, to Kelli Lopardo and Elaine Kwan for help with monoclonal antibody preparation, and to Ian Oliver for technical assistance. Scanning electron micrographs were taken at the California Department of Health Services and we gratefully acknowledge the assistance of Dr. Don Scales. We thank Ron Blackman, Felix Karim, Kevin Moses, Kathryn Anderson, Monica Boyle, Peter Bokor, and Claude Desplan for fly stocks and reagents. The manuscript was improved by the critical comments of Alex Schier, Mark Van Doren, and Scott Dougan, and by the editing of Donald Haumant. J.E.T. was supported by a Jane Coffin Childs Memorial Fund postdoctoral fellowship. G.M.R. is a HHMI investigator. This work was supported in part by National Institutes of Health grant GM33135 to G.M.R. and in part by NIH grant EY11410 to U.H.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL treisman@saturn.med.nyu.edu; FAX (212) 263-7760.
References
- Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- Axelrod JD, Matsuno K, Artavanis-Tsakonas S, Perrimon N. Interaction between wingless and Notch signaling pathways mediated by dishevelled. Science. 1996;271:1826–1832. doi: 10.1126/science.271.5257.1826. [DOI] [PubMed] [Google Scholar]
- Baker NE. Embryonic and imaginal requirements for wingless, a segment-polarity gene in Drosophila. Dev Biol. 1988;125:96–108. doi: 10.1016/0012-1606(88)90062-0. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bejsovec A, Martinez-Arias A. Roles of wingless in patterning the larval epidermis of Drosophila. Development. 1991;113:471–485. doi: 10.1242/dev.113.2.471. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh J-C, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Binari R, Perrimon N. Stripe-specific regulation of pair-rule genes by hopscotch, a putative Jak family tyrosine kinase in Drosophila. Genes & Dev. 1994;8:300–312. doi: 10.1101/gad.8.3.300. [DOI] [PubMed] [Google Scholar]
- Blackman RK, Sanicola M, Raftery LA, Gillevet T, Gelbart WM. An extensive 3′ cis-regulatory region directs the imaginal disc expression of decapentaplegic, a member of the TGF-β family in Drosophila. Development. 1991;111:657–665. doi: 10.1242/dev.111.3.657. [DOI] [PubMed] [Google Scholar]
- Blair SS. shaggy (zeste-white 3) and the formation of supernumerary bristle precursors in the developing wing blade of Drosophila. Dev Biol. 1992;152:263–278. doi: 10.1016/0012-1606(92)90134-3. [DOI] [PubMed] [Google Scholar]
- Bourouis M, Heitzler P, El Messal M, Simpson P. Mutant Drosophila embryos in which all cells adopt a neural fate. Nature. 1989;341:442–444. doi: 10.1038/341442a0. [DOI] [PubMed] [Google Scholar]
- Bourouis M, Moore P, Ruel L, Grau Y, Heitzler P, Simpson P. An early embryonic product of the gene shaggy encodes a serine/threonine protein kinase related to the CDC28/cdc2+ subfamily. EMBO J. 1990;9:2877–2884. doi: 10.1002/j.1460-2075.1990.tb07477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NL, Sattler CA, Paddock SW, Carroll SB. Hairy and emc negatively regulate morphogenetic furrow progression in the Drosophila eye. Cell. 1995;80:879–887. doi: 10.1016/0092-8674(95)90291-0. [DOI] [PubMed] [Google Scholar]
- Brunner E, Peter O, Schweizer L, Basler K. pangolin encodes a Lef-1 homolog that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature. 1997;385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- Burke R, Basler K. Hedgehog-dependent patterning in the Drosophila eye can occur in the absence of dpp signaling. Dev Biol. 1996;179:360–368. doi: 10.1006/dbio.1996.0267. [DOI] [PubMed] [Google Scholar]
- Campbell G, Weaver T, Tomlinson A. Axis specification in the developing Drosophila appendage: The role of wingless, decapentaplegic, and the homeobox gene aristaless. Cell. 1993;74:1113–1123. doi: 10.1016/0092-8674(93)90732-6. [DOI] [PubMed] [Google Scholar]
