Abstract
The rhesus macaque model is currently the best available model for HIV-AIDS with respect to understanding the pathogenesis as well as for the development of vaccines and therapeutics1,2,3. Here, we describe a method for the detailed phenotypic and functional analyses of cellular immune responses, specifically intracellular cytokine production by CD4+ and CD8+ T cells as well as the individual memory subsets. We obtained precise quantitative and qualitative measures for the production of interferon gamma (INF-) and interleukin (IL) -2 in both CD4+ and CD8+ T cells from the rhesus macaque PBMC stimulated with PMA plus ionomycin (PMA+I). The cytokine profiles were different in the different subsets of memory cells. Furthermore, this protocol provided us the sensitivity to demonstrate even minor fractions of antigen specific CD4+ and CD8+ T cell subsets within the PBMC samples from rhesus macaques immunized with an HIV envelope peptide cocktail vaccine developed in our laboratory. The multicolor flow cytometry technique is a powerful tool to precisely identify different populations of T cells 4,5 with cytokine-producing capability6 following non-specific or antigen-specific stimulation 5,7.
Protocol
The procedures for the detailed phenotypic and functional analyses of total as well as memory subsets of T cells can be divided into four parts: 1) Cell preparation and antigenic stimulation, 2) Surface marker staining, 3) Intracellular cytokine staining and 4) Flow cytometry analyses.
1. Cell preparation and antigenic stimulation
Both freshly isolated as well as cryo-preserved peripheral blood mononuclear cells (PBMC) were used in this protocol. The PBMC were isolated from heparinized or citrated venous blood samples of rhesus macaques by density gradient sedimentation using Ficoll-Hypaque (Histopaquen-1077, Sigma-Aldrich, ST. Louis, MO). Aliquots of PBMC were stored frozen in 90% FCS and 10% DMSO in liquid nitrogen.
When using the cryo-preserved PBMC, the vials of frozen PBMC were removed from liquid nitrogen and rapidly thawed in a 37 C water bath, gently mixed, washed with RPMI-1640 (HyClone laboratories, logan, UT) to remove the freezing medium and re-suspended in complete media [CM; RPMI-1640 supplement with 10% heat-inactivated FCS (HyClone laboratories), 2mM L-glutamine (Sigma-Aldrich), 100U/ml penicillin/streptomycin (Invitrogen), and cultured in 6-well tissue culture plates overnight at 37°C in a humidified 5% CO2 atmosphere. The next morning, viable cell counts were determined by the trypan-blue dye exclusion method and re-suspended in CM.
The cells were treated differently for non-specific versus antigen-specific stimulation followed by cytokine analyses: (a) For non-specific stimulation with phorbol 12-myristate 13-acetate (PMA) and Ionomycin (I) (Sigma-Aldrich, St. Louis, MO), aliquots of cells in a volume of 0.1ml (1.0 x 106 cells/well) were plated in individual wells of 96-well tissue culture plates (BD Biosciences, Franklin lake, NJ). The PMA and Ionomycin were used at a final concentration of 50ng/ml and 500ng/ml, respectively. The final total volume for 96-well tissue culture plate is 0.2ml/well: (b) For antigen specific stimulation, 1.0 x 106 cells in a volume 1.0 ml were plated in individual wells of 24-well tissue culture plates (BD Biosciences, Franklin lake, NJ), where the wells were pre-treated as previously described with modification9. Briefly, the 24-well tissue culture plates were coated with 2.5 μg/ml/well goat anti-mouse IgG (H + L) (Kierkegaard and Perry Laboratory, Gaithersburg, MD) in 50mM Tris solution (PH 8.6) for 4°C overnight. The next morning the plates were washed twice with sterile PBS and co-stimulatory mAb CD49d, clone 9F10 (BD Biosciences; San Jose, CA) were added at 10 μg/ml/well followed by incubating for one hour at 37°C. After the incubation, the plates were washed twice with sterile PBS at room temperature. The antigens of interest (including cocktail of six HIV envelope peptides 8 were added at a final concentration of 10μg/ml of each peptide. Additional wells with cells in CM alone were prepared as negative control. The final total volume for each well of the 24-well tissue culture plate was 1.0 ml.
