Abstract
Transverse aortic constriction (TAC) in the mouse is a commonly used experimental model for pressure overload-induced cardiac hypertrophy and heart failure.1 TAC initially leads to compensated hypertrophy of the heart, which often is associated with a temporary enhancement of cardiac contractility. Over time, however, the response to the chronic hemodynamic overload becomes maladaptive, resulting in cardiac dilatation and heart failure.2 The murine TAC model was first validated by Rockman et al.1, and has since been extensively used as a valuable tool to mimic human cardiovascular diseases and elucidate fundamental signaling processes involved in the cardiac hypertrophic response and heart failure development. When compared to other experimental models of heart failure, such as complete occlusion of the left anterior descending (LAD) coronary artery, TAC provides a more reproducible model of cardiac hypertrophy and a more gradual time course in the development of heart failure. Here, we describe a step-by-step procedure to perform surgical TAC in mice. To determine the level of pressure overload produced by the aortic ligation, a high frequency Doppler probe is used to measure the ratio between blood flow velocities in the right and left carotid arteries.3, 4 With surgical survival rates of 80-90%, transverse aortic banding is an effective technique of inducing left ventricular hypertrophy and heart failure in mice.
Protocol
Part 1: Preparation of Operating Field
The operating field is disinfected with 75% isopropyl alcohol.
Make sure the heating pad is on and at the right temperature. A recommended system is a Gaymar circulating water pump connected to a therapy pad that is maintained at 37 °C ± 1 °C. It is important to maintain normal body temperature during surgery as to avoid a rapid decrease in heart rate.
Surgical tools are sterilized in a hot bead sterilizer before surgery. For this procedure, you will need the following surgical instruments: blunt scissors, course curved forceps X 2, fine 45° angled forceps X 2, angled spring scissors, chest retractor, and needle holder.
Cotton applicators should be on hand in case of bleeding.
Part 2: Preparation and Intubation of Mice
Mice are anesthetized in an induction chamber with 2% isoflurane mixed with 0.5 -1.0 L/min 100% O2.
Hair clippers are used to shave the fur from the neckline to mid chest level.
The mouse is placed in a supine position atop a heating pad in order to maintain body temperature.
A rubber band is placed over the animal s front teeth to extend the neck. Using curved forceps in one hand, the tongue is gently manipulated to the side. With the other hand, endotracheal intubation is performed using PE 90 tubing. The endotracheal tube is then connected to a Harvard volume-cycled rodent ventilator cycling at 125-150 breaths/minute and a tidal volume of 0.1-0.3ml. During the surgical procedure, anesthesia is maintained at 1.5-2% isoflurane with 0.5 - 1.0 L/min 100% O2. Correct level of anesthesia is verified by applying pressure on the mouse nail bed (toe-pinch reflex).
The surgical field is disinfected with betadine solution followed by 70% alcohol. This procedure is repeated three times.
To prevent contamination of the surgical field during the operation, a sterile drape is placed over the mouse leaving only the operation field exposed.
A set of sterile gloves is used for each individual mouse.
Part 3: Ligation of the Transverse Aorta
Partial thoracotomy to the second rib is performed under a surgical microscope and the sternum retracted using a chest retractor.
Fine tip 45° angled forceps are used to gently separate the thymus and fat tissue from the aortic arch.
Following identification of the transverse aorta, a small piece of a 6.0 silk suture is placed between the innominate and left carotid arteries (Figure 1).
Two loose knots are tied around the transverse aorta and a small piece of a 27½ gauge blunt needle is placed parallel to the transverse aorta. The first knot is quickly tied against the needle, followed by the second and the needle promptly removed in order to yield a constriction of 0.4mm in diameter. In sham control mice, the entire procedure is identical except for the ligation of the aorta.
The chest retractor is removed and the outflow of the ventilator pinched off for 2 seconds to re-inflate the lungs.
The rib cage is closed using a 6.0 prolene suture with an interrupted suture pattern.
The skin is closed using a 6.0 prolene suture with a continuous suture pattern.