- Chanut F, Heberlein U. Role of decapentaplegic in initiation and progression of the morphogenetic furrow in the developing Drosophila retina. Development. 1997;124:559–567. doi: 10.1242/dev.124.2.559. [DOI] [PubMed] [Google Scholar]
- Chou TB, Perrimon N. Use of a yeast site-specific recombinase to produce female germline chimeras in Drosophila. Genetics. 1992;131:643–653. doi: 10.1093/genetics/131.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B, Sincox AA, Cohen SM. Allocation of the imaginal primordia in the Drosophila embryo. Development. 1993;117:597–608. doi: 10.1242/dev.117.2.597. [DOI] [PubMed] [Google Scholar]
- Couso JP, Martinez-Arias A. Notch is required for wingless signaling in the epidermis of Drosophila. Cell. 1994;79:259–272. doi: 10.1016/0092-8674(94)90195-3. [DOI] [PubMed] [Google Scholar]
- Couso JP, Bate M, Martinez-Arias A. A wingless-dependent polar coordinate system in Drosophila imaginal discs. Science. 1993;259:484–489. doi: 10.1126/science.8424170. [DOI] [PubMed] [Google Scholar]
- Couso JP, Bishop SA, Martinez-Arias A. The wingless signaling pathway and the patterning of the wing margin in Drosophila. Development. 1994;120:621–636. doi: 10.1242/dev.120.3.621. [DOI] [PubMed] [Google Scholar]
- Couso JP, Knust E, Martinez-Arias A. Serrate and wingless cooperate to induce vestigial gene expression and wing formation in Drosophila. Curr Biol. 1995;5:1437–1448. doi: 10.1016/s0960-9822(95)00281-8. [DOI] [PubMed] [Google Scholar]
- Coyle-Thompson CA, Banerjee U. The strawberry notch gene functions with Notch in common developmental pathways. Development. 1993;119:377–395. doi: 10.1242/dev.119.2.377. [DOI] [PubMed] [Google Scholar]
- de Celis JF, Ruiz-Gomez M. groucho and hedgehog regulate engrailed expression in the anterior compartment of the Drosophila wing. Development. 1995;121:3467–3476. doi: 10.1242/dev.121.10.3467. [DOI] [PubMed] [Google Scholar]
- de Celis JF, Garcia-Bellido A, Bray SJ. Activation and function of Notch at the dorsal-ventral boundary of the wing imaginal disc. Development. 1996;122:359–369. doi: 10.1242/dev.122.1.359. [DOI] [PubMed] [Google Scholar]
- Delidakis C, Preiss A, Hartley DA, Artavanis-Tsakonas S. Two genetically and molecularly distinct functions involved in early neurogenesis reside within the Enhancer of split locus of Drosophila melanogaster. Genetics. 1991;129:803–823. doi: 10.1093/genetics/129.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplan C, Theis J, O’Farrell PH. The sequence specificity of homeodomain-DNA interaction. Cell. 1988;54:1081–1090. doi: 10.1016/0092-8674(88)90123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Benjumea FJ, Cohen SM. Serrate signals through Notch to establish a wingless-dependent organizer at the dorsal-ventral compartment boundary of the Drosophila wing. Development. 1995;121:4215–4225. doi: 10.1242/dev.121.12.4215. [DOI] [PubMed] [Google Scholar]
- DiNardo S, Sher E, Heemskerk-Jongens J, Kassis JA, O’Farrell PH. Two-tiered regulation of spatially patterned engrailed gene expression during Drosophila embryogenesis. Nature. 1988;332:604–609. doi: 10.1038/332604a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S, Heemskerk J, Dougan S, O’Farrell PH. The making of a maggot: Patterning the Drosophila embryonic epidermis. Curr Opin Genet Dev. 1994;4:529–534. doi: 10.1016/0959-437x(94)90068-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty D, Feger G, Younger-Shepherd S, Jan LY, Jan YN. Delta is a ventral to dorsal signal complementary to Serrate, another Notch ligand, in Drosophila wing formation. Genes & Dev. 1996;10:421–434. doi: 10.1101/gad.10.4.421. [DOI] [PubMed] [Google Scholar]
