Abstract
Aims
Coxsackievirus B3 (CVB3)-induced myocarditis, initially considered a sole immune-mediated disease, also results from a direct CVB3-mediated injury of the cardiomyocytes. Mesenchymal stem cells (MSCs) have, besides immunomodulatory, also anti-apoptotic features. In view of clinical translation, we first analysed whether MSCs can be infected by CVB3. Next, we explored whether and how MSCs could reduce the direct CVB3-mediated cardiomyocyte injury and viral progeny release, in vitro, in the absence of immune cells. Finally, we investigated whether MSC application could improve murine acute CVB3-induced myocarditis.
Methods and results
Phase contrast pictures and MTS viability assay demonstrated that MSCs did not suffer from CVB3 infection 4–12–24–48 h after CVB3 infection. Coxsackievirus B3 RNA copy number decreased in this time frame, suggesting that no CVB3 replication took place. Co-culture of MSCs with CVB3-infected HL-1 cardiomyocytes resulted in a reduction of CVB3-induced HL-1 apoptosis, oxidative stress, intracellular viral particle production, and viral progeny release in a nitric oxide (NO)-dependent manner. Moreover, MSCs required priming via interferon-γ (IFN-γ) to exert their protective effects. In vivo, MSC application improved the contractility and relaxation parameters in CVB3-induced myocarditis, which was paralleled with a reduction in cardiac apoptosis, cardiomyocyte damage, left ventricular tumour necrosis factor-α mRNA expression, and cardiac mononuclear cell activation. Mesenchymal stem cells reduced the CVB3-induced CD4− and CD8− T cell activation in an NO-dependent way and required IFN-γ priming.
Conclusion
We conclude that MSCs improve murine acute CVB3-induced myocarditis via their anti-apoptotic and immunomodulatory properties, which occur in an NO-dependent manner and require priming via IFN-γ.
Keywords: Mesenchymal stem cells, Coxsackievirus, Apoptosis, Immunoregulation, Nitric oxide, Interferon-γ
Introduction
Myocarditis is a common inflammatory cardiomyopathy which can lead to a chronic impaired left ventricular (LV) function. Many different infectious agents have been considered the cause of viral myocarditis, including enteroviruses, adenovirus, cytomegalovirus, hepatitis C virus, parvovirus B19, and others. Among the most commonly identified infectious agents are the coxsackie B viruses (CVB), members of the enteroviral family. A direct CVB3-mediated injury of the cardiomyocytes in infected hearts1,2 as well as cardiac inflammation3 underlies CVB3-induced viral myocarditis.
Under present pharmacological treatment, which is mainly focused on decreasing the activity of the neuroendocrine system, the prognosis against virus-induced inflammatory cardiomyopathy is not improved. This might be due to the lack of effects on the direct virus-induced components of the disorder. Therefore, different strategies, including gene therapeutic approaches and pharmaca directed at blocking viral replication or stimulating the anti-viral directed immune response,4 are under investigation in experimental5 and/or clinical studies.6
There is accumulating experimental2 and clinical support7,8 for the application of cellular transplantation as a strategy to improve myocardial function. Mesenchymal stem cells (MSCs) have anti-apoptotic,9 anti-fibrotic,10 and pro-angiogenic11 features. They have the advantage over other stem cells that they are non-immunogenic, enabling the use of allogeneic MSCs for clinical application.8 Moreover, MSCs have immunomodulatory properties,12 which make them an attractive cell source for the treatment of virus-induced inflammatory cardiomyopathy, given the importance of the inflammatory component in this disorder. Among others, MSCs suppress T cell responses,13,14 induce apoptosis of activated T cells,15 and increase T regulatory cells.16 Interferon-γ (IFN-γ) has been shown to play an important role in priming MSC-mediated immunoregulatory effects12,17 as well as in inducing nitric oxide (NO) production in MSCs.18 On the other hand, NO exerts anti-apoptotic effects on cardiomyocytes19,20 and has anti-viral properties.21 Moreover, MSCs have been shown to exert their immunomodulatory effects in an NO-dependent manner.12
The present study focuses at investigating whether MSCs are potential candidates for the treatment of acute CVB3-induced inflammatory cardiomyopathy. In view of clinical translation, the safety aspect whether MSCs can be infected by CVB3 is investigated. In addition, the study focuses at evaluating whether and how MSCs can (i) reduce the direct CVB3-mediated cardiomyocyte injury in vitro, and (ii) decrease cardiac apoptosis and improve LV function in an experimental model of murine acute CVB3-induced myocarditis. Furthermore, the role of NO and of IFN-γ priming in the MSC-mediated protective effects is investigated.
Methods
For detailed methodology, please see Supplementary material online. Human adult MSCs were isolated from iliac crest bone marrow aspirates of normal male donors (n = 6) after their written approval. Mesenchymal stem cells were characterized by flow cytometry analysis according to Binger et al.22 To investigate whether MSCs can be infected with CVB3, MSCs were serum starved or exposed to CVB3 at a multiplication of infection (m.o.i.) of 5 for 1 h under serum-starvation conditions. Next, cell morphology, cell viability, and CVB3 RNA copy number were evaluated 4, 12, 24, and 48 h after serum starvation/infection via phase contrast pictures, MTS viability assay, and real-time PCR, respectively. Next, to determine whether MSCs can protect against direct CVB3-induced cardiomyocyte damage, MSCs were co-cultured with uninfected or CVB3-infected HL-1 cardiomyocytes at a ratio of 1 MSC to 10 HL-1. The effect of MSC supplementation on CVB3-induced HL-1 cardiomyocyte apoptosis, oxidative stress, virion progeny release, and intracellular virion production was determined via annexin V/7AAD flow cytometry and caspase 3/7 activity analysis, DCF flow cytometry, and plaque assay, respectively. To analyse whether the MSC-mediated effects were NO dependent, MSCs were pre-treated with nitro-l-argininmethylesterhydrochloride (L-NAME) for 24 h. To investigate whether MSCs require IFN-γ to exert their protective effects, MSCs were co-cultured with uninfected or CVB3-infected HL-1 cells in the presence of 1 µg/mL of anti-murine IFN-γ antibody. NOx and IFN-γ levels in HL-1 monocultures as well as in co-cultures with MSCs were analysed. Furthermore, the effect of IFN-γ supplementation on NOx production in uninfected or CVB3-infected MSCs was evaluated.
