Abstract
Ocular allergy is an inflammatory response of the conjunctival mucosa that also affects the cornea and eyelids. Allergic conjunctivitis includes seasonal allergic conjunctivitis (SAC), perennial allergic conjunctivitis (PAC), vernal keratoconjunctivitis (VKC), atopic keratoconjunctivitis (AKC) and giant papillary conjunctivitis (GPC). In general, allergic conditions involve mast cell degranulation that leads to release of inflammatory mediators and activation of enzymatic cascades generating pro-inflammatory mediators. In chronic ocular inflammatory disorders associated with mast cell activation such as VKC and AKC constant inflammatory response is observed due to predominance of inflammatory mediators such as eosinophils and Th2-generated cytokines. Antihistamines, mast-cell stabilizers, non-steroidal anti-inflammatory agents, corticosteroids and immunomodulatory agents are commonly indicated for the treatment of acute and chronic allergic conjunctivitis. In recent years newer drug molecules have been introduced in the treatment of allergic conjunctivitis. This article reviews recent patents and emerging therapeutics in the treatment of allergic conjunctivitis.
Keywords: Allergy, conjunctivitis, mast-cell, cytokines, antihistamines, mast-cell stabilizers, non-steroidal anti-inflammatory agents, corticosteroids, immunomodulatory agents
Introduction
Ocular allergy is a disease primarily characterized by an inflammatory response of the conjunctival mucosa [1]. It is a hypersensitivity response that affects 15-20% of the population [2, 3]. Allergic eye disorders are primarily characterized as IgE mediated; T- Lymphocyte mediated disorders or both [4]. These disorders primarily affect the conjunctiva, the cornea and the eyelids. Ocular allergies can be chronic or acute type. Acute allergy is caused by IgE mediated mast cell degranulation whereas chronic allergies are associated with continuous activation of mast cell [5]. Acute form involves transient symptoms of itching, tearing and swelling. Chronic allergies are sight threatening and exhibit symptoms such as severe pain and visual disturbances. In both acute and chronic allergic conditions mast cell degranulation leads to release of inflammatory mediators and activation of enzymatic cascades generating pro-inflammatory mediators [6]. However, in chronic allergic conditions a constant inflammatory response is observed due to predominance of mediators such as eosinophils and Th2-generated cytokines [6]. The most frequent forms of ocular allergy such as seasonal allergic conjunctivitis (SAC) and perennial allergic conjunctivitis (PAC) are characterized as acute allergic disorders. Severe forms of allergy having more complex pathophysiological phenomenon such as vernal keratoconjunctivitis (VKC) and atopic keratoconjunctivitis (AKC) are categorized as chronic ocular allergic disorders [7].
In recent years advancement in the understanding of the pathophysiology of ocular allergies has paved way for the development of newer drug candidates. Genetic variation in the expression of IL-10 levels is a contributing factor in the susceptibility of mast cells to allergens [8]. The role of β-chemokines in mast cell activation and leukocyte migration has also been explored recently. In addition, other mediators such as eotaxin as co-stimulating factor for mast cell were studied as newer targets for allergic treatment [9]. In this review an update on recent patents and the development of emerging therapeutics in the treatment of ocular allergy has been presented with a brief outline on various allergic diseases.
Types of Ocular Allergies
Based on clinical manifestations six major forms of anterior ocular disorders exist. These are summarized in Table 1 as SAC, PAC, VKC, AKC, GPC and contact allergy.
Table 1. Characteristic Symptoms and Treatment Options for Allergic Conjunctivitis.
| Allergic Condition | Cell Types Involved | Clinical Symptoms | Drugs for the Treatment |
|---|---|---|---|
| Seasonal Allergic Conjunctivitis (SAC) and Perennial Allergic Conjunctivitis (PAC) | Eosinophil and mast cells are major inflammatory mediators | Itching, redness, inflammation and tarsal papillae | Antihistaminic, NSAIDs and mast cell stabilizers |
| Vernal Keratoconjunctivitis (VKC) | Lymphocytes, eosinophil and mast cells | Severe symptoms such as persistent itching, corneal ulceration and cobblestone papillae | Immunomodulators, corticosteroids, NSAIDs, antihistaminic and mast cell stabilizers |
| Atopic Keratoconjunctivitis (AKC) | Lymphocytes, basophils, eosinophil and mast cells | Conjunctival damage, severe ocular itching and cataract | Antihistaminic and mast cell stabilizers, NSAIDs. In severe cases Immunomodulators and corticosteroids |
| Giant Papillary Conjunctivitis (GPC) | Mast cells and lymphocytes | Formation of giant papillae and mild ocular irritation due to contact lens | Mast cell stabilizers, Antihistaminic and NSAIDs |
| Contact Dermatitis | Lymphocytes and dendritic cells | Erythema and mild ocular itching | Antihistaminic, NSAIDs and mast cell stabilizers |
1. Seasonal/Perennial Conjunctivitis (SAC/PAC)
SAC/PAC represents 25-50% of the total cases of ocular allergy [4]. It is initiated by the binding of environment air borne allergens such as pollen to the IgE receptor in mast cells. This binding leads to the degranulation of mast cells and subsequent release of pro-inflammatory mediators Fig. (1). PAC represents hypersensitive response from indoor antigens such as dust mites while SAC is due to the exposure from environmental antigens such as pollens [4]. Clinical symptoms of itching, redness, chemosis and tearing are observed which lead to conjunctival redness and edema [10]. In SAC; conjunctival hyperemia and sign of papillae in tarsal conjunctiva is observed. Symptoms of SAC are generally more severe in comparison to PAC [10]. Histological analysis of tear film reveals elevated level of MCT mast cells, IgE antibodies, histamine and tryptase, eotaxin and eosinophil cationic protein [10].
Fig. (1).
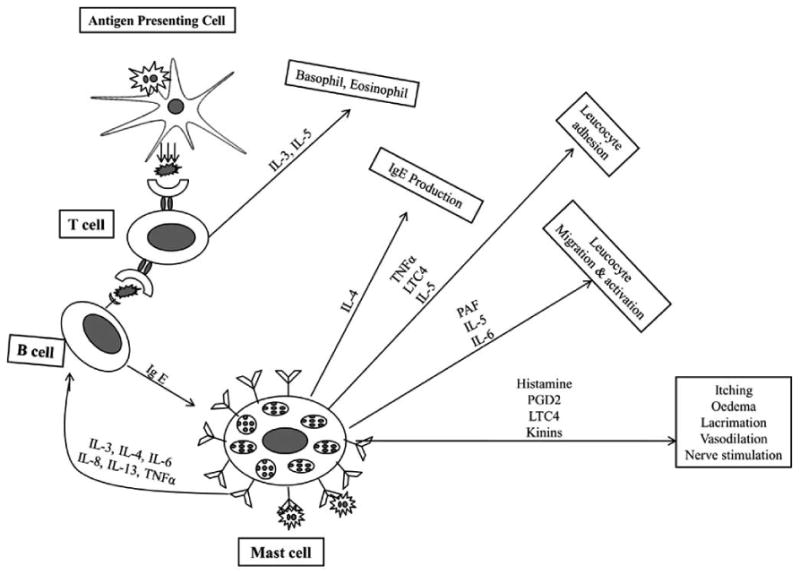
Mast cell mediated allergic response mechanisms.
2. Atopic Keratoconjunctivitis (AKC)
AKC represents a severe chronic ocular allergy primarily in adult population. It exhibits symptoms such as itching, burning, tearing, erythematous and swollen eye lids. AKC may progress to corneal scarring commonly known as corneal opacity and may also leads to vascularization of the cornea. It exhibits unique papillary hypertrophy on the lower tarsal conjunctiva [10]. It also shows conjunctival scarring which eventually may effects the lids. The symptoms may become more severe in the presence of chronic blepharitis. Cytokines derived from Th1 and Th2 cell types and inflammatory cells such as mast cells and eosinophils are the major mediators of inflammatory response [4].
