Abstract
Western society has an increasing proportion of older adults. Increasing age is associated with a general decrease in the control over task-relevant mental processes. In the present study we investigated the possibility that successful transfer of game-based cognitive improvements to untrained tasks in elderly people is modulated by preexisting neuro-developmental factors as genetic variability related to levels of the brain-derived neurotrophic factor (BDNF), an important neuromodulator underlying cognitive processes. We trained participants, genotyped for the BDNF Val66Met polymorphism, on cognitive tasks developed to improve dynamic attention. Pre-training (baseline) and post-training measures of attentional processes (divided and selective attention) were acquired by means of the useful field of view task. As expected, Val/Val homozygous individuals showed larger beneficial transfer effects than Met/-carriers. Our findings support the idea that genetic predisposition modulates transfer effects.
Keywords: videogame, brain-derived neurotrophic factor, useful field of view
Introduction
The mean age of Western societies is growing rapidly: the proportion of people of 65 years and older will rise from 15% in 2009 to 26% in 2039 and the ratio of retired to working adults will increase from 25 to 49% Central Bureau Statistiek (CBS, 2011). Given this growing population of elderly people it will be important in the near future to understand how cognitive functioning can be preserved and promoted in old age. We already know that some cognitive processes (e.g., feature perception, speech) are less impaired while other cognitive processes such a working memory (WM) and reasoning show large decrements with increasing age (Ball et al., 2004; Brehmer et al., 2007; Shing et al., 2010). Attention abilities and speed of processing also deteriorate in aging (Faust and Balota, 1997; Ball et al., 1998). A crucial deficit that triggers these problems is a general decrease in the control over task-relevant mental processes: with increasing age it is increasingly difficult to apply new rules, to coordinate multiple rules, and to maintain relevant information in the context of interfering ones (Ball et al., 2004). From a meta-analysis of aging studies relevant to selective and divided attention, Verhaeghen and Cerella (2002) drew the more cautious conclusion that specific age effects were largely confined to conditions that require the concurrent handling of multiple task sets, such as in divided attention tasks, consistent with a resource-limitation view of cognitive aging. Interestingly, individual differences in cognitive performance increase from early to late adulthood reflecting genetic differences. In particular, Lindenberger et al. (2008) suggested the function relating brain resources to cognitive performance is non-linear, so that genetic variability is more likely to result in performance differences when resources move away from close-to-optimal levels, as in aging. In other words, the genetic setup of an individual matters more the older he or she gets.
What are useful cognitive interventions to preserve executive functioning in old age? The evidence that the regular use of brain trainers may compensate for cognitive decline with old age is still controversial. Very recently, in a provocative study, Owen et al. (2010) trained 11,430 participants online over a period of 6 weeks on cognitive tasks developed to improve reasoning, memory, planning, visuo-spatial skills, and attention. Even though the participants improved in every one of the trained tasks, the benefit unfortunately did not stretch to transfer effects to untrained tasks. The authors came to the rather pessimistic conclusion that this provides: “no evidence to support the widely held belief that the regular use of computerized brain trainers improves general cognitive functioning in healthy participants beyond those tasks that are actually being trained.”
The purpose of the present study was to investigate whether conclusions of that sort might come out somewhat less pessimistically if one considers the fact that people are not all the same. In particular, it is possible that successful transfer to untrained tasks in elderly people is modulated by, among other things, preexisting neuro-developmental factors. Particularly promising in this respect seems to be genetic variability related to levels of the brain-derived neurotrophic factor (BDNF), an important neuromodulator underlying cognitive processes. BDNF is a critical regulator of the formation, plasticity, and integrity of neurons (Angelucci et al., 2005). An interesting functional single nucleotide polymorphism that leads to an exchange of amino acids from valine (Val) to methionine (Met) has been found at codon 66 in the 5′ pro domain of the BDNF gene (Egan et al., 2003). The Met allele is associated with a decrease in activity-dependent secretion of BDNF compared to the Val allele (Egan et al., 2003). Given that the Met/Met homozygote variant occurs in only 2–3% of the Caucasian population, most studies have compared carriers of a Met allele (Val/Met) with individuals who are homozygous for the Val allele (Val/Val). Val homozygotes show a greater task-related brain activation, as well as higher performance in memory tasks than Met carriers (Egan et al., 2003; Hariri et al., 2003; Pezawas et al., 2004). Interestingly, Li et al. (2010) observed a BDNF Val allele benefit in an episodic memory performance (backward serial recall) in old age but not in young age, supporting the hypothesis of Lindenberger et al. (2008) that genetic variability plays a greater role with increasing age.
