Abstract
OBJECTIVE:
Long-chain polyunsaturated fatty acids such as docosahexaenoic acid (DHA) influence immune function and inflammation; however, the influence of maternal DHA supplementation on infant morbidity is unknown. We investigated the effects of prenatal DHA supplementation on infant morbidity.
METHODS:
In a double-blind randomized controlled trial conducted in Mexico, pregnant women received daily supplementation with 400 mg of DHA or placebo from 18 to 22 weeks' gestation through parturition. In infants aged 1, 3, and 6 months, caregivers reported the occurrence of common illness symptoms in the preceding 15 days.
RESULTS:
Data were available at 1, 3, and 6 months for 849, 834, and 834 infants, respectively. The occurrence of specific illness symptoms did not differ between groups; however, the occurrence of a combined measure of cold symptoms was lower in the DHA group at 1 month (OR: 0.76; 95% CI: 0.58–1.00). At 1 month, the DHA group experienced 26%, 15%, and 30% shorter duration of cough, phlegm, and wheezing, respectively, but 22% longer duration of rash (all P ≤ .01). At 3 months, infants in the DHA group spent 14% less time ill (P < .0001). At 6 months, infants in the DHA group experienced 20%, 13%, 54%, 23%, and 25% shorter duration of fever, nasal secretion, difficulty breathing, rash, and “other illness,” respectively, but 74% longer duration of vomiting (all P < .05).
CONCLUSIONS:
DHA supplementation during pregnancy decreased the occurrence of colds in children at 1 month and influenced illness symptom duration at 1, 3, and 6 months.
Keywords: DHA, omega-3 fatty acids, prenatal, infant, morbidity
WHAT'S KNOWN ON THIS SUBJECT:
Intake of long-chain polyunsaturated fatty acids influences immune cell function; however, the influence on infant morbidity of maternal docosahexaenoic acid (DHA) intake during pregnancy is unknown. In 1 study, allergic responses and immunologic profiles in infants were affected by DHA supplementation during pregnancy in atopic pregnant women.
WHAT THIS STUDY ADDS:
Maternal DHA supplementation (400 mg/day) during pregnancy decreased the occurrence of colds in infants at 1 month and influenced illness symptom duration at 1, 3, and 6 months. This dose of DHA can be achieved through diet.
Long-chain polyunsaturated fatty acids (LCPUFAs), particularly docosahexaenoic acid (DHA) (22:6n-3) and arachidonic acid (20:4n-6), are integral to fetal neural and retinal development and accrete extensively in the last trimester of pregnancy.1,2 Inflammatory cells of the immune system contain membrane-bound LCPUFAs, which have been shown to modulate immune function and inflammation.3–8
Trials in adults and children who received supplementation with fish oil or LCPUFAs have shown inconsistent effects on immune function, inflammation, and morbidity.9–17 Furthermore, the specific clinical effects of n-3 PUFA supplementation in pregnancy on child morbidity are unknown. The determination of these effects is important because the infant immune system begins to develop and become functional in utero and DHA intake is lower than recommended in many populations.18,19 Worldwide, >50% of mortality in children younger 5 years is caused by infection, exacerbated by malnutrition, lack of safe water and sanitation, and poor maternal health; hence, healthy infant immune function is essential for child health and survival.20
In utero insults such as maternal nutritional deficiencies can impair the development of the fetal and subsequent infant immune system, but the influence of prenatal maternal LCPUFA nutrition on infant health and immune function is unclear.19,21 Prenatal n-3 PUFA supplementation improves maternal status in pregnancy, breast milk LCPUFA concentrations, and infant LCPUFA status.22–26 We hypothesized that these resultant modifications in LCPUFA status would enhance infant immune function. We therefore investigated the effect of DHA intake during the second half of pregnancy on infant illness symptoms in the context of a large randomized controlled trial in Mexico.27
SUBJECTS AND METHODS
Study Population and Setting
We recruited study participants at the Mexican Institute of Social Security (Instituto Mexicano del Seguro Social [IMSS]) General Hospital I, Cuernavaca, Mexico, and 3 small health clinics within the IMSS system in Cuernavaca during routine prenatal care visits between February 2005 and February 2007. Women were considered for inclusion in the study if they were in gestation week 18 to 22, were aged 18 to 35 years, planned to deliver at the IMSS General Hospital in Cuernavaca, planned to predominantly breastfeed for at least 3 months, and planned to live in the area for 2 years after delivery. Exclusion criteria included (1) high-risk pregnancy, (2) lipid metabolism/absorption disorders, (3) regular intake of fish oil or DHA supplements, or (4) chronic use of certain medications. Study team members who were employed by the Instituto Nacional de Salud Pública and IMSS conducted the field work.
The study protocol and all informed consent documents were approved by Emory University's institutional review board and by the Instituto Nacional de Salud Pública biosafety and ethics committees. Written informed consent was obtained from each participant after a thorough explanation of the study details, and participants were free to withdraw from the study at any time, without consequence to their care or treatment. The welfare of the subjects was monitored by an external data safety-monitoring committee.