- Dougan S, DiNardo S. Drosophila wingless generates cell type diversity among engrailed expressing cells. Nature. 1992;360:347–350. doi: 10.1038/360347a0. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Anderson KV. decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell. 1992a;71:451–461. doi: 10.1016/0092-8674(92)90514-d. [DOI] [PubMed] [Google Scholar]
- ————— Localized enhancement and repression of the activity of the TGF-beta family member, decapentaplegic, is necessary for dorsal-ventral pattern formation in the Drosophila embryo. Development. 1992b;114:583–597. doi: 10.1242/dev.114.3.583. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A, Ripoll P, Morata G. Developmental compartmentalization of the wing disc of Drosophila. Nature New Biol. 1973;245:251–253. doi: 10.1038/newbio245251a0. [DOI] [PubMed] [Google Scholar]
- Golic K. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- Gregory SL, Kortschak D, Kalionis B, Saint R. Characterization of the dead ringer gene identifies a novel, highly conserved family of sequence-specific DNA-binding proteins. Mol Cell Biol. 1996;16:792–799. doi: 10.1128/mcb.16.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Pflugfelder GO. Control of the gene optomotor-blind in Drosophila wing development by decapentaplegic and wingless. Science. 1996;271:1601–1604. doi: 10.1126/science.271.5255.1601. [DOI] [PubMed] [Google Scholar]
- Han K, Manley JL. Transcriptional repression by the Drosophila even-skipped protein: Definition of a minimal repression domain. Genes & Dev. 1993;7:491–503. doi: 10.1101/gad.7.3.491. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Rubinfeld B, Souza B, Polakis P, Wieschaus E, Levine AJ. A Drosophila homolog of the tumor suppressor gene adenomatous polyposis coli down-regulates β-catenin but its zygotic expression is not essential for the regulation of Armadillo. Proc Natl Acad Sci. 1997;94:242–247. doi: 10.1073/pnas.94.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein U, Wolff T, Rubin GM. The TGFβ homolog dpp and the segment polarity gene hedgehog are required for propagation of a morphogenetic wave in the Drosophila retina. Cell. 1993;75:913–926. doi: 10.1016/0092-8674(93)90535-x. [DOI] [PubMed] [Google Scholar]
- Heberlein U, Singh CM, Luk AY, Donohoe TJ. Growth and differentiation in the Drosophila eye coordinated by hedgehog. Nature. 1995;373:709–711. doi: 10.1038/373709a0. [DOI] [PubMed] [Google Scholar]
- Heemskerk J, DiNardo S, Kostriken R, O’Farrell PH. Multiple modes of engrailed regulation in the progression towards cell fate determination. Nature. 1991;352:404–410. doi: 10.1038/352404a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrscher RF, Kaplan MH, Lelsz DL, Das C, Scheuermann R, Tucker PW. The immunoglobulin heavy-chain matrix-associating regions are bound by Bright: A B cell-specific trans-activator that describes a new DNA-binding protein family. Genes & Dev. 1995;9:3067–3082. doi: 10.1101/gad.9.24.3067. [DOI] [PubMed] [Google Scholar]
- Hou XS, Melnick MB, Perrimon N. marelle acts downstream of the Drosophila HOP/JAK kinase and encodes protein similar to the mammalian STATs. Cell. 1996;84:411–419. doi: 10.1016/s0092-8674(00)81286-6. [DOI] [PubMed] [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R. Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- Immergluck K, Lawrence PA, Bienz M. Induction across germ layers in Drosophila mediated by a genetic cascade. Cell. 1990;62:261–268. doi: 10.1016/0092-8674(90)90364-k. [DOI] [PubMed] [Google Scholar]
- Karim FD, Chang HC, Therrien M, Wassarman DA, Laverty T, Rubin GM. A screen for genes that function downstream of Ras1 during Drosophila eye development. Genetics. 1996;143:315–329. doi: 10.1093/genetics/143.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis JA. Spatial and temporal control elements of the Drosophila engrailed gene. Genes & Dev. 1990;4:433–443. doi: 10.1101/gad.4.3.433. [DOI] [PubMed] [Google Scholar]
