Abstract
The activity of the alternate ς-factor ςE of Escherichia coli is induced by several stressors that lead to the extracytoplasmic accumulation of misfolded or unfolded protein. The ςE regulon contains several genes, including that encoding the periplasmic protease DegP, whose products are thought to be required for maintaining the integrity of the cell envelope because cells lacking ςE are sensitive to elevated temperature and hydrophobic agents. Selection of multicopy suppressors of the temperature-sensitive phenotype of cells lacking ςE revealed that overexpression of the lipoprotein NlpE restored high temperature growth to these cells. Overexpression of NlpE has been shown previously to induce DegP synthesis by activating the Cpx two-component signal transduction pathway, and suppression of the temperature-sensitive phenotype by NlpE was found to be dependent on the Cpx proteins. In addition, a constitutively active form of the CpxA sensor/kinase also fully suppressed the temperature-sensitive defect of cells lacking ςE. DegP was found to be necessary, but not sufficient, for suppression. Activation of the Cpx pathway has also been shown to alleviate the toxicity of several LamB mutant proteins. Together, these results reveal the existence of two partially overlapping regulatory systems involved in the response to extracytoplasmic stress in E. coli.
Keywords: rpoE, stress response, cpxRA, outer membrane, nlpE, periplasm
The response to misfolded or unfolded protein is one of the most highly conserved regulatory responses among all organisms. When exposed to stressors such as heat or ethanol, all cells undergo the transcriptional induction of a conserved set of genes known as the heat shock genes, which are required to combat cellular stress (Bukau 1993; Yura et al. 1993; Becker and Craig 1994; Georgopoulos et al. 1994; Gross 1996). The majority of these genes encode proteins such as chaperones or proteases that act to either refold, prevent aggregation, or degrade misfolded protein (Bukau 1993; Yura et al. 1993; Becker and Craig 1994; Georgopoulos et al. 1994; Gross 1996).
In addition to being highly conserved, the stress response is compartmentalized into separate pathways that regulate protein-folding processes in different subcellular compartments (McMillan et al. 1994). For example, the cytoplasmic stress response in eukaryotes is coordinated by a family of transcriptional activators known as the heat shock transcription factors (HSFs) (Wu 1995; Morimoto et al. 1996). A separate pathway for the response to the accumulation of misfolded protein in the endoplasmic reticulum, known as the unfolded protein response (UPR) (McMillan et al. 1994; Shamu et al. 1994), is controlled by a novel signal transduction pathway (Cox et al. 1993; Mori et al. 1993; Cox and Walter 1996; Sidrauski et al. 1996). In this way, cells can respond to insults that affect only a single subcellular compartment without inducing a cell-wide response.
In Escherichia coli, the stress response is compartmentalized into cytoplasmic and extracytoplasmic responses. The cytoplasmic response is coordinated by the alternate σ-factor σ32 (Grossman et al. 1984; Landick et al. 1984; Yura et al. 1984), which responds to the accumulation of misfolded protein by directing the transcription of a well-characterized set of genes, including those encoding the DnaK/DnaJ chaperone complex (Bukau 1993; Yura et al. 1993; Becker and Craig 1994; Georgopoulos et al. 1994; Gross 1996). These chaperones, in turn, are thought to down-regulate σ32 activity upon relief of cytoplasmic stress (Straus et al. 1989, 1990; Gamer et al. 1992, 1996; Liberek et al. 1992; Liberek and Georgopoulos 1993).
In contrast, the extracytoplasmic response is less well defined and is believed to be controlled by at least two signal transduction systems, the Cpx two-component system and the σE-mediated system. The Cpx system, composed of an inner membrane sensor kinase encoded by cpxA and a response regulator encoded by cpxR, is activated by either overexpression of the outer membrane lipoprotein NlpE (Gupta et al. 1995; Snyder et al. 1995) or mutational activation of CpxA (Cosma et al. 1995; Danese et al. 1995; Snyder et al. 1995). Upon activation of the Cpx system, the envelope-associated toxicity of certain LamB mutant proteins is suppressed by inducing synthesis of the periplasmic protease DegP (Cosma et al. 1995; Snyder et al. 1995). The physiological signal inducing this pathway remains to be definitively identified. Because cells lacking the Cpx pathway have no obvious phenotype (Danese et al. 1995; L. Connolly, unpubl.), the cellular role of this signal transduction system remains obscure.
The second periplasmic stress response system is coordinated by the heat and ethanol responsive alternate σ-factor, σE. In addition to being induced by these general stresses, the σE pathway is uniquely induced in response to alterations in the expression or maturation of a class outer membrane proteins (OMPs) called the porins (Mecsas et al. 1993). The porins undergo a complex series of folding and oligomerization steps prior to being inserted into the outer membrane (Pugsley 1993), and alterations in this pathway lead to a buildup of folding intermediates that are believed to be sensed by the σE pathway (Mecsas et al. 1993; Rouvière and Gross 1996). This signal is transduced to σE by RseA, a σE-specific anti-σ-factor located in the inner membrane, and a periplasmic protein, RseB (De Las Peñas et al. 1997; Missiakas et al. 1997). Activation of σE, in turn, leads to the induction of at least 10 different proteins, 4 of which have been definitively identified: the periplasmic protease DegP, the second heat shock σ-factor, σ32, the periplasmic peptidyl prolyl isomerase FkpA, and σE itself (Erickson et al. 1987; Lipinska et al. 1988; Erickson and Gross 1989; Wang and Kaguni 1989; Raina et al. 1995; Rouvière et al. 1995; Danese and Silhavy 1997). Genes under the control of σE are believed to encode functions required for the maintenance of envelope integrity under stress conditions because cells lacking σE are sensitive to elevated temperature, SDS/EDTA, and crystal violet (Hiratsu et al. 1995; Raina et al. 1995; Rouvière et al. 1995). In an attempt to isolate either genes downstream of σE or components of other signal transduction cascades capable of relieving periplasmic stress, we isolated genes that when overexpressed were capable of restoring growth at high temperature to cells lacking σE.
Results
Selection and identification of a suppressor plasmid
To isolate genes capable of restoring growth at high temperature to cells lacking σE, rpoE− cells were transformed with a genomic library containing DNA prepared from the prototypic wild-type E. coli strain MG1655. Following phenotypic expression at 30°C, one-tenth of the cells were plated at 30°C for viable count, and the remainder at 42°C for the selection of temperature-resistant candidates. Of a total of 3.7 × 105 possible transformants, 423 temperature-resistant suppressor candidates were identified. Because our recipient strain contains a lacZ reporter gene, which is exclusively transcribed by holoenzyme containing σE (Mecsas et al. 1993), we were able to identify and discard transformants containing wild-type rpoE sequences by screening on X-gal. Plasmid DNA was then purified from white candidate colonies and retransformed into the original rpoE− strain to ensure that the temperature-resistant phenotype was plasmid dependent. After retransformation, one candidate plasmid, pBA25, retained suppressor ability. Remarkably, pBA25 raised the plating efficiency of the parental strain four orders of magnitude, from 4.2 × 10−5 for cells containing vector alone to 0.6 (Fig. 1).