Intervention
Women were assigned to receive 2 DHA or placebo capsules daily, from gestation weeks 18 through 22 through parturition. The DHA capsules contained 200 mg DHA each, which was derived from an algal source (Martek Biosciences Corporation, Columbia, MD). Algal-derived DHA supplements have a less distinct taste than fish oil. The placebo capsules, which were similar in appearance and taste to the DHA capsules, contained a corn and soy oil blend with no added antioxidants. We deemed this an appropriate placebo because it is considered safe in pregnancy and the very small dose was not expected to influence study outcomes. Fieldworkers visited the women's homes and/or workplaces weekly to deliver a new bottle of 14 capsules, and compliance was monitored by counting any remaining pills and through interviews with participants.
Blinding
All participants and members of the study team were blinded to the treatment scheme throughout the intervention period of the study. Data were unblinded for the analytical study team after the last infant in the study was born and had reached the age of 6 months.
Randomization and Sample Size
All eligible women were randomly assigned to either the treatment or the control group by use of a computer-generated list created by the study biostatistician at Emory University. We used block randomization to create balanced replicates of 4 treatments (2 colors for DHA and 2 for control) using a block size of 8. Success of randomization was assessed by comparison of maternal characteristics between the 2 treatment groups. In the context of the parent study design, 1094 pregnant women were recruited, which allowed for detection of differences in birth weight of 100 g (SD: 0.2 g) with at least 80% power.27 A posthoc power analysis indicates that our study had >95% power (assuming a 2-sided, 2-tailed test and a type-1 error of 0.05) to detect differences in duration of symptoms (Power Analysis and Sample Size [PASS] software [NCSS, Kaysville, UT]).
Outcomes
Primary study outcomes, including birth outcomes, and information about safety and adverse effects of the intervention are described elsewhere.27 We assessed the secondary outcome of child morbidity using a 15-day recall questionnaire administered when mothers brought their infants to the hospital for study visits at 1, 3, and 6 months. Mothers were queried about the occurrence and duration of illness symptoms, and whether they sought medical care for the symptoms. We created a variable of aggregate symptoms (henceforth denoted as “cold”), a priori, including phlegm, nasal congestion, nasal secretion, or cough, which characterize an upper respiratory tract infection. Mothers were asked at the 1- and 3-month study visits whether they were currently breastfeeding their child, and if they gave their child any supplementary milk, formula, other liquids, or other foods. We defined infants as exclusively breastfed if the mother reported providing only breast milk (no water, formula, etc). We did not assess breastfeeding at 6 months.
Statistical Analysis
We conducted the analysis according to a modified intention-to-treat principle, including in our analysis all children with data at 1, 3, and 6 months postpartum, regardless of treatment compliance. Continuous baseline characteristics and characteristics at birth were tested for normality, and group differences were calculated by using t tests. Differences in categorical characteristics were tested by using χ2 and Fisher's exact tests. We used logistic regression models to generate odds ratios and 95% confidence intervals. Differences in duration of child illness were assessed by using unadjusted Poisson regression models that included only children who experienced the specific illness symptom being tested and provided risk ratios and 95% confidence intervals. If duration of illness for a reported symptom was missing, we did not include that child in that particular analysis. All children were included in the calculation of percentage time ill from “all illnesses.” We did not control for breastfeeding status or any demographic or outcome measures because these characteristics were well balanced across the treatment arms. Statistical significance was defined as P ≤ .05. We conducted statistical analyses using the LOGISTIC and GENMOD procedures in the Statistical System Software 9.2 (SAS Institute, Cary, NC).
RESULTS
Consort Statement
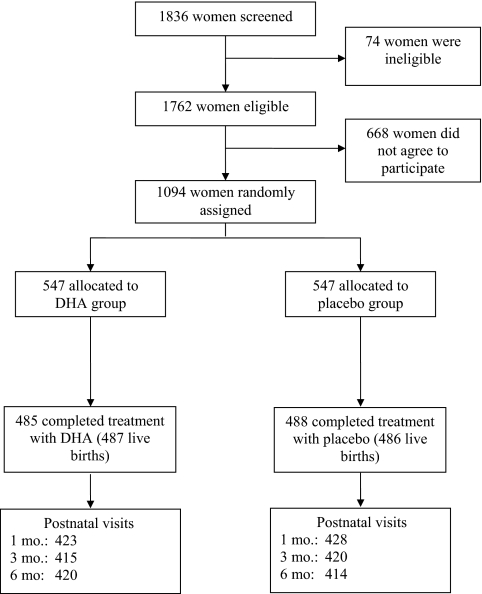
Fig 1 shows the trial profile. Among 1836 women screened, 1094 were randomly assigned to treatment and 89% remained in the study through parturition.27 Overall, loss to follow-up of women who initially received treatment through 6 months postpartum was 19.8%, and was similar across treatment groups (P = .7). Compliance, measured as the proportion of capsules consumed of the capsules distributed, was high (>94%) and was similar between the 2 groups (P = .6). Maternal plasma DHA concentrations at delivery and 1 month and breast milk DHA concentrations at 1 month were higher in the DHA group compared with the control group.28
FIGURE 1.
Trial profile with descriptions of recruitment and follow-up procedures.
Baseline Demographics and Birth Outcomes
Characteristics at randomization for the 851 mothers who attended the 1-month postpartum visit were similar between treatment groups (Table 1). Mean maternal BMI at randomization was ∼26 kg/m2, and mean height was ∼155 cm.27 Birth outcomes among the 851 infants were also similar across groups (Table 2).27 The prevalence of low birth weight was <6%. There were no significant between-group differences in the rates of stillbirths and infant deaths.27 The prevalence of breastfeeding was similar in the 2 treatment groups.