3. Vernal Keratoconjunctivitis (VKC)
VKC represents 0.5% of allergic conditions. It usually occurs in children between 8 and 12 years with preponderance in female with a ratio of 2:1 [10]. It is characterized by symptoms such as severe ocular itching, tearing, photophobia and mucus discharge. It represents chronic ocular allergy in which Th-2 lymphocyte mediated generation of IgE and activation of mast cell occurs [4]. An increased level of TNF-α, histamine, tryptase, IgE and IgG antibodies is observed on pathological examination of tears. The symptoms of AKC and VKC are usually similar in nature. However, VKC exhibits a unique symptom of giant papillae on the tarsal conjunctiva. In severe condition, a non-healing ulcer of mucus and fibrin develops which eventually leads to corneal scarring [11].
4. Giant Papillary Conjunctivitis (GPC)
GPC is usually not considered as an ocular allergy but symptoms resemble the other allergic conditions. It generally occurs due to the presence of foreign bodies such as contact lens, prostheses and corneal sutures [12]. Allergic response to contact lens occurs due to the preservatives incorporated in the solution and foreign particles adsorbed on the surface of contact lens. Immune response ensues through chronic irritation of tarsal conjunctival epithelium. Mechanical trauma of the conjunctival epithelium stimulates Th-2 lymphocyte mediated immune response. In this allergic condition a higher level of inflammatory cells such as MTC mast cells are observed. Although tears tryptase level is elevated, no significant increase in histamine is observed. Symptoms of this allergy subside upon removal of foreign body [12].
5. Contact Allergy
Contact allergy is primarily due to allergen exposure from cosmetics or chemicals [12]. It is a Th-1 cell mediated immune response, which primarily affects the ocular surface and eyelids. Such allergy is a delayed hypersensitivity response with symptoms such as itching and conjunctival hyperemia [4]. In some cases skin surrounding the eye also demonstrates contact dermatitis.
Role of Mast Cell and T-Lymphocyte in Allergic Conjunctivitis
Conjunctiva is the most common site for allergic reactions. It is a vascularized tissue consisting of a large number of dendritic cells and macrophages [4]. These cells regulate innate and adaptive immunity of the conjunctival epithelium. Antigen presenting cells such as dendritic cells, B cells and macrophages play a key role in antigen recognition and processing by T- lymphocytes. B lymphocytes produce the immunological response by expressing receptors for antigen recognition on the cell membrane. Macrophages are another important component that regulates the production of major cytokines and removal of pathogens. Dendritic cells are involved in the T-helper type 2 (Th-2) mediated allergic responses. These cells present antigen to memory T-lymphocytes, which produce Th-2 cytokines and also regulate the production of IL-12. In addition, conjunctival epidermal dendritic cells, commonly known as langerhans cells are also involved in the antigen processing and presenting function. Two major cell types involved in the allergic immunopathological response are mast cell and T-cells. The normal conjunctival epithelium is composed of CD4+ and CD8+ T lymphocytes. In a chronic allergic state memory T-lymphocyte increases dramatically [5].
1. Role of Mast Cells in Allergic Eye Diseases
Two different types of mast cells exist in humans, classified as MCT and MCTc subtypes on the basis of granule and protease content. MCT subtype contains single neutral protease or typtase, whereas MCTc carries both typtase and chymase [13]. MCT subtype rises in acute inflammation while an increased number of MCTc subtype is found in chronic inflammatory symptom. Mast cell mediated acute allergic eye diseases such as SAC and PAC are categorized as type-1 hypersensitivity reactions. These reactions occur due to the exposure of allergens on the conjunctival mucus membrane and follow three phases, i.e. sensitization, early and late phases [14-17].
In sensitization phase, allergens are processed and localized by antigen presenting cells (APC) as a complex of peptide fragments with MHC class-II molecules on their surface. The allergen-MHC class II complexes on the APC then promote the maturation of T-helper (TH) cells into Th2 cells, which result in the production of cytokines. These cytokines further promote the synthesis of IgE from B-cells. IgE binds to FcεRI receptors on mast cell, which upon subsequent exposure to allergens results in the cross linking of these antibodies. It changes the mast cell membrane permeability and results in the release of inflammatory mediators. Such inflammatory mediators include histamine, serotonin, leukotriene (LTC4), prostaglandin, carboxypeptidase A, platelet activating factor (PAF), cathepsin G, neutrophil and eosinophil Fig. (1).
These inflammatory mediators contribute to the early phase response that begins within seconds of allergen exposure. The early phase clinical symptoms are itching, tearing, swelling, edema and redness. In the last phase response aggregation of FcεRI receptor initiates the breakdown of membrane phospholipids into inflammatory mediators such as prostaglandin, leukotriene and thromboxanes that lead to late phase response via recruitment of additional inflammatory cells. The late phase response involves the infiltration of eosinophils, neutrophils and basophils [18].
2. Role of T Lymphocytes in Allergic Eye Diseases
T cell mediated reactions are commonly known as type-IV delayed hypersensitivity response. A type-IV reaction occurs in chronic allergic conditions such as AKC, VKC or giant papillary conjunctivitis (GPC). In combination with mast cell mediated inflammatory responses there is also an abundance of memory T-cells. Such hypersensitivity response occurs through interaction of antigens with Th1 and Th2 cell subsets followed by release of cytokines [19]. Type-IV reaction involves two phases, i.e. sensitization and elicitation. In the sensitization phase APC processed antigen-MHC class II complex interacts with T-lymphocytes, resulting in the differentiation of CD4+ T-lymphocyte into memory T-lymphocyte. In the elicitation phase interaction between the antigen-MHC-II complex and memory T-cells stimulates the proliferation of T-cells. The memory T-lymphocytes during proliferation release cytokines [20].
Th1 or Th2 derived cytokines perform different functions. Cytokines such as IL-2, IL-3, IFN-γ; are Th1 derived cytokines, which mediates recruitment of macrophages. Th2 derived cytokine, such as IL-4 and IL-5, participates in the activation and chemotaxis of eosinophils. In chronic allergy such as VKC, only Th2 activation occurs, whereas in AKC both Th1 and Th2 cells are involved in the immunological response [21, 22]. Two novel Th cell subsets, IL-17-producing Th cells (Th17 cells) and regulatory T cells (Treg cells) are also found to be contributors in the pathogenesis of conjunctivitis. However, the role of these cells in the activation of mast cells has not been identified clearly [23].
Drugs for the Treatment of Allergic Conjunctivitis
1. Antihistamines
Topical antihistamines are the most preferred option for the treatment of ocular allergies. These drugs act by inhibiting the action of histamine on H1 receptors. Oral H1 antagonists may cause systemic adverse effects and have slower onset of action. Therefore a topical H1 antagonist is preferred in the treatment of conjunctivitis. H1 receptor antagonists are categorized into first and second generation antagonists. First generation antagonists produce side effects such as sedation due to the lipophilic nature of the molecules that favor easy penetration across the blood-brain barrier as a result these compound blocks the effect of histamine [10]. Moreover, these agents nonspecifically bind to H2 receptors, which cause cardiac arrhythmia [10]. Drugs such as chlorpheniramine or cyclizine are classified as first generation antihistamines. Second generation H1 receptor antagonists are non-sedating and commonly employed in the treatment of acute allergic conjunctivitis. The most common drugs in this class are levocabastine, azelastine and emedastine. Levocabastine is available as 0.5% ophthalmic suspension. It is recommended for the treatment of SAC. A newer antihistaminic agent bepostatine is available in the United States for the treatment of allergic conjunctivitis. It is a nonsedating H1 receptor antagonist. It is available as 1.5% ophthalmic solution which is effective in the clearing of ocular itch. Alcaftadine is newly approved H1 receptor antagonist that acts by inhibiting the release of histamine from mast cells. The most common adverse reactions associated with the eye are irritation, burning and/or stinging on instillation, redness, pruritus and nonocular adverse effects are nasopharyngitis, headache, and influenza. Alcaftadine ophthalmic solution was more efficient than plecebo in preventing ocular itching in patients with severe allergic conjunctivitis, based on the results obtained following ocular allergen challenge at 3 minutes and 16 hours after dosing. Emedastine difumarate 0.05% is highly selective H1 receptor antagonist. It possesses more potent H1 receptor binding affinity in comparison to ketotifen and levocabastine [24]. Olopatadine (oral) is a selective H1 receptor blocking drug having effectiveness in the treatment of SAC and PAC [25]. In clinical trials olopatadine demonstrated significantly higher effectiveness than the placebo [26, 27]. Epinastine has effect on both H1 and H2 receptors. However, H2 receptor antagonist effect may be more beneficial in reducing the eye-lid swelling. It also has mast-cell stabilizing and anti-inflammatory effects [28]. Table 2 summarizes the recent ongoing clinical trials (compiled from ClinicalTrials.gov database) in the treatment of allergic conjunctivitis.