In vivo, 106 MSCs or phosphate buffered saline (PBS) was i.v. injected23 in 6–8-week-old C57BL/6 mice 1 day after i.p. infection with 5 × 105 plaque-forming units of CVB3 (Nancy strain). Controls received PBS instead of CVB3. Seven days after CVB3 infection, contractility parameters were analysed as described previously.24 Left ventricular damage, apoptosis, and tumour necrosis factor-α (TNF-α) mRNA expression were determined via haematoxylin eosin staining, TUNEL staining, and real-time PCR, respectively. Furthermore, the effect of MSC application on cardiac mononuclear cell (MNC) proliferation/activation was evaluated via isolation of cardiac MNCs, followed by carboxyfluorescein succinimidyl ester-staining and flow cytometry. Finally, it was evaluated how MSCs reduce the proliferation of CVB3-induced CD4− and CD8− T cell proliferation via (co)-culture of carboxyfluorescein succinimidyl ester-labelled MNCs stimulated with inactivated CVB3, with or without MSCs (untreated or pre-treated with L-NAME for 24 h) in the presence or absence of 1 µg/mL of anti-murine IFN-γ antibody, followed by CD4 and CD8 staining and flow cytometry. NOx and IFN-γ levels in MNC monocultures as well as in co-cultures with MSCs were analysed.
Results
Mesenchymal stem cells cannot be infected by coxsackievirus B3
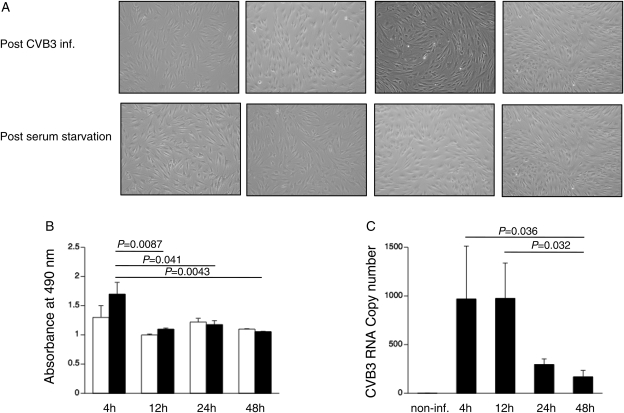
Human adult MSCs were isolated from iliac crest bone marrow aspirates of normal male donors (n= 6) after their written approval. A representative picture of a flow cytometry analysis of MSC is shown in Supplementary material online, Figure S1. To investigate whether MSCs can be infected with CVB3, whether replication of CVB3 takes place, and whether MSCs suffer from CVB3, a time-frame experiment was performed. Phase contrast pictures did not show any significant changes in cell morphology between serum-starved and CVB3-infected MSCs under serum-starvation conditions (Figure 1A). In line with this observation, no significant differences were found in MSC viability between post-infection and post-serum starvation at all time points (Figure 1B). Coxsackievirus B3 RNA copy number, expressed as CVB3 towards L32, decreased over time in MSCs, with 4.1-fold (P= 0.057) and 7.2-fold (P= 0.036) lower levels at 24 and 48 h post-infection vs. 4 h post-infection, respectively (Figure 1C). No plaques were detected on HeLa cells incubated with the medium collected from MSCs 24 h after CVB3 infection, not at dilutions 10−5 and 10−4, neither at dilution 10−3. In addition, the inability to infect MSCs with CVB3 was further underscored by the finding that the beneficial effects of MSCs were not impaired after CVB3 infection compared with serum starvation (Supplementary material online, Figure S2).
Figure 1.
Mesenchymal stem cells cannot be infected by coxsackievirus B3 (CVB3). Mesenchymal stem cells were plated in a 6-well plate for phase contrast pictures or in a 96-well plate for MTS assay, respectively. After 24 h, reaching 80% confluence, mesenchymal stem cells were serum starved or infected with coxsackievirus B3 under serum-starvation conditions at a m.o.i. of 5. Four, 12, 24, and 48 h after infection or serum starvation, phase contrast pictures were taken or MTS assay was performed. (A) Phase contrast pictures of mesenchymal stem cells, 4, 12, 24, and 48 h after coxsackievirus B3 infection (upper panel) or serum starvation (lower panel), at ×100 magnification. (B) Bar graphs representing the absorbance at 490 nm from non-infected (open bar graphs) and coxsackievirus B3-infected (closed bar graphs) mesenchymal stem cells 4, 12, 24, and 48 h after serum starvation or coxsackievirus B3 infection, respectively. n= 6/condition. (C) Bar graphs representing coxsackievirus B3 copy number expressed as coxsackievirus B3 vs. L32 in coxsackievirus B3-infected (closed bar graphs) mesenchymal stem cells 4, 12, 24, and 48 h after coxsackievirus B3 infection. n= 6/condition.
Mesenchymal stem cells reduce coxsackievirus B3-induced apoptosis
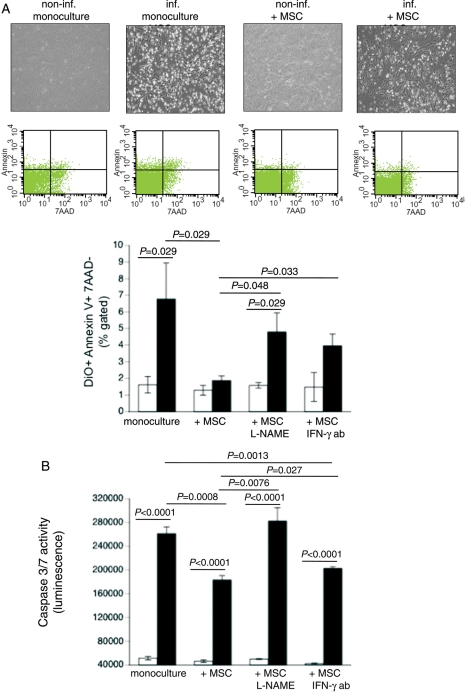
Coxsackievirus B3 targets cardiomyocytes, leading to cardiomyocyte apoptosis.2 To investigate whether MSCs exert anti-apoptotic effects on CVB3-infected HL-1 cardiomyocytes, we co-cultured MSCs in the presence of CVB3-infected HL-1 and performed annexin V/7AAD FACS analysis. Phase contrast pictures showed that MSCs reduce the amount of CVB3-induced floating cells, indicative for necrotic cells (Figure 2A, upper panel). In addition, MSCs decreased the 4.2-fold (P= 0.029) CVB3-induced HL-1 apoptosis to levels not significantly different from non-infected cells (Figure 2A, middle and lower panels). Mesenchymal stem cells have been shown to exert immunomodulatory effects in an NO-dependent manner and to require IFN-γ priming.12 We evaluated whether these underlying mechanisms could be extrapolated to the MSC-mediated protective effects in the context of CVB3-infected HL-1 cells. Therefore, MSCs were pre-treated with L-NAME or co-cultured with CVB3-infected HL-1 cells in the presence of an anti-mouse IFN-γ-neutralizing antibody. Under these conditions, the anti-apoptotic MSC-mediated effects were abrogated. Next, we analysed whether the MSC-mediated reduction in HL-1 apoptosis was associated with decreased caspase 3/7 activity. Supplementation of MSCs declined the CVB3-induced caspase 3/7 activity by 1.4-fold (P< 0.0008). This effect was abrogated when MSCs were pre-treated with L-NAME and was less pronounced in the presence of an anti-mouse IFN-γ antibody (Figure 2B). NOx levels were not increased in the medium of HL-1 and MSC co-cultures compared with HL-1 monocultures, suggesting that at a ratio of 1 MSC to 10 HL-1 cells, the increase in NO is not detectable (Supplementary material online, Figure S3A). Mesenchymal stem cell supplementation did not affect murine IFN-γ levels of HL-1 cells (Supplementary material online, Figure S3B).