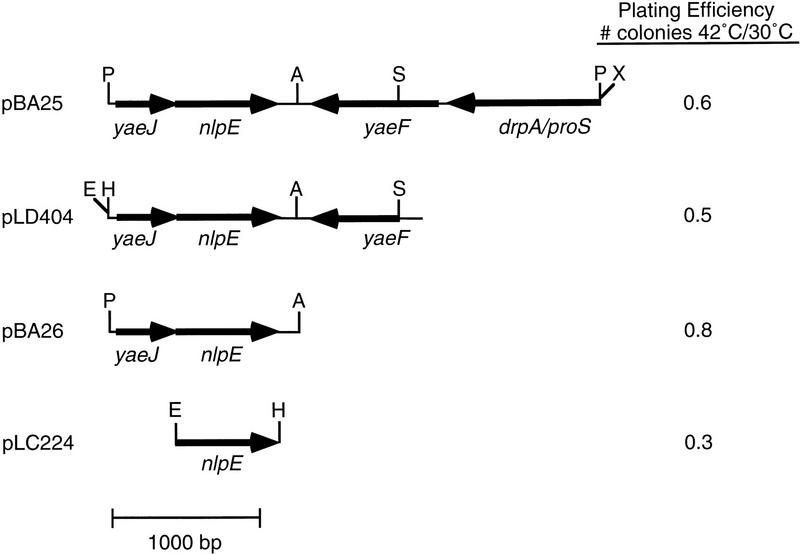
Figure 1.
Maps and suppressor activity of plasmid subclones. pBA25 is the original suppressor plasmid, pBA26 was created by the removal of the represented sequences and religating, pLD404 has been described previously (Snyder et al. 1995), and pLC224 was generated by PCR amplification and subcloning of a fragment containing nlpE alone from pBA25. Restriction enzyme site abbreviations: (P) PstI; (A) AflII; (X) XbaI; (E) EcoRI; (H) HindIII; (S) StyI. Note that the plating efficiency of cells containing vector alone was 4.2 × 10−5 (pUC18 for pBA25 and pBA26), 4.0 × 10−6 (pBR322 for pLD404), and 7.1 × 10−7 (pLC222 for pLC224).
Mapping and sequence analysis
To determine the region of the genome located on the suppressor plasmid, sequence was obtained from either end of the insert using vector-based primers. The sequence was then subjected to a BLAST search (Altschul et al. 1990). This analysis revealed that the plasmid contained three full open reading frames (ORF) and a partial ORF mapping to minute 4.7 of the E. coli chromosome. The insert size, restriction map (data not shown), and identity of sequences at either end of the insert indicated that only one insert was contained in the plasmid. The insert encodes the ORFs yaeJ, yaeF, and the recently described gene nlpE in addition to a partial copy of the drpA/proS gene (Fig. 1).
Deletion of yaeF and drpA/proS by removal of the AflII–XbaI fragment revealed that the segment containing yaeJ and nlpE was responsible for suppression of the temperature-sensitive defect (Fig. 1). Overexpression of nlpE has recently been shown to induce expression of the periplasmic protease DegP by activating the Cpx two-component signal transduction pathway (Danese et al. 1995). degP is also a member of the σE regulon, and we reasoned that nlpE might be suppressing rpoE− cells by activating this pathway, leading to the induction of degP.
To determine whether nlpE alone was capable of restoring growth to rpoE− cells at high temperature in the absence of yaeJ, nlpE was PCR amplified from the original suppressor plasmid and placed under the control of the lac promoter. nlpE alone retained the ability to rescue the temperature-sensitive phenotype of the parental rpoE− strain (Fig. 1), indicating that overexpression of nlpE is responsible for suppression.
During the course of characterizing pBA25, we discovered a single restriction digest change in nlpE that could potentially change the coding sequence of the protein. To ensure that any mutations in the nlpE allele we isolated were not responsible for suppressor activity, we introduced plasmid pLD404 (Snyder et al. 1995), which carries a known wild-type allele of nlpE, into the original rpoE− strain. rpoE− cells containing pLD404 plated with similar efficiency at 42°C as cells containing plasmids derived from the original suppressor plasmid (Fig. 1). This result indicates that wild-type nlpE suppresses the temperature-sensitive phenotype of rpoE− cells and that any mutations present within the allele of nlpE originally isolated are not responsible for suppression.
Mechanism of suppression
To determine whether nlpE requires the Cpx pathway for suppression, we constructed rpoE− cpxA− mutant strains and asked whether nlpE-containing plasmids could restore growth at high temperature to these double mutant cells. As shown in Table 1, although cells lacking both σE and the Cpx pathway plate with equal efficiency at 42°C as cells lacking σE alone (1.9 × 10−5 vs. 1.1 × 10−5), plasmids containing nlpE were no longer able to suppress the temperature sensitive phenotype. These results demonstrate that NlpE requires the Cpx pathway for suppression of the temperature-sensitive phenotype of cells lacking σE. One caveat to these experiments is that the vector alone decreased the plating efficiency of the double mutant strain 1000-fold. β-Lactamase expression from these ApR plasmids may constitute a second periplasmic stressor that might interfere with any residual suppression by nlpE in the Cpx− background.
Table 1.
Plating efficiency of rpoE− strains lacking the Cpx pathway
| Strain
|
Genotype
|
Plasmid
|
Plating efficiency (no. of colonies at 42°C/30°C)
|
|---|---|---|---|
| CAG22700 | rpoE::Ωcm recA56 | none | 1.1 × 10−5 |
| CAG33126 | CAG22700 | vector | 4.2 × 10−5 |
| CAG33127 | CAG22700 | pnlpE | 0.6 |
| CAG33091 | rpoE::ΩCm cpxR::ΩSp recA56 | none | 1.9 × 10−5 |
| CAG33239 | CAG33091 | vector | <1.6 × 10−8 |
| CAG33240 | CAG33091 | pnlpE | <3.9 × 10−8 |
The plating efficiency of each strain was determined as described in Materials and Methods. Vector-containing cells carry pUC18; pnlpE represents pBA25.
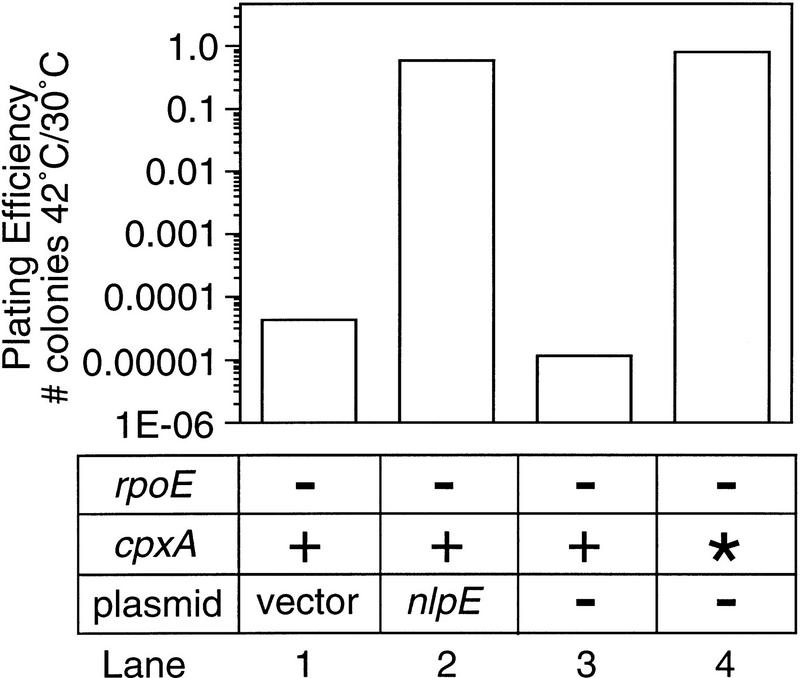
The requirement of the Cpx pathway for suppression by NlpE indicated that NlpE was probably acting via the Cpx proteins to suppress the rpoE− temperature-sensitive phenotype. If this were true, then other conditions that activate this pathway should similarly suppress the rpoE− temperature-sensitive phenotype. To test this hypothesis, we introduced a gain-of-function allele of cpxA (cpxA*) (Cosma et al. 1995; Danese et al. 1995) into the original rpoE− strain and asked whether mutational activation of the Cpx pathway would suppress the rpoE− cells to the same extent as overexpression of nlpE. rpoE− cells containing the cpxA* allele plated with the same efficiency at 42°C as cells overexpressing nlpE (Fig. 2, lanes 2,4), indicating that activation of the Cpx pathway suppresses the temperature-sensitive phenotype.