Table 2. Clinical Trials of Drugs for the Treatment of Allergic Conjunctivitis*.
| Clinical Trial ID | Trail Purpose | Drug | Sponsor | Indicated for Allergic Condition | Phase |
|---|---|---|---|---|---|
| NCT01037179 | An open-label, long-term study, with al-4943a ophthalmic solution, 0.2% in patients with allergic conjunctivitis | Olopatadine | Alcon Research | Allergic conjunctivitis | Phase III |
| NCT00891436 | Study of the effect of fluticasone furoate nasal spray on spring allergy eye symptoms | Fluticasone furoate | Rush University Medical Center | Allergic conjunctivitis to tree pollen or grass pollen | Phase IV |
| NCT01012752 | A multicenter study to evaluate safety and efficacy of specific immunotherapy with modified allergen extracts | Modified allergen extract | Roxall Medizin | Allergic rhinitis and conjunctivitis | Phase III |
| NCT00932607 | SUBLIVAC® birch probe study | Sublingual immunotherapy | HAL Allergy | Seasonal rhinitis, rhinoconjunctivitis and birch pollen allergy | Phase II |
| NCT00831025 | Olea europaea subcutaneous immunotherapy | Biological: immunotherapy with modified extract of Olea europaea pollen | Laboratories Leti, S.L. | Allergy rhinoconjunctivitis | Phase III |
2. Mast-cell Stabilizers
Mast-cell stabilizers inhibit the release of histamine from mast cells. These therapeutic agents prevent the mast-cell degranulation process and eventually inhibit the inflammatory cascade in allergic conjunctivitis [29]. These agents are effective in both acute and chronic allergic disorders. Drugs, such as sodium cromoglycate, nedocromil sodium, pemirolast, and lodoxamide act by stabilizing mast cells. These drugs exhibit fewer local or systemic side effects [30]. Sodium cromoglycate is the oldest therapeutic agent. It acts by inhibiting secretion of mast cells. The action of this drug is concentration dependent. Sodium cromoglycate in combination with steroids or oral histamine is more effective and reduces the dose [25, 30]. Another agent, nedocromil sodium, exerts its effect by inhibiting chloride ion influx in mast cells [30]. Pemirolast potassium 0.1% ophthalmic solution is used to alleviate the signs and symptoms associated with seasonal allergic conjunctivitis [31]. Pemirolast potassium is a more potent mast cell stabilizer than cromolyn sodium and tranilast. It acts by inhibiting type-1 immediate hypersensitivity reaction. Pemirolast inhibits eosinophil chemotaxis and blocks the antigen induced release of inflammatory agents such as histamine, leukotrienes C4, D4 and E4 [32, 33]. Lodoxamide is approximately 2500 times more potent than sodium cromoglycate in different animal models. It inhibits both acute and chronic phase response by blocking histamine release from mast cells and eosinophil chemotaxis. Lodoxamide 0.1% ophthalmic solution is indicated in the treatment of vernal conjunctivitis and keratoconjunctivitis [11].
3. Dual Acting Agents
These drugs act as H1 receptor antagonist. These agents also stabilize mast cells. Drugs such as olopatadine, ketotifen, azelastine and epinastine and bepostatine are included in this category. These agents exert multiple pharmacological effects such as histamine receptor antagonist action, stabilization of mast-cell degranulation and suppression of activation and infiltration of eosinophils. Ketotifen is widely utilized for allergic conjunctivitis. It is a bezocyclohep-tathiophane derivative that inhibits eosinophil activation, generation of leukotrienes and cytokine release [34, 35]. Azelastine is a selective second generation H1 receptor antagonists. Azelastine also acts by inhibiting platelet activating factor (PAF) and blocking expression of intercellular adhesion molecule 1 (ICAM-1), thereby demonstrating its efficacy in the treatment of perennial allergic conjunctivitis [36]. Both drugs are indicated in the treatment of SAC and other allergic condition [28, 37]. Epinastine has effect on both H1 and H2 receptors. However, H2 receptor antagonist effect may be more beneficial in reducing the eyelid swelling. It also has mast-cell stabilizing and anti-inflammatory effects [28].
4. Non-Steroidal Anti-Inflammatory Agents (NSAIDS)
These drugs alleviate the symptoms of pain and inflammation associated with allergic response. These agents inhibit the production of inflammatory mediators such as prostaglandins and leukotrienes by acting on cyclooxygenase enzymes. Generally NSAIDS employed in ocular allergy treatment inhibit both COX-1 (cyclooxygenase) and COX-2 enzymes [38, 39]. These drugs have shown effectiveness in conjunctival hyperemia and pruritus. Agents such as ketorolac, diclofenac and flurbiprofen are commonly indicated in ocular allergic conditions. These drugs neither induce cataract formation nor increase intraocular pressure (IOP) thus are preferred over steroidal agents. Ketorolac 0.5%, is prescribed in the treatment of SAC and VKC, is approved by US-FDA [40]. However, ketorolac has shown limited efficacy in the treatment of allergic conjunctivitis in comparision to olopatadine and emedastine [41, 42]. Application of NSAIDS is limited due to stinging and burning sensation on topical administration. In addition oral administration of these agents can cause gastrointestinal ulceration and hypersensitivity response. Despite these facts ketorolac tromethamine formulation has shown significant effectiveness in the treatment of acute allergic conjunctivitis [10]. Drugs such as indomethacin 1%, ketorolac 0.5%, diclofenac 0.1% have shown effectiveness in the treatment of VKC [40].
5. Corticosteroids
Corticosteroids are potent anti-inflammatory agents that have gained important attention as therapeutic candidates for the treatment of allergic conjunctivitis. Topical ophthalmic corticosteroids inhibit the production of various inflammation-causing mediators that are released when the eye reacts to allergens. These inflammation-causing mediators include prostaglandins and other inflammatory substances. Corticosteroids like hydrocortisone, triamcinolone, clobeta-sonebutyrate, fluromethalone, rimexalone, prednisolone, dexamethasone have been widely used in the treatment of allergic conjunctivitis [43-46]. However, draw-backs with topical corticosteroids are increased intraocular pressure and risk of cataract formation. Prednisolone acetate ophthalmic suspension 1% is a topical anti-inflammatory ophthalmic solution which is 3-5 times more potent than hydrocortisone. More recently, two new topical ophthalmic corticosteroids, loteprednol etabonate and difluprednate have been approved by FDA. Loteprednol etabonate is an ester corticosteroid with a high therapeutic index. It acts by blocking the release of inflammatory mediators. It is effective in the treatment of steroid-responsive inflammatory conditions such as allergic conjunctivitis and uveitis. Owing to its rapid deesterification into inactive metabolites, loteprednol etabonate appears to have superior safety profile when compared to ketone corticosteroids. However, further comparative safety studies are required to confirm this strategy [47, 48]. Loteprednol etabonate is available in the form of 0.2% and 0.5% ophthalmic suspensions and 0.5% eye drops. Loteprednol etabonate is usually indicated for 7-8 days in the treatment of severe allergic conditions [49]. Difluprednate is a derivative of prednisolone indicated in post-operative ocular inflammation and pain. Desonide 0.25% is another ophthalmic corticosteroid solution which was studied for the management of SAC. Desonide is effective in the treatment of SAC and it is safe with no side effects reported such as increased intraocular pressure [50]. Clobetasone is another glucocorticosteroid often prescribed for the treatment of allergic conjunctivitis. A study was conducted to compare the clinical efficacy of topical clobetasone butyrate compared to placebo. The steroid group had shown considerable clinical improvement at the end of the trial [51]. A statistically significant difference was noticed between the active drug treated group and placebo treated group. The results suggest that clobetasone butyrate can be used successfully in the treatment of allergic conjunctivitis [51]. In eye clinics, corticosteroids are widely applied through conventional dosage forms such as eye drops and ointments. However, topical administration of corticosteroids often results in increased intraocular pressure and cataract formation. Durezol is a sterile ophthalmic emulsion containing difluprednate. It is indicated for inflammation caused by ocular allergic conditions. Topically applied corticosteroids hamper the inflammatory response to a wide range of inciting agents that may slowdown healing. They act by inhibiting the edema, leukocyte migration, fibrin deposition, capillary proliferation, capillary dilation, fibroblast proliferation, deposition of collagen, and scar formation which are related to inflammation [52].