The cells were cultured for 6 hours at 37°C in a humidified 5% CO2 atmosphere. Brefeldin A (Sigma-Aldrich, St. Louis, MO) was added to the culture at 10μg/ml for the final 4.5 hours of stimulation. Subsequently, the cells were transferred to 5 ml polypropylene tubes and washed with cold (4°C) flow wash buffer (Dulbecco's PBS (DPBS, Ca2/Mg2-free; Life technologies, Rockville, MD) with 2% heat-inactivated FCS), and then processed for staining with the various fluorescence labeled antibodies.
2. Immunofluorescence surface markers staining
Stimulated cells were first stained with 1ul live/dead fixable aqua fluorescent reactive dye at 4°C in the dark for 30 minutes, washed once with cold flow wash buffer.
Then, the following appropriately titrated fluorescence-labeled monoclonal antibodies were added to the cells: anti-CD3 PE-Cy7 (SP34-2), anti-CD8 Alexa700 (RPA-T8), anti-CD28 PerCP-cy5.5 (L293), anti-CD95 APC (DX2) and anti CD4 pacific blue (OKT4), all from BD Biosciences (Franklin lake, NJ), except CD4 pacific blue (OKT4) from eBioscience (San Diego, CA). The cells were incubated for 30 minutes at 4°C in the dark.
For each experiment both compensation controls and fluorescence minus one (FMO) controls10,11 were utilized. Compensation controls were utilized to determine the spillover coeffecients of each individual stain into other detectors. For the compensation controls, a complete set of tubes containing cell suspensions from one of monkey were stained with fluorescent conjugated mAb individually as single color stains. FMO is a multicolor staining combination that contains all reagents but the one of interest and is used to determine the boundary between a positive and negative population by duplicating auto-fluorescence level and data present in fully stained sample.
Stained cells were then washed with cold flow wash buffer, and fixed in fixation/permeabilization solution (BD Biosciences; San Jose, CA) for at least 10 minutes (at this stage the cells can be stored overnight at 4°C in the dark) before performing intracellular cytokine staining.
3. Intracellular cytokine staining
Fixed cells were washed with flow wash buffer, and then Perm/Wash buffer (BD Biosciences; San Jose, CA) was added according to the instructions of the manufacturer. Briefly, cells were incubated in 0.1ml of the 1X Perm/Wash buffer for 10 minutes at 4°C in the dark.
Subsequently, the cells were incubated with the appropriately titrated FITC-labeled anti-IFN-γ (B27) and PE-labeled anti-IL-2 (MQ1-17H12) for 60 minutes at 4°C in the dark.
Then the cells were washed two more times with the permeabilization solution
After the final wash, the cells were re-suspended in 1% paraformaldehyde in DPBS and subjected to Flow cytometry analysis within 24 hours.
4. Flow cytometry analysis
The stained cells were acquired on a LSR II flow cytometer (BD Biosciences; San Jose, CA) or CyAn ADP (Dako, Carpinteria, CA). FACS data were analyzed by using FlowJo software (Tree Star, Ashland, OR).
Figure. 1 shows the gating scheme utilized for the analyses of the different T cell subsets from a representative animal. The lymphocytes were first gated via forward scatter (FSC) versus side scatter (SSC), and then live lymphocytes were identified based on SSC and aqua-negative population. The T cells were then positively identified by CD3 expression followed by the detection of the CD4+ CD8- (CD4+ T cells) and CD4- CD8+ (CD8+ T cells) populations within the CD3+ T cell population. The CD4+ and CD8+ T cells were further distinguished into different subsets on the basis of CD28 and CD95 expression as naíve (Tn, CD28+ CD95-), central memory (Tcm, CD28+ CD95+) and effector memory (Tem, CD28- CD95+) cells as described in the literature5, 10.
Figure. 2 shows how the different subsets of CD4+ and CD8+ T cells were assessed for functional capacity in terms of cytokine production (INF-γ and/or IL-2) in response to stimulation with PMA and ionomycin (PMA+I).
