Abstract
Adolescent offspring of schizophrenia patients (HR-S) are an important group in whom to study impaired brain function and structure, particularly of the frontal cortices. Studies of working memory have suggested behavioral deficits and fMRI-measured hypoactivity in fronto-parietal regions in these subjects. Independent structural MRI (sMRI) studies have suggested exaggerated frontal gray matter decline. Therefore the emergent view is that fronto-parietal deficits in function and structure characterize HR-S. However, it is unknown if fronto-parietal sub-regions in which fMRI-measured hypo-activity might be observed are precisely those regions of the cortex in which gray matter deficits are also observed. To investigate this question we conducted conjoint analyses of fronto-parietal function and structure in HR-S (n=19) and controls (n=24) with no family history of psychoses using fMRI data during a continuous working memory task (2 Back), and sMRI collected in the same session. HR-S demonstrated significantly reduced BOLD activation in left dorso-lateral prefrontal cortex (BA 9/46) and bilateral parietal cortex (BA 7/40). Sub-regions of interest were created from the significant fronto-parietal functional clusters. Analyses of gray matter volume from volume-modulated gray matter segments in these clusters did not reveal significant gray matter differences between groups. The results suggest that functional impairments in adolescent HR-S can be independent of impairments in structure, suggesting that the relationship between impaired function and structure is complex. Further studies will be needed to more closely assess whether impairments in function and structure provide independent or interacting pathways of vulnerability in this population.
Keywords: Schizophrenia, Adolescent Offspring, Working Memory, Vulnerability, fMRI, Structural MRI
1. Introduction
Adolescent offspring of schizophrenia patients are at increased risk for schizophrenia and psychiatric disorders, and therefore provide an interesting and important group in whom to assess vulnerabilities in developmentally mediated brain dysfunction and structure (Diwadkar et al., 2004). Whereas documented conversion rates to psychosis are relatively low, the incidence of psychopathology (Keshavan et al., 2008), cognitive and affective impairments and in vivo imaging related abnormalities (Diwadkar et al., 2006; Keshavan et al., 2002a) is relatively high or significantly different from controls (with no immediate family history of the disorder). The emerging view is that this high risk group (henceforth HR-S) while heterogeneous, nevertheless represents a developmentally vulnerable cohort, characterized by impaired brain function, structure and neurochemistry.
Recent investigations have targeted the understanding of disordered function of prefrontal tasks, in particular working memory (Casey et al., 1995) in HR-S. Working memory is the ability to retain and in the case of specific tasks such as the n-back to manipulate information for brief intervals (Baddeley, 1986; Rypma et al., 1999). In general, load-dependent processing in working memory is generally associated with fronto-parietal activity (Braver et al., 1997; Chafee and Goldman-Rakic, 2000). Primate studies have indicated the presence of single cell units within the superior frontal sulcus that specifically code for the retention of memoranda for brief intervals in time (Fuster, 1989). In vivo studies of both spatial and non-spatial working memory suggest that working memory implementation in humans has similarities with primates. For example, both the prefrontal and parietal cortices are sensitive to increases in verbal working memory load (Braver et al., 1997), analogous to increases in phasic activity in prefrontal neurons as a function of memory delay (Funahashi et al., 1989). Further, memory guided saccades in the macaque are implemented in large part by interdependent activity between fronto-parietal units (Chafee and Goldman-Rakic, 2000). Analogously in humans, fronto-parietal connectivity is positively modulated by increases in working memory load (Diwadkar et al., 2000). Though the functional architecture of working memory is not confined to fronto-parietal interactions (Wager and Smith, 2003), the previously cited and other studies (Edin et al., 2007) suggest that fronto-parietal interactions may underlie the development of working memory mechanisms in the brain. Furthermore, working memory deficits have long been thought to lie at the center of cognitive deficits in the schizophrenia diathesis (Goldman-Rakic, 1999) and have been related to independently observed deficits in frontal structure.
1.1 Deficits in HR-S
In HR-S, working memory deficits have been demonstrated when memory maintenance interval increase from brief to longer delays (Diwadkar et al., 2001). Preliminary fMRI studies during working memory tasks suggesting reduced response of the dorsal prefrontal cortex (Keshavan et al., 2002b). Independently of the fMRI results, structural MRI (sMRI) studies have documented fronto-parietal reductions in gray matter (Diwadkar et al., 2006) suggesting an emergent structural impairment related to the ongoing developmental processes of cortical pruning or thinning that are particularly active in fronto-parietal cortex through adolescence (Gogtay et al., 2004). Therefore, in general, it is assumed that impaired fMRI-related fronto-parietal function in HR-S is related to reduced volume or structural integrity of these regions. This has lead to the general notion that, as in the case with the schizophrenia spectrum (Manoach, 2003; Weinberger et al., 2001), HR-S are also characterized by disordered functionality of the frontal lobe. However, it is unknown whether in HR-S, reduced gray matter volume or micro-structure is a necessary condition for the presence of functional alterations in frontal and parietal cortex during working memory. The answer to this question may a) help address the functional relevance of gray matter deficits in HR-S and b) elucidate the relative contributions of fMRI and sMRI related vulnerabilities in these subjects.