6. Immunomodulatory Agents for Allergic Conjunctivitis
Immunomodulatory agents are the substances that interact with immune system. These compounds cause immunostimulation and immunosuppression. Immunomodulatory agents are associated with fewer side effects. Hence, these compounds are preferred over corticosteroids in the treatment of allergic conjunctivitis. Several immunomodulatory agents such as cyclosporine A (CsA), tacrolimus (FK 506), mycophenolatemofetil (MMF), leflunomid, rapamycin (sirolimus), capoxone, laquinimod, infliximab have also shown efficacy in the treatment of ocular immune-mediated diseases [53]. Cyclosporin A is an immunosuppressant that acts by inhibiting eosinophilic infiltration by interfering with the type IV allergic reactions in the conjunctiva [54]. Ebihara et.al evaluated the effectiveness and safety of 0.1% aqueous ophthalmic solution of cyclosporine in a large population suffering from VKC and AKC. This clinical study is a prospective and observational in about 594 patients suffering from VKC and AKC. This was the first study conducted to follow up on the effects of low-concentration cyclosporine eye drops on severe VKC and AKC in such a large group. This study concluded that topical cyclosporine 0.1% is effective for the treatment of severe VKC and AKC and can be used safely [55]. Tacrolimus acts by inhibiting the action of T-cells. Tacrolimus ointment 0.03% is effective, well tolerated, and safe in the treatment of allergic conjunctivitis following application into the conjunctival sac [56]. Ohashi et al. conducted a randomized, placebo-controlled clinical trial with 0.1% tacrolimus ophthalmic suspension in patients with severe allergic conjunctivitis. Fifty six patients were chosen for this study and it was a multicenter, randomized, double masked, placebo-controlled clinical trial. These patients were treated with topical anti-allergic agents and corticosteroids and they were randomized to tacrolimus or placebo. All patients in the clinical trial were treated for about 4 weeks with tacrolimus and placebo twice daily. The study has two efficacy endpoints; first one is change in the total score of objective signs and the second endpoint is change in the visual analog assessment (VAS) for each subjective symptom. Safety was also evaluated based on the severity and prevalence of adverse effects. The total score for objective signs was significantly greater in tacrolimus treated group compared to placebo group. All the subjective symptoms like itching, discharge, hyperemia, lacrimation and foreign body sensation were improved in tacrolimus treated group compared to placebo group. Ocular irritation was very less in tacrolimus treated group compared to placebo and tacrolimus 0.1% was well-tolerated by the patients. Tacrolimus was effective in severe allergic conjunctivitis [57].
Most of the above mentioned immunomodulatory drugs have shown limited therapeutic success due to lipophilic nature and low water solubility. Even conventional routes of administration (oral or intravenous) of immunomodulatory agents such as rapamycin are associated with severe side effects. Because of the multilayer structure of the cornea, a molecule should possess optimal hydrophilicity/lipophilicity balance to generate maximum permeability across the cornea and to be formulated into eye drops. Various novel drug delivery systems such as nanoparticles, liposomes and nanomicelles have been utilized in the delivery of rapamycin. Forrest et al. studied the in vitro release of rapamycin from poly (ethylene glycol)-b-poly (epsilon-caprolactone) micelles [58]. This study concluded that PEG-PCL micelles contained rapamycin at 7-10% by weight with solubility >1 mg/mL [58]. Infliximab and daclizumab are the antibodies which were showing promising results in some patients suffering from conjunctivitis [59]. New DNA sequences (ISS or CpG motifs) with immunostimulatory activity can hamper an on-going Th2/allergic reaction and stimulate a de novo Th1 reaction. ISS oligonucleotide (ISS-ODN) had inhibited instantaneous hypersensitivity response and the late-phase cellular infiltration in allergic conjunctivitis induced in a mice model. Treatment with ISS was more successful than dexamethasone in inhibiting allergic response. Immunomodulatory effects induced by ISS-ODN are eliminated when treated with anti-IL-12 neutralizing antibodies, signifying a critical role for type 1 cytokines in inhibiting both immediate hypersensitivity and the late-phase cellular infiltration. Hence, ISS-ODN can be considered as a novel anti-inflammatory and immunomodulatory agent that can be extensively utilized for the treatment of ocular allergic conjunctivitis [60].
Recent Patents on the Treatment Options of Conjunctivitis
As described earlier, antihistamines, mast cell stabilizers and anti-inflammatory agents including steroids and nonsteroidal drugs are conventionally used for the treatment of allergic conjunctivitis. However, antihistamines and mast cell stabilizers cannot suppress the activation of free neutrophils and eosinophils. Long-term use of glucocorticosteroids can cause serious side effects such as glaucoma, cataract, Cushing's syndrome, osteoporosis and bacterial infections. Therefore, newer therapeutic agents that possess anti-inflammatory action and exhibit lower side effects are desired for the treatment of allergic conjunctivitis [61, 62]. In this section we have discussed patents selected from year 2008-2010 compiled from various patent databases.
A recent invention discloses a method of treating conjunctivitis, caused by contact with a pathogen or allergen, by administrating lipid-conjugates [63]. This patent discloses the lipid or phospholipid moiety conjugated to a monomer, dimer, oligomer or polymer. Lipid-conjugates can prevent or treat conjunctivitis via inhibitory effect of phospholipase A2 (PLA2) enzyme activity. PLA2 enzyme catalyzes the breakdown of phospholipids at sn-2 position and produces the fatty acids, a lysophospholipid and other lipid mediators. Lipid-conjugates are claimed to suppress the expression of PLA2 enzyme, which in turn reduces the production of lipid mediators such as eicosanoids. They also control the production of inflammatory mediators such as platelet-activating factor (PAF), cysteinyl leukotrienes, tumor necrosis factor-α (TNF-α), IL-8, oleic acid release, oxidants and nitric oxide. Lipid-conjugates act by protecting the cell membrane from the injurious compounds. The inventors reported that administration of lipid conjugates has reduced early and late post-provocation corneal opacity and ocular levels of inflammatory modulators such as leukotriene B4 and prostaglandin E2 in a delayed-type hypersensitivity conjunctivitis model of guinea pigs. Lipid-conjugates also prevent or treat conjunctivitis via their membrane stabilizing effects. These compounds can be used to suppress, inhibit and prevent pathologic conditions associated directly with conjunctivitis and also reduce the symptoms [63].