The influence of BDNF on brain and cognition is not restricted to long-term memory processes: Met carriers show reduced gray-matter volume in prefrontal cortex (Xu et al., 2007) and perform worse on the Wisconsin Card Sorting Test (Rybakowski et al., 2003). The study by Bueller et al. (2006) indicated a hippocampal reduction in Met carriers compared to individuals homozygous for the Val allele. Given that the hippocampus has been suggested to be involved in the rapid, flexible switching of attention (Fenton et al., 2010), individual differences regarding BDNF may thus mediate performance in, and perhaps the transfer to, tasks drawing on these cognitive functions.
In order to test whether individual differences regarding BDNF may mediate the efficiency of brain training in preserving cognitive control in old age, we trained elderly healthy participants, genotyped for the BDNF Val66Met polymorphism, on cognitive tasks developed to improve dynamic attention. Pre-training (baseline) and post-training measures of attentional processes (divided attention and selective attention) were acquired by means of the useful field of view (UFOV™) task.
The UFOV concept was originally defined by Sanders (1970) as the visual-field area over which information can be acquired in a brief glance without eye or head movements. In contrast to clinical visual-field tests, which commonly require the detection of threshold targets, the UFOV task requires both identification and localization of suprathreshold targets through subtests that primarily tap one’s ability to process rapidly presented, increasingly complex information within a single glimpse through three increasingly difficult visual subtests incorporating stimulus identification, divided attention, and selective attention, respectively. The UFOV task is considered a useful test in guiding diagnosis and treatment of older adults who are experiencing functional visual problems without a clinical basis (Ball and Owsley, 1993). Consistent with this, poorer UFOV task performance has been found to be associated with poor driving performance in older adults (Clay et al., 2005) and other impairments of everyday activities.
The first UFOV subtest requires the identification of a target (silhouette of a car or truck) presented in a central fixation box. The second subtest taps divided attention and involves the identification of a central target along with localization of a simultaneous peripheral target (silhouette of a car). The third subtest consists of these same two tasks, but also includes visual distractors (triangles of the same size and luminance as the targets) that fill the rest of the visual display. Given that the first UFOV subtest has been found to be not sensitive to cognitive intervention (Ball et al., 2007), in the current study participants were asked to perform only the second and third subtest.
Considering the evidence of a hippocampal reduction in BDNF Met carriers as compared to Val/Val homozygous (Bueller et al., 2006) and the suggestion that the hippocampus is involved in the rapid, flexible switching of attention (Fenton et al., 2010), we expected that BDNF variability accounts for individual differences in the transfer to performance in the second and third UFOV subtest. Accordingly, we expected Val/Val homozygous individuals to show larger transfer effects than Met/-carriers.
Materials and Methods
Participants
Ninety participants were recruited through advertisements in a local newspaper and on the internet. Eight participants withdrew from the study and another 12 could not complete the intervention due to technical issues, time constraints, or medical problems. For 10 participants the BDNF Val66Met polymorphism was not available. The data of the remaining 60 participants (32 males, 28 females), with a mean age of 67.6 years (SD = 3.7), 112.9 IQ (SD = 3.0, range 100–130, as determined by a Raven Standard Progressive Matrices (SPM) task, with raw scores transformed into age-specific IQ scores on the basis of Peck, 1970), and with a mean score of 28.8 (SD = 1.2, range 27–30) in the mini mental state examination (MMSE; Folstein et al., 1975), were analyzed. Forty participants were randomly assigned to the experimental group and 20 to the control group. Full participation was rewarded with €100. Written informed consent was obtained from all participants after the nature of the study was explained to them; the protocol was approved by the institutional review board (Leiden University, Institute of Psychology).
Apparatus and stimuli
Computerized tests were controlled by a Windows-operated computer attached to a Philips 17′ monitor in the Leiden University laboratories. Games were played through internet at home.