- Kassis JA, Noll E, VanSickle EP, Odenwald WF, Perrimon N. Altering the insertional specificity of a Drosophila transposable element. Proc Natl Acad Sci. 1992;89:1919–1923. doi: 10.1073/pnas.89.5.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Irvine KD, Carroll SB. Cell recognition, signal induction, and symmetrical gene activation at the dorsal-ventral boundary of the developing Drosophila wing. Cell. 1995;82:795–802. doi: 10.1016/0092-8674(95)90476-x. [DOI] [PubMed] [Google Scholar]
- Kimmel BE, Heberlein U, Rubin GM. The homeodomain protein rough is expressed in a subset of cells in the developing Drosophila eye where it can specify photoreceptor cell subtype. Genes & Dev. 1990;4:712–727. doi: 10.1101/gad.4.5.712. [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Nusse R. Signaling by wingless in Drosophila. Dev Biol. 1994;166:396–414. doi: 10.1006/dbio.1994.1325. [DOI] [PubMed] [Google Scholar]
- Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes & Dev. 1994;8:118–130. doi: 10.1101/gad.8.1.118. [DOI] [PubMed] [Google Scholar]
- Ma C, Moses K. wingless and patched are negative regulators of the morphogenetic furrow and can affect tissue polarity in the developing Drosophila compound eye. Development. 1995;121:2279–2289. doi: 10.1242/dev.121.8.2279. [DOI] [PubMed] [Google Scholar]
- Ma C, Zhou Y, Beachy PA, Moses K. The segment polarity gene hedgehog is required for progression of the morphogenetic furrow in the developing Drosophila eye. Cell. 1993;75:927–938. doi: 10.1016/0092-8674(93)90536-y. [DOI] [PubMed] [Google Scholar]
- Masucci JD, Miltenberger RJ, Hoffmann FM. Pattern-specific expression of the Drosophila decapentaplegic gene in imaginal discs is regulated by 3′ cis-regulatory elements. Genes & Dev. 1990;4:2011–2023. doi: 10.1101/gad.4.11.2011. [DOI] [PubMed] [Google Scholar]
- Mlodzik M, Baker NE, Rubin GM. Isolation and expression of scabrous, a gene regulating neurogenesis in Drosophila. Genes & Dev. 1990;4:1848–1861. doi: 10.1101/gad.4.11.1848. [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Morata G, Ripoll P. Minutes: Mutants autonomously affecting cell division rate in Drosophila. Dev Biol. 1975;42:211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- Morata G, Lawrence PA. The development of wingless, a homeotic mutation of Drosophila. Dev Biol. 1977;56:227–240. doi: 10.1016/0012-1606(77)90266-4. [DOI] [PubMed] [Google Scholar]
- Neumann CJ, Cohen SM. Distinct mitogenic and cell specification functions of wingless in different regions of the wing. Development. 1996;122:1781–1789. doi: 10.1242/dev.122.6.1781. [DOI] [PubMed] [Google Scholar]
- Ng M, Diaz-Benjumea FJ, Vincent J-P, Wu J, Cohen SM. Specification of the wing by localized expression of wingless protein. Nature. 1996;381:316–318. doi: 10.1038/381316a0. [DOI] [PubMed] [Google Scholar]
- Noordemeer J, Klingensmith J, Perrimon N, Nusse R. dishevelled and armadillo act in the Wingless signaling pathway in Drosophila. Nature. 1994;367:80–83. doi: 10.1038/367080a0. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- Orsulic S, Peifer M. An in vivo structure-function study of armadillo, the beta-catenin homologue, reveals both separate and overlapping regions of the protein required for cell adhesion and for wingless signaling. J Cell Biol. 1996;134:1283–1300. doi: 10.1083/jcb.134.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroush ZF, Finley Jr RL, Kidd T, Wainwright SM, Ingham PW, Brent R, Ish-Horowicz D. Groucho is required for Drosophila neurogenesis, segmentation, and sex determination and interacts directly with hairy-related bHLH proteins. Cell. 1994;79:805–815. doi: 10.1016/0092-8674(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Peifer M, Wieschaus E. The segment polarity gene armadillo encodes a functionally modular protein that is the Drosophila homolog of human plakoglobin. Cell. 1990;63:1167–1176. doi: 10.1016/0092-8674(90)90413-9. [DOI] [PubMed] [Google Scholar]