Here we used an established verbal working memory task (Casey et al., 1995; Cohen et al., 1997), the n-back to assess working memory related activation differences in HR-S compared to HC specifically in the frontal and parietal cortices. Then, fMRI-based region of interest masks were created to include clusters with significant differences in activation. Finally, fMRI-based masks were used to extract gray matter volume (based on T1-weighted MRI images) from these functionally defined regions of interest. The noted advantage of this approach is that it allows us to identify working memory related hypo-activity in fronto-parietal cortex in HR-S, and specifically assess whether significantly different clusters are characterized by reductions in gray matter. In short, the convergent approach permits an assessment of whether gray matter deficits in HR-S are necessary for differences in fMRI-measured fronto-parietal function.
2. Methods
2.1 Subjects
Forty three subjects gave informed consent or assent to participate in the fMRI studies. MRI and behavioral protocols were approved by the Human Investigative Committee (HIC) of Wayne State University. Subjects received monetary compensation for their participation. The twenty four controls (HC; age:10–19, mean=14.5 yrs; 8 females) had no family history of psychiatric illness to the 2nd degree. Nineteen HR-S had at least one parent with schizophrenia (HR-S; age:8–19, mean=14.16 yrs; 7 females). Subjects were recruited from the greater Detroit area through advertisements and through out-patient services at the University Psychiatric Center, Wayne State University School of Medicine. Rule outs were achieved through telephone and personal interview, and screening questionnaires, to ascertain if subjects had a history of psychotic illness in first-degree relatives. Diagnoses for parents of HR-S were reached using the Structured Clinical Interview for DSM-IV schizophrenia (First et al., 1997) and consensus meetings chaired by a senior clinician (M.S.K.) discussing all available data. Subjects younger than 15 years were clinically evaluated using the Schedule for Affective Disorders and Schizophrenia -Child Version (K-SADS) (Kaufman et al., 1997); those aged 15 years or above were assessed using the SCID, and all subjects were assessed with the Structured Interview for Prodromal Symptoms (Miller et al., 2003; Miller et al., 2002). Assessments were administered by a trained interviewer (U.R., A.J.). Four HR-S were diagnosed with disorders (which were not exclusionary criteria) including Separation Anxiety
Disorder (n=1), Attention Deficit Hyperactivity Disorder hyperactive type (n=1) and Social phobia (n=1). Table 1 presents the general characteristics of the sample, including the average Global Assessment of Function (GAF) sub-scale of the SIPS. The GAF is an index of the psychological, social, and occupational functioning of individuals providing a measure of the extent of clinically assessed impairment in individuals.
Table 1.
Demographic, clinical and behavioral information subjects in the study.
| SCZ-Off (n=19) | HC (n=24) | |
|---|---|---|
| Gender (F/M) | 7/12 | 8/16 |
| Age range (yrs) | 8–19 | 10–19 |
| Mean age (± sd) | 14.1 ± 2.85 | 14.91± 2.84 |
| Mean FSIQ (± sd) | 92.05 ± 13.39 | 92.3 ± 16.46 |
| Mean GAF Score (± sd) | 76.2 ± 10.7 | 85.8 ± 6.6 |
| Performance d’ | 2.91 ± .56 | 3.05 ± .88 |
| Performance (hit rate) | .86 ± .08 | .88 ± .09 |
| Response Latency (s) | .81 ± .29 | .68 ± .17 |
2.2 fMRI and sMRI
MR images were acquired on a Bruker MedSpec 4.0 T full-body scanner with an 8-channel head coil. For fMRI, gradient echo EPI was collected over an 11.5 minute scan time (TR = 2000ms, TE = 30 ms, matrix = 64×64, slices =24, FOV = 240 mm, voxel size = 3.8×3.8×4.0mm). In the same subjects, high resolution sMRI images were axially acquired using a whole brain 3D T1-weighted MPRAGE sequence (TR = 2200ms, TE = 2.56 ms, Flip angle = 13º, FOV = 208×256 mm, voxel size = 1×1×1 mm).