Novel compositions comprised of chlorogenic acid or derivative thereof exhibiting anti-inflammatory effect comparable to steroidal drugs are provided. Chlorogenic acid and its ester derivatives are obtained from natural or synthetic sources. These compounds produce an inhibitory effect on transglutaminase and are highly effective in the treatment of conjunctivitis through anti-inflammatory effects. The inventors found a significant decrease in the degree of conjunctival edema and redness in guinea pigs pretreated with short ragweed pollen with the use of chlorogenic acid [61]. This invention discloses the use of a pharmaceutical composition comprising chlorogenic acid or a derivative thereof alone or in combination with other antiinflammatory drugs. The pharmaceutical composition lowers the side effects associated with various anti-inflammatory drugs and provides synergistic therapeutic effects. It can be formulated as an oral dosage form, into injections for subcutaneous, intravenous or intramuscular route, suppositories or in the form of inhalation sprays [61]. The same investigators have also filed another patent for use of glucosamine and its derivatives in the treatment of allergic and irritant conjunctivitis. The applicants mentioned that pharmaceutical compositions of glucosamine and its derivatives can be applied topically as ophthalmic eye drops or ointment. It can also be delivered in the oral capsule [64].
It is now known that the second messenger cyclic adenosine monophosphate (cAMP) regulates the activity of neutrophils, eosinophils and mast cells. A phosphodiesterase (PDE) IV enzyme is commonly present in these cells that hydrolyze the cAMP. Therefore, drugs that increase intracellular concentration of cAMP exhibit PDE IV inhibitory activity. These compounds are effective in alleviating the allergic eye diseases. New compounds containing 3-anilino-2-cycloalkenonne derivatives are claimed to have a PDE IV inhibitory activity. These new compounds are expected to increase intracellular cAMP activity that suppresses the activation of the inflammatory cells and provide anti-inflammatory activity [62].
Another patent discloses a pharmaceutical composition comprising of aqueous solution of epinastine for topical administration [65]. Early phase ocular allergic reactions occur by the release of toxic eosinophilic granule protein, enzymes and mast cell mediators such as histamine. The influx of neutrophils and eosinophils into conjunctiva starts the late phase reactions (LPR). Epinastine inhibits influx of these mediators into the conjunctiva and prevents occurrence of LPR; thereby provides long lasting effect in the treatment of allergic conjunctiva. After topical administration of a solution containing epinastine to the passive anaphylaxis model of rats, a significantly lower content of eosinophils and lymphocytes was detected in conjunctiva. In addition, almost 35% inhibition of mast cell degranulation was observed in animals pretreated with epinastine solution [65].
It was recently discovered that Toll like receptors (TLR) are present in the donor ocular tissues and cultured cells of the retinal pigment epithelium, cornea and conjunctiva [66]. TLR play a major role in the activation of the innate immune system. Mast cells and circulating leukocytes such as neutrophil, eosinophil, basophil, monocytes and macrophages express TLR. Pathogenic ligands bind to the TLR of these immune cells and initiate the production of cytokines and inflammatory mediators that cause allergic inflammation. TLR are hypothesized to modulate Th1/Th2 lymphocyte equilibrium that leads to allergic diseases. TLR alone and with other coreceptors such as CD14 and MD-2 initiate inflammatory cascades that eliminate the foreign materials. Novel compositions with inhibitory action on human TLR and coreceptors have been discovered. These compositions contain the compound that can down regulate the human TLR signaling pathway. This compound acts as TLR antagonist or TLR-coreceptor antagonist that prevents the binding of pathogenic ligands to the TLR or coreceptors and generates anti-allergic activity. The inventors disclosed many compounds with anti-allergic effects including oligodeoxynucleoside, antihistamines, leukotriene antagonists, mast cell stabilizers, immunomodulators, anti-IgE agents, a ligand of Vit D receptor, an antimicrobial agent, a quinazoline derivative or an antibody that inhibits the activity of TLR [66]. Another patent application has disclosed a class of pyrimidine derivatives that act on TLR7 for the treatment of viral or allergic diseases and cancers [67].
In another invention, compositions with small interfering RNA which act by silencing spleen tyrosine kinase (Syk) are disclosed [68]. Syk is activated by signaling through immune receptors such as FcεRI. That causes the activation of PLCγ and PI3K pathways and results in mast cell degranulation. This disclosure provides small interfering RNA containing 19 to 49 nucleotides, which silences the expression of Syk mRNA, a non-receptor tyrosine kinase thereby interrupting the FcεRI, PLCγ and PI3K pathways. Interference of these pathways blocks a series of reactions relavent to mast cell degranulation and prevents the release of histamine and other pro-inflammatory mediators. Interfering RNA alleviates the Syk-related allergic conditions while avoiding undesirable side effects associated with systemic use of antihistamines [68]. The inventors have filed another patent for the use of medicament containing interfering RNA that targets histamine receptor H1 (HRH1) mRNA. This RNA attenuates the expression of HRH1 mRNA and interrupts the G-protein-coupled receptor signaling pathway, thus inhibiting histamine mediated inflammatory responses in an HRH1-related condition. The inventors described this treatment as most potent and efficacious for prevention or intervention of an HRH1-related condition while circumventing the side effects associated with other treatments [69]. Recent patents on the treatment of allergic conjunctivitis are summarized in Table 3 [63-77].
Table 3. Recent Patents on the Treatment of Allergic Conjunctivitis.
| Patent No. | Publication Name | Description | Ref. |
|---|---|---|---|
| US20100087397 | Use of lipid-conjugates in the treatment of conjunctivitis | Lipid-conjugates to inhibit PLA2 enzyme activity control the production of inflammatory mediators and modulators Have membrane stabilizing effects |
[63] |
| WO2009045054 | A composition for treatment of conjunctivitis comprising chlorogenic acid and derivatives thereof | Chlorogenic acid or derivative thereof exhibiting anti-inflammatory effects comparable to steroidal drugs have less side effects and provide synergistic action when used in combination with known anti-inflammatory agents | [61] |
| WO2008035922 | A composition comprising glucosamine and derivatives thereof and a method for treatment of conjunctivitis using the same | Glucosamine and derivatives thereof exhibiting anti-inflammatory effects comparable to steroidal drugs have less side effects and provide synergistic action when used in combination with known anti-inflammatory agents | [64] |
| US20080306163 | Agent for treatment of allergic eye disease | 3-Anilino-2-cycloalkenonne derivatives for the treatment of allergic conjunctivitis, vernal conjunctivitis and vernal catarrh by having PDE IV inhibitory activity increase intracellular cAMP activity that suppress the activation of the inflammatory cells and provide anti-inflammatory activity | [62] |