Figure 2.
Mesenchymal stem cells (MSCs) reduce coxsackievirus B3-induced apoptosis in a nitric oxide-dependent way and require priming via interferon-γ (IFN-γ). DiO-labelled HL-1 cells were cultured in a six-well plate and 24 h later infected with coxsackievirus B3 under serum-starvation conditions at a m.o.i. of 5. Four hours later, MSCs were added at a ratio of 1 to 10 HL-1 cells. After 24 h, cells were collected for annexin V/7AAD FACS analysis. (A) Upper panel shows representative pictures of non-infected HL-1, non-infected HL-1 co-cultured with MSCs, infected HL-1, and infected HL-1 co-cultured with MSCs; middle panel demonstrates representative pictures of annexin V/7AAD dot plots on pre-selected DiO+ HL-1 cells. Lower panel: bar graphs represent the mean ± SEM of DiO+ annexin V+/7AAD- HL-1 cells in cultures of HL-1 with or without untreated or L-NAME-treated MSCs or MSCs in the presence or absence of 1 µg/mL of IFN-γ antibody (ab); n= 4/group. (B) Bar graphs represent the mean ± SEM of caspase 3/7 activity in cultures of HL-1 with or without untreated or L-NAME-treated MSCs or MSCs in the presence or absence of 1 µg/mL of IFN-γ antibody (ab); n= 6/group.
Mesenchymal stem cells reduce coxsackievirus B3-induced oxidative stress in HL-1 cardiomyocytes
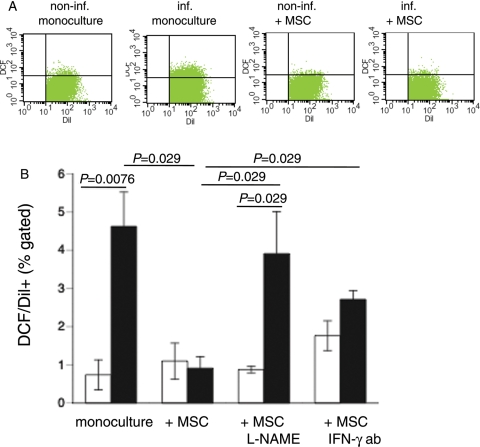
Given the importance of the intracellular oxidation status in the pathogenesis of CVB3 infections,25 we investigated whether MSCs exert anti-oxidative effects on CVB3-infected HL-1 cells. Therefore, we analysed the presence of ROS in MSC-HL-1 co-cultures by CM-H2DCFDA flow cytometry. To distinguish the presence of ROS in HL-1 cells or MSCs in the MSC-HL-1 co-cultures, HL-1 cells were pre-labelled with the fluorescence dye Dil before plating. Flow cytometry analysis showed that CVB3 induced ROS production in HL-1 cells by 6.3-fold (P= 0.0076) (Figure 3), whereas MSCs reduced the increased ROS production by 5.1-fold (P= 0.029) to levels not significantly different from non-infected controls. The anti-oxidative effects of MSCs were abrogated when MSCs were pre-treated with L-NAME or co-cultured in the presence of anti-mouse IFN-γ-neutralizing antibody (Figure 3).
Figure 3.
Mesenchymal stem cell (MSCs) reduce coxsackievirus B3-induced oxidative stress in a nitric oxide-dependent way and require priming via interferon-γ (IFN-γ). Dil-labelled HL-1 cells were cultured in a six-well plate and 24 h later infected with coxsackievirus B3 under serum-starvation conditions at a m.o.i. of 5. Four hours later, mesenchymal stem cells were added at a ratio of 1 to 10 HL-1 cells. After 24 h, cells were collected for DCF FACS analysis. Upper panel demonstrates representative pictures of Dil/DCF dot plots of non-infected HL-1, non-infected HL-1 co-cultured with MSCs, infected HL-1, and infected HL-1 co-cultured with MSCs. Lower panel: bar graphs represent the mean ± SEM of DCF+/Dil+ HL-1 cells in cultures of HL-1 with or without untreated or L-NAME-treated MSCs or with MSCs in the presence or absence of 1 µg/mL of IFN-γ antibody (ab); n= 4/group.
Mesenchymal stem cells reduce viral progeny release and intracellular viral particle production in HL-1 cardiomyocytes
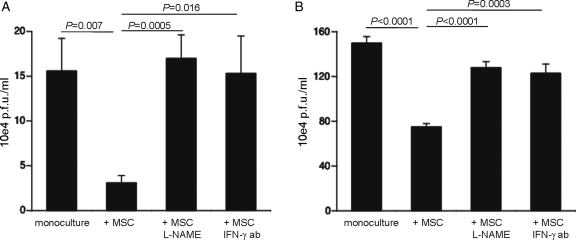
We next evaluated whether the anti-apoptotic and anti-oxidative effects of MSCs on CVB3-infected HL-1 cells were paralleled with a reduction in viral progeny release. Therefore, medium from co-culture experiments was collected and plaque assay was performed. CAPs reduced the viral progeny release by 5.1-fold (P= 0.007), an effect which was blunted in the presence of L-NAME and by blocking murine IFN-γ (Figure 4A). Further analysis demonstrated that besides reducing the release of CVB3 virions, MSCs also decreased the production of intracellular CVB3 particles in an NO-dependent manner and required priming via IFN-γ (Figure 4B).