TABLE 1.
Selected Characteristics at Randomization of 851 Women Who Attended the 1-Month Postpartum Study Visit
| DHA (n = 423) | Placebo (n = 428) | |
|---|---|---|
| Age, mean ± SD, ya | 26.3 ± 4.9 | 26.4 ± 4.6 |
| Gestational age, mean ± SD, wka | 20.5 ± 1.9 | 20.6 ± 2.0 |
| Primigravida, %b | 37.4 | 39.0 |
| SES, %b | ||
| Low | 29.3 | 31.5 |
| Medium | 35.7 | 31.1 |
| High | 35.0 | 37.4 |
| Schooling, ya | 12.0 ± 3.5 | 12.0 ± 3.6 |
P values were calculated by t test for equality of means.
P values were calculated by χ2 test for equality of proportions.
TABLE 2.
Selected Characteristics of 851 Infants of Women Who Attended the 1-Month Postpartum Study Visit
| DHA (n = 423) | Placebo (n = 428) | P | |
|---|---|---|---|
| Gender, male, %a | 53.7 | 53.0 | .85 |
| Birth weight, mean ± SD, gb | 3225 ± 425 | 3224 ± 451 | .98 |
| Birth length, mean ± SD, cmb | 50.4 ± 2.1 | 50.4 ± 2.6 | .79 |
| Gestational age at birth, mean ± SD, wkb | 39.1 ± 1.7 | 39.1 ± 1.7 | .81 |
| Premature (<37 wk), %a | 9.5 | 8.2 | .52 |
| Breastfed at 1 mo, %a | 93.1 | 93.8 | .68 |
| Exclusively breastfed at 1 mo, %a | 19.4 | 20.3 | .73 |
| Breastfed at 3 mo, %a,c | 82.2 | 83.0 | .78 |
| Exclusively breastfed at 3 mo, %a,c | 15.2 | 14.8 | .92 |
P values were calculated by χ2 test for equality of proportions.
P values were calculated by t test for equality of means.
Among 833 infants with breastfeeding data at 3 months.
Numbers Analyzed
Morbidity data were available for 849, 834, and 834 infants, respectively, at 1, 3 and 6 months. Duration of illness was missing for ≤3% of ill infants who were reported to have experienced illness. Baseline characteristics of the subset of children who experienced an illness were similar to those who did not experience illness, and hence the likelihood of bias is low (data not shown).
Outcomes
History of specific illness symptoms such as cough, nasal congestion, and other symptoms in the previous 15 days did not differ significantly between the treatment groups at 1, 3, or 6 months (Table 3). At 1 and 3 months, the DHA group experienced a lower occurrence of cold symptoms than the placebo group (37.6% vs 44.6%; P < .05; and 37.8 vs 44.1; P > .05, respectively). There was no between-group difference in the number of symptoms experienced; specifically, occurrence of 2 or more or 3 or more symptoms, etc, did not differ according to group (data not shown).
TABLE 3.
History of Illness Symptoms Among Infants as Reported by the Infant's Mother in the Previous 15 Days at 1, 3, and 6 Months
| Symptoms | 1 mo |
3 mo |
6 mo |
||||||
|---|---|---|---|---|---|---|---|---|---|
| DHA (n = 422), % | Placebo (n = 427), % | OR (95% CI) | DHA (n = 415), % | Placebo (n = 419), % | OR (95% CI) | DHA (n = 420), % | Placebo (n = 414), % | OR (95% CI) | |
| Cough | 9.5 | 11.0 | 0.85 (0.54–1.32) | 19.3 | 23.9 | 0.76 (0.55–1.06) | 33.1 | 32.9 | 1.01 (0.76–1.35) |
| Phlegm | 16.8 | 19.2 | 0.85 (0.60–1.21) | 19.5 | 18.6 | 1.06 (0.75–1.50) | 23.9 | 24.2 | 0.98 (0.72–1.35) |
| Nasal congestion | 28.2 | 32.8 | 0.81 (0.60–1.08) | 25.1 | 28.4 | 0.83 (0.62–1.15) | 29.6 | 28.0 | 1.08 (0.80–1.46) |
| Nasal secretion | 7.1 | 10.8 | 0.64 (0.39–1.03) | 14.9 | 17.2 | 0.85 (0.58–1.23) | 28.2 | 29.5 | 0.94 (0.70–1.27) |
| Colda | 37.6 | 44.6 | 0.76 (0.58–1.00) | 37.8 | 44.1 | 0.77 (0.59–1.02) | 46.2 | 46.6 | 0.98 (0.75–1.29) |
| Wheezing | 8.3 | 7.0 | 1.19 (0.72–1.98) | 7.0 | 8.1 | 0.85 (0.51–1.43) | 11.9 | 10.9 | 1.11 (0.72–1.70) |
| Difficulty breathing | 2.4 | 2.3 | 1.01 (0.42–2.46) | 2.9 | 2.4 | 1.22 (0.52–2.85) | 1.4 | 1.7 | 0.85 (0.28–2.54) |
| Fever | 3.6 | 3.3 | 1.09 (0.52–2.28) | 8.4 | 10.5 | 0.79 (0.49–1.25) | 18.3 | 18.6 | 0.98 (0.69–1.39) |
| Vomiting | 5.5 | 3.5 | 1.58 (0.81–3.08) | 4.1 | 2.9 | 1.45 (0.68–3.07) | 5.5 | 4.1 | 1.36 (0.71–2.58) |
| Diarrhea | 3.3 | 4.0 | 0.83 (0.40–1.70) | 4.6 | 5.5 | 0.83 (0.44–1.54) | 7.6 | 7.5 | 1.02 (0.61–1.71) |
| Rash | 29.0 | 26.1 | 1.16 (0.86–1.57) | 8.4 | 10.3 | 0.81 (0.50–1.29) | 10.7 | 9.4 | 1.16 (0.74–1.82) |
| Other illness | 6.9 | 5.9 | 1.19 (0.69–2.07) | 5.1 | 5.3 | 0.96 (0.52–1.78) | 6.7 | 5.8 | 1.16 (0.66–2.04) |
Logistic regression was used to assess differences in the occurrence of reported illness symptom. OR indicates odds ratio.