| US20080009476 | Treating conjunctivitis by topically administering an epinastine solution to the conjunctiva | Topically administered aqueous solution of epinastine inhibits the influx of neutrophils and tosinophils in to the tissue of ocular conjunctiva and nasal mucous membrane | [65] |
| WO2009089401 | Compositions comprising toll-like receptor or coreceptor antagonists and methods for treating or controlling ocular allergy using same | Novel compositions that bind to TLRs and coreceptors and inhibit the production of cytokines and inflammatory mediators. | [66] |
| WO2009067081 | Pyrimidine derivatives for the treatment of asthma, COPD, allergic rhinitis, allergic conjunctivitis, atopic dermatitis, cancer, hepatitis B, hepatitis C, HIV, HPV, bacterial infections and dermatosis | Invention provides pyrimidine derivatives that act on TLR7 receptor and provide immune-modulating properties in the treatment of viral or allergic diseases and cancers | [67] |
| US20090324507 | RNAi-mediated inhibition of spleen tyrosine kinase-related inflammatory conditions | Interfering RNA which acts by silencing spleen tyrosine kinase (SyK) and blocks a cascade of events related to mast cell degranulation and prevents the release of histamine and other pro-inflammatory mediators | [68] |
| US20090274631 | RNAi-mediated inhibition of histamine receptor h1-related conditions | Interfering RNA that attenuates the expression of histamine receptor H1 mRNA | [69] |
| US20090182035 | Use of a combination of olopatadine and cilomilast to treat non-infectious rhinitis and allergic conjunctivitis | Pharmaceutical composition containing effective amount of olopatadine and cilomilast for topical administration in the treatment of allergic conjunctivitis and allergic rhinitis | [70] |
| US20100120741 | Heterocyclic inhibitors of histamine receptors for the treatment of disease | Novel heterocyclic compounds are synthesized which have antagonistic effect specifically towards histamine type-1 (H1R) and/or type-4 (H4R) receptors | [71] |
| US20100081646 | Bicyclic heteroaryl inhibitors of PDE4 | Bicyclic heteroaryl compounds are provided, which have PDE4 selective effect and have less adverse effects in comparison to other PDE4 inhibitors Regulate the production of cytokines and increase the cAMP levels at the target site |
[72] |
| US20100063047 | Aminopyrimidine inhibitors of histamine receptors for the treatment of disease | Novel compounds and pharmaceutical composition having antihistamine effect on H1 and/or H4 receptors are provided for the treatment of histamine-receptor mediated diseases | [73] |
| US20090324691 | Methods and ophthalmic devices used in the treatment of ocular allergies | Provides method for preparing polymerized ophthalmic device with or without minimum effective amount of anti-allergic agent | [74] |
| US20080051385 | Ocular allergy treatments | Method for preventing and treating ocular allergic conditions by applying effective amount of alcaftadine topically Invention provides new therapy that is effective in the treatment of allergic symptoms such as ocular redness, chemosis and eyelid swelling and have longer duration of action |
[75] |
| US7687539 | Method of treating ocular allergy | Method of treating allergic conjunctivitis by the use of 5,6,7-trihydroxyheptanoic acid and its analogs alone or in combination with histamine receptor inhibitior and/or a mast cell degranulation inhibitor | [76] |
| US20100022470 | Method for treating allergic diseases | Use of Dendrobii Herba polysaccharides for the treatment of allergic diseases including allergic conjunctivitis via oral administration Polysaccharides used in the present invention increase the number of T regulatory cells in the intestinal lamina propria site that suppress the activation of immune system |
[77] |
Current & Future Developments
Current therapies based on antihistamines, mast cell inhibitors and anti-inflammatory drugs are effective in the treatment of allergic conjunctivitis. However, identification of newer targets provides a major impetus for the discovery of novel potent drug molecules for the treatment of allergic conjunctivitis. A novel target spleen tyrosine kinase (Syk) which regulates the phosphorylation of enzymes such as phopholipase-C, phosphotidylinositol-3 kinase and protein kinase which regulate the release of histamine. Future investigation will be focused on designing novel Syk inhibitors. Another target histamine H4 receptor was identified for regulating T-cell mediated response. The development of novel H4 inhibitors will be of paramount significance in the treatment of ocular allergies. NSAIDs are effective in the treatment of SAC, VKC and GPC. However, these agents exhibit limited effectiveness in immediate hypersensitive response. Cyclosporine-A is the most effective anti-lymphocyte therapy for the treatment of VKC and AKC. Another novel drug mycophenolic acid inhibit T and B lymphocyte replication and recently under investigation for the conjunctivitis therapy. Another target Janus protein kinase-3 is involved in the activation and proliferation of T-cells. Future drug design approach would be based on evaluating novel inhibitors of JAK-3 which may be an effective therapy for AKC and VKC. A study also suggests the application of monoclonal antibody i.e. the humanized anti-eotaxin 1 for the inhibition of human conjunctival mast cell by acting on eotaxin receptor. Monoclonal antibody based therapy can be explored further to evaluate its potential in the treatment of allergic conjunctivitis [78]. Therefore, development of novel drug molecules will be a valuable tool in the treatment of allergic conjunctivitis [79].
Acknowledgments
This study was supported by NIH R01EY09171-16 and NIH RO1EY10659-14
Footnotes
Conflict of Interest: Authors declare no conflict of interest in this study.
References
- 1.Origlieri C, Bielory L. Emerging drugs for conjunctivitis. Expert Opin Emerg Drugs. 2009;14:523–36. doi: 10.1517/14728210903103818. [DOI] [PubMed] [Google Scholar]
- 2.Butrus S, Portela R. Ocular allergy: Diagnosis and treatment. Ophthalmol Clin North Am. 2005;18:485–92. doi: 10.1016/j.ohc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Singh K, Axelrod S, Bielory L. The epidemiology of ocular and nasal allergy in the United States, 1988-1994. J Allergy Clin Immunol. 2010;126:778–83. e6. doi: 10.1016/j.jaci.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 4.Chigbu DI. The pathophysiology of ocular allergy: A review. Cont Lens Anterior Eye. 2009;32:3–15. doi: 10.1016/j.clae.2008.07.003. quiz 43-4. [DOI] [PubMed] [Google Scholar]
- 5.McGill JI, H S, Church MK, Anderson DF, Bacon A. Allergic eye disease mechanisms. Br J Ophthalmol. 1998;82:1203–14. doi: 10.1136/bjo.82.10.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonardi A. Pathophysiology of allergic conjunctivitis. Acta Ophthalmol Scand. 1999;228:21–3. doi: 10.1111/j.1600-0420.1999.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 7.Abelson MB, Leonardi A, Smith L. The mechanisms, diagnosis and treatment of allergy. Rev Ophthalmol. 2002;9:74–84. [Google Scholar]
- 8.Bundoc VG, Keane-Myers A. Animal models of ocular allergy. Curr Opin Allergy Clin Immunol. 2003;3:375–9. doi: 10.1097/00130832-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Toda M, Ohbayashi M, Ono SJ. Detailed criteria for the assessment of clinical symptoms in a new murine model of severe allergic conjunctivitis. Cornea. 2003;22:S13–8. doi: 10.1097/00003226-200310001-00003. [DOI] [PubMed] [Google Scholar]