Figure 4.
Mesenchymal stem cells (MSCs) reduce coxsackievirus B3 viral progeny release and intracellular viral particle building in a nitric oxide-dependent way and require priming via interferon-γ (IFN-γ). HeLa cells were incubated for 30 min with 1 mL of diluted medium/supernatant. Then, cells were washed with PBS and covered with agar consisting 50% of 1.3% noble agar and 50% of 2 MEM, supplemented with 4% FBS. (A) Bar graphs represent the mean ± SEM of counted plaques in six wells/condition, 72 h after incubation with 10−4 diluted medium of cultures of coxsackievirus B3-infected HL-1 with or without untreated or L-NAME-treated MSCs or with MSCs in the presence or absence of 1 µg/mL of IFN-γ antibody (ab). (B) Bar graphs represent the mean ± SEM of counted plaques in six wells/condition, 72 h after incubation with 10−4 diluted supernatant, which is obtained after four freeze–thaw cycles of cell pellets collected 8 h post-infection from coxsackievirus B3-infected HL-1 with or without untreated or L-NAME-treated MSCs or with MSCs in the presence or absence of 1 µg/mL of IFN-γ ab.
Interferon-γ increases nitric oxide in coxsackievirus B3-infected mesenchymal stem cells
Recently, Oh et al.18 demonstrated that IFN-γ is critical for NO production by MSCs. Since our data indicate that MSCs exert their protective effects on CVB3-infected HL-1 cells in an NO-dependent manner and require IFN-γ, we next investigated whether the concentration of IFN-γ present in the co-culture of HL-1 cells with MSCs can lead to NO induction in MSCs. Supplementation of murine IFN-γ to non-infected MSCs did not increase NO production, whereas CVB3 infection combined with murine IFN-γ administration raised NO production by 1.2-fold (P= 0.031) vs. non-infected MSCs (Supplementary material online, Figure S4).
Mesenchymal stem cells improve murine acute coxsackievirus B3-induced myocarditis
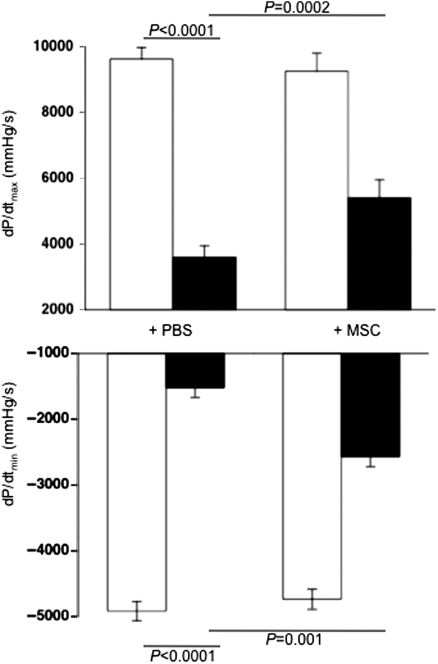
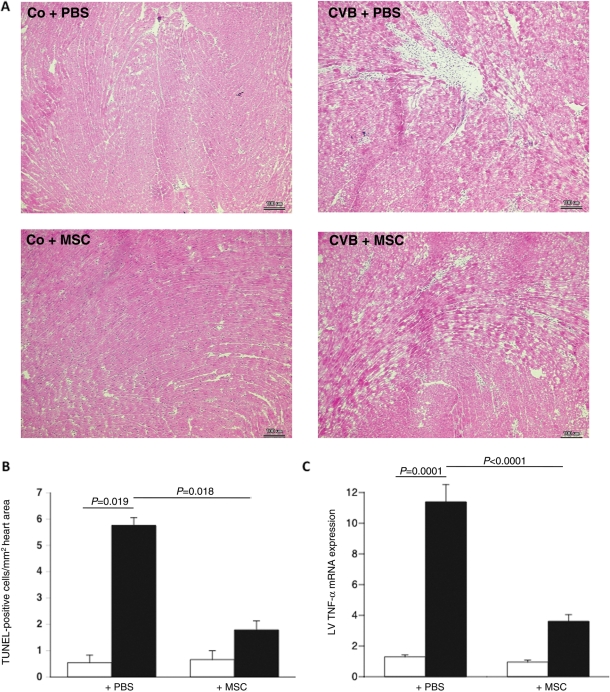
After evaluating in vitro whether and how MSCs can reduce the direct CVB3-induced cardiomyocyte damage, we finally investigated whether MSC application could improve murine acute CVB3-induced myocarditis in vivo. Cardiac contractility and relaxation were significantly ameliorated in MSC-treated compared with PBS-injected CVB3-infected mice, as indicated by a 1.5-fold (P= 0.0002) and 1.7-fold (P= 0.001) improvement in the contractility parameters dP/dtmax and dP/dtmin, respectively (Figure 5). This was associated with a reduction in cardiomyocyte necrosis and fibrosis, as indicated by haematoxylin and eosin staining (Figure 6A). Furthermore, MSC application decreased the CVB3-induced cardiac apoptosis (Figure 6B) and LV TNF-α mRNA expression (Figure 6C) by 3.2-fold (P= 0.018) and 3.2-fold (P< 0.0001), respectively.
Figure 5.
Mesenchymal stem cells (MSCs) improve left ventricular function in an experimental model of coxsackievirus B3-induced myocarditis. Bar graphs represent the mean ± SEM of the contractility parameters (A) dP/dtmax and (B) dP/dtmin in control (open bar graphs) or coxsackievirus B3-infected (closed bar graphs) mice injected with PBS or MSCs, with n= 9/group.
Figure 6.