Useful field of view test
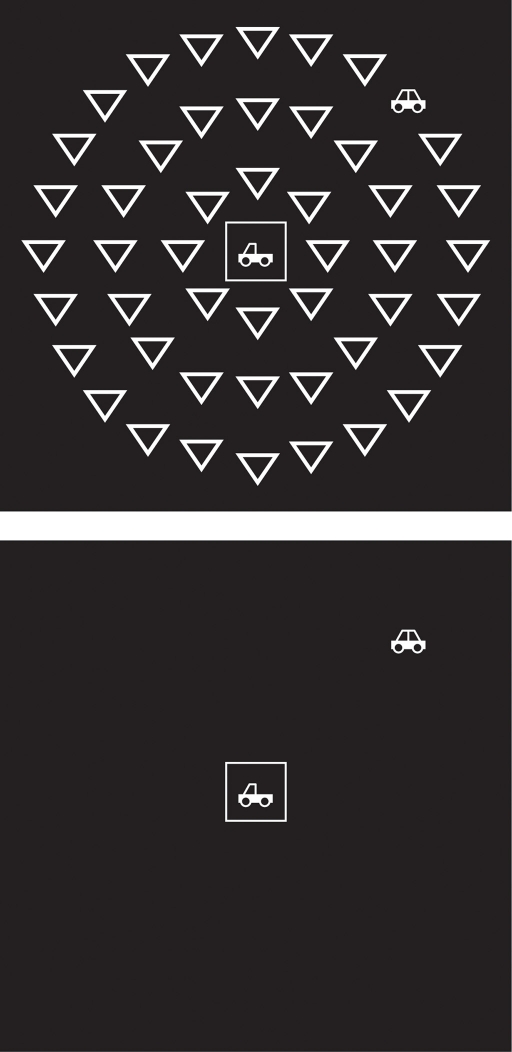
In the divided attention subtest and a selective attention subtest of the UFOV™ (Edwards et al., 2005) the central stimulus was either a picture of a car or a picture of a truck. The peripheral target was a picture of a car or a truck. Stimuli were simple schematic line drawings. The two stimuli were exactly the same except for two of the lines constituting the car stimulus being absent in the truck stimulus. The peripheral target could appear at one of eight radial locations. The sequence of events was identical for both subtests, except that in the selective attention subtest, the empty parts of the stimulus display were filled up with distracters (triangles), while in the divided subtest there were no distracters, see Figure 1. After the stimuli, two response prompts appeared consecutively. First, participants were requested to indicate the perceived identity of the central stimulus by clicking on one of the two response alternatives. Second, the participant was prompted to click one of the peripheral locations to indicate where the peripheral stimulus had been presented.
Figure 1.
Example of trial in the selective subtest (upper panel) and in the divided subtest (lower panel).
In both conditions, a fixation box was presented at the beginning of a trial, followed by both relevant stimuli. The screen was filled with a white-noise visual mask immediately after stimulus presentation. The duration of stimulus presentation was determined by a staircase algorithm. After two correct responses, stimulus presentation time for the next item was shortened, whereas stimulus presentation time for the next item was lengthened if the previous response was incorrect. Scores were expressed as the duration of stimulus presentation at which the participant could correctly perform each subtest 75% of the time. Note that lower scores indicate better performance.
Video games of cognitive intervention
The online intervention games were developed using Adobe Authorware 7 (©Adobe Systems Incorporated, 2011) and were inspired by Nintendo Brain Training for Nintendo DS.
Giving change
In this game the player is presented with a price to be cashed and a payment that is made. The player’s task is to return change by pressing the optimal combination of bills and coins. As players advance, the presented prices and payments increase. Also, the price and payment are presented increasingly briefly.
Firemen
In this game an animation of stick figures walking into and out of a house is presented. After the animation, the player must indicate how many people are inside the house. As players advance, stick figures will walk into and out of the house with greater frequency, faster, and in larger numbers.
Falling bricks
In this game an animation of bricks falling behind an occluding grid structure of rows and columns is presented. After the animation, the player must indicate how high the stack of bricks in a cued column is. As players advance, the number and speed of falling bricks and the number of columns to monitor increase.
Anagrams
In this game a string of letters is presented. The player’s task is to spell a new word using all the presented letters. As players advance, the length of the presented letter string increases.
Clock
In this game an analog clock is presented. The player must indicate what time it will be in a certain amount of time, given the current time indicated by the clock. As players advance, the amount of time to be added to the presented time increases.