During fMRI, stimuli were projected from a computer onto a screen mounted over the subject’s head which they could view through a mirror. An MRI-compatible two-button box was provided to subjects for them to indicate their responses. Foam padding was packed around each subject’s head to minimize movement, and subjects were given earplugs to reduce noise. Working Memory Paradigm. Subjects participated in an established verbal n-back paradigm (Casey et al., 1995). Letters were projected in sequence (Presentation Time: 500 ms; ISI: 2500 ms) and subjects signaled if a letter was a target letter (0-back condition) or the same as one shown two letters ago in the sequence (2-back condition). Conditions were blocked (30 s/block) and the paradigm cycled between rest epochs (20 s), 0-back and 2-back conditions.
2.3 fMRI and sMRI Processing
MR images were preprocessed and analyzed using SPM (Statistical Parametric Mapping, Wellcome Department of Imaging and Neuroscience, London, UK). For fMRI, all images were manually oriented to the AC-PC line, realigned to correct for head movement, spatially normalized to the MNI (Montreal Neurological Institute) template brain, resliced (2 mm3) and smoothed spatially by a Gaussian filter of 8mm full-width half maximum (FWHM). An autoregressive AR(1) model was used to account for serial correlation and regressors modeled as a 30 sec box-car vectors (for each of the task-related conditions) were convolved with a canonical hemodynamic reference wave form, with the six motion parameters included as co-variates of no interest. To isolate memory related processing, first level contrasts were computed based on 2Back>0Back contrasts for each subject, and were submitted to second-level random effects analyses of covariance (age and gender as covariates) to identify group differences in memory-related activity.
To identify differences from controls in HR-S, data were spatially thresholded in the dorsal prefrontal cortex (Brodmann Area 9/46) and the superior parietal cortex (BA 7/40) based on structural regions of interest in stereotactic space (Maldjian et al., 2003). Because we wanted to optimize sensitivity to detect clusters with significant voxels (pu<.05) in order to specifically interrogate fMRI-defined regions of interest, cluster extent thresholds (pc<.01) were derived based on 104 Monte Carlo simulations from voxels across the individual regions of interest (Ward, 2000). The bilateral clusters of peak significance (see Results) within each of the regions were saved as spatial masks (2 mm3 voxel sizes) in MNI space. sMRI analyses were focused specifically on estimated gray matter underlying these functionally derived masks.
sMRI images were processed using SPM’s diffeomorphic image registration algorithm (DARTEL)(Ashburner, 2007). DARTEL optimizes the fidelity of shape-based deformations applied to fit native images in stereotactic space, outperforming all or most competing non-linear deformation algorithms (Klein et al., 2009). It is therefore optimized for assessing structural changes within a stereotactic framework, and is ideally suited for convergent fMRI and sMRI analyses. In brief, following resampling (2 mm3) and segmentation of MPRAGE images, a rigid gray matter template was created representing the average shape and size of the brains of the 44 subjects included in the study. Subjects' grey matter maps were warped to the coordinate system of the template, with Jacobian modulation used to scale native gray matter volume from native to MNI space. This process of volume-modulation (Good et al., 2001) has been extensively used in voxel-based analyses of gray matter images within the framework of random field methods, and can be used to extract estimated volumes from regions of interest using structural masks. Subsequent sMRI analyses was based on the extraction and summation of voxel-wise volumes across all locations within the functionally defined region of interests.