Figure 2.
Plating efficiency of rpoE::ΩCm cells activated for the Cpx pathway. The efficiency of plating of rpoE::ΩCm cells containing vector alone (lane 1) or pBA25 (lane 2), or no plasmid and a wild-type (lane 3) or constitutively activated (lane 4) cpxA allele was determined as described in Materials and Methods. The numerical values of the plating efficiency of each strain were vector alone, 4.2 × 10−5; pBA25, 0.6; cpxA+, 1.1 × 10−5; cpxA*, 0.8.
DegP is required, but not sufficient, for suppression
Cpx and σE both regulate the expression of the periplasmic protease DegP. We reasoned that activation of the Cpx pathway might be suppressing the rpoE− phenotype by simply restoring expression of a single gene in the rpoE regulon, degP, and decided to test whether degP was both required and sufficient for suppression.
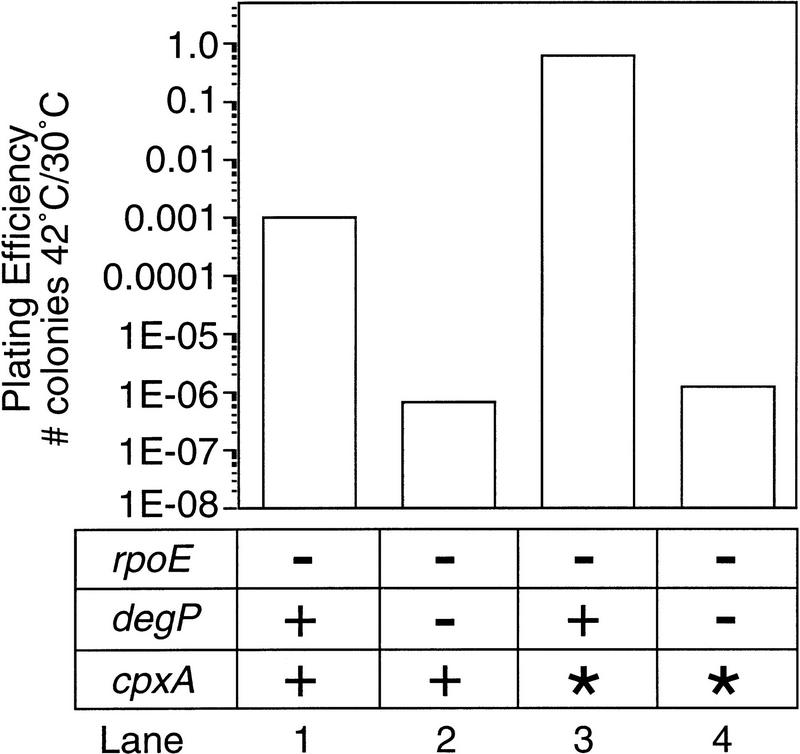
To test whether degP was required for suppression of the rpoE− phenotype by the Cpx pathway, we introduced a degP null allele into rpoE− cells containing the cpxA* allele. Introduction of a degP null allele into rpoE− cells reduced the plating efficiency 1500-fold, and subsequent introduction of the cpxA* allele did not restore growth at high temperature (Fig. 3, lanes 2,4). In addition, the plating efficiency of rpoE− degP− double mutant strains was not restored by overexpression of NlpE, as double mutant cells containing vector alone or a plasmid overexpressing NlpE plated with virtually the same efficiency (<1.8 × 10−7 vs. <5.0 × 10−7). These results indicate that degP is an essential component of suppression of the rpoE− temperature-sensitive phenotype by the Cpx pathway.
Figure 3.
Plating efficiency of rpoE::ΩCm cells lacking degP. The plating efficiency of rpoE::ΩCm cpxA+ (lane 1), rpoE::ΩCm cpxA+ degP::Km (lane 2), rpoE::ΩCm cpxA* (lane 3), and rpoE::ΩCm cpxA* degP::Km (lane 4) strains was determined as described in Materials and Methods. The numerical values of each plating efficiency were rpoE::ΩCm cpxA+, 1.0 × 10−3; rpoE::ΩCm cpxA+ degP::Km, 6.7 × 10−7; rpoE::ΩCm cpxA*, 0.6; and rpoE::ΩCm cpxA* degP::Km, 1.2 × 10−6. Note that all strains used in this experiment were recA+.
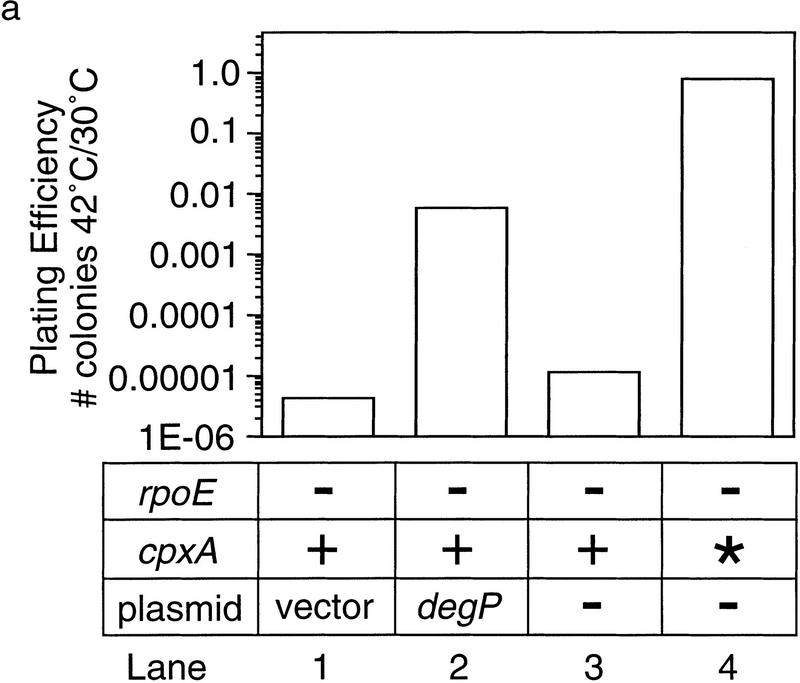
To determine whether overexpression or restoration of degP expression was sufficient to rescue rpoE− cells, we introduce a plasmid encoding degP into the original rpoE− strain. Introduction of the degP plasmid partially restores growth at high temperature to rpoE− cells, as cells containing this plasmid exhibit a 103-fold higher plating efficiency than a similar strain containing vector alone (Fig. 4a, lanes 2,1). However, degP did not restore plating efficiency to the same extent as full activation of the Cpx pathway (Fig. 4a, lanes 2,4).