Documentaries in the control intervention
In the control intervention, participants viewed a new documentary of 30 min on each session, delivered by streaming video through internet (cf. Smith et al., 2009). The topics of the documentaries were of interest to the population (e.g., politics, music, tourism, recent history), and the documentaries did not tax executive control functions. Each documentary was followed by 3–5 multiple-choice quiz questions.
DNA laboratory analysis
Genomic DNA was extracted from saliva samples using the Oragene™ DNA self-collection kit following the manufacturer’s instructions (DNA Genotek, Inc., 2006). Following Colzato et al. (2010a,b), the genotype was scored by two independent readers by comparison to sequence-verified standards. The Val66Met polymorphism (rs 6265) was extracted from whole genome data using PLINK software (http://pngu.mgh.harvard.edu/∼purcell/plink). Val66Met was in the equilibrium as stated by Hardy and Weinberg (p = 0.29). Individuals who were homozygous for the Met allele were merged with the heterozygous individuals into a group of Met carriers (n = 13 in the experimental group and n = 7 in the control group) and compared to homozygous Val carriers (n = 27 in the experimental Group and n = 13 in the control group). Genotype frequencies were similar to those reported in previous studies on Caucasian populations (Lang et al., 2005; Colzato et al., 2011).
Procedure and design
All participants were tested individually. The intervention in the experimental group consisted of the five computer games taxing executive functions. The games were randomly changed approximately every 3 min, and the level of difficulty was adjusted to the performance of the participant, both in order to maximize the challenge of executive function. After each game or documentary round, data were saved.
The documentaries were presented in a fixed order. Before and after the 7-week intervention, the UFOV task was administered. In addition, participants were subjected to a shortened, computerized version of the Raven’s SPM (Raven et al., 1988), as used in previous studies (Keizer et al., 2010), to estimate individual IQs. Out of the original, full SPM set of 60 items, half of the items (odd or even) was administered in the pre-test, the other half in the post-test. The order of subsets was balanced between-participants. The SPM assesses the individual’s ability to create perceptual relations and to reason by analogy independent of language and formal schooling; it is a standard, widely used test to measure Spearman’s g factor and of fluid intelligence in particular. Each participant performed the tests in the same order at pre- and post-test.
The MMSE was administered only during pre-test. It is the most widely used assessment of global cognitive function. It is often used to screen for dementia or monitor its progression. A Dutch version (adapted from Folstein et al., 1975) of the test was used. The MMSE consists of several questions and problems related to arithmetic, memory, orientation, language use and comprehension, and basic motor skills. Participants were instructed to answer all questions and perform all requested actions to the best of their ability.
The testing session took approximately 40 min to complete. The pre- and post-test assessment was separated by an intervention period of 7 weeks. Participants were instructed to complete one 30-min intervention session per day, resulting in a total of approximately 24.5 h of training. Compliance was confirmed by analysis of the recorded progression data.
Statistical analysis
First, independent samples t-tests were performed to test Age, MMSE, and IQ values between groups. Second, for both the divided attention subtest and a selective attention subtest of the UFOV mean scores were analyzed by means of ANOVAs using assessment (pre- and post-test) as within-, and BDNF (Val/Val vs. Met/-) and Group (Experimental vs. Control) as between-participants factors. Third, mean scores of the five intervention games were analyzed by means of ANOVAs using BDNF (Val/Val vs. Met/) as between-participants factors. A significance level of α = 0.05 was adopted for all tests.
Results
Participants
Participants trained 49 times during the 7-weeks of intervention. Participants in the experimental group were not significantly different from participants in the control group with respect to age, sex, IQ, and MMSE score (all ps > 0.05).
UFOV2 (divided attention)
Mean scores for both groups and assessment are shown in Table 1. Mean scores analysis showed a main effect of assessment, F(1,56) = 7.47, p = 0.008, MSE = 6385.66, , due to a general improvement after the cognitive intervention. More important for present purposes, assessment was involved in a three-way interaction with group and BDNF, F(1,56) = 3.77, p = 0.049, MSE = 6385.66, . As expected, Val/Val homozygous exhibited a significant main effect of assessment, F(1,38) = 15.78, p = 0.0001, MSE = 5338.54, , which was involved in a two-way interaction with group, F(1,38) = 4.04, p = 0.048, MSE = 5338.54, . Only Val/Val homozygous in the experimental, F(1,23) = 23.50, p = 0.0001, MSE = 5084.30, , but not in the control group, significantly benefited from the cognitive intervention, F(1,15) = 1.15, p = 0.24, MSE = 5728.38, In contrast, Met/-carriers, did not show either a significant main effect of assessment, Fs < 1. To be certain that the three-way interaction involving assessment, group, and BDNF was driven by the beneficial effect of Val/Val homozygous in the experimental and not by an eventual benefit of Met/-carriers in the control group, we ran separate ANOVAs for the experimental and control group. The two-way interaction involving BDNF and assessment was significant for the experimental group, F(1,38) = 7.52, p = 0.009, MSE = 51584.53, , but not for the control group, F < 1, suggesting that indeed Val/Val homozygous showed a successful transfer to untrained tasks.