Cold (any of the following: cough, phlegm, nasal congestion, nasal secretion).
At 1 month, 42%, 71%, and 10% of infants who had diarrhea had mucus in their stool, liquid stool, or bloody stool, respectively. The corresponding percentages at 3 and 6 months were 71%, 79%, and 5%, and 52%, 73%, and 5%, respectively. There was no difference in severity of diarrhea between treatment groups at 1, 3, and 6 months.
Care seeking for illness symptoms, used as a measure of perceived illness severity, was high among families with ill infants. For infants aged 3 and 6 months, >60% of caregivers sought care if their infant had fever, cough, phlegm, nasal congestion, wheezing, vomiting, or diarrhea. All caregivers (100%) sought care if their infant had difficulty breathing, and care seeking for wheezing was higher at 3 and 6 months compared with 1 month. There were no significant differences in care seeking between treatment groups.
Median duration of illness symptoms is shown in Table 4. At 1 month, the DHA group experienced shorter duration of cough, phlegm, and wheezing, but had longer duration of rash, compared with the placebo group (Table 5). At 6 months, infants in the DHA group experienced shorter duration of fever, nasal secretion, difficulty breathing, rash and “other illness,” and longer duration of vomiting than the placebo group. Percentage of time ill from all illnesses in the previous 15 days among all children was similar between groups at 1 (n = 851) and 6 months (n = 834); at 3 months (n = 835), infants in the DHA group spent 14% less time ill than children in the placebo group.
TABLE 4.
Duration of Symptoms in Infants Who Experienced Illness
| Symptoms | No. of Days at Ill at 1 mo |
No. of Days Ill at 3 mo |
No. of Days Ill at 6 mo |
|||
|---|---|---|---|---|---|---|
| DHA | Placebo | DHA | Placebo | DHA | Placebo | |
| Cough | 4.0 (3, 5) | 5.0 (3, 9) | 5.0 (3, 7) | 4.0 (3, 7) | 5.0 (3, 9) | 5.0 (3, 9) |
| Phlegm | 4.0 (3, 10) | 6.0 (3, 14) | 5.5 (3, 10) | 5.0 (3, 10) | 5.0 (3, 10) | 5.0 (3, 9) |
| Nasal congestion | 4.0 (3, 9) | 5.0 (3, 12) | 5.0 (3, 9) | 4.0 (3, 8) | 4.0 (3, 7) | 5.0 (3, 7) |
| Nasal secretion | 3.0 (2, 4) | 4.0 (1, 6) | 4.0 (3, 7) | 3.0 (2, 6) | 3.0 (3, 5) | 4.0 (3, 7) |
| Wheezing | 3.0 (2, 7) | 5.0 (3, 15) | 4.5 (2, 8) | 4.0 (2, 8) | 4.0 (3, 10) | 3.0 (2, 8) |
| Difficulty breathing | 3.5 (2, 10) | 3.0 (2, 9) | 3.0 (2, 7) | 2.0 (1, 4) | 2.0 (2, 3) | 3.5 (1, 10) |
| Fever | 2.0 (1, 3) | 2.0 (1, 3) | 2.0 (1, 3) | 2.0 (1, 3) | 2.0 (o1, 3) | 2.0 (1, 4) |
| Vomiting | 3.0 (2, 15) | 2.0 (1, 4) | 2.0 (2, 8) | 4.0 (2, 12) | 3.0 (1, 4) | 2.0 (1, 4) |
| Diarrhea | 3.5 (3, 5) | 2.5 (2, 10) | 3.0 (2, 3) | 3.0 (2, 6) | 3.0 (1, 4) | 3.5 (2, 5) |
| Rash | 11.0 (6, 15) | 7.0 (4, 15) | 5.0 (2.5, 10) | 5.0 (3, 10) | 3.5 (2, 7) | 4.0 (3, 9) |
| Other illness | 12.0 (5, 15) | 10.5 (5, 15) | 7.0 (4, 12) | 11.0 (5, 15) | 5.0 (3, 6) | 8.0 (4, 14) |
All values are expressed as median (25th, 75th percentiles).
TABLE 5.