Figure 4.
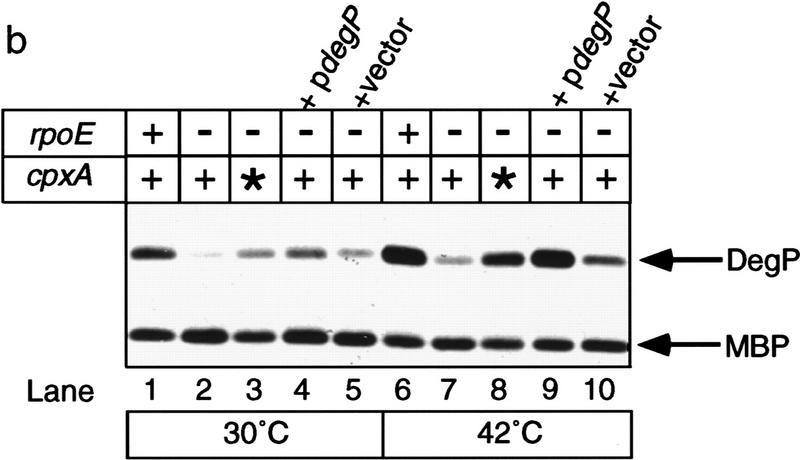
Phenotype of rpoE::ΩCm cells overexpressing DegP. (a) Plating efficiency. The efficiency of plating at 42°C of rpoE::ΩCm cells containing vector alone (lane 1) or a plasmid overexpressing degP (lane 2) was determined. For comparison, the plating efficiencies of rpoE− cells containing a wild-type (lane 3) or fully activated (lane 4) cpxA allele are also shown. The numerical values of each plating efficiency were vector alone, 4.1 × 10−6; pdegP, 6.0 × 10−3; cpxA+, 1.1 × 10−5; and cpxA*, 0.8. (b) Western blot analysis of DegP expression. Wild-type cells (lanes 1,6), rpoE::ΩCm cells containing wild-type (cpxA+, lanes 2,7) or activated (cpxA*, lanes 3,8) cpxA alleles, or rpoE::ΩCm cells containing either a plasmid overexpressing degP (pdegP, lanes 4,9) or vector alone (lanes 5,10) were grown at 30°C to mid-log phase and then shifted to 42°C and grown for 20 min. The steady-state level of DegP expression in cells grown before and after heat shock was then determined by Western blot analysis as described in Materials and Methods. As a loading control, the blot was also probed with antibodies against maltose-binding protein (MBP).
To ensure that degP expression in cells carrying the plasmid was at least as high as cells fully suppressed by activation of the Cpx pathway, Western blot analysis of DegP expression was carried out (Fig. 4b). The steady-state level of DegP in cells grown at 30°C or 42°C was determined, and these studies revealed that rpoE− cells containing the degP plasmid (Fig. 4b, lanes 4,9) expressed at least as much, if not more, DegP as cells carrying the cpxA* allele (Fig. 4b, lanes 3,8) at both temperatures. Together, these analyses show that overexpression of DegP alone does not restore growth to the same levels as does activation of the Cpx pathway, indicating that other Cpx-controlled genes are required for full suppression of the rpoE− temperature-sensitive defect.
Discussion
The activity of the alternate σ-factor σE of E. coli is induced by several stressors, including elevated temperature, ethanol, and alterations in the expression and maturation of OMPs, which lead to the periplasmic accumulation of misfolded or unfolded protein species (Erickson et al. 1987; Erickson and Gross 1989; Raina et al. 1995; Rouvière et al. 1995; Missiakas et al. 1996; Rouvière and Gross 1996). The σE regulon includes several genes whose products are likely to be required for envelope integrity because cells lacking σE are sensitive to several conditions that potentially disrupt outer membrane function (Hiratsu et al. 1995; Raina et al. 1995; Rouvière et al. 1995). Here we show that activation of a second signal transduction cascade, the Cpx pathway, can restore viability to rpoE− cells at elevated temperature. Suppression of the temperature-sensitive phenotype requires the expression of at least two Cpx-dependent genes, one of which encodes for the periplasmic protease DegP, whose expression is also under σE control. These results suggest that E. coli has at least two partially overlapping signal transduction cascades capable of relieving extracytoplasmic stress.
Cellular role of the Cpx pathway
Although the cpx genes were first identified over a decade ago (McEwen and Silverman 1980a), a clear-cut role for the Cpx pathway in E. coli physiology has proven more elusive. The Cpx pathway has been described in relation to pleiotropic phenotypes, including defective conjugative plasmid transfer (McEwen and Silverman 1980a,b), low level resistance to aminoglycoside antibiotics (Thorbjarnardottir et al. 1978), and alterations in the protein composition of the outer membrane (McEwen and Silverman 1982; McEwen et al. 1983), that result from constitutive activation of this pathway. It is unclear whether these phenotypes reflect the normal function of the Cpx pathway or result from aberrant activation (Danese et al. 1995). In addition, cells lacking the Cpx pathway show no obvious phenotypes (Danese et al. 1995; L. Connolly, unpubl.).
The observation that the Cpx pathway modulates expression of the periplasmic protease DegP (Danese et al. 1995) led to the idea that this pathway is involved in combating extracytoplasmic stress, and activation of the Cpx pathway by either mutational induction or overexpression of NlpE has been shown to relieve the toxicity of several periplasmic LamB mutant proteins (Cosma et al. 1995; Snyder et al. 1995). The recent finding that the Cpx pathway modulates the expression of other periplasmic proteins involved in folding supports the idea that this pathway is involved in regulating protein folding and turnover in the extracytoplasmic compartment (Danese and Silhavy 1997; Pogliano et al. 1997).
What does the Cpx pathway sense?
The ability of the Cpx pathway to relieve extracytoplasmic stress due to protein misfolding has been described only in relation to mutational activation of this pathway (Cosma et al. 1995) or activation by nlpE in multicopy (Snyder et al. 1995), suggesting that CpxA may not directly sense the folding state of this compartment. The mechanism of induction by overexpression of NlpE remains unclear. Overexpression of several other lipoproteins did not activate the Cpx pathway (Danese et al. 1995), indicating that NlpE is a specific activator. In addition, the fast kinetics of activation by NlpE (Pogliano et al. 1997) suggest that this activator acts directly on the Cpx pathway and not through slowly altering some physical or biochemical property of the envelope. NlpE has a putative serine protease inhibitor motif, and it has been proposed that NlpE may modulate or monitor protease activity in periplasm (Snyder et al. 1995). For instance, NlpE may use the serine protease inhibitor to monitor free levels of DegP activity in the periplasm. Upon an increase in substrates, DegP may be titrated off of NlpE, and an increase in the levels of free NlpE may signal the need for increased transcription of degP to the Cpx pathway.
In this regard, it is interesting to note that the temperature-sensitive phenotype of cells lacking degP can be suppressed by null mutations in the gene encoding a second envelope-associated protease, OmpT (N. McFarland, unpubl.). An imbalance in the protease activities of the envelope may lead to a similar imbalance of key substrates, which in turn might alter envelope physiology. For example, if OmpT were a substrate of DegP, then deletion of DegP would lead to an increase in OmpT activity and a subsequent decrease in OmpT substrates. It has been proposed that several of the phenotypes of cells containing constitutive alleles of cpxA result from such an imbalance of proteolytic activity that leads to alterations in the protein composition of the outer membrane (Danese et al. 1995).