Mesenchymal stem cells (MSCs) reduce cardiac damage, cardiac apoptosis, and left ventricular tumour necrosis factor-α mRNA expression levels in an experimental model of coxsackievirus B3 (CVB3)-induced myocarditis. (A) Haematoxylin-and-eosin-stained heart sections of control mice receiving PBS (Co + PBS; upper left panel) or MSCs (Co + MSC; lower left panel), or of CVB3 infected mice receiving PBS (CVB3 + PBS; upper right panel) or MSCs (CVB3 + MSC; lower right panel), at a magnification of ×200. (B) Bar graphs represent the mean ± SEM of TUNEL-positive cells per square millimetre in heart cryosections of control mice (open bar), CVB3-infected mice (closed bar) injected with PBS or MSCs, with n= 10/group. (C) Bar graphs represent the mean ± SEM of left ventricular TNF-α mRNA expression levels normalized towards 18S, of control mice (open bar), CVB3-infected mice (closed bar) injected with PBS or MSCs, with n= 9/group.
Mesenchymal stem cells reduce cardiac mononuclear cell activity in coxsackievirus B3-infected mice
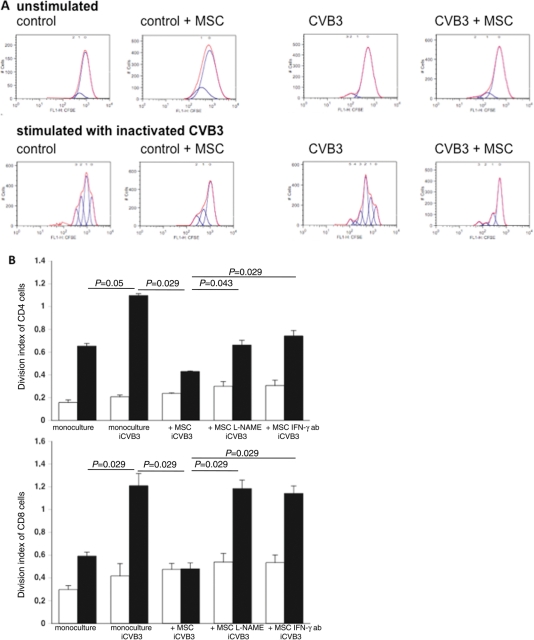
Finally, we analysed whether MSC application could reduce the activity of cardiac MNCs in murine acute CVB3-induced myocarditis. Therefore, cardiac MNC isolated from control and CVB3-infected mice, injected with PBS or MSCs, was carboxyfluorescein succinimidyl ester-labelled and cultured for 72 h in the presence or absence of inactivated CVB3, followed by flow cytometry analysis. The amount of peaks, indicative for cell proliferation, was reduced in cardiac MNCs isolated from CVB3-infected mice receiving MSCs compared with PBS-injected CVB3-infected mice, under unstimulated as well as stimulated conditions (Figure 7A). To further evaluate how MSCs exert these anti-proliferative effects, MNCs were isolated from the spleen from control and CVB3-infected mice, carboxyfluorescein succinimidyl ester-labelled, and next cultured with or without untreated or L-NAME-treated MSCs, or with MSCs in the presence or absence of 1 µg/mL of IFN-γ antibody. Supplementation of MSCs to MNCs at a ratio of 1 to 10 reduced the inactivated CVB3-stimulated division index of CD4− and CD8− T cells from CVB3-infected mice by 2.5-fold (P= 0.029) and 2.5-fold (P= 0.029), respectively. This effect was less pronounced or abrogated when MSCs were pre-treated with L-NAME or co-cultured with MNCs in the presence of an IFN-γ antibody (Figure 7B). Similar to HL-1-MSC co-cultures, no increase in NOx levels could be observed in MNC-MSC co-cultures compared with MNC monocultures, suggesting that at a ratio of 1 MSC to 10 MNCs, the increase in NO is not detectable (Supplementary material online, Figure S5A). Interferon-γ levels in the medium of MNCs derived from CVB3-infected mice were significantly induced upon stimulation with inactivated CVB3 and decreased when MSCs were supplemented (Supplementary material online, Figure S5B).
Figure 7.
Mesenchymal stem cells (MSCs) decrease cardiac mononuclear cell activation in coxsackievirus B3 (CVB3)-infected mice. (A) Cardiac mononuclear cells were isolated from control mice receiving PBS (Co + PBS) or MSCs (Co + mesenchymal stem cell), and of CVB3-infected mice receiving PBS (CVB3 + PBS) or mesenchymal stem cells (CVB3 + MSC). A minimum of n= 8 hearts/group were pooled to perform the experiment. Next, cardiac mononuclear cells were labelled with 10 µM of carboxyfluorescein succinimidyl ester to be able to measure cell proliferation and cultured for 72 h in the absence (unstimulated; upper panel) or presence of heat-inactivated CVB3 (stimulated: lower panel), followed by flow cytometry and analysis with FlowJo 8.7. software. Peaks are indicative for the amount of cell divisions. Representative peaks per group and condition ((un)stimulated) are shown. (B) Mononuclear cells were isolated from the spleen of control mice (open bar graph) or CVB3-infected (closed bar graph) mice. Next, carboxyfluorescein succinimidyl ester-labelled mononuclear cells were directly cultured, in the presence or absence of inactivated CVB3, with or without MSCs (untreated or 24 h pre-treated with L-NAME), in the presence or absence of 1 µg/mL of anti-murine interferon-γ (IFN-γ) antibody for 72 h. Then, cells were stained with monoclonal anti-CD4 or anti-CD8 antibodies, followed by flow cytometry and analysis with FlowJo 8.7. software. Bar graphs represent the division index of CD4− (upper panel) and of CD8− T cells (lower panel) with n= 4/group.
Discussion
The findings of the present study are that MSCs (i) cannot be infected by CVB3; (ii) reduce direct CVB3-induced cardiomyocyte injury in an NO-dependent way and require priming by IFN-γ, and (iii) improve experimental murine acute CVB3-induced myocarditis.
Coxsackievirus B3-induced viral myocarditis, initially considered a sole immune-mediated disease,3 also results from a direct CVB3-mediated injury of the cardiomyocytes in infected hearts.1,2 Besides its immunomodulatory effects, MSCs have also been shown to have anti-apoptotic features. The aim of our study was to investigate whether MSCs are potential candidates for the treatment of acute CVB3-induced inflammatory cardiomyopathy. In detail, we analysed whether and how MSCs can (i) reduce the direct CVB3-mediated cardiomyocyte injury in vitro, and (ii) decrease cardiac apoptosis and improve LV function in an experimental model of murine acute CVB3-induced myocarditis. Furthermore, the role of NO and IFN-γ priming in the MSC-mediated protective effects was investigated.