3. Results
3.1 Behavioral Results
Behavioral results are presented in Table 1. Sensitivity to the task for both groups was compared using d’ (Macmillan and Creelman, 2005) which accounts for performance characteristics including correct hits and rejections, false alarms and misses. These sensitivity data were submitted to an analysis of covariance with group as single factor and age and gender as covariates. No significant differences in performance were observed (HC:d’=3.05, sd=.88; HR-S: d’=2.91, sd=.56, F1,39<1) suggesting that behavioral measures were equivalent across groups. An analyses of hit rates alone confirmed the sensitivity analyses; HR-S did not differ from HC, F1,39<1. By comparison, HR-S responded significantly more quickly, F1,39=5.45, p<.023, MSe=.043
3.2 fMRI & sMRI Results
Whole Brain Analyses: Main effect of Group
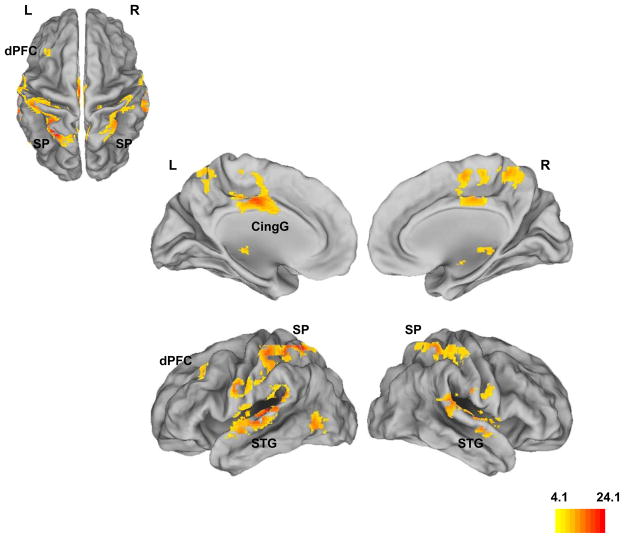
Figure 1 shows clusters under a significant main effect of group with peak loci indicated in Table 2. As seen, four principle regions in the whole brain analyses distinguished HR-S from HC, with the overall peak observed in the left superior parietal cortex. The other regions of significant difference have been implicated in control mechanisms in working memory (dorsal anterior cingulated) (Bakshi et al., In Press), aberrant hyper-activation in the schizophrenia prodrome (superior temporal gyrus)(Crossley et al., 2009), and reduced activation in adult subjects with prodromal symptoms (Fusar-Poli et al., 2010).
Figure 1. Main effect of group (HR-S, HC).
Whole brain analyses of activation differences between HRS-S and HC depict a main effect of group in the dorsal prefrontal cortex (dPFC), superior parietal cortex (SP), cingulate gyrus (CingG), superior temporal gyrus (STG) and middle temporal gyrus (MTG; See Table 2 for statistics). The results are largely consistent with the limited fMRI studies in clinical risk populations within the schizophrenia diathesis (see text for references).
Table 2.
Significance peaks (pu<.10−4, cluster extent:100 voxels) and locations from whole brain analyses under the overall main effect of group (HC vs. SCZ-Offspring). See Figure 1 for cluster renditions.
| Peak Statistic (F1,39) | kE (voxels) | Peak (MNI) | Region |
|---|---|---|---|
| 23.99 | 3383 | −26, −52, 60 | Superior Parietal (BA 7) |
| 18.20 | 2375 | −42, −28, 8 | Superior Temporal Gyrus |
| 17.61 | 970 | −4, −12, 36 | Cingulate Gyrus (BA 24) |
| 15.01 | 2158 | 62, −34, 12 | Superior Temporal Gyrus |
| 12.92 | 210 | −50, −66, 0 | Middle Temporal Gyrus (BA 37) |
| 8.39 | 128 | −34, 26, 42 | Dorsal Prefrontal Cortex (BA 9) |
Contrast Analyses
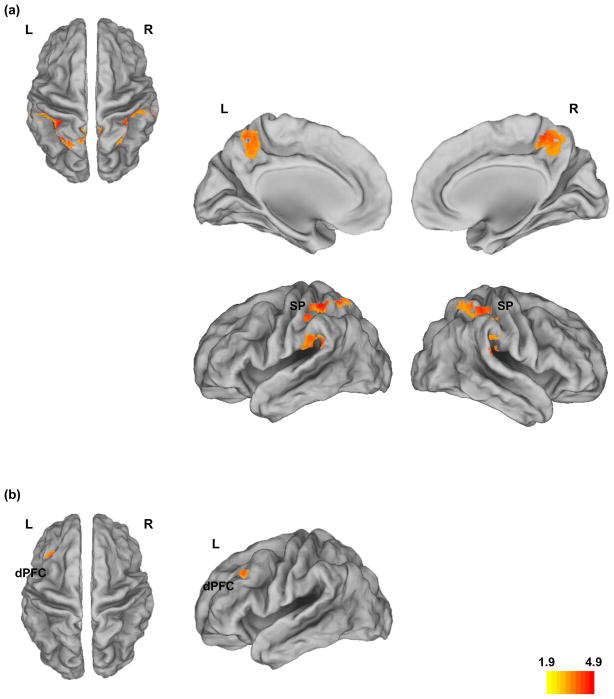
Bidirectional contrast analyses (HR-S > HC; HC > HR-S) were conducted to investigate directional differences in activation. No significant clusters were observed for HR-S > HC. For the HC > HR-S contrast, within the parietal cortex, clusters with reduced activity in HR-S relative to HC during working memory were observed bilaterally. Figure 2a depicts bilateral superior parietal hypo-activity in HR-S compared to HC (pc<.01, extent: 123 voxels) projected to dorsal (a), medial (b) and lateral (c) surfaces of the brain. Significance peaks for the contrasts of interest (HR-S < HC) were observed in left, t39=4.9, x=−26, y=−52, z=60, kE=346 voxels, and right superior parietal cortex, t39=3.49, x=32, y=−50, z=62, kE=255 voxels. Figure 2b depicts significant hypo-activity in the left dorsal prefrontal cortex in HR-S relative to HC (pc<.01, extent: 51 voxels), with significance peak at t39=2.9, pc<.05, x=−34, y=26, z=42, kE=137 voxels.