Part 4: Post-operative Recovery
For post-operative analgesia, the mouse is injected with buprenorphine (0.1 mg/kg) intraperitoneally. If there are signs of dehydration after surgery, sterile saline is given intraperitoneally.
Anesthesia is gradually lowered to the off position and the endotracheal tube removed when signs of spontaneous breathing occur.
The mouse is moved to the prone position and allowed to recover on a heating pad.
Part 5: Confirmation of Successful Ligation of the Transverse Aorta
One week after TAC, the mouse is re-anesthetized to determine the degree of pressure overload induced by ligation of the transverse aorta. As described above, anesthesia is maintained at 1.5-2% isoflurane with 0.5 - 1.0 L/min 100% O2 and body temperature maintained at 37 °C ± 1 °C.
A 20 MHz Doppler probe is placed on the left and right sides of the neck at a 45° angle to detect flow velocities. A computer-based Doppler signal processor (Indus Instruments, Houston, TX) was used to display and store Doppler signals.5
Depending on the degree of pressure overload required for the experimental protocol, only mice with a right carotid (RC)/left carotid (LC) flow ratio within a certain range are included for further analysis. For example, a moderate degree of pressure overload leads to a ratio of 5-8, whereas a tighter constriction resulting in severe pressure overload leads to a ratio of 8-10 (Figure 5.3A). A sham animal (operated but not ligated), however, is expected to have a ratio of ~1 (Figure 5.3B).
6: Representative Results
The typical surgical survival following TAC in wild-type mice is about 80-90%. Successful surgical ligation of the transverse aorta will lead to a Doppler flow velocity ratio between right and left carotid artery (RC/LC) of 5-10 (Figure 1). Compared to sham-operated mice (Figure 2A), mice with pressure overload are expected to develop cardiac hypertrophy within 1-2 weeks, and cardiac dilatation after 6-8 week, depending on the tightness of the constriction (Figure 2B). Mortality during the first month after TAC is typically low (<20%) in wild-type mice, although genetically-modified mice may exhibit different survival rates.6,7
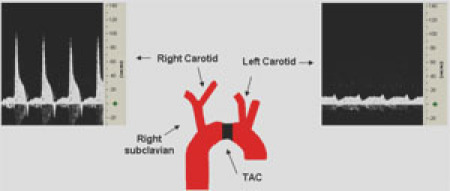 Figure 1. Confirmation of Transverse Aorta Ligation using Doppler Velocity Measurement. Representative Doppler velocity signals of the right (RC) and left carotid (LC) arteries 6 days post-TAC. These recordings demonstrate a successful ligation with a RC/LC flow ratio of ~6.8. Please click here to see a larger version of figure 1.
Figure 1. Confirmation of Transverse Aorta Ligation using Doppler Velocity Measurement. Representative Doppler velocity signals of the right (RC) and left carotid (LC) arteries 6 days post-TAC. These recordings demonstrate a successful ligation with a RC/LC flow ratio of ~6.8. Please click here to see a larger version of figure 1.
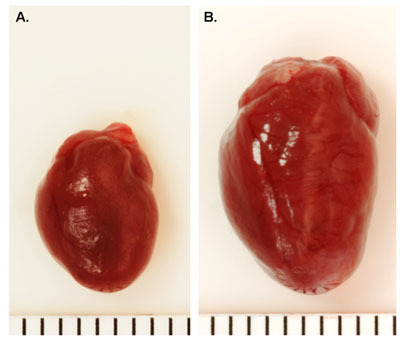 Figure 2. Representative Whole Heart Images Following TAC. A. Sham-operated wild type mouse heart. B. Wild type mouse heart 16 weeks post-TAC. Each line = 1mm.
Figure 2. Representative Whole Heart Images Following TAC. A. Sham-operated wild type mouse heart. B. Wild type mouse heart 16 weeks post-TAC. Each line = 1mm.