Relative Risk of Longer Duration of Illness at 1, 3, and 6 Months in Children Who Experienced Illness
| Symptoms | 1 mo |
3 mo |
6 mo |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | RR | 95% CI | P | n | RR | 95% CI | P | n | RR | 95% CI | P | |
| Cough | 75 | 0.74 | 0.61–0.90 | <.01 | 164 | 1.00 | 0.88–1.14 | .95 | 251 | 1.00 | 0.91–1.10 | .93 |
| Phlegm | 133 | 0.85 | 0.74–0.96 | .01 | 144 | 1.06 | 0.93–1.20 | .40 | 184 | 1.01 | 0.90–1.14 | .83 |
| Nasal congestion | 228 | 0.98 | 0.89–1.09 | .72 | 197 | 1.15 | 1.03–1.28 | .02 | 227 | 0.94 | 0.84–1.05 | .25 |
| Nasal secretion | 65 | 0.69 | 0.46–1.03 | .07 | 123 | 1.07 | 0.92–1.25 | .38 | 220 | 0.87 | 0.77–0.98 | .02 |
| Wheezing | 59 | 0.70 | 0.57–0.86 | <.01 | 54 | 0.99 | 0.79–1.24 | .93 | 91 | 1.12 | 0.94–1.33 | .21 |
| Difficulty breathing | 16 | 1.05 | 0.69–1.58 | .83 | 19 | 1.59 | 0.96–2.62 | .07 | 11 | 0.46 | 0.24–0.87 | .02 |
| Fever | 24 | 0.99 | 0.57–1.73 | .98 | 67 | 1.14 | 0.85–1.52 | .40 | 133 | 0.80 | 0.66–0.98 | .03 |
| Vomiting | 31 | 1.51 | 0.64–3.59 | .35 | 23 | 0.73 | 0.51–1.04 | .08 | 38 | 1.74 | 1.19–2.54 | <.01 |
| Diarrhea | 26 | 0.74 | 0.52–1.06 | .10 | 35 | 0.90 | 0.65–1.24 | .51 | 57 | 0.86 | 0.65–1.13 | .27 |
| Rash | 216 | 1.22 | 1.05–1.41 | <.01 | 70 | 0.95 | 0.79–1.14 | .56 | 79 | 0.77 | 0.64–0.94 | <.01 |
| Other illness | 45 | 1.01 | 0.84–1.22 | .89 | 39 | 0.77 | 0.62–0.95 | .02 | 42 | 0.75 | 0.59–0.94 | .01 |
| All illnessesa | 518 | 1.04 | 0.98–1.1 | .21 | 433 | 0.97 | 0.90–1.05 | .46 | 484 | 1.01 | 0.94–1.1 | .75 |
| All illnessesb | 851 | 1.04 | 0.97–1.1 | .26 | 835 | 0.86 | 0.80–0.93 | <.0001 | 834 | 1.01 | 0.94–1.1 | .87 |
Relative risk (RR) was estimated by exponentiating β by using Poisson regression modeling.
Relative risk of total days ill in previous 15 days; includes only children who experienced an illness.
Relative risk of total days ill in previous 15 days; includes all children.
DISCUSSION
In a large double-blind randomized controlled trial we found that 400 mg/day DHA from 18 to 22 weeks' gestation through parturition reduced the occurrence of colds in offspring at 1 month of age and influenced illness duration at 1, 3, and 6 months. At 1 month, infants in the DHA group experienced shorter duration of cough, phlegm, and wheezing, but longer duration of rash. At 3 months, infants in the DHA group spent 14% less time ill from any illness compared with the placebo group and experienced shorter duration of “other illnesses” such as ear infections and sore throats, but longer duration of nasal congestion. At 6 months, infants in the DHA group experienced shorter duration of nasal secretion, difficulty breathing, fever, rash, and “other illnesses,” but longer duration of vomiting. We noted a general trend that infants in the DHA group were less likely to experience upper respiratory symptoms at 1 and 3 months; however, not all differences were statistically significant.
Overall, infants in the DHA group were determined to be healthier on the basis of the observation that fewer of these infants experienced a cold at 1 month, and they experienced a significantly shorter duration of all illnesses at 3 months, but longer duration of a few symptoms at certain time points. The increased duration of vomiting in the DHA group at 6 months of age may have been caused by either viral or a bacterial illness, or by gastrointestinal upset (“spitting up”) attributable to sensitive stomach or acid reflux. Longer duration of rash, perhaps atopic dermatitis, or a simple diaper rash caused by sensitive skin, diet, or wearing a soiled diaper for too long, occurred more frequently in the DHA group than in the placebo group at 1 month of age.
We conducted 36 statistical tests of differences in the duration of various symptoms. The number of significant results (12) is greater than would be expected because of chance alone. We are therefore confident that the significant findings are not a result of multiple testing alone. Although we observed some heterogeneity in our results, 9 of the 12 significant estimates were in the direction of a beneficial effect of DHA. The slight heterogeneity in results may be explained by the differing etiology of symptoms (ie, viral infection, bacterial infection, atopic response) and DHA's mechanism of action on their etiologies, which is unknown. Our study was sufficiently powered to demonstrate clinically relevant differences between treatment groups.
Illness symptoms were reported by the mother and not confirmed by a health care professional; therefore, we were unable to distinguish between, for example, diaper rash and clinical atopic dermatitis. A high proportion of mothers sought care for their infant's illness symptoms, likely because of the free access to health care within the IMSS hospital system. Self-reporting of morbidity data using recall questionnaires can introduce bias attributable to memory loss.29,30 Because treatment groups shared similar baseline characteristics and double-blinding was maintained throughout the study, recall was unlikely to be biased by treatment group.31 In addition, the use of health calendars/diaries and the high rate of literacy in this study likely greatly aided in maternal recall of child illness during interviews.32 The high occurrence of certain illness symptoms may be related to the low rate of exclusive breastfeeding.33 Breastfeeding was common because intent to breastfeed was an inclusion criterion for participation, but the prevalence of exclusive breastfeeding was low; however, there were no differences between the groups.