Figure 2. Contrast Differences, HC > HR-S in parietal and frontal cortex.
a) Bilateral Parietal Hypo-activity (pc<.01) during working memory in HR-S. Significantly reduced activity in bilateral superior parietal cortex (BA 7 & 40) during the 2 back task in HR-S relative to controls is projected on dorsal, medial and lateral surfaces of the brain. b) Dorsal Prefrontal Hypoactivity in HR-S. Significantly reduced activity (pc<.01) in left dorso-lateral prefrontal cortex during the 2 back task in HR-S relative to controls is projected on dorsal and lateral. Peak statistics and cluster extents are shown in the results.
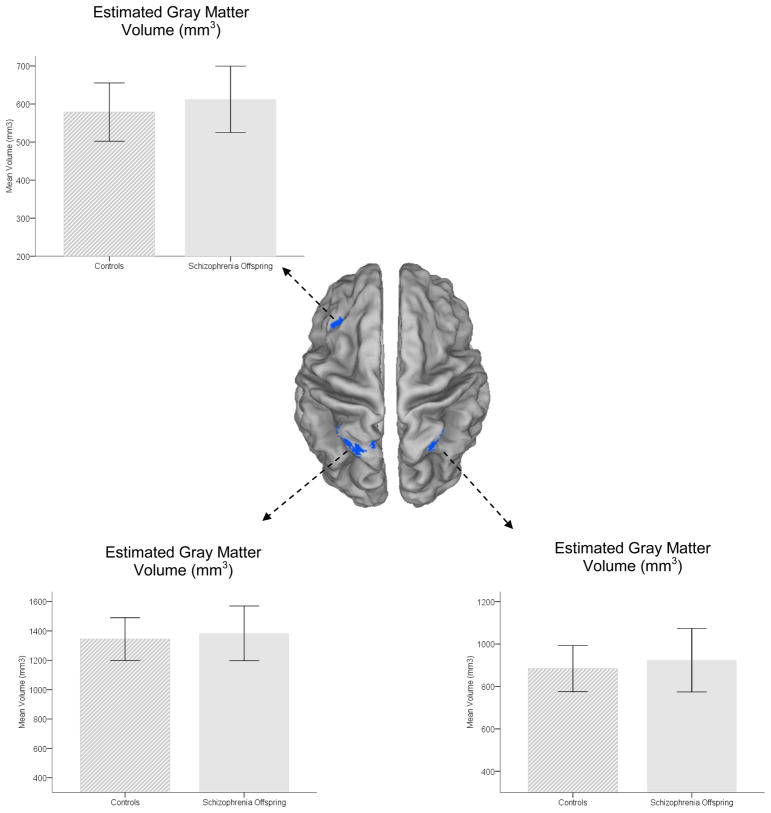
Estimated gray matter (in mm3) for each of the clusters of maximal significance in the three structural regions of interest was extracted using custom designed scripts written in Matlab (MathWorks, 2007). Extracted volumes were submitted to analyses of covariance with group as single factor and age and gender as covariates. Figure 3 depicts estimated gray matter volume in the functional regions of interest (center) drawn from the fMRI results. Graphs depicting gray matter for each of the hypo-active parietal clusters are depicted in right, F1,39=1.28, p>.25, MSe=12665, or left parietal cortex F1,39=.82, p>.35, MSe=18291. Effect sizes (partial η2)(Cohen, 1988) on the main effect were small (η 2=.03 and η 2=.02 respectively). Similarly, analyses of prefrontal data were also negative, F1,39=1.63, p>.2, MSe=6211 with a small effect size (η 2=.04) on the main effect of group. The fMRI, sMRI and behavioral results were unaffected when excluding the HR-S (n=4) with non-exclusionary disorders.
Figure 3. Estimate gray matter under clusters of significance (Figure 2).