The Cpx pathway has also been shown to be required for the pH-dependent activation of the Shigella sonnei virF gene (Nakayama and Watanabe 1995), which encodes a positive regulator of the ipaBCD invasion genes. This observation suggests that the Cpx system may be responsive to changes in pH and play a role in bacterial pathogenesis. It will be interesting to determine whether known Cpx-dependent genes are also modulated in response to pH alterations and whether any of these genes encode functions required for survival under such conditions.
Finally, the Cpx pathway is activated in cells lacking phosphatidyl–ethanolamine (Milekovskaya and Dowhan 1997), suggesting that this pathway may also sense alterations in the structural or physical integrity of the cell envelope. Deletion of nlpE from these strains did not alter Cpx activation, suggesting that the Cpx pathway may directly sense these envelope changes (Milekovskaya and Dowhan 1997). It remains to be seen whether each of the conditions described above generates a single, common signal that is sensed by CpxA or whether this pathway is capable of responding to diverse signals.
What is the relationship between the Cpx and ςE pathways?
Several lines of evidence suggest that the Cpx and σE pathways represent distinct stress–response systems that do not sense or regulate redundant functions (Fig. 5). Deletion of either the Cpx pathway or nlpE leads to no obvious phenotype (Danese et al. 1995; Snyder et al. 1995; L. Connolly, unpubl.), suggesting that this pathway may contribute to envelope homeostasis under a specific set of conditions not normally achieved in the laboratory or that Cpx-controlled genes exhibit a basal level of transcription that is sufficient for cell growth. In contrast, the σE pathway appears to be involved in envelope homeostasis under most growth conditions. Cells lacking σE are sensitive to membrane-disrupting agents and fail to grow at high temperature (Hiratsu et al. 1995; Raina et al. 1995; Rouvière et al. 1995). Recent work suggests that σE-directed functions are required at all temperatures, as we have discovered that our strains lacking σE contain an unidentified suppressor that is required for low temperature growth (A. De Las Peñas, L. Connolly, and C.A. Gross, in prep.). The presence of a cpxA* allele does not alleviate the requirement for this suppressor (L. Connolly, unpubl.), indicating that the Cpx pathway cannot substitute for σE under all conditions. In addition, the suppressor alone does not confer a temperature-sensitive phenotype (L. Connolly, unpubl.), indicating that the temperature-sensitive phenotype observed in rpoE− strains is attributable to a loss of σE-dependent gene products. Activation of the Cpx pathway compensates for this loss of σE-dependent gene expression normally required at high temperature, as evidenced by the observation that restoration of expression of a single σE-dependent gene product, DegP, by activation of the Cpx pathway accounts for the majority of suppression (Fig. 4a).
Figure 5.
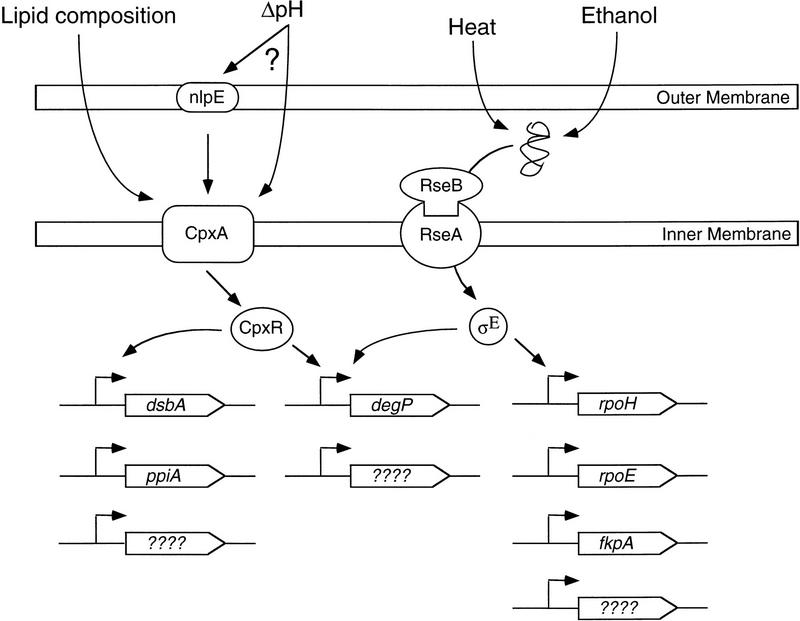
The extracytoplasmic stress response is controlled by partially overlapping pathways. The Cpx pathway controls the expression of several resident periplasmic folding proteins (DegP, PpiA, and DsbA) in response to overproduction of the outer membrane lipoprotein NlpE, and perhaps structural or physical alterations in the envelope induced by changes in pH or lipid composition. The alternate σ-factor σE controls the expression of degP, rpoH, rpoE, fkpA, and several other unidentified genes in response to the accumulation of misfolded OMP precursors in the periplasmic space. (See Discussion for further details).
In addition to exhibiting distinct phenotypes, each system appears to respond to different signals. Overexpression of NlpE does not induce σE activity in general, as measured from a minimal σE-dependent promoter (Danese et al. 1995), and several types of stressors that induce σE activity do not induce the Cpx pathway (Danese et al. 1995). For example, alterations in the maturation of OMPs, either by overexpression or the titration or periplasmic folding agents, uniquely induce σE (Mecsas et al. 1993; Danese et al. 1995; Missiakas et al. 1996; Rouvière and Gross 1996). Alterations in the lipid composition of the envelope have been shown recently to induce the Cpx pathway (Milekovskaya and Dowhan 1997), and it remains to be seen whether these conditions similarly alter σE activity.
The Cpx and σE regulons overlap at degP; however, several observations suggest that the two regulons are not identical. First, the Cpx system does not rely exclusively on holoenzyme containing σE for transcriptional activation. Although CpxR and σE appear to act together at the degP promoter, CpxR is capable of inducing degP transcription approximately threefold in the absence of σE (Danese et al. 1995). In addition, new members unique to either the Cpx or σE regulons have been described (Fig. 5) (Danese and Silhavy 1997; Pogliano et al. 1997). The Cpx proteins regulate expression of the disulfide bond isomerase dsbA (Danese and Silhavy 1997; Pogliano et al. 1997), and the peptidyl prolyl isomerase (PPIase), ppiA (Pogliano et al. 1997), whereas σE induces the expression of a second PPIase, FkpA (Danese and Silhavy 1997). Although each regulon encodes similar biochemical activities, and activation of the Cpx pathway can rescue the temperature-sensitive defect of rpoE− cells, activation of the σE pathway cannot substitute for the Cpx system under some conditions. For example, activation of σE by overexpression of OMPs does not relieve the toxicity of LamB mutant proteins (Snyder et al. 1995). These results suggest that the two pathways can be induced independently in response to unique stressors and that they may integrate these disparate signals arising in the face of multifaceted insults. A complete understanding of the relationship between these two systems awaits further molecular characterization of their regulon members and inducing signals.