To our knowledge, 1 randomized controlled trial has been conducted to examine the effect on infant immune function of n-3 PUFA supplementation in pregnancy.34–36 We did not identify any studies that examined the influence of prenatal DHA on infant morbidity. Unlike our study, that study included only atopic pregnant women, the primary outcome of interest was allergic immune response, and the supplement was fish oil, rather than DHA. In that study, fish oil supplementation in pregnancy lowered cord blood concentration of the cytokine interleukin 13, modified neonatal neutrophil production, did not influence neonatal immunoglobulin E concentrations, and lowered the risk of a positive reaction to a specific skin prick test at 1 year. The investigators also found a positive correlation between maternal n-3 PUFA concentration and immunoglobulin A concentration in breast milk, indicating that n-3 PUFA in pregnancy might modulate infant immune function by means of breast milk immunoglobulin protection.37 Our findings extend those results to the clinical manifestations of infant immune function.
Several studies have evaluated the influence of LCPUFA supplementation during childhood on illness occurrence and severity and immune function.9,11,14–17,38 Children aged 18 to 36 months who were given formula containing DHA for 60 days had a lower occurrence of respiratory illness.16 Thai schoolchildren aged 9 to 12 years who consumed fish oil–enriched milk for 6 months experienced fewer episodes and shorter duration of illness compared with controls.15 Infants fed formula enriched with DHA and arachidonic acid during the first year of life experienced fewer upper respiratory infections than did the placebo group.17 Infants who consumed an LCPUFA-supplemented formula had a decreased incidence of bronchiolitis/bronchitis compared with controls.9 Term infants who were given formula supplemented with LCPUFA had enhanced presence and function of infant CD3+ and CD44++ cells compared with controls.38 Children aged 5 to 7 years who were given a dietary supplement containing arachidonic acid and DHA for 7 months had improved immune cell phenotypes, compared with children who were given a placebo.14 Danish infants who were provided fish oil from 9 to 12 months of age had higher Lactobacillus paracasei–induced interferon γ amounts than controls, suggesting that fish oil may influence maturation of the infant immune system.39 Dietary EFA supplementation in children aged 36 to 49 months at risk of recurrent respiratory infections reduced the number of infective episodes and days with fever.11 A systematic review of randomized controlled trials of LCPUFA supplementation of infant formula in preterm infants demonstrated no effect on severe adverse outcomes such as sepsis or necrotizing enterocolitis.12 In summary, numerous studies have shown that n-3 PUFA supplementation in childhood reduced the occurrence and duration of illness, particularly respiratory illness.
Results from our study could be generalized to other populations of pregnant women of middle-to-lower socioeconomic status who have similarly low dietary DHA intakes. DHA intakes in our study population were low (median: 80 mg/day) compared with the recommended intake of at least 200 mg/day.26,40 Intake of n-3 PUFA in the United States is an estimated 1.6 g/day, and intake of DHA is ∼100 to 200 mg/day.41 Our study population experienced lower infectious disease rates than are reported from many developing countries, in particular for diarrhea and fever.
CONCLUSIONS
Our findings contribute to the accumulating evidence base for a relationship of prenatal n-3 PUFA nutrition to the development of fetal and neonatal immune function. We demonstrated that DHA supplementation in pregnancy influenced the duration of illness symptoms and reduced occurrence of colds in Mexican infants. n-3 PUFA may influence fetal and infant immune function development in utero, via breast milk, or by a combination of both prenatal and postnatal factors.42 Additional studies that are designed specifically to examine the influence of perinatal n-3 PUFA nutriture on infant immune function and include both biologically and clinically relevant outcomes are necessary to evaluate the potential value of dietary modification or n-3 PUFA supplementation during pregnancy and/or lactation.
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Health (HD043099) and the March of Dimes Foundation (6FY04-69).
The authors acknowledge the guidance and contributions of Drs Ricardo Uauy and Maria Makrides, who served as study consultants for the overall planning and implementation of the larger study, and the contributions of Ms Meng Wang and Ms Clara Dominguez, who served as data managers for the study at Emory and Instituto Nacional de Salud Pública, respectively.
Dr Imhoff-Kunsch contributed to the design of the morbidity-specific aspects of the study, conducted the statistical analysis, and wrote the manuscript. Dr Stein contributed to the study design, reviewed the accuracy of the statistical methods and interpretation of results, and reviewed the initial article and subsequent revisions. Dr Martorell contributed to the study design, interpretation of results and review of the article. Dr Parra-Cabrera contributed to the study design, recruitment of subjects, supervision of data collection, and review of the article. Dr Romieu contributed to the development of data collection methods for the morbidity-specific aspect of the study and review of the manuscript. Dr Ramakrishnan (principal investigator) obtained research funding and contributed to study design, development of study protocols, statistical methods, interpretation of results, and review of the article.
This trial is registered at clinicaltrials.gov (identifier NCT00646360).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH) and March of Dimes Foundation.