- 10.Manzouri B, Flynn TH, Larkin F, Ono SJ, Wyse R. Pharmacotherapy of allergic eye disease. Expert Opin Pharmacother. 2006;7:1191–200. doi: 10.1517/14656566.7.9.1191. [DOI] [PubMed] [Google Scholar]
- 11.Schmid KL, Schmid LM. Ocular allergy: Causes and therapeutic options. Clin Exp Optom. 2000;83:257–70. doi: 10.1111/j.1444-0938.2000.tb05014.x. [DOI] [PubMed] [Google Scholar]
- 12.Mcgill JI, Holgate ST, Church MK. Allergic eye disease mechanisms. Br J Ophthalmol. 1998;82:1203–14. doi: 10.1136/bjo.82.10.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irani AM, Butrus SI, Tabbara KF, Schwartz LB. Human conjunctival mast cells: Distribution of MCT and MCTC in vernal conjunctivitis and giant papillary conjunctivitis. J Allergy Clin Immunol. 1990;86:34–40. doi: 10.1016/s0091-6749(05)80120-4. [DOI] [PubMed] [Google Scholar]
- 14.Abelson MB, Chambers WA, Smith LM. Conjunctival allergen challenge. A clinical approach to studying allergic conjunctivitis. Arch Ophthalmol. 1990;108:84–8. doi: 10.1001/archopht.1990.01070030090035. [DOI] [PubMed] [Google Scholar]
- 15.Anderson DF. The conjunctival late-phase reaction and allergen provocation in the eye. Clin Exp Allergy. 1996;26:1105–7. [PubMed] [Google Scholar]
- 16.Choi SH, Bielory L. Late-phase reaction in ocular allergy. Curr Opin Allergy Clin Immunol. 2008;8:438–44. doi: 10.1097/ACI.0b013e32830e6b3a. [DOI] [PubMed] [Google Scholar]
- 17.Izushi K, Nakahara H, Tai N, Mio M, Watanabe T, Kamei C. The role of histamine H(1) receptors in late-phase reaction of allergic conjunctivitis. Eur J Pharmacol. 2002;440:79–82. doi: 10.1016/s0014-2999(02)01304-3. [DOI] [PubMed] [Google Scholar]
- 18.Metz DP, Bacon AS, Holgate S, Lightman SL. Phenotypic characterization of T cells infiltrating the conjunctiva in chronic allergic eye disease. J Allergy Clin Immunol. 1996;98:686–96. doi: 10.1016/s0091-6749(96)70103-3. [DOI] [PubMed] [Google Scholar]
- 19.Niederkorn JY. Immune regulatory mechanisms in allergic conjunctivitis: Insights from mouse models. Curr Opin Allergy Clin Immunol. 2008;8:472–6. doi: 10.1097/ACI.0b013e32830edbcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niederkorn JY, Chen PW, Mellon J, Stevens C, Mayhew E. Allergic conjunctivitis exacerbates corneal allograft rejection by activating Th1 and Th2 alloimmune responses. J Immunol. 2010;184:6076–83. doi: 10.4049/jimmunol.0902300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosmann TR, Coffman RL. Th1 and Th2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 22.Bielory L. Ocular allergy and dry eye syndrome. Curr Opin Allergy Clin Immunol. 2004;4:421–4. doi: 10.1097/00130832-200410000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Oboki K, Ohno T, Saito H, Nakae S. Th17 and allergy. Allergol Int. 2008;57:121–34. doi: 10.2332/allergolint.R-07-160. [DOI] [PubMed] [Google Scholar]
- 24.Sharif NA, Su SX, Yanni JM. Emedastine: A potent, high affinity histamine H1-receptor-selective antagonist for ocular use: Receptor binding and second messenger studies. J Ocul Pharmacol. 1994;10:653–64. doi: 10.1089/jop.1994.10.653. [DOI] [PubMed] [Google Scholar]
- 25.Bielory L. Update on ocular allergy treatment. Expert Opin Pharmacother. 2002;3:541–53. doi: 10.1517/14656566.3.5.541. [DOI] [PubMed] [Google Scholar]
- 26.Abelson MB. A review of olopatadine for the treatment of ocular allergy. Expert Opin Pharmacother. 2004;5:1979–94. doi: 10.1517/14656566.5.9.1979. [DOI] [PubMed] [Google Scholar]
- 27.Abelson MB. Evaluation of olopatadine, a new ophthalmic antiallergic agent with dual activity, using the conjunctival allergen challenge model. Ann Allergy Asthma Immunol. 1998;81:211–8. doi: 10.1016/S1081-1206(10)62814-1. [DOI] [PubMed] [Google Scholar]
- 28.Bielory L, Lien KW, Bigelsen S. Efficacy and tolerability of newer antihistamines in the treatment of allergic conjunctivitis. Drugs. 2005;65:215–28. doi: 10.2165/00003495-200565020-00004. [DOI] [PubMed] [Google Scholar]
- 29.Avunduk AM, Tekelioglu Y, Turk A, Akyol N. Comparison of the effects of ketotifen fumarate 0.025% and olopatadine HCl 0.1% ophthalmic solutions in seasonal allergic conjunctivities: A 30-day, randomized, double-masked, artificial tear substitute-controlled trial. Clin Ther. 2005;27:1392–402. doi: 10.1016/j.clinthera.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Solomon A, Pe'er J, Levi-Schaffer F. Advances in ocular allergy: Basic mechanisms, clinical patterns and new therapies. Curr Opin Allergy Clin Immunol. 2001;1:477–82. doi: 10.1097/01.all.0000011063.28808.cc. [DOI] [PubMed] [Google Scholar]
- 31.Miyake-Kashima M, Takano Y, Tanaka M, Satake Y, Kawakita T, Dogru M, et al. Comparison of 0.1% bromfenac sodium and 0.1% pemirolast potassium for the treatment of allergic conjunctivitis. Jpn J Ophthalmol. 2004;48:587–90. doi: 10.1007/s10384-004-0127-2. [DOI] [PubMed] [Google Scholar]
- 32.Minami K, Hossen MA, Kamei C. Increasing effect by simultaneous use of levocabastine and pemirolast on experimental allergic conjunctivitis in rats. Biol Pharm Bull. 2005;28:473–6. doi: 10.1248/bpb.28.473. [DOI] [PubMed] [Google Scholar]
- 33.Gous P, Ropo A. A comparative trial of the safety and efficacy of 0.1 percent pemirolast potassium ophthalmic solution dosed twice or four times a day in patients with seasonal allergic conjunctivitis. J Ocul Pharmacol Ther. 2004;20:139–50. doi: 10.1089/108076804773710812. [DOI] [PubMed] [Google Scholar]
- 34.Woerly G, Loiseau S, Loyens M, Schoch C, Capron M. Inhibitory effects of ketotifen on eotaxin-dependent activation of eosinophils: Consequences for allergic eye diseases. Allergy. 2003;58:397–406. doi: 10.1034/j.1398-9995.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 35.Nabe M, Miyagawa H, Agrawal DK, Sugiyama H, Townley RG. The effect of ketotifen on eosinophils as measured at LTC4 release and by chemotaxis. Allergy Proc. 1991;12:267–71. doi: 10.2500/108854191778879313. [DOI] [PubMed] [Google Scholar]
- 36.Canonica GW, Ciprandi G, Petzold U, Kolb C, Ellers-Lenz B, Hermann R. Topical azelastine in perennial allergic conjunctivitis. Curr Med Res Opin. 2003;19:321–9. doi: 10.1185/030079903125001794. [DOI] [PubMed] [Google Scholar]
- 37.Chand N, Pillar J, Diamantis W, Sofia RD. Inhibition of IgE-mediated allergic histamine release from rat peritoneal mast cells by azelastine and selected antiallergic drugs. Agents Actions. 1985;16:318–22. doi: 10.1007/BF01982866. [DOI] [PubMed] [Google Scholar]
- 38.Blaho K. Non-steroidal anti-inflammatory drugs: Current trends in pharmacology and therapeutics. J Am Optom Assoc. 1992;63:875–8. [PubMed] [Google Scholar]
- 39.Masferrer JL, Kulkarni PS. Cyclooxygenase-2 inhibitors: A new approach to the therapy of ocular inflammation. Surv Ophthalmol. 1997;41(Suppl 2):S35–40. doi: 10.1016/s0039-6257(97)80005-7. [DOI] [PubMed] [Google Scholar]
- 40.Kim SJ, Flach AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol. 2010;55:108–33. doi: 10.1016/j.survophthal.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 41.Yaylali V, Demirlenk I, Tatlipinar S, Ozbay D, Esme A, Yildirim C, et al. Comparative study of 0.1% olopatadine hydrochloride and 0.5% ketorolac tromethamine in the treatment of seasonal allergic conjunctivitis. Acta Ophthalmol Scand. 2003;81:378–82. doi: 10.1034/j.1600-0420.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 42.Discepola M, Deschenes J, Abelson M. Comparison of the topical ocular antiallergic efficacy of emedastine 0.05% ophthalmic solution to ketorolac 0.5% ophthalmic solution in a clinical model of allergic conjunctivitis. Acta Ophthalmol Scand Suppl. 1999;(228):43–6. doi: 10.1111/j.1600-0420.1999.tb01173.x. [DOI] [PubMed] [Google Scholar]