In view of clinical translation, it is a prerequisite that no replication of CVB3 can take place in MSCs or in any other cell used for (cardiac) cell therapy. We could demonstrate that MSCs did not suffer from CVB3 infection and that CVB3 copy number decreased over time, suggesting that CVB3 did not replicate. Moreover, no viral progeny release occurred. These findings are in line with the described limited expression of the coxsackievirus adenovirus receptor, which is a critical determinant for cellular uptake and pathogenesis of CVB3,26 on MSCs.27 Furthermore, CVB3 infection did not alter the beneficial effects of MSCs, suggesting that the functionality of MSCs upon application in patients with acute CVB3 infection would not be impaired. Recent studies show that the protective effects of bone marrow-derived MSCs from patients with co-morbidities28,29 are less pronounced compared with those of MSCs from healthy donors. The investigation of the characteristics of MSCs from patients with virus-induced inflammatory cardiomyopathy was beyond the scope of this study. The successful use of allogeneic MSCs in clinical studies for the treatment of non-cardiac30 as well as cardiac8 disorders provides consisting support to use allogeneic instead of autologous bone marrow-derived MSCs for the treatment of virus-induced inflammatory cardiomyopathy.
Apoptosis plays an important role in the CVB3 life cycle by facilitating viral progeny release and propagation.31 CVB3 also induces ROS production, which takes place secondary to virus-induced apoptosis, following caspase cleavage32 and can contribute to the activation of stress-activated protein kinases, which in turn are required for viral replication and progeny release.33 In our study, we found that MSCs significantly reduced apoptosis and ROS production in HL-1 cells. We suggest that the MSC-mediated decrease in caspase activity underlies the reduction in ROS. The intracellular oxidation status has been implicated in the pathogenesis of CVB3 infections25 by which the ROS-mediated release of host cell nuclear transcription factor-κ-B results in increased viral replication. Our data indicate that MSCs reduce the intracellular viral production, suggesting that the MSC-mediated decrease in ROS production underlies the reduction in viral replication. Furthermore, MSCs impaired the viral progeny release. Since the intracellular virion particle production affects the amount of viral particle release, on the one hand, and the liberation of viral particles requires apoptosis of infected cells,31 on the other hand, we suggest that the MSC-mediated decline in cardiomyocyte ROS production and apoptosis underlies the attenuation in viral progeny release.
Moreover, we could demonstrate that the anti-apoptotic and anti-oxidative features of MSCs and their effect on viral progeny release and viral production were abrogated when MSCs were pre-treated with the iNOS inhibitor L-NAME, suggesting an NO-dependent mechanism. This observation is supported by the evidence that stem cells conduct their cardioprotective effects in an NO-dependent manner,34 that NO exerts anti-apoptotic effects on cardiomyocytes,19,20 and that NO has anti-viral properties.21,35 In detail, NO donors have been shown to reduce viral replication35 and to decrease signs of myocarditis in CVB3-infected mice.20
Furthermore, the MSC-mediated protective effects were less pronounced in the presence of a murine anti-IFN-γ antibody, suggesting that MSCs require IFN-γ priming to exert their anti-apoptotic, anti-oxidative, and anti-viral features. This hypothesis is supported by the finding that IFN-γ is crucial for priming MSCs to conduct their immunomodulatory effects.12,17 Furthermore, Oh et al.18 recently demonstrated that IFN-γ is critical for NO production by MSCs. We could show that supplementation of IFN-γ to CVB3-infected MSCs increased NO vs. non-infected MSC controls. This suggests that in the context of CVB3 and upon activation with IFN-γ, MSCs produce NO via which they exert their anti-apoptotic and anti-oxidative effects, leading to a decrease in viral progeny release and viral production.
Besides priming MSCs, IFN-γ has been shown to reduce CVB3 replication and CVB3-induced cytopathogenicity, in part via inducing the release of NO by macrophages.36,37 However, we can exclude that the worsened condition of CVB3-infected HL-1 supplemented with an IFN-γ antibody and MSCs was due to the reduced presence of unbound IFN-γ, since supplementation of an IFN-γ antibody alone by CVB3 infection was not associated with an impaired condition of HL1 cells (data not shown).
In vivo, we demonstrated that MSC application resulted in an improvement in contractility, which was paralleled with an impaired cardiac damage and cardiac apoptosis, reduced TNF-α mRNA levels, and cardiac MNC activation. The pathogenesis of CVB3-induced myocarditis is besides a direct CVB3-induced injury also characterized by the infiltration of inflammatory cells, by which the infiltration of T cells coincides with the most severe acute pathological damage in the myocardium.38 The importance of T cells in the severity and development of myocarditis follows from the marked reduction in myocardial damage in CVB3-infected mice treated with a monoclonal antibody against total T cells.39 We foresee that the reduced cardiac injury and apoptosis in CVB3-infected mice receiving MSCs compared with PBS are due to the direct anti-apoptotic and anti-viral effects of MSCs, as shown in vitro, and to MSC-mediated immunomodulatory effects. Injection of MSCs resulted in a decrease in cardiac mRNA levels of the pro-inflammatory cytokine TNF-α, which is known to induce cardiomyocyte apoptosis.40 Furthermore, MSC injection reduced the activity of cardiac MNCs from CVB3-infected mice. Since T cells are crucial for the severity of cardiac damage in CVB3-induced myocarditis,39 we suggest that the impaired cardiac MNC activity contributed to the decreased cardiac damage and apoptosis. In agreement with Ren et al.,12 we demonstrated that the MSC-mediated reduction in CVB3-induced CD4− and CD8− T cell proliferation was NO dependent and required IFN-γ priming. The importance of IFN-γ priming for the functionality of MSCs, on the one hand, and the increased cardiac IFN-γ levels in acute CVB3-induced myocarditis, on the other hand,41 support the hypothesis that MSCs will exert their protective effects in the cardiac inflammatory environment by acute CVB3-induced myocarditis.
In conclusion, our data suggest that MSCs have the potential to be used to treat acute CVB3-induced inflammatory cardiomyopathy since MSCs (i) cannot be infected with CVB3, (ii) have anti-apoptotic and anti-viral effects, and (iii) improve cardiac function in an experimental model of acute CVB3-induced myocarditis. However, further investigation is still required to assess the potential of MSCs for the treatment of chronic CVB3-induced inflammatory cardiomyopathy.