- LCPUFA
- long-chain polyunsaturated fatty acid
- DHA
- docosahexaenoic acid
- IMSS
- Instituto Mexicano del Seguro Social
REFERENCES
- 1. Uauy R, Hoffman DR, Peirano P, Birch DG, Birch E. Essential fatty acids in visual and brain development. Lipids. 2001;36(9):885–895 [DOI] [PubMed] [Google Scholar]
- 2. McCann JC, Ames BN. Is docosahexaenoic acid, an n-3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am J Clin Nutr. 2005;82(2):281–295 [DOI] [PubMed] [Google Scholar]
- 3. Calder PC. Immunomodulation by omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids. 2007;77(5–6):327–335 [DOI] [PubMed] [Google Scholar]
- 4. Gottrand F. Long-chain polyunsaturated fatty acids influence the immune system of infants. J Nutr. 2008;138(9):1807S–1812S [DOI] [PubMed] [Google Scholar]
- 5. Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids. 2003;38(4):343–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calder PC, Grimble RF. Polyunsaturated fatty acids, inflammation and immunity. Eur J Clin Nutr. 2002;56(suppl 3):S14–S19 [DOI] [PubMed] [Google Scholar]
- 7. Deckelbaum RJ, Worgall TS, Seo T. n-3 fatty acids and gene expression. Am J Clin Nutr. 2006;83(6 suppl):1520S–1525S [DOI] [PubMed] [Google Scholar]
- 8. Field CJ, Clandinin MT, Van Aerde JE. Polyunsaturated fatty acids and T-cell function: implications for the neonate. Lipids. 2001;36(9):1025–1032 [DOI] [PubMed] [Google Scholar]
- 9. Pastor N, Soler B, Mitmesser SH, Ferguson P, Lifschitz C. Infants fed docosahexaenoic acid- and arachidonic acid-supplemented formula have decreased incidence of bronchiolitis/bronchitis the first year of life. Clin Pediatr (Phila). 2006;45(9):850–855 [DOI] [PubMed] [Google Scholar]
- 10. Merchant AT, Curhan GC, Rimm EB, Willett WC, Fawzi WW. Intake of n-6 and n-3 fatty acids and fish and risk of community-acquired pneumonia in US men. Am J Clin Nutr. 2005;82(3):668–674 [DOI] [PubMed] [Google Scholar]
- 11. Venuta A, Spano C, Laudizi L, Bettelli F, Beverelli A, Turchetto E. Essential fatty acids: the effects of dietary supplementation among children with recurrent respiratory infections. J Int Med Res. 1996;24(4):325–330 [DOI] [PubMed] [Google Scholar]
- 12. Smithers LG, Gibson RA, McPhee A, Makrides M. Effect of long-chain polyunsaturated fatty acid supplementation of preterm infants on disease risk and neurodevelopment: a systematic review of randomized controlled trials. Am J Clin Nutr. 2008;87(4):912–920 [DOI] [PubMed] [Google Scholar]
- 13. Rees D, Miles EA, Banerjee T, et al. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. Am J Clin Nutr. 2006;83(2):331–342 [DOI] [PubMed] [Google Scholar]
- 14. Mazurak VC, Lien V, Field CJ, Goruk SD, Pramuk K, Clandinin MT. Long-chain polyunsaturated fat supplementation in children with low docosahexaenoic acid intakes alters immune phenotypes compared with placebo. J Pediatr Gastroenterol Nutr. 2008;46(5):570–579 [DOI] [PubMed] [Google Scholar]
- 15. Thienprasert A, Samuhaseneetoo S, Popplestone K, West AL, Miles EA, Calder PC. Fish oil n-3 polyunsaturated fatty acids selectively affect plasma cytokines and decrease illness in Thai schoolchildren: a randomized, double-blind, placebo-controlled intervention trial. J Pediatr. 2009;154(3):391–395 [DOI] [PubMed] [Google Scholar]
- 16. Minns LM, Kerling EH, Neely MR, et al. Toddler formula supplemented with docosahexaenoic acid (DHA) improves DHA status and respiratory health in a randomized, double-blind, controlled trial of US children less than 3 years of age. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4–6):287–293 [DOI] [PubMed] [Google Scholar]
- 17. Birch EE, Khoury JC, Berseth CL, et al. The impact of early nutrition on incidence of allergic manifestations and common respiratory illnesses in children. J Pediatr. 2010;156(6):902–906 [DOI] [PubMed] [Google Scholar]
- 18. Hibbeln JR, Nieminen Levi RG, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr. 2006;83(6 suppl):1483S–1493S [DOI] [PubMed] [Google Scholar]
- 19. Calder PC, Krauss-Etschmann S, de Jong EC, et al. Early nutrition and immunity: progress and perspectives. Br J Nutr. 2006;96(4):774–790 [PubMed] [Google Scholar]
- 20. Christian P. Infant mortality. In: Semba RD, Bloem MW. eds. Nutrition and Health in Developing Countries. Totowa, NJ: Humana Press; 2008:87–112 [Google Scholar]
- 21. Prescott SL, Dunstan JA. Prenatal fatty acid status and immune development: the pathways and the evidence. Lipids. 2007;42(9):801–810 [DOI] [PubMed] [Google Scholar]
- 22. Dunstan JA, Mitoulas LR, Dixon G, et al. The effects of fish oil supplementation in pregnancy on breast milk fatty acid composition over the course of lactation: a randomized controlled trial. Pediatr Res. 2007;62(6):689–694 [DOI] [PubMed] [Google Scholar]