Table 1.
Mean UFOV2 and UFOV3 scores for Met/-carriers and Val/Val homozygous individuals for the pre- and post-test assessment, and effect sizes (Δ).
| Met/-carriers (n = 20) | Val/Val homozygous (n = 40) | |||
|---|---|---|---|---|
| UFOV2 | UFOV3 | UFOV2 | UFOV3 | |
| Experimental group | ||||
| Pre-test | 164 (32) | 277 (27) | 178 (27) | 267 (21) |
| Post-test | 168 (25) | 308 (25) | 78 (21) | 248 (19) |
| Δ | +4 | +31 | −100* | −19 |
| Control group | ||||
| Pre-test | 196 (66) | 265 (44) | 144 (33) | 277 (24) |
| Post-test | 126 (52) | 242 (40) | 114 (26) | 199 (22) |
| Δ | −70 | −23 | −30 | −78 |
SE in parentheses. Note that lower scores indicate better performance. Significant group difference; *p < 0.05.
UFOV3 (Selective attention)
Mean scores for both groups and assessment are shown in Table 1. Mean scores analysis showed a not yet significant main effect of assessment, F(1,56) = 2.85, p = 0.097, MSE = 4259.88, , which was involved in a two-way interaction with BDNF, F(1,56) = 4.98, p = 0.045, MSE = 4259.88, Val/Val homozygous benefited from the cognitive intervention, F(1,38) = 14.82, p = 0.0001, MSE = 4052.06, , while Met/-carriers did not, F < 1. However, this interaction did not depend on the intervention. BDNF was not further involved in any significant effect and no other interaction was reliable.
Video games of cognitive intervention
In all five video games Met carriers reported comparable scores as Val/Val homozygotes, Fs < 1 (for Giving Change, Firemen, Clock, Anagrams), and F(1,38) = 2.69, p = 0.11, MSE = 206.83, (for Falling Bricks), see Table 2.
Table 2.
Mean video games of cognitive intervention scores for Met/-carriers and Val/Val homozygous individuals.
| Met/-carriers (n = 13) | Val/Val homozygous (n = 27) | |
|---|---|---|
| Video games | ||
| Giving change | 42 (3.6) | 38 (2.9) |
| Firemen | 27 (3.0) | 25 (2.5) |
| Falling bricks | 16 (3.6) | 24 (2.9) |
| Anagrams | 244 (18) | 327 (14) |
| Clock | 53 (8.8) | 52 (7.2) |
SE in parentheses.
Discussion
Our findings suggest that successful transfer to untrained tasks in elderly people is modulated by preexisting neuro-developmental factors as genetic variability related to levels of BDNF – an important neuromodulator underlying attentional processes. As expected, in the experimental group, Val/Val homozygous individuals showed larger beneficial transfer effects than Met/-carriers. This effect was clearly observable in divided attention, but not in the selective attention subtest of the UFOV. This suggests that the BDNF Val66Met polymorphism affects different, separable components of attention. Fossella et al. (2002) suggested that the efficiency of neural networks related to different aspects of attention rely on different anatomical and neuromodular networks.
Alternatively, the finding that elderly Val homozygotes were able to improve divided, but not selective attention by training might be a reflection of how difficult each task was and how much room for improvement it left. That is, selective attention may be much less sensitive to the adverse effects of old age than divided attention is (e.g., Madden et al., 1997; Verhaeghen and Cerella, 2002). We interpret the data to suggest that following the game training it had become easier for older adults to recruit control, which is crucial for managing the burden of a divided attention task. Similar recruitment may not be necessary, or at least not to the same extent, for older adults performing on the selective attention subtask of the UFOV. Thus, game training seems to have helped Val homozygous older adults to extend, or make more efficient use of the resources for handling multiple tasks concurrently. Given the explanatory value that the UFOV has been shown to possess for accident proneness (Clay et al., 2005), the game-based enhancement that we have demonstrated is likely to transfer beyond the simplified setting of a laboratory.