Regulation of DegP
From these experiments and those of others, it is clear that DegP plays a key role in combating envelope stress (Lipinska et al. 1989; Strauch et al. 1989; Cosma et al. 1995; Snyder et al. 1995). During the original characterization of the degP promoter, it became evident that degP was a heat shock gene (Lipinska et al. 1988). This heat induction appeared to be independent of the main heat shock σ-factor, σ32, and was instead found to depend on a second heat shock σ, σE (Lipinska et al. 1988; Erickson and Gross 1989). These experiments provided some of the first evidence that σE controlled a second heat shock regulon. However, it became clear during the course of our experiments that the expression of DegP is still induced by heat shock in the absence of σE (Fig. 4b). The heat shock regulation of degP has been shown recently to be independent of the Cpx pathway in cells containing σE (Pogliano et al. 1997), and we have observed heat shock induction of DegP in cells lacking both of these systems (L. Connolly, unpubl.). These results raise the possibility that a third regulatory cascade modulates DegP expression and help to explain the seemingly contradictory observation that cells lacking σE and, by extension the entire σE regulon, plate 100-fold better than cells lacking DegP alone (10−3 vs. 10−5; L. Connolly, unpubl.). It is also clear that this residual expression contributes substantially to the survival of rpoE− cells at high temperature (Fig. 3), underscoring the key role that DegP plays in maintaining envelope integrity. From these experiments, it is unclear at what level this σE-independent induction of DegP is occurring, and it will be interesting to determine how DegP levels are regulated in the absence of the σE and Cpx systems.
Materials and methods
Media, reagents, and enzymes
Luria-Bertani (LB) and M9 minimal media were prepared as described (Sambrook et al. 1989). Where needed, media were supplemented with 100 μg/ml of ampicillin (Ap100), 3 μg/ml of amikacin, 50 μg/ml of spectinomycin (Sp50), 10 μg/ml of tetracycline (Tc10), 30 μg/ml kanamycin (Km30), 12 μg/ml of chloramphenicol (Cm12), or 0.4% glucose.
Strains
Bacterial strains used in this study are described in Table 2.
Table 2.
Strains and plasmids used in this study
|
|
Relevant genotype
|
Reference
|
|---|---|---|
| Strain | ||
| CAG16037 | MC1061 φ(λ rpoH P3::lacZ) | Mecsas et al. (1993) |
| CAG37193 | CAG16037 srl-300::Tn10 recA56 | this work |
| CAG22216 | CAG16037 rpoE::ΩCm | Rouvière et al. (1995) |
| CAG22700 | CAG22216 srl-300::Tn10 recA56 | this work |
| CAG33126 | CAG22700, pUC18 | this work |
| CAG33127 | CAG22700, pBA25 | this work |
| CAG33183 | CAG22700, pBA26 | this work |
| CAG33267 | CAG22700, pBR322 | this work |
| CAG33268 | CAG22700, pLD404 | this work |
| CAG33190 | CAG22700, pLC222 | this work |
| CAG33191 | CAG22700, pLC224 | this work |
| CAG33113 | CAG22700, pAP87 | this work |
| CAG33114 | CAG22700, pACYC177 | this work |
| PND325 | MC4100 λRS88(degP–lacZ) cpxR::ΩSp | Danese et al. (1995) |
| CAG18636 | MG1655 zii-3088::Tn10Km | Singer et al. (1989) |
| CAG33064 | PND325 zii-3088::Tn10Km | this work |
| CAG33069 | CAG22216 zii-3088::Tn10Km cpxR::ΩSp | this work |
| CAG33091 | CAG33069 srl-300::Tn10 recA56 | this work |
| CAG33239 | CAG33091, pUC18 | this work |
| CAG33240 | CAG33091, pBA25 | this work |
| CAG33241 | CAG33091, pLC222 | this work |
| CAG33242 | CAG33091, pLC224 | this work |
| CAG16237 | CAG16037 degP::Km | Mecsas et al. (1993) |
| CAG33245 | CAG33104 degP::Km | this work |
| CAG33067 | CAG22216 degP::Km | this work |
| CAG33070 | CAG33067 srl-300::Tn10 recA56 | this work |
| CAG33163 | CAG33070, pUC18 | this work |
| CAG33164 | CAG33070, pBA25 | this work |
| CLC145 | MC4100 cpx103 lamBA23D | Cosma et al. (1995) |
| CAG33104 | CAG22216 cpx103 | this work |
| CAG33131 | CAG33104 srl-300::Tn10 recA56 | this work |
| Plasmid | ||
| pUC18 | cloning vector, ColE1 ori, Ap | Norrander et al. (1983) |
| pBA25 | yaeJ, nlpE, yaeF, drpA/proS in pUC, Ap | this work |
| pBA26 | yaeJ and nlpE in pUC, Ap | this work |
| pSU18 | cloning vector, p15A ori, Cm | Bartolomé et al. (1991) |
| pLC222 | bla gene from pUC cloned into pSU18, Ap | this work |
| pLC224 | nlpE in pLC2222, Ap | this work |
| pBR322 | cloning vector, ColE1 ori, Ap, Tc | Bolivar et al. (1977) |
| pLD404 | yaeJ, nlpE, and yaeF in pBR322 | Snyder et al. (1995) |
| pACYC177 | cloning vector, p15A ori, Km, Ap | Rose (1988) |
| pAP87 | degP in pACYC177, Km | this work |
Plasmids
Plasmids used in this study are listed in Table 2. The E. coli genomic library was a generous gift of Alan Derman (University of California, San Francisco) and contains PstI-digested chromosomal DNA from MG1655 cloned into the PstI site of pUC19 (Norrander et al. 1983) as described (Neumann et al. 1992). pBA25 was isolated directly from this library. During the course of subcloning nlpE from this plasmid, we noticed a difference in a single restriction site versus a previously reported sequence derived from MC4100 (Snyder et al. 1995), suggesting that sequence heterogeneity of this gene may exist among different E. coli isolates. pBA26 was constructed by deleting a 2.1-kb AflII–XbaI fragment from pBA25. pLC224 was constructed by PCR amplification of nlpE from pBA25 using primers NLPE28 (5′-CAGCGGTCGGGAATTCAAAGAAGGAATG) and NLPE29 (5′-GGGGGGAAGCTTACGCCTTATCCGGCCTAC). The resulting product was then cloned into the EcoRI–HindIII sites of pLC222 [an ApR version of pSU18 (Bartolomé et al. 1991), containing the bla gene from pBS SK+ cloned into the SspI sites of pSU18]. pAP87 was constructed by subcloning a 3.0-kb DraI–BamHI fragment from pKS17 into pACYC177 (Rose 1988).
DNA sequencing
Sequence was obtained from either end of the insert in pBA25 using vector-derived primers (M13 Sequencing Primer and M13 Reverse Sequencing Primer) and a cycle sequencing kit according to the manufacturer’s directions (GIBCO BRL, Gaithersburg, MD).
Plating efficiencies
recA56 versions of all strains were used to determine plating efficiencies, with the exception of the data in Fig. 3 (CAG22216, CAG33104, CAG33067, CAG33245).
Tenfold dilutions of the desired strain were made in 1 ml of LB from fresh overnight cultures grown at 30°C in LB plus the appropriate antibiotic and 0.4% glucose for rpoE::ΩCm cpxR::ΩSp strains containing the following plasmids: pUC18, pBA25, pLC222, and pLC224. Each dilution (100 μl) was plated in duplicate on LB or Ap100 for plasmid-containing strains. One plate was incubated at 30°C, the other at 42°C for 24–48 hr, and the resulting number of colonies counted. Plating efficiency was scored as the number of colonies arising at 42°C divided by the number at 30°C, and the values reported represent the average of at least three independent determinations.