Volumetric estimates of gray matter in clusters of maximal difference. Mean estimates of gray matter volume under the significant clusters of interest (depicted on dorsal surface views in the center are depicted. See results for statistical analyses and effect sizes on the main effects. Error bars are ± s.d.
4. Discussion
Using a simple working memory task, we studied the response of the frontal and parietal cortices during working memory (2 back) in HR-S relative to controls. Three principal findings were observed. In general behavioral performance as measured by sensitivity was not compromised in HR-S. fMRI analyses revealed significantly fronto-parietal hypoactivity in HR-S compared to HC suggesting reduced memory-related engagement in offspring compared to controls. The fMRI results confirm previous preliminary fMRI results (Keshavan et al., 2002b). Finally, within clusters of significant fMRI hypo-activity, no significant differences in estimated gray matter volume were observed.
4.1 Activation differences in the schizophrenia diathesis
Early functional studies with PET suggested a pattern of resting as well as task-based “hypo-frontality” that may be related to the pathophysiological effects of the illness on frontal function (Andreasen et al., 1992). However, the fMRI literature on activation differences is characterized by conflicting findings. In general, when performance differences are not accounted for, the engagement of fronto-parietal regions appears to be reduced, leading to a pattern of hypo-activation in schizophrenia and HR-S subjects relative to controls (Broome et al., 2009; Cannon et al., 2005; Keshavan et al., 2002b; Perlstein et al., 2001; Schneider et al., 2007). The direction of activation differences however is affected by considerations of performance. Thus, when fMRI data are compared under conditions of equivalent behavioral performance, schizophrenia is characterized by relative “hyper-frontality” (Jansma et al., 2004; Karlsgodt et al., 2009). These data suggest that working memory implementation in schizophrenia may be characterized by inefficient recruitment, with greater neural resource allocation need to subserve performance levels comparable to controls (Callicott et al., 2003b; Manoach, 2003). A consequent of these results is the suggestion that working memory engagement in the schizophrenia diathesis is not by domain-related, but rather performance-related (Van Snellenberg et al., 2006). The current results accrue from the analyses of block-design data and do not permit the assessment of event-wise changes in activation as a function of performance, though previous event-related analyses have also suggested patterns of hypo-frontality in schizophrenia (Barch et al., 2001).
The literature on individuals with a family relationship to, or clinical risk for, schizophrenia is heterogeneous with studies specifically documenting frontal or parietal dysfunction involving diverse groups and age-ranges. Among these are studies involving adolescent offspring (Diwadkar et al., 2006; Keshavan et al., 2002b), adult siblings (Callicott et al., 2003a; Woodward et al., 2009), and adult or young adult clinically and genetically high risk groups (Crossley et al., 2009; Whalley et al., 2004). In general, the effect sizes of prefrontal impairment based on fMRI studies is moderate to large (Fusar-Poli et al., 2007) and is shared across different vulnerability groups. The specific bases of these differences between groups and controls and the convergence of the results within subjects remain unclear. This may also be because the biological bases of these imaging data are quite different.
4.2 Bases of fMRI and sMRI
fMRI activity measured using the Blood Oxygen Level Dependent (BOLD) effect most closely correlates to local field potential activity in the 10–300 Hz (Logothetis, 2002, 2003) or gamma range (Niessing et al., 2005). This relatively low frequency neural signal corresponds most closely to the synaptic inputs to regions reflecting a weighted average of synchronized “dendrosomatic” components of a neuronal population (Juergens et al., 1999). Findings of reduced prefrontal BOLD response in schizophrenia or vulnerability for schizophrenia are consistent with independent investigations showing reduced frontal gamma band power in the illness (Cho et al., 2006). In general reduced functional activity measured with BOLD may relate to reduced synaptic inputs and coherence resulting from altered prefrontal GABA-ergic neurotransmission (Lewis and Gonzalez-Burgos, 2006). This vulnerability trait (evident in our fMRI data) may be inherited by adolescent offspring, though whether they eventually progress to development or disease may depend on complex processes of development, adaptation or regression (Lewis and Levitt, 2002)
The specific biological correlates of sMRI-related changes in gray matter are less clear. The T1-weighted signal that is used by most investigations of gray matter is related to the degree of MR-visible water; least visible in white matter, intermediately so in gray matter, and most visible in cerebrospinal fluid (Diwadkar and Keshavan, 2002). Segmentation methods parcel sMRI volumes based primarily (though not entirely) on this single measure of signal intensity (Pommert et al., 2002). However, MRI data have neither the resolution not the specificity to explain the relationship between estimated gray matter volume or density and complex cellular processes including but not restricted to, dendritic remodeling, cell death, synaptic pruning, or plausible encroachment from myelination (Toga et al., 2006). The developmental literature has been most sensitive to these shortcomings in assessing the relevance of MR-based findings. In general, the correspondence between MR-documented developmental changes in gray matter (Thompson et al., 2001), and post-mortem investigations of synaptic density and morphology (Huttenlocher, 1979, 1990) have been consistent in documenting heterochronous cortical development. Nevertheless the neural bases of gray matter estimates and of BOLD appear to be independent. Recent evidence corroborates the non-linear or complex nature of this relationship (Kannurpatti et al.). In assessing BOLD variability and its relationship to estimated gray matter, reductions in BOLD with age during motor and digit symbol tasks outpaced reductions in gray matter estimated within the same subjects. Furthermore, correlations between measured BOLD and estimated gray matter volume were sub-linear, with changes in BOLD activation volume being only weakly or unrelated to gray matter. Our results provide a measure of methodological corroboration of these published studies.