It interesting to note that the training-induced benefit that Val carriers showed in the divided attention task was not accompanied by comparable benefits in the videogame tasks being trained. On the one hand, it is easy to see that the videogame tasks are so complex that they were likely to draw on numerous abilities and resources of the participants, including sensory and motor processes. Therefore, getting better on a task is likely to involve improvements on various types of processes, with many of them being unrelated to cognitive control. In contrast, the UFOV tasks are tailored to assess only a small number of cognitive functions, which makes it easier to make improvements on these functions visible in performance measures. In that sense, finding more reliable effects in a task that has been conceived to be more selective may not be surprising. On the other hand, however, this reinforces the need for sensitive testing and the employment of tasks that are suited to assess well-defined cognitive functions. Without such selective tasks cognitive enhancements may very well come and go unnoticed.
Even though our findings, based on a rather small sample, may be limited to attentional processes and need thus be interpreted with caution, they do provide preliminary support for the idea that genetic predispositions might modulate transfer effects. This raises the possibility that the pessimistic conclusions of Owen et al. (2010) regarding the efficiency of brain trainers might have been premature, at least for attentional resources. The consideration of individual differences might help to tailor training programs better to the needs and abilities of elderly people and thereby improve the efficiency of brain training. Our findings suggest that at least for some individuals all hope for general cognitive improvement is not lost.
Conclusion
The present findings reopen the question whether brain training is a useful cognitive intervention to preserve cognitive control – the ability to control one’s thoughts and goal-directed behavior – in old age. Of particular interest would be to use genetic markers related to cognitive control to predict individual differences in both predicting cognitive aging deficits and identifying predispositions that are sensitizing individuals for brain training in old age. Future research, with a greater sample size, is needed to extend these preliminary findings to the three major control functions: the “inhibition” of unwanted responses, the “shifting” between tasks and mental sets (also called “flexibility”), and the “updating” (and monitoring of) WM representations (Miyake et al., 2000).
Our study is the first to combine cognition, intervention studies, and behavioral genetics from an aging neuroscience perspective to establish a proof-of-principle regarding personalized training procedures toward retention of cognition in healthy elderly. Given the great societal relevance of prevention of neurocognitive aging, these kinds of proofs-of-principle are particularly important for optimizing societal intelligence and mental welfare as a whole.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Jan Duijndam, Astrid van Houten, Marjolein Boele for their enthusiasm and invaluable assistance in recruiting, testing the participants of this study and collecting the data. The research of Lorenza S. Colzato and Bernhard Hommel is supported by NWO (Netherlands Organization for Scientific Research). This study was made possible with a NWO grant and a Gratama/LUF grant to Band.
References
- Angelucci F., Brene S., Mathe A. A. (2005). BDNF in schizophrenia, depression and corresponding animal models. Mol. Psychiatry 10, 345–352 10.1038/sj.mp.4001637 [DOI] [PubMed] [Google Scholar]
- Ball K., Edwards J. D., Ross L. A. (2007). The impact of speed of processing training on cognitive and everyday functions. J. Gerontol. Psychol. Sci. 62B, 19–31 [DOI] [PubMed] [Google Scholar]
- Ball K., Owsley C. (1993). The useful field of view test: a new technique for evaluating age – related declines in visual function. J. Am. Optom. Assoc. 64, 71–79 [PubMed] [Google Scholar]