Funding
This study was supported by the Berlin–Brandenburg Center for Regenerative Therapies—BCRT (Bundesministerium für Bildung und Forschung—0313911) to M.S. and C.T., by DFG funding through the Berlin-Brandenburg School for Regenerative Therapies, and by the DFG Sonderforschungsbereich Transregio-19 B5 to C.T. and B6 to C.S.-L. Funding to pay the Open Access publication charges for this article was provided by the DFG Sonderforschungsbereich Transregio-19.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
We thank Annika Koschel for excellent technical assistance.
References
- 1.Chow LH, Beisel KW, McManus BM. Enteroviral infection of mice with severe combined immunodeficiency. Evidence for direct viral pathogenesis of myocardial injury. Lab Invest. 1992;66:24–31. [PubMed] [Google Scholar]
- 2.Yuan JP, Zhao W, Wang HT, Wu KY, Li T, Guo XK, Tong SQ. Coxsackievirus B3-induced apoptosis and caspase-3. Cell Res. 2003;13:203–209. doi: 10.1038/sj.cr.7290165. doi:10.1038/sj.cr.7290165. [DOI] [PubMed] [Google Scholar]
- 3.Mason JW, O'Connell JB, Herskowitz A, Rose NR, McManus BM, Billingham ME, Moon TE. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333:269–275. doi: 10.1056/NEJM199508033330501. doi:10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 4.Kuhl U, Schultheiss HP. Viral myocarditis: diagnosis, aetiology and management. Drugs. 2009;69:1287–1302. doi: 10.2165/00003495-200969100-00001. doi:10.2165/00003495-200969100-00001. [DOI] [PubMed] [Google Scholar]
- 5.Pinkert S, Westermann D, Wang X, Klingel K, Dorner A, Savvatis K, Grossl T, Krohn S, Tschope C, Zeichhardt H, Kotsch K, Weitmann K, Hoffmann W, Schultheiss HP, Spiller OB, Poller W, Fechner H. Prevention of cardiac dysfunction in acute coxsackievirus B3 cardiomyopathy by inducible expression of a soluble coxsackievirus-adenovirus receptor. Circulation. 2009;120:2358–2366. doi: 10.1161/CIRCULATIONAHA.108.845339. doi:10.1161/CIRCULATIONAHA.108.845339. [DOI] [PubMed] [Google Scholar]
- 6.Kuhl U, Pauschinger M, Schwimmbeck PL, Seeberg B, Lober C, Noutsias M, Poller W, Schultheiss HP. Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation. 2003;107:2793–2798. doi: 10.1161/01.CIR.0000072766.67150.51. doi:10.1161/01.CIR.0000072766.67150.51. [DOI] [PubMed] [Google Scholar]
- 7.Yerebakan C, Kaminski A, Liebold A, Steinhoff G. Safety of intramyocardial stem cell therapy for the ischemic myocardium: results of the Rostock trial after 5-year follow-up. Cell Transplant. 2007;16:935–940. doi: 10.3727/096368907783338280. doi:10.3727/096368907783338280. [DOI] [PubMed] [Google Scholar]
- 8.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. doi:10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li JH, Zhang N, Wang JA. Improved anti-apoptotic and anti-remodeling potency of bone marrow mesenchymal stem cells by anoxic pre-conditioning in diabetic cardiomyopathy. J Endocrinol Invest. 2008;31:103–110. doi: 10.1007/BF03345575. [DOI] [PubMed] [Google Scholar]
- 10.Ohnishi S, Sumiyoshi H, Kitamura S, Nagaya N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Lett. 2007;581:3961–3966. doi: 10.1016/j.febslet.2007.07.028. doi:10.1016/j.febslet.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 11.Halkos ME, Zhao ZQ, Kerendi F, Wang NP, Jiang R, Schmarkey LS, Martin BJ, Quyyumi AA, Few WL, Kin H, Guyton RA, Vinten-Johansen J. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol. 2008;103:525–536. doi: 10.1007/s00395-008-0741-0. doi:10.1007/s00395-008-0741-0. [DOI] [PubMed] [Google Scholar]
- 12.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. doi:10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. doi:10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 14.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. doi:10.1182/blood.V99.10.3838. [DOI] [PubMed] [Google Scholar]
- 15.Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19:1597–1604. doi: 10.1038/sj.leu.2403871. doi:10.1038/sj.leu.2403871. [DOI] [PubMed] [Google Scholar]
- 16.Di Ianni M, Del Papa B, De Ioanni M, Moretti L, Bonifacio E, Cecchini D, Sportoletti P, Falzetti F, Tabilio A. Mesenchymal cells recruit and regulate T regulatory cells. Exp Hematol. 2008;36:309–318. doi: 10.1016/j.exphem.2007.11.007. doi:10.1016/j.exphem.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, Shen B, Yin S, Liu W, Cui L, Li N. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18:846–857. doi: 10.1038/cr.2008.80. doi:10.1038/cr.2008.80. [DOI] [PubMed] [Google Scholar]
- 18.Oh I, Ozaki K, Sato K, Meguro A, Tatara R, Hatanaka K, Nagai T, Muroi K, Ozawa K. Interferon-gamma and NF-kappaB mediate nitric oxide production by mesenchymal stromal cells. Biochem Biophys Res Commun. 2007;355:956–962. doi: 10.1016/j.bbrc.2007.02.054. doi:10.1016/j.bbrc.2007.02.054. [DOI] [PubMed] [Google Scholar]
- 19.Razavi HM, Hamilton JA, Feng Q. Modulation of apoptosis by nitric oxide: implications in myocardial ischemia and heart failure. Pharmacol Ther. 2005;106:147–162. doi: 10.1016/j.pharmthera.2004.11.006. doi:10.1016/j.pharmthera.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Szelid Z, Pokreisz P, Liu X, Vermeersch P, Marsboom G, Gillijns H, Pellens M, Verbeken E, Van de Werf F, Collen D, Janssens SP. Cardioselective nitric oxide synthase 3 gene transfer protects against myocardial reperfusion injury. Basic Res Cardiol. 2009;105:169–179. doi: 10.1007/s00395-009-0077-4. [DOI] [PubMed] [Google Scholar]
- 21.Zell R, Markgraf R, Schmidtke M, Gorlach M, Stelzner A, Henke A, Sigusch HH, Gluck B. Nitric oxide donors inhibit the coxsackievirus B3 proteinases 2A and 3C in vitro, virus production in cells, and signs of myocarditis in virus-infected mice. Med Microbiol Immunol. 2004;193:91–100. doi: 10.1007/s00430-003-0198-6. doi:10.1007/s00430-003-0198-6. [DOI] [PubMed] [Google Scholar]