- 43.Doggrell SA. Triamcinolone: New and old indications. Expert Opin Pharmacother. 2001;2:1177–86. doi: 10.1517/14656566.2.7.1177. [DOI] [PubMed] [Google Scholar]
- 44.Hyvarinen L. Local treatment of allergic conjunctivitis. Duodecim. 1982;98:717–9. [PubMed] [Google Scholar]
- 45.Frankland AW, Walker SR. A clinical comparison of topical clobetasone butyrate and sodium cromoglycate in allergic conjunctivitis. Clin Allergy. 1981;11:473–8. doi: 10.1111/j.1365-2222.1981.tb01621.x. [DOI] [PubMed] [Google Scholar]
- 46.Foulds WS, Greaves DP, Herx-Heimer H, Kingdom LG. Hydrocortisone in treatment of allergic conjunctivitis, allergic rhinitis, and bronchial asthma. Lancet. 1955;268:234–5. doi: 10.1016/s0140-6736(55)90164-1. [DOI] [PubMed] [Google Scholar]
- 47.Pavesio CE, Decory HH. Treatment of ocular inflammatory conditions with loteprednol etabonate. Br J Ophthalmol. 2008;92:455–9. doi: 10.1136/bjo.2007.132621. [DOI] [PubMed] [Google Scholar]
- 48.Ilyas H, Slonim CB, Braswell GR, Favetta JR, Schulman M. Long-term safety of loteprednol etabonate 0.2% in the treatment of seasonal and perennial allergic conjunctivitis. Eye Contact Lens. 2004;30:10–3. doi: 10.1097/01.ICL.0000092071.82938.46. [DOI] [PubMed] [Google Scholar]
- 49.Klosterhalfen B, Offner FA, Kirkpatrick CJ, Mittermayer C. Pathology of multiple organ failure. Contrib Nephrol. 1991;93:71–5. doi: 10.1159/000420189. [DOI] [PubMed] [Google Scholar]
- 50.Leonardi A, Papa V, Milazzo G, Secchi AG. Efficacy and safety of desonide phosphate for the treatment of allergic conjunctivitis. Cornea. 2002;21:476–81. doi: 10.1097/00003226-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Cerqueti PM, Prosio PE, Bosia S, Buscaglia S, Ciprandi G. Clobetasone treatment of allergic conjunctivitis: A comparative double blind study. Allergol Immunopathol (Madr) 1993;21:67–70. [PubMed] [Google Scholar]
- 52.Korenfeld MS, Silverstein SM, Cooke DL, Vogel R, Crockett RS. Difluprednate ophthalmic emulsion 0.05% for postoperative inflammation and pain. J Cataract Refract Surg. 2009;35:26–34. doi: 10.1016/j.jcrs.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 53.Bertelmann E, Pleyer U. Immunomodulatory therapy in ophthalmology - Is there a place for topical application? Ophthalmologica. 2004;218:359–67. doi: 10.1159/000080937. [DOI] [PubMed] [Google Scholar]
- 54.Fukushima A, Yamaguchi T, Ishida W, Fukata K, Liu FT, Ueno H. Cyclosporin A inhibits eosinophilic infiltration into the conjunctiva mediated by type IV allergic reactions. Clin Exp Ophthalmol. 2006;34:347–53. doi: 10.1111/j.1442-9071.2006.01221.x. [DOI] [PubMed] [Google Scholar]
- 55.Ebihara N, Ohashi Y, Uchio E, Okamoto S, Kumagai N, Shoji J, et al. A large prospective observational study of novel cyclosporine 0.1% aqueous ophthalmic solution in the treatment of severe allergic conjunctivitis. J Ocul Pharmacol Ther. 2009;25:365–72. doi: 10.1089/jop.2008.0103. [DOI] [PubMed] [Google Scholar]
- 56.Attas-Fox L, Barkana Y, Iskhakov V, Rayvich S, Gerber Y, Morad Y, et al. Topical tacrolimus 0.03% ointment for intractable allergic conjunctivitis: an open-label pilot study. Curr Eye Res. 2008;33:545–9. doi: 10.1080/02713680802149115. [DOI] [PubMed] [Google Scholar]
- 57.Ohashi Y, Ebihara N, Fujishima H, Fukushima A, Kumagai N, Nakagawa Y, et al. A randomized, placebo-controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis. J Ocul Pharmacol Ther. 2010;26:165–74. doi: 10.1089/jop.2009.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forrest ML, Won CY, Malick AW, Kwon GS. In vitro release of the mTOR inhibitor rapamycin from poly(ethylene glycol)-b-poly(epsilon-caprolactone) micelles. J Control Release. 2006;110:370–7. doi: 10.1016/j.jconrel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 59.de Carvalho JF, Ribeiro AC, de Moraes JC, Goncalves C, Goldenstein-Schainberg C, Bonfa E. Infliximab: A promising alternative therapy for refractory arthritis/urethritis/conjunctivitis triad. Isr Med Assoc J. 2009;11:511–3. [PubMed] [Google Scholar]
- 60.Magone MT, Chan CC, Beck L, Whitcup SM, Raz E. Systemic or mucosal administration of immunostimulatory DNA inhibits early and late phases of murine allergic conjunctivitis. Eur J Immunol. 2000;30:1841–50. doi: 10.1002/1521-4141(200007)30:7<1841::AID-IMMU1841>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 61.Kim S, Sohn J. A composition for treatment of conjunctivitis comprising chlorogenic acid and derivatives thereof. WO2009045054. 2009
- 62.Ina S, Takahama A. Agent for treatment of allergic eye disease. US20080306163. 2008
- 63.Yedgar S. Use of lipid-conjugates in the treatment of conjunctivitis. US20100087397. 2010
- 64.Kim S, Kim T, Sohn J. A composition comprising glucosamine and derivatives thereof and a method for treatment of conjuctivitis using the same. WO2008035922. 2008
- 65.Trach V, Duschler G. Treating conjunctivitis by topically administering an epinastine solution to the conjunctiva. US20080009476. 2008
- 66.Zhang J, Ward KW, Vo TP. Compositions comprising toll-like receptor or coreceptor antagonists and methods for treating or controlling ocular allergy using same. WO2009089401. 2009
- 67.Bennett NJ, McInally T, Mochel T, Thom S, Tiden A. Pyrimidine derivatives for the treatment of asthma, copd, allergic rhinitis, allergic conjunctivitis, atopic dermatitis, cancer, hepatitis B, hepatitis C, HIV, HPV, bacterial infections and dermatosis. WO2009067081. 2009
- 68.Yanni JM, Chatterton JE, Gamache DA, Miller ST. RNAi-mediated inhibition of spleen tyrosine kinase-related inflammatory conditions. US20090324507. 2009
- 69.Yanni JM, Chatterton JE, Gamache DA, Miller ST. RNAi-mediated inhibition of histamine receptor h1-related conditions. US20090274631. 2009
- 70.Yanni JM, Gamache DA, Miller ST, Beauregard C. Use of a combination of olopatadine and cilomilast to treat non-infectious rhinitis and allergic conjunctivitis. US20090182035. 2009
- 71.Borchardt AJ, Beauregard C, Cook T, Davis RL, Gamache DA, Yanni JM. Heterocyclic inhibitors of histamine receptors for the treatment of disease. US20100120741. 2010
- 72.Govek SP, Beauregard C, Gamache DA, Hellberg MR, Noble SA, Shiau AK, Thomas DJ, Yanni M. Bicyclic heteroaryl inhibitors of PDE4. US20100081646. 2010
- 73.Borchardt AJ, Beauregard C, Davis RL, Gamache DA, Yanni JM. Aminopyrimidine inhibitors of histamine receptors for the treatment of disease. US20100063047. 2010
- 74.Mahadevan S, Menezes EV. Methods and ophthalmic devices used in the treatment of ocular allergies. US20090324691. 2009
- 75.Parasrampuria J, Ingerman A, Janssens F, Megens A. Ocular allergy treatments. US20080051385. 2008
- 76.Klimko PG, Beauregard C. Method of treating ocular allergy. US7687539. 2010
- 77.Wu R. Method for treating allergic diseases. US20100022470. 2010
- 78.Ono SJ, Abelson MB. Allergic conjunctivitis: Update on pathophysiology and prospects for future treatment. J Allergy Clin Immunol. 2005;115:118–22. doi: 10.1016/j.jaci.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 79.Yanni JM, Barney NP. Ocular Allergy: Clinical, Therapeutic and Drug Discovery Consideration. In: Yorio T, Clark A, Wax M, editors. Ocular Therapeutics: Eye on new discoveries. Elsevier; USA: 2008. [Google Scholar]