Discussion
TAC, which mimics human aortic stenosis, is a common method to induce cardiac hypertrophy and heart failure in mice. Alternative sites for aortic constriction include the ascending and abdominal aorta. Ascending aortic constriction provides an extreme and more rapid overload on the left ventricle (LV). In contrast, abdominal aortic constriction leaves intact a larger portion of the circulation as a means of possible compensation.8 Therefore, TAC is often the preferred model as it provides for adequate LV overload in a time-dependant manner more amenable to investigation. Although this procedure can be technically challenging, with practice, we have achieved a survival rate of 80-90% in wild type mice.
The tightness of transverse aortic constriction determines the degree of hypertrophy development and the time frame in which cardiac failure and dilatation develops. Moreover, the age of the mice affects the recovery rate and kinetics of heart failure development. Older mice (>12 months of age) take longer to develop an adaptive response of the carotid arteries to TAC, and are likely to develop dilated cardiomyopathy faster than younger mice (3-4 months of age).5
Non-invasive intubation of mice takes some practice. An alternative method is as follows:
A small midline cervical incision is made with blunt scissors and tissue separated around the trachea using curved forceps in order to expose the trachea.
A piece of suture is placed around the front teeth and gently pulled away from the animal's body to extend the neck.
Using curved forceps in one hand, the tongue is gently manipulated to the side.
With the other hand, endotracheal intubation is performed using PE 90 tubing beveled on the edge for ease of entry.
The endotracheal tube is then connected to a Harvard volume-cycled rodent ventilator cycling at 125-150 breaths/minute and a tidal volume of 0.1-0.3ml.
Acknowledgments
X.H.T.W. is a W.M. Keck Foundation Distinguished Young Scholar in Medical Research, and is also supported by NIH/NHLBI grants R01-HL089598 and R01- R01HL091947, and Muscular Dystrophy Association grant #69238. R.J.v.O. is the recipient of the 2008-2010 American Physiological Society Postdoctoral Fellowship in Physiological Genomics. This work is also supported in part by the Fondation Leducq Alliance for CaMKII Signaling in Heart.
References
- Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J, Jr, Chein KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc. Natl. Acad. Sci. USA. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signaling pathways. Nat. Rev. Mol. Cell. Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- Hartley CJ, Reddy AK, Madala S, Michael LH, Entman ML, Taffett GE. Doppler estimation of reduced coronary flow reserve in mice with pressure overload cardiac hypertrophy. Ultrasound Med. Biol. 2008;34:892–901. doi: 10.1016/j.ultrasmedbio.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AK, Taffett GE, Li Y-H, Lim S-W, Pham TT, Pocius JS, Entman ML, Michael LH, Hartley CJ. Doppler signal processing for use in mice: applications. IEEE Trans. Biomed. Eng. 2005;52:1771–1783. doi: 10.1109/TBME.2005.855709. [DOI] [PubMed] [Google Scholar]
- Li Y-H, Reddy AK, Ochoa LN, Pham TT, Hartley CJ, Micheal LH, Entman ML, Taffett GE. Effect of age on peripheral vascular response to transverse aortic banding in mice. J. Gerontol. 2003;58A:895–899. doi: 10.1093/gerona/58.10.b895. [DOI] [PubMed] [Google Scholar]
- Bourajjaj M, Armand AS, Martins daCosta, Weijts PA, Nagel BVander, Heeneman R, Wehrens S, Windt XHDe, J L. NFATc2 is a necessary mediator of calcineurin-dependent cardiac hypertrophy and heart failure. J. Biol. Chem. 2008;28:22295–22303. doi: 10.1074/jbc.M801296200. [DOI] [PubMed] [Google Scholar]
- Oort RJvan, Respress JL, Li N, Reynolds C, Almeida ACDe, Skapura DG, Windt LJDe, Wehrens XH. Accelerated development of pressure overload induced cardiac hypertrophy and dysfunction in an RyR2-R176Q knockin mouse model. Hypertension. 2010;55 doi: 10.1161/HYPERTENSIONAHA.109.146449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics. 2004;16:349–360. doi: 10.1152/physiolgenomics.00041.2003. [DOI] [PubMed] [Google Scholar]