- Peifer M, Sweeton D, Casey M, Wieschaus E. wingless signal and zeste-white 3 kinase trigger opposing changes in the intracellular distribution of armadillo. Development. 1994a;120:369–380. doi: 10.1242/dev.120.2.369. [DOI] [PubMed] [Google Scholar]
- Peifer M, Pai L-M, Casey M. Phosphorylation of the Drosophila adherens junction protein armadillo: Roles for wingless signal and zeste-white 3 kinase. Dev Biol. 1994b;166:543–556. doi: 10.1006/dbio.1994.1336. [DOI] [PubMed] [Google Scholar]
- Perrimon N, Smouse D. Multiple functions of a Drosophila homeotic gene, zeste-white3, during segmentation and neurogenesis. Dev Biol. 1989;135:287–305. doi: 10.1016/0012-1606(89)90180-2. [DOI] [PubMed] [Google Scholar]
- Phillips RG, Whittle JRS. wingless expression mediates determination of peripheral nervous system elements in late stages of Drosophila wing disc development. Development. 1993;118:427–438. doi: 10.1242/dev.118.2.427. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Zipursky SL. Induction of Drosophila eye development by Decapentaplegic. Development. 1997;124:271–278. doi: 10.1242/dev.124.2.271. [DOI] [PubMed] [Google Scholar]
- Ready DF, Hanson TE, Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976;53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Riese J, Yu X, Munnerlyn A, Eresh S, Hsu S-C, Grosschedl R, Bienz M. LEF-1, a nuclear factor coordinating signaling inputs from wingless and decapentaplegic. Cell. 1997;88:777–788. doi: 10.1016/s0092-8674(00)81924-8. [DOI] [PubMed] [Google Scholar]
- Robinow S, White K. Characterization and spatial distribution of the ELAV protein during Drosophila melanogaster development. J Neurobiol. 1991;22:443–461. doi: 10.1002/neu.480220503. [DOI] [PubMed] [Google Scholar]
- Ronchi E, Treisman JE, Dostatni N, Struhl G, Desplan C. Down- regulation of the Drosophila morphogen bicoid by the torso receptor-mediated signal transduction cascade. Cell. 1993;74:347–355. doi: 10.1016/0092-8674(93)90425-p. [DOI] [PubMed] [Google Scholar]
- Royet J, Finkelstein R. hedgehog, wingless and orthodenticle specify adult head development in Drosophila. Development. 1996;122:1849–1858. doi: 10.1242/dev.122.6.1849. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz RH, Munemitsu S, Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Blair SS. Notch regulates wingless expression and is not required for reception of the paracrine wingless signal during wing margin neurogenesis in Drosophila. Development. 1995;121:2813–2824. doi: 10.1242/dev.121.9.2813. [DOI] [PubMed] [Google Scholar]
- Rulifson EJ, Micchelli CA, Axelrod JD, Perrimon N, Blair SS. wingless refines its own expression domain on the Drosophila wing margin. Nature. 1996;384:72–74. doi: 10.1038/384072a0. [DOI] [PubMed] [Google Scholar]
- Rushlow CA, Hogan A, Pinchin SM, Howe KM, Lardelli M, Ish-Horowicz D. The Drosophila hairy protein acts in both segmentation and bristle patterning and shows homology to N-myc. EMBO J. 1989;8:3095–3103. doi: 10.1002/j.1460-2075.1989.tb08461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual, second ed. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Siegfried E, Perrimon N. Drosophila wingless: A paradigm for the function and mechanism of Wnt signaling. BioEssays. 1994;16:395–404. doi: 10.1002/bies.950160607. [DOI] [PubMed] [Google Scholar]