- 23. Dunstan JA, Mori TA, Barden A, et al. Effects of n-3 polyunsaturated fatty acid supplementation in pregnancy on maternal and fetal erythrocyte fatty acid composition. Eur J Clin Nutr. 2004;58(3):429–437 [DOI] [PubMed] [Google Scholar]
- 24. Krauss-Etschmann S, Shadid R, Campoy C, et al. Effects of fish-oil and folate supplementation of pregnant women on maternal and fetal plasma concentrations of docosahexaenoic acid and eicosapentaenoic acid: a European randomized multicenter trial. Am J Clin Nutr. 2007;85(5):1392–1400 [DOI] [PubMed] [Google Scholar]
- 25. Bergmann RL, Haschke-Becher E, Klassen-Wigger P, et al. Supplementation with 200 mg/day docosahexaenoic acid from midpregnancy through lactation improves the docosahexaenoic acid status of mothers with a habitually low fish intake and of their infants. Ann Nutr Metab. 2008;52(2):157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koletzko B, Lien E, Agostoni C, et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med. 2008;36(1):5–14 [DOI] [PubMed] [Google Scholar]
- 27. Ramakrishnan U, Stein AD, Parra-Cabrera S, et al. Effects of docosahexaenoic acid supplementation during pregnancy on gestational age and size at birth: randomized, double-blind, placebo-controlled trial in Mexico. Food Nutr Bull. 2010;31(2 suppl):S108–S116 [DOI] [PubMed] [Google Scholar]
- 28. Imhoff-Kunsch B, Stein AD, Villalpando S, Martorell R, Ramakrishnan U. Docosahexaenoic acid supplementation from mid-pregnancy to parturition influenced breast milk fatty acid concentrations at 1 month postpartum in Mexican women. J Nutr. 2011;141(2):321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramakrishnan R, Venkatarao T, Koya PK, Kamaraj P. Influence of recall period on estimates of diarrhoea morbidity in infants in rural Tamilnadu. Indian J Public Health. 1999;43(4):136–139 [PubMed] [Google Scholar]
- 30. Martorell R, Habicht JP, Yarbrough C, Lechtig A, Klein RE. Underreporting in fortnightly recall morbidity surveys. J Trop Pediatr Environ Child Health. 1976;22(3):129–134 [DOI] [PubMed] [Google Scholar]
- 31. Manesh AO, Sheldon TA, Pickett KE, Carr-Hill R. Accuracy of child morbidity data in demographic and health surveys. Int J Epidemiol. 2008;37(1):194–200 [DOI] [PubMed] [Google Scholar]
- 32. Goldman N, Vaughan B, Pebley AR. The use of calendars to measure child illness in health interview surveys. Int J Epidemiol. 1998;27(3):505–512 [DOI] [PubMed] [Google Scholar]
- 33. Duijts L, Jaddoe VW, Hofman A, Moll HA. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126(1). Available at: www.pediatrics.org/cgi/content/full/126/1/e18 [DOI] [PubMed] [Google Scholar]
- 34. Dunstan JA, Mori TA, Barden A, et al. Maternal fish oil supplementation in pregnancy reduces interleukin-13 levels in cord blood of infants at high risk of atopy. Clin Exp Allergy. 2003;33(4):442–448 [DOI] [PubMed] [Google Scholar]
- 35. Dunstan J, Mori TA, Barden A, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol. 2003;112(6):1178–1184 [DOI] [PubMed] [Google Scholar]
- 36. Prescott SL, Barden AE, Mori TA, Dunstan JA. Maternal fish oil supplementation in pregnancy modifies neonatal leukotriene production by cord-blood-derived neutrophils. Clin Sci (Lond). 2007;113(10):409–416 [DOI] [PubMed] [Google Scholar]
- 37. Dunstan JA, Roper J, Mitoulas L, Hartmann PE, Simmer K, Prescott SL. The effect of supplementation with fish oil during pregnancy on breast milk immunoglobulin A, soluble CD14, cytokine levels and fatty acid composition. Clin Exp Allergy. 2004;34(8):1237–1242 [DOI] [PubMed] [Google Scholar]
- 38. Field CJ, Van Aerde JE, Robinson LE, Clandinin MT. Effect of providing a formula supplemented with long-chain polyunsaturated fatty acids on immunity in full-term neonates. Br J Nutr. 2008;99(1):91–99 [DOI] [PubMed] [Google Scholar]
- 39. Damsgaard CT, Lauritzen L, Kjaer TM, et al. Fish oil supplementation modulates immune function in healthy infants. J Nutr. 2007;137(4):1031–1036 [DOI] [PubMed] [Google Scholar]
- 40. Parra-Cabrera S, Stein AD, Wang M, Martorell R, Rivera J, Ramakrishnan U. Dietary intakes of polyunsaturated fatty acids among pregnant Mexican women. Maternal Child Nutr. 2011;7(2):140–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kris-Etherton PM, Taylor DS, Yu-Poth S, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71(1 suppl):179S–188S [DOI] [PubMed] [Google Scholar]
- 42. Laitinen K, Hoppu U, Hamalainen M, Linderborg K, Moilanen E, Isolauri E. Breast milk fatty acids may link innate and adaptive immune regulation: analysis of soluble CD14, prostaglandin E2, and fatty acids. Pediatr Res. 2006;59(5):723–727 [DOI] [PubMed] [Google Scholar]