5. Representative FACS data
PBMC composition in rhesus macaques was determined using FACS analysis. Figure 1 shows the gating scheme utilized for the analyses of the different T cell subsets from a representative animal. The lymphocytes were first gated via forward scatter (FSC) versus side scatter (SSC), and then live lymphocytes were identified based on SSC and aqua-negative population. The T cells were then identified by CD3 expression. CD4+ CD8- (CD4+ T cells) and CD4- CD8+ (CD8+ T cells) populations within the CD3+ T cell population were also determined. As shown in Figure 2, the CD4+ and CD8+ T cells were further distinguished into different subsets on the basis of CD28 and CD95 expression as naíve (Tn, CD28+ CD95-), central memory (Tcm, CD28+ CD95+) and effector memory (Tem, CD28- CD95+) cells. Figure 2A shows INF- γ and IL-2 production in unstimulated and PMA / ionomycin stimulated CD4+ T cells, and subsets, and Figure 2B shows INF- γ and IL-2 production in unstimulated and PMA / ionomycin stimulated CD8+ T cells, and subsets Note the distinct cytokine profiles elicited by stimulation in each subset.
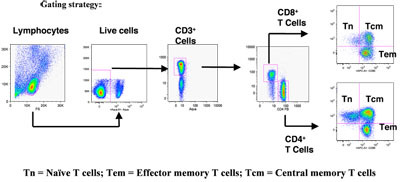 Figure 1. Gating strategy used for flow cytometric analysis of PBMC composition in rhesus macqaues. Please click here to see a larger version of figure 1.
Figure 1. Gating strategy used for flow cytometric analysis of PBMC composition in rhesus macqaues. Please click here to see a larger version of figure 1.
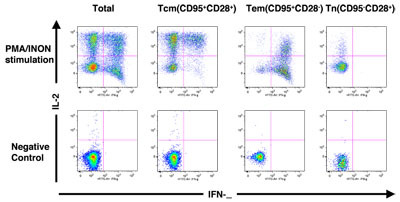
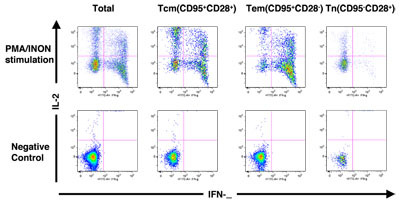 Figure 2. (A) Cytokine production profile of total CD4+ T cells and subsets. (B) Cytokine production profile of total CD8+ T cells and subsets. Please click here to see a larger version of 2a, or here for a larger version of figure 2b.
Figure 2. (A) Cytokine production profile of total CD4+ T cells and subsets. (B) Cytokine production profile of total CD8+ T cells and subsets. Please click here to see a larger version of 2a, or here for a larger version of figure 2b.
Acknowledgments
Dr. Pramod Nehete and Mrs. Bharti Nehete for macaque blood and PBMC samples; This work was partially supported by funds from NIH grant AI 46969, and all the cell culture media were produced by the Central Media Laboratory, which is funded by NIH grant CA 16672.
References
- Ogg GS. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup RA. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Maecker HT, Maino VC, Picker LJ. Multicolor flow cytometric analysis in SIV-infected rhesus macaque. Methods Cell Biol. 2004;75:535–557. doi: 10.1016/s0091-679x(04)75022-0. [DOI] [PubMed] [Google Scholar]
- Pitcher CJ. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- Keeney TS, Nomura LE, Maecker HT, Sastry KJ. Flow cytometric analysis of macaque whole blood for antigen-specific intracellular cytokine production by T lymphocytes. J Med Primatol. 2003;32:23–30. doi: 10.1034/j.1600-0684.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- Gauduin MC. Optimization of intracellular cytokine staining for the quantitation of antigen-specific CD4+ T cell responses in rhesus macaques. J Immunol Methods. 2004;288:61–79. doi: 10.1016/j.jim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Baumgarth N, Roederer MA. A practical approach to multicolor flow cytometry for immunophenotyping. J Immunol Methods. 2000;243:77–97. doi: 10.1016/s0022-1759(00)00229-5. [DOI] [PubMed] [Google Scholar]
- De Rosa SC, Herzenberg LA, Roederer M11-color. 13-parameter flow cytometry: identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nat Med. 2001;7:245–248. doi: 10.1038/84701. [DOI] [PubMed] [Google Scholar]
- Tung JW, Parks DR, Moore WA, Herzenberg LA. New approaches to fluorescence compensation and visualization of FACS data. Clin Immunol. 2004;110:277–283. doi: 10.1016/j.clim.2003.11.016. [DOI] [PubMed] [Google Scholar]