Detection of DegP and maltose-binding protein by Western blot
Fifteen milliliters of LB were inoculated with 150 μl of a fresh overnight culture of the desired strains. Cells were grown at 30°C to an OD600 of 0.5, and 0.9 ml of culture was sampled directly into 0.1 ml of 50% TCA on ice, vortexed, and placed at −20°C overnight. The remainder of the culture was shifted to 42°C, and grown for 20 min. After 20 min of growth, the OD600 of the culture was determined and 0.9 ml sampled directly into TCA as above. The next day, TCA precipitates were pelleted at 17,500g for 30 min at 4°C. The pellets were washed twice with 100% acetone, air-dried, and resuspended in 1× protein sample buffer (50 mm Tris-Cl at pH 6.8, 100 mm DTT, 2% SDS, 0.2% bromophenol blue) to a concentration of 107 cells/μl. Cells (2.5 × 107) were run on 10% SDS-polyacrylamide gels and transferred to a nitrocellulose membrane. The membrane was blocked overnight at room temperature with 3% nonfat dry milk in TBST (10 mm Tris at pH 8.0, 150 mm NaCl, 0.2% Tween 20). A mixture of anti-DegP and maltose-binding protein (MBP) primary antibodies (provided by Jon Beckwith, Harvard Medical School, Boston, MA) were used at 1:10,000 concentration in TBST plus 1% milk for 1 hr at room temperature. The blot was washed three times for 5 min each with TBST, then incubated with 1:10,000 dilution of sheep anti-rabbit peroxidase (Boehringer Mannheim, Indianapolis, IN) for 1 hr at room temperature, and washed again as before. It was then developed with enhanced chemiluminescence (ECL) reagents (Amersham, Arlington Heights, IL) and Hyperfilm ECL (Amersham, Arlington Heights, IL) and exposed to film from 5–30 sec.
Acknowledgments
We give special thanks to Chris Cosma for sharing strains. We also thank Paul Danese and Tom Silhavy for strains and helpful discussions concerning this manuscript, Alan Derman for the gift of the genomic library, and Joe Pogliano, Jon Beckwith, Paul Danese, and Tom Silhavy for sharing results prior to publication. We also thank Christophe Herman for critical reading of this manuscript. This work was supported by U.S. Public Service grant GM36278 from the National Institutes of Health. L.C. was also supported by the UCSF Medical Scientist Training Program GM07618.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL cgross@cgl.ucsf.edu; FAX (415) 476-4204.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bartolomé B, Jubete Y, Martinez E, de la Cruz F. Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene. 1991;102:75–78. doi: 10.1016/0378-1119(91)90541-i. [DOI] [PubMed] [Google Scholar]
- Becker J, Craig EA. Heat-shock proteins as molecular chaperones. Eur J Biochem. 1994;219:11–23. doi: 10.1007/978-3-642-79502-2_2. [DOI] [PubMed] [Google Scholar]
- Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heyneker HL, Boyer HW. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- Bukau B. Regulation of the Escherichia coli heat-shock response. Mol Microbiol. 1993;9:671–680. doi: 10.1111/j.1365-2958.1993.tb01727.x. [DOI] [PubMed] [Google Scholar]
- Cosma CL, Danese PN, Carlson JH, Silhavy TJ, Snyder WB. Mutational activation of the Cpx signal transduction pathway of Escherichia coli suppresses the toxicity conferred by certain envelope-associated stresses. Mol Microbiol. 1995;18:491–505. doi: 10.1111/j.1365-2958.1995.mmi_18030491.x. [DOI] [PubMed] [Google Scholar]
- Cox JS, Walter P. A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell. 1996;87:391–404. doi: 10.1016/s0092-8674(00)81360-4. [DOI] [PubMed] [Google Scholar]
- Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- Danese P, Silhavy T. The σE and Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes & Dev. 1997;11:1183–1193. doi: 10.1101/gad.11.9.1183. [DOI] [PubMed] [Google Scholar]
- Danese PN, Snyder WB, Cosma CL, Davis LJ, Silhavy TJ. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes & Dev. 1995;9:387–398. doi: 10.1101/gad.9.4.387. [DOI] [PubMed] [Google Scholar]
- De Las Peñas A, Connolly L, Gross CA. The σE-mediated response to extracytoplasmic stress in Escherichia coli is transduced by RseA and RseB, two negative regulators of σE. Mol Microbiol. 1997;24:373–386. doi: 10.1046/j.1365-2958.1997.3611718.x. [DOI] [PubMed] [Google Scholar]
- Erickson JW, Gross CA. Identification of the σE subunit of Escherichia coli RNA polymerase: A second alternate σ factor involved in high-temperature gene expression. Genes & Dev. 1989;3:1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- Erickson JW, Vaughn V, Walter WA, Neidhardt FC, Gross CA. Regulation of the promoters and transcripts of rpoH, the Escherichia coli heat shock regulatory gene. Genes & Dev. 1987;1:419–432. doi: 10.1101/gad.1.5.419. [DOI] [PubMed] [Google Scholar]
- Gamer J, Bujard H, Bukau B. Physical interaction between heat shock proteins DnaK, DnaJ, and GrpE and the bacterial heat shock transcription factor σ32. Cell. 1992;69:833–842. doi: 10.1016/0092-8674(92)90294-m. [DOI] [PubMed] [Google Scholar]
- Gamer J, Multhaup G, Tomoyasu T, McCarty JS, Rudiger S, Schonfeld HJ, Schirra C, Bujard H, Bukau B. A cycle of binding and release of the DnaK, DnaJ, and GrpE chaperones regulates activity of the Escherichia coli heat shock transcription factor σ32. EMBO J. 1996;15:607–617. [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C, Liberek K, Zylicz M, Ang D. Properties of the heat shock proteins of Escherichia coli and the autoregulation of the heat shock response. In: Morimoto RI, Tissieres A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 209–249. [Google Scholar]
- Gross CA. Function and regulation of the heat shock proteins. In: Curtiss R, et al., editors. Escherichia coli and Salmonella typhimurium. Cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1382–1399. [Google Scholar]
- Grossman AD, Erickson JW, Gross CA. The htpR gene product of E. coli is a σ factor for heat-shock promoters. Cell. 1984;38:383–390. doi: 10.1016/0092-8674(84)90493-8. [DOI] [PubMed] [Google Scholar]
- Gupta SD, Lee BT, Camakaris J, Wu HC. Identification of cutC and cutF (nlpE) genes involved in copper tolerance in Escherichia coli. J Bacteriol. 1995;177:4207–4215. doi: 10.1128/jb.177.15.4207-4215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsu K, Amemura M, Nashimoto H, Shinagawa H, Makino K. The rpoE gene of Escherichia coli, which encodes σE, is essential for bacterial growth at high temperature. J Bacteriol. 1995;177:2918–2922. doi: 10.1128/jb.177.10.2918-2922.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landick R, Vaughn V, Lau ET, VanBogelen RA, Erickson JW, Neidhardt FC. Nucleotide sequence of the heat shock regulatory gene of E. coli suggests its protein product may be a transcription factor. Cell. 1984;38:175–182. doi: 10.1016/0092-8674(84)90538-5. [DOI] [PubMed] [Google Scholar]