- Ball K., Vance D. E., Edwards J. D., Wadley V. G. (2004). “Aging and the brain,” in Principles and Practice of Behavioral Neurology and Neuropsychology, Chapt. 36 (Philadelphia: W.B. Saunders; ), 795–809 [Google Scholar]
- Ball K. K., Beard B. L., Roenker D. L., Miller R. L., Griggs D. S. (1998). Age and visual search: expanding the useful field of view. J. Opt. Soc. Am. A 5, 2210–2219 10.1364/JOSAA.5.002210 [DOI] [PubMed] [Google Scholar]
- Brehmer Y., Li S.-C., Müller V., von Oertzen T., Lindenberger U. (2007). Memory plasticity across the lifespan: uncovering children’s latent potential. Dev. Psychol. 43, 465–478 10.1037/0012-1649.43.2.465 [DOI] [PubMed] [Google Scholar]
- Bueller J. A., Aftab M., Sen S., Gomez-Hassan D., Burmeister M., Zubieta J. K. (2006). BDNF val66met allele is associated with reduced hippocampal volume in healthy subjects. Biol. Psychiatry 59, 812–815 10.1016/j.biopsych.2005.09.022 [DOI] [PubMed] [Google Scholar]
- CBS, Central Bureau Statistiek (2011). Bevolkingsprognose 2010–2060: sterkere vergrijzing, langere levensduur. Available at: http://www.cbs.nl/NR/rdonlyres/389D62E2-7205-42C3-956F-33E17CFE6433/0/2011-k1b15p16art.pdf
- Clay O. J., Wadley V. G., Edwards J. D., Roth D. L., Roenker D. L., Ball K. K. (2005). Cumulative meta-analysis of the relationship between useful field of view and driving performance in older adults: current and future implications. Optom. Vis. Sci. 82, 724–731 10.1097/01.opx.0000175009.08626.65 [DOI] [PubMed] [Google Scholar]
- Colzato L. S., van den Wildenberg W., van der Does W. A. J., Hommel B. (2011). BDNF val66met polymorphism is associated with higher anticipatory cortisol stress response, anxiety, and alcohol consumption in healthy adults. Psychoneuroendocrinology. [Epub ahead of print]. 10.1016/j.psyneuen.2011.04.010 [DOI] [PubMed] [Google Scholar]
- Colzato L. S., Waszak F., Nieuwenhuis S. T., Posthuma D., Hommel B. (2010a). The flexible mind is associated with the catechol-O-methyltransferase (COMT) Val158Met polymorphism: evidence for a role of dopamine in the control of task switching. Neuropsychologia 48, 2764–2768 10.1016/j.neuropsychologia.2010.04.023 [DOI] [PubMed] [Google Scholar]
- Colzato L. S., van den Wildenberg W., van der Does W. A. J., Hommel B. (2010b). Genetic markers of striatal dopamine predict individual differences in dysfunctional, but not functional impulsivity. Neuroscience 170, 782–788 10.1016/j.neuroscience.2010.07.050 [DOI] [PubMed] [Google Scholar]
- Edwards J., Vance D., Wadley V., Cissell G., Roenker D., Ball K. (2005). Reliability and validity of Useful Field of View test scores as administered by personal computer. J. Clin. Exp. Neuropsychol. 27, 529–543 10.1080/13803390490515432 [DOI] [PubMed] [Google Scholar]
- Egan M. F., Kojima M., Callicott J. H., Goldberg T. E., Kolachana B. S., Bertolino A., Zaitsev E., Gold B., Goldman D., Dean M., Lu B., Weinberger D. L. (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269 10.1016/S0092-8674(03)00035-7 [DOI] [PubMed] [Google Scholar]
- Faust M. E., Balota D. A. (1997). Inhibition of return and visuospatial attention in healthy older adults and individuals with dementia of the Alzheimer type. Neuropsychology 11, 13–29 10.1037/0894-4105.11.1.13 [DOI] [PubMed] [Google Scholar]
- Fenton A. A., Lytton W. W., Barry J. M., Lenck-Santini P. P., Zinyuk L. E., Kubík S., Bures J., Poucet B., Muller R. U., Olypher A. V. (2010). Attention-like modulation of hippocampus place cell discharge. J. Neurosci. 30, 4613–4625 10.1523/JNEUROSCI.5576-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M., Folstein S., McHugh P. (1975). “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Fossella J. A., Sommer T., Fan J., Wu Y., Swanson J. M., Pfaff D. W., Posner M. I. (2002). Assessing the molecular genetics of attention networks. BMC Neurosci. 3, 14. 10.1186/1471-2202-3-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A. R., Goldberg T. E., Mattay V. S., Kolachana B. S., Callicott J. H., Egan M. F., Weinberger D. R. (2003). Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J. Neurosci. 23, 6690–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keizer A. W., Verschoor M., Verment R., Hommel B. (2010). The effect of gamma enhancing neurofeedback on measures of feature-binding flexibility and intelligence. Int. J. Psychophysiol. 75, 25–32 10.1016/j.ijpsycho.2009.10.011 [DOI] [PubMed] [Google Scholar]