4.3 Conclusions
Adolescence remains one of the most neurodevelopmentally active phases in the human lifespan, characterized by both the rapid ascent of cognitive proficiency in multiple domains with an accent on prefrontal development (Case, 1992; Luna et al., 2004), shaping of cortical morphology (Gogtay et al., 2004; Lenroot et al., 2009), and an increase in the connective strength of cortical and sub-cortical regions (Asato et al., 2010). The extended dynamic range of human neurodevelopment is unique across species (Johnson, 2001), providing both opportunities of expansive function and vulnerabilities to disorders when development is derailed (Dahl, 2004). HR-S appear to be characterized by increased vulnerability evidenced from data from several domains, including the incidence of psychopathology (Keshavan et al., 2008), cognitive deficits and MRI-based assessments of brain structure and function. Reduced fronto-parietal engagement during working memory may be a direct expression of latent neurodevelopmental vulnerability in a critical sub-circuit that may be lead to manifest expressions of psychopathology later in young adulthood (Lewis and Levitt, 2002). Our results indicate that latent vulnerabilities can be manifestly present in the function but not in the structure within this circuit. The precise meaning of this discordance is elusive. As noted earlier, studies indicate that the relationship between gray matter measures and fMRI-measured function is unclear, and the biological information conveyed by imaging modalities is complementary. Consequently there may be complementary pathways to vulnerability in HR-S which future studies will need to assess using multiple imaging modalities. Finally, a latent vulnerability in function during adolescence is not predictive of eventual pathology, given the brain’s enormous reserve of adaptive plasticity and ability to reorganize functional networks, particularly in adolescence (Meunier et al., 2009). However, the relatively high frequency of some form of Axis I psychopathology in HR-S suggests that neuroimaging offers significant potential for identifying signatures of vulnerability in the adolescent brain, and identifying emergent vulnerability may be essential for prospective identification of disorders in adolescents.
Acknowledgments
We thank R. Rajarathinem, A. Amirsadri, L. Haddad, A. Jenrow and R. Marciano for assistance in subject recruitment and characterization. We also thank Jeffrey Stanley for helpful discussions, Valentina Gumenyuk, Mark Benton and Serguei Fedorov for assistance in experimental design and programming, and Dalal Khatib for help in data acquisition.