- Liberek K, Georgopoulos C. Autoregulation of the Escherichia coli heat shock response by the DnaK and DnaJ heat shock proteins. Proc Natl Acad Sci. 1993;90:11019–11023. doi: 10.1073/pnas.90.23.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberek K, Galitski TP, Zylicz M, Georgopoulos C. The DnaK chaperone modulates the heat shock response of Escherichia coli by binding to the σ32 transcription factor. Proc Natl Acad Sci. 1992;89:3516–3520. doi: 10.1073/pnas.89.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska B, Sharma S, Georgopoulos C. Sequence analysis and regulation of the htrA gene of Escherichia coli: a σ32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 1988;16:10053–10067. doi: 10.1093/nar/16.21.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska B, Fayet O, Baird L, Georgopoulos C. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol. 1989;171:1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen J, Silverman P. Chromosomal mutations of Escherichia coli that alter expression of conjugative plasmid functions. Proc Natl Acad Sci. 1980a;77:513–517. doi: 10.1073/pnas.77.1.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Genetic analysis of Escherichia coli K-12 chromosomal mutants defective in expression of F-plasmid functions: Identification of genes cpxA and cpxB. J Bacteriol. 1980b;144:60–67. doi: 10.1128/jb.144.1.60-67.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Mutations in genes cpxA and cpxB alter the protein composition of Escherichia coli inner and outer membranes. J Bacteriol. 1982;151:1553–1559. doi: 10.1128/jb.151.3.1553-1559.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen J, Sambucetti L, Silverman PM. Synthesis of outer membrane proteins in cpxA cpxB mutants of Escherichia coli K-12. J Bacteriol. 1983;154:375–382. doi: 10.1128/jb.154.1.375-382.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DR, Gething MJ, Sambrook J. The cellular response to unfolded proteins: Intercompartmental signaling. Curr Opin Biotechnol. 1994;5:540–545. doi: 10.1016/0958-1669(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Mecsas J, Rouvière PE, Erickson JW, Donohue TJ, Gross CA. The activity of σE, an Escherichia coli heat-inducible sigma-factor, is modulated by expression of outer membrane proteins. Genes & Dev. 1993;7:2618–2628. doi: 10.1101/gad.7.12b.2618. [DOI] [PubMed] [Google Scholar]
- Milekovskaya E, Dowhan W. The Cpx two-component signal transduction pathway is activated in Escherichia coli mutant strains lacking phosphatidylethanolamine. J Bacteriol. 1997;179:1029–1034. doi: 10.1128/jb.179.4.1029-1034.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiakas D, Betton JM, Raina S. New components of protein folding in extracytoplasmic compartments of Escherichia coli SurA, FkpA and Skp/OmpH. Mol Microbiol. 1996;21:871–884. doi: 10.1046/j.1365-2958.1996.561412.x. [DOI] [PubMed] [Google Scholar]
- Missiakas D, Lemaire M, Mayer M, Georgopoulos C, Raina S. Modulation of the Escherichia coli σE (RpoE) heat shock transcription factor activity by the RseA, RseB, and RseC proteins. Mol Microbiol. 1997;24:355–372. doi: 10.1046/j.1365-2958.1997.3601713.x. [DOI] [PubMed] [Google Scholar]
- Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74:743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Kroeger PE, Cotto JJ. The transcriptional regulation of heat shock genes: A plethora of heat shock factors and regulatory conditions. Exs. 1996;77:139–163. doi: 10.1007/978-3-0348-9088-5_10. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Watanabe H. Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J Bacteriol. 1995;177:5062–5069. doi: 10.1128/jb.177.17.5062-5069.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann B, Pospiech A, Schairer HU. Rapid isolation of genomic DNA from gram-negative bacteria. Trends Genet. 1992;8:332–333. doi: 10.1016/0168-9525(92)90269-a. [DOI] [PubMed] [Google Scholar]
- Norrander J, Kempe T, Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983;26:101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Pogliano J, Lynch AS, Belin D, Lin ECC, Beckwith J. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes & Dev. 1997;11:1169–1182. doi: 10.1101/gad.11.9.1169. [DOI] [PubMed] [Google Scholar]
- Pugsley AP. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina S, Missiakas D, Georgopoulos C. The rpoE gene encoding the σE (σ24) heat shock sigma factor of Escherichia coli. EMBO J. 1995;14:1043–1055. doi: 10.1002/j.1460-2075.1995.tb07085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RE. The nucleotide sequence of pACYC177. Nucleic Acids Res. 1988;16:356. doi: 10.1093/nar/16.1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière PE, Gross CA. SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes & Dev. 1996;10:3170–3182. doi: 10.1101/gad.10.24.3170. [DOI] [PubMed] [Google Scholar]
- Rouvière PE, De Las Peñas A, Mecsas J, Lu CZ, Rudd KE, Gross CA. rpoE, the gene encoding the second heat-shock sigma factor, σE, in Escherichia coli. EMBO J. 1995;14:1032–1042. doi: 10.1002/j.1460-2075.1995.tb07084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T. Molecular cloning: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Shamu CE, Cox JS, Walter P. The unfolded-protein-response pathway in yeast. Trends Cell Biol. 1994;4:56–60. doi: 10.1016/0962-8924(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Sidrauski C, Cox JS, Walter P. tRNA ligase is required for regulated mRNA splicing in the unfolded protein response. Cell. 1996;87:405–413. doi: 10.1016/s0092-8674(00)81361-6. [DOI] [PubMed] [Google Scholar]
- Singer M, Baker TA, Schnitzler G, Deischel SM, Goel M, Dove W, Jaacks KJ, Grossman AD, Erickson JW, Gross CA. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989;53:1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder WB, Davis LJ, Danese PN, Cosma CL, Silhavy TJ. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J Bacteriol. 1995;177:4216–4223. doi: 10.1128/jb.177.15.4216-4223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch KL, Johnson K, Beckwith J. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol. 1989;171:2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus DB, Walter WA, Gross CA. The activity of σ32 is reduced under conditions of excess heat shock protein production in Escherichia coli. Genes & Dev. 1989;3:2003–2010. doi: 10.1101/gad.3.12a.2003. [DOI] [PubMed] [Google Scholar]
- ————— DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of σ32. Genes & Dev. 1990;4:2202–2209. doi: 10.1101/gad.4.12a.2202. [DOI] [PubMed] [Google Scholar]
- Thorbjarnardottir SH, Magnusdottir RA, Eggertsson G. Mutations determining generalized resistance to aminoglycoside antibiotics in Escherichia coli. Mol & Gen Genet. 1978;161:89–98. doi: 10.1007/BF00266619. [DOI] [PubMed] [Google Scholar]
- Wang QP, Kaguni JM. A novel sigma factor is involved in expression of the rpoH gene of Escherichia coli. J Bacteriol. 1989;171:4248–4253. doi: 10.1128/jb.171.8.4248-4253.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. Heat shock transcription factors: Structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- Yura T, Tobe T, Ito K, Osawa T. Heat shock regulatory gene (htpR) of Escherichia coli is required for growth at high temperature but is dispensable at low temperature. Proc Natl Acad Sci. 1984;81:6803–6807. doi: 10.1073/pnas.81.21.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura T, Nagai H, Mori H. Regulation of the heat-shock response in bacteria. Annu Rev Microbiol. 1993;47:321–350. doi: 10.1146/annurev.mi.47.100193.001541. [DOI] [PubMed] [Google Scholar]