- Siegfried E, Perkins LA, Capaci TM, Perrimon N. Putative protein kinase product of the Drosophila segment-polarity gene zeste-white3. Nature. 1990;345:825–829. doi: 10.1038/345825a0. [DOI] [PubMed] [Google Scholar]
- Siegfried E, Chou TB, Perrimon N. wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell. 1992;71:1167–1179. doi: 10.1016/s0092-8674(05)80065-0. [DOI] [PubMed] [Google Scholar]
- Siegfried E, Wilder EL, Perrimon N. Components of wingless signaling in Drosophila. Nature. 1994;367:76–80. doi: 10.1038/367076a0. [DOI] [PubMed] [Google Scholar]
- Simpson P, El Messal W, Moscoso del Prado J, Ripoll P. Stripes of positional homologies across the wing blade of Drosophila melanogaster. Development. 1988;103:391–401. [Google Scholar]
- Spradling AC, Stern DM, Kiss I, Roote J, Laverty T, Rubin GM. Gene disruptions using P transposable elements: An integral component of the Drosophila genome project. Proc Natl Acad Sci. 1995;92:10824–10830. doi: 10.1073/pnas.92.24.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- Tamkun JW, Deuring R, Scott MP, Kissinger M, Pattatucci AM, Kaufman TC, Kennison JA. brahma: A regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2. Cell. 1992;68:561–572. doi: 10.1016/0092-8674(92)90191-e. [DOI] [PubMed] [Google Scholar]
- Theisen H, Purcell J, Bennett M, Kansagara D, Syed A, Marsh JL. dishevelled is required during wingless signaling to establish both cell polarity and cell identity. Development. 1994;120:347–360. doi: 10.1242/dev.120.2.347. [DOI] [PubMed] [Google Scholar]
- Treisman JE, Rubin GM. wingless inhibits morphogenetic furrow movement in the Drosophila eye disc. Development. 1995;121:3519–3527. doi: 10.1242/dev.121.11.3519. [DOI] [PubMed] [Google Scholar]
- Treisman JE, Lai Z, Rubin GM. shortsighted acts in the decapentaplegic pathway in Drosophila eye development and has homology to a mouse TGF-β responsive gene. Development. 1995;121:2835–2845. doi: 10.1242/dev.121.9.2835. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Ready DF. Cell fate in the Drosophila ommatidium. Dev Biol. 1987;123:264–275. doi: 10.1016/0012-1606(87)90448-9. [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ympa A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–800. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- Vincent JP, Girdham CH, O’Farrell PH. A cell-autonomous, ubiquitous marker for the analysis of Drosophila genetic mosaics. Dev Biol. 1994;164:328–331. doi: 10.1006/dbio.1994.1203. [DOI] [PubMed] [Google Scholar]
- Wiersdorff V, Lecuit T, Cohen SM, Mlodzik M. Mad acts downstream of dpp receptors, revealing a differential requirement for dpp signaling in initiation and propagation of morphogenesis in the Drosophila eye. Development. 1996;122:2153–2162. doi: 10.1242/dev.122.7.2153. [DOI] [PubMed] [Google Scholar]
- Wilder EL, Perrimon N. Dual functions of wingless in the Drosophila leg imaginal disc. Development. 1995;121:477–488. doi: 10.1242/dev.121.2.477. [DOI] [PubMed] [Google Scholar]
- Williams JA, Paddock SW, Carroll SB. Pattern formation in a secondary field: A hierarchy of regulatory genes subdivides the developing Drosophila wing disc into discrete subregions. Development. 1993;117:571–584. doi: 10.1242/dev.117.2.571. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Yan R, Small S, Desplan C, Dearolf CR, Darnell JE. Identification of a Stat gene that functions in Drosophila development. Cell. 1996;84:421–430. doi: 10.1016/s0092-8674(00)81287-8. [DOI] [PubMed] [Google Scholar]
- Yanagawa S, van Leeuwen F, Wodarz A, Klingensmith J, Nusse R. The Dishevelled protein is modified by Wingless signaling in Drosophila. Genes & Dev. 1995;9:1087–1097. doi: 10.1101/gad.9.9.1087. [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–844. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]