- 22.Binger T, Stich S, Andreas K, Kaps C, Sezer O, Notter M, Sittinger M, Ringe J. Migration potential and gene expression profile of human mesenchymal stem cells induced by CCL25. Exp Cell Res. 2009;315:1468–1479. doi: 10.1016/j.yexcr.2008.12.022. doi:10.1016/j.yexcr.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, Sobel BE, Delafontaine P, Prockop DJ. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354:700–706. doi: 10.1016/j.bbrc.2007.01.045. doi:10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westermann D, Rutschow S, Van Linthout S, Linderer A, Bucker-Gartner C, Sobirey M, Riad A, Pauschinger M, Schultheiss HP, Tschope C. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetologia. 2006;49:2507–2513. doi: 10.1007/s00125-006-0385-2. doi:10.1007/s00125-006-0385-2. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz KB. Oxidative stress during viral infection: a review. Free Radic Biol Med. 1996;21:641–649. doi: 10.1016/0891-5849(96)00131-1. doi:10.1016/0891-5849(96)00131-1. [DOI] [PubMed] [Google Scholar]
- 26.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. doi:10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 27.Kawabata K, Sakurai F, Koizumi N, Hayakawa T, Mizuguchi H. Adenovirus vector-mediated gene transfer into stem cells. Mol Pharm. 2006;3:95–103. doi: 10.1021/mp0500925. doi:10.1021/mp0500925. [DOI] [PubMed] [Google Scholar]
- 28.Kasper G, Mao L, Geissler S, Draycheva A, Trippens J, Kuhnisch J, Tschirschmann M, Kaspar K, Perka C, Duda GN, Klose J. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells. 2009;27:1288–1297. doi: 10.1002/stem.49. doi:10.1002/stem.49. [DOI] [PubMed] [Google Scholar]
- 29.Khan M, Akhtar S, Mohsin S, Khan SN, Riazuddin S. Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem Cells Dev. 2010 doi: 10.1089/scd.2009.0397. doi:10.1089/scd.2009.0397. Published online ahead of print 12 October 2010. [DOI] [PubMed] [Google Scholar]
- 30.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. doi:10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carthy CM, Yanagawa B, Luo H, Granville DJ, Yang D, Cheung P, Cheung C, Esfandiarei M, Rudin CM, Thompson CB, Hunt DW, McManus BM. Bcl-2 and Bcl-xL overexpression inhibits cytochrome c release, activation of multiple caspases, and virus release following coxsackievirus B3 infection. Virology. 2003;313:147–157. doi: 10.1016/s0042-6822(03)00242-3. doi:10.1016/S0042-6822(03)00242-3. [DOI] [PubMed] [Google Scholar]
- 32.Si X, McManus BM, Zhang J, Yuan J, Cheung C, Esfandiarei M, Suarez A, Morgan A, Luo H. Pyrrolidine dithiocarbamate reduces coxsackievirus B3 replication through inhibition of the ubiquitin-proteasome pathway. J Virol. 2005;79:8014–8023. doi: 10.1128/JVI.79.13.8014-8023.2005. doi:10.1128/JVI.79.13.8014-8023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Si X, Luo H, Morgan A, Zhang J, Wong J, Yuan J, Esfandiarei M, Gao G, Cheung C, McManus BM. Stress-activated protein kinases are involved in coxsackievirus B3 viral progeny release. J Virol. 2005;79:13875–13881. doi: 10.1128/JVI.79.22.13875-13881.2005. doi:10.1128/JVI.79.22.13875-13881.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ii M, Nishimura H, Iwakura A, Wecker A, Eaton E, Asahara T, Losordo DW. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via ‘imported’ nitric oxide synthase activity. Circulation. 2005;111:1114–1120. doi: 10.1161/01.CIR.0000157144.24888.7E. doi:10.1161/01.CIR.0000157144.24888.7E. [DOI] [PubMed] [Google Scholar]
- 35.Zaragoza C, Ocampo CJ, Saura M, McMillan A, Lowenstein CJ. Nitric oxide inhibition of coxsackievirus replication in vitro. J Clin Invest. 1997;100:1760–1767. doi: 10.1172/JCI119702. doi:10.1172/JCI119702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henke A, Zell R, Martin U, Stelzner A. Direct interferon-gamma-mediated protection caused by a recombinant coxsackievirus B3. Virology. 2003;315:335–344. doi: 10.1016/s0042-6822(03)00538-5. doi:10.1016/S0042-6822(03)00538-5. [DOI] [PubMed] [Google Scholar]
- 37.Jarasch N, Martin U, Kamphausen E, Zell R, Wutzler P, Henke A. Interferon-gamma-induced activation of nitric oxide-mediated antiviral activity of macrophages caused by a recombinant coxsackievirus B3. Viral Immunol. 2005;18:355–364. doi: 10.1089/vim.2005.18.355. doi:10.1089/vim.2005.18.355. [DOI] [PubMed] [Google Scholar]
- 38.Kishimoto C, Kuribayashi K, Masuda T, Tomioka N, Kawai C. Immunologic behavior of lymphocytes in experimental viral myocarditis: significance of T lymphocytes in the severity of myocarditis and silent myocarditis in BALB/c-nu/nu mice. Circulation. 1985;71:1247–1254. doi: 10.1161/01.cir.71.6.1247. [DOI] [PubMed] [Google Scholar]
- 39.Kishimoto C, Abelmann WH. Monoclonal antibody therapy for prevention of acute coxsackievirus B3 myocarditis in mice. Circulation. 1989;79:1300–1308. doi: 10.1161/01.cir.79.6.1300. [DOI] [PubMed] [Google Scholar]
- 40.Bajaj G, Sharma RK. TNF-alpha-mediated cardiomyocyte apoptosis involves caspase-12 and calpain. Biochem Biophys Res Commun. 2006;345:1558–1564. doi: 10.1016/j.bbrc.2006.05.059. doi:10.1016/j.bbrc.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 41.Fuse K, Chan G, Liu Y, Gudgeon P, Husain M, Chen M, Yeh WC, Akira S, Liu PP. Myeloid differentiation factor-88 plays a crucial role in the pathogenesis of Coxsackievirus B3-induced myocarditis and influences type I interferon production. Circulation. 2005;112:2276–2285. doi: 10.1161/CIRCULATIONAHA.105.536433. doi:10.1161/CIRCULATIONAHA.105.536433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.