- Lang U. E., Hellweg R., Kalus P., Bajbouj M., Lenzen K. P., Sander T., Kunz D., Gallinat J. (2005). Association of a functional BDNF polymorphism and anxiety-related personality traits. Psychopharmacology (Berl.) 180, 95–99 10.1007/s00213-004-2137-7 [DOI] [PubMed] [Google Scholar]
- Li S.-C., Chicherio C., Nyberg L., von Oertzen T., Nagel I. E., Papenberg G., Sander T., Heekeren H. R., Lindenberger U., Bäckman L. (2010). Ebbinghaus revisited: influences of the BDNF val66met polymorphism on backward serial recall are modulated by human aging. J. Cogn. Neurosci. 10, 2164–2173 10.1162/jocn.2009.21374 [DOI] [PubMed] [Google Scholar]
- Lindenberger U., Nagel I. E., Chicherio C., Li S.-C., Heekeren H. R., Bäckman L. (2008). Age-related decline in brain resources modulates genetic effects on cognitive functioning. Front. Neurosci. 2:39. 10.3389/neuro.01.039.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden D. J., Turkington T. G., Provenzale J. M., Hawk T. C., Hoffman J. M., Coleman R. E. (1997). Selective and divided visual attention: age-related changes in regional cerebral blood flow measured by H215O PET. Hum. Brain Mapp. 5, 389–409 [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N., Emerson M., Witzki A., Howerter A. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn. Psychol. 41, 49–100 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- Owen A. M., Hampshire A., Grahn J. A., Stenton R., Dajani S., Burns A. S., Howard R. J., Ballard C. G. (2010). Putting brain training to the test. Nature 465, 775–778 10.1038/nature09042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck D. F. (1970). The conversion of progressive matrices and Mill Hill vocabulary raw scores into deviation IQs. J. Clin. Psychol. 26, 67–70 [DOI] [Google Scholar]
- Pezawas L., Verchinski B. A., Mattay V. S., Callicott J. H., Kolachana B. S., Straub R. E., Egan M. F., Meyer- Lindenberg A., Weinberger D. R. (2004). The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J. Neurosci. 24, 10099–10102 10.1523/JNEUROSCI.2680-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J. C., Court J. H., Raven J. (1988). Manual for Raven’s Progressive Matrices and Vocabulary Scales. London: Lewis [Google Scholar]
- Rybakowski J. K., Borkowska A., Czerski P. M., Skibinska M., Hauser J. (2003). Polymorphism of the brain-derived neurotrophic factor gene and performance on a cognitive prefrontal test in bipolar patients. Bipolar Disord. 5, 468–472 10.1046/j.1399-5618.2003.00071.x [DOI] [PubMed] [Google Scholar]
- Sanders A. F. (1970). Some aspects of the selective process in the functional visual field. Ergonomics 13, 101–117 10.1080/00140137008931124 [DOI] [PubMed] [Google Scholar]
- Shing Y. L., Werkle-Bergner M., Brehmer Y., Muller V., Li S. C., Lindenberger U. (2010). Episodic memory across the lifespan: the contributions of associative and strategic components. Neurosci. Biobehav. Rev. 34, 1080–1091 10.1016/j.neubiorev.2009.11.002 [DOI] [PubMed] [Google Scholar]
- Smith G. E., Housen P., Yaffe K., Ruff R., Kennison R. F., Mahncke H. W., Zelinski E. M. (2009). A cognitive training program based on principles of brain plasticity: results from the improvement in memory with plasticity-based adaptive cognitive training (IMPACT) study. J. Am. Geriatr. Soc. 57, 594–603 10.1111/j.1532-5415.2008.02081.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P., Cerella J. (2002). Aging, executive control, and attention: a review of meta-analyses. Neurosci. Biobehav. Rev. 26, 849–857 10.1016/S0149-7634(02)00071-4 [DOI] [PubMed] [Google Scholar]
- Xu X., Mill J., Zhou K., Brookes K., Chen C. K., Asherson P. (2007). Family-based association study between brain-derived neurotrophic factor gene polymorphisms and attention deficit hyperactivity disorder in UK and Taiwanese samples. Am. J. Med. Genet. B Neuropsychiatr. Genet. 144, 83–86 [DOI] [PubMed] [Google Scholar]