Funding Sources. This research was supported by grants from the National Institute of Mental Health (MH68680) and the Children’s Research of Michigan (CRCM) to VAD, and the Joseph Young Jr. Fund to the Dept of Psychiatry & Behavioral Neuroscience. The funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Conflicts of Interest. None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC, Rezai K, Alliger R, Swayze VW, 2nd, Flaum M, Kirchner P, Cohen G, O'Leary DS. Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia. Assessment with xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry. 1992;49:943–958. doi: 10.1001/archpsyc.1992.01820120031006. [DOI] [PubMed] [Google Scholar]
- Asato MR, Terwilliger R, Woo J, Luna B. White Matter Development in Adolescence: A DTI Study. Cereb Cortex. 2010 doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Oxford University Press; Oxford: 1986. [Google Scholar]
- Bakshi N, Pruitt P, Radwan J, Keshavan MS, Rajan U, Zajac-Benitez C, Diwadkar VA. Inefficiently increased anterior cingulate modulation of cortical systems during working memory in young offspring of schizophrenia patients. J Psychiatr Res. doi: 10.1016/j.jpsychires.2011.01.002. In Press. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, 3rd, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Broome MR, Matthiasson P, Fusar-Poli P, Woolley JB, Johns LC, Tabraham P, Bramon E, Valmaggia L, Williams SC, Brammer MJ, Chitnis X, McGuire PK. Neural correlates of executive function and working memory in the 'at-risk mental state'. Br J Psychiatry. 2009;194:25–33. doi: 10.1192/bjp.bp.107.046789. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Egan MF, Mattay VS, Bertolino A, Bone AD, Verchinksi B, Weinberger DR. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am J Psychiatry. 2003a;160:709–719. doi: 10.1176/appi.ajp.160.4.709. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003b;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Glahn DC, Kim J, Van Erp TG, Karlsgodt K, Cohen MS, Nuechterlein KH, Bava S, Shirinyan D. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Arch Gen Psychiatry. 2005;62:1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- Case R. The role of the frontal lobes in the regulation of cognitive development. Brain and Cognition. 1992;20:51–73. doi: 10.1016/0278-2626(92)90061-p. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, Giedd J, Kaysen D, Hertz-Pannier L, Rapoport JL. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. J Neurophysiol. 2000;83:1550–1566. doi: 10.1152/jn.2000.83.3.1550. [DOI] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for behavioral sciences. 2. Laurence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Fusar-Poli P, Broome MR, Matthiasson P, Johns LC, Bramon E, Valmaggia L, Williams SC, McGuire PK. Superior temporal lobe dysfunction and frontotemporal dysconnectivity in subjects at risk of psychosis and in first-episode psychosis. Hum Brain Mapp. 2009;30:4129–4137. doi: 10.1002/hbm.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann N Y Acad Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Diwadkar V, Sweeney J, Boarts D, Montrose D, Keshavan M. Oculomotor delayed response abnormalities in young offspring and siblings at risk for schizophrenia. CNS Spectrums. 2001;6(11):899–903. doi: 10.1017/s109285290000095x. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Carpenter PA, Just MA. Collaborative activity between parietal and dorso-lateral prefrontal cortex in dynamic spatial working memory revealed by fMRI. Neuroimage. 2000;12:85–99. doi: 10.1006/nimg.2000.0586. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Keshavan MS. Newer techniques in magnetic resonance imaging and their potential for neuropsychiatric research. J Psychosom Res. 2002;53:677–685. doi: 10.1016/s0022-3999(02)00422-1. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Montrose DM, Dworakowski D, Sweeney JA, Keshavan MS. Genetically predisposed offspring with schizotypal features: an ultra high-risk group for schizophrenia? Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:230–238. doi: 10.1016/j.pnpbp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Prasad KM, Keshavan MS. Approaches for adolescents with an affected family member with schizophrenia. Curr Psychiatry Rep. 2004;6:296–302. doi: 10.1007/s11920-004-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin F, Macoveanu J, Olesen P, Tegner J, Klingberg T. Stronger synaptic connectivity as a mechanism behind development of working memory-related brain activity during childhood. J Cogn Neurosci. 2007;19:750–760. doi: 10.1162/jocn.2007.19.5.750. [DOI] [PubMed] [Google Scholar]
- First MD, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured clinical interview for DSM-IV Axis II personality disorders. Biometrics Research Department, NYSPI; New York: 1997. [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Broome MR, Matthiasson P, Woolley JB, Johns LC, Tabraham P, Bramon E, Valmaggia L, Williams SC, McGuire P. Spatial working memory in individuals at high risk for psychosis: Longitudinal fMRI study. Schizophr Res. 2010 doi: 10.1016/j.schres.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Perez J, Broome M, Borgwardt S, Placentino A, Caverzasi E, Cortesi M, Veggiotti P, Politi P, Barale F, McGuire P. Neurofunctional correlates of vulnerability to psychosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2007;31:465–484. doi: 10.1016/j.neubiorev.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex : anatomy, physiology, and neuropsychology of the frontal lobe. 2. Raven Press; New York: 1989. [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry. 1999;46:650–661. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex. Developmental changes and effects of aging. Brain Res. 1979;163:195. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, van der Wee NJ, Kahn RS. Working memory capacity in schizophrenia: a parametric fMRI study. Schizophr Res. 2004;68:159–171. doi: 10.1016/S0920-9964(03)00127-0. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in humans. Nat Rev Neurosci. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Juergens E, Guettler A, Eckhorn R. Visual stimulation elicits locked and induced gamma oscillations in monkey intracortical- and EEG-potentials, but not in human EEG. Exp Brain Res. 1999;129:247–259. doi: 10.1007/s002210050895. [DOI] [PubMed] [Google Scholar]
- Kannurpatti SS, Motes MA, Rypma B, Biswal BB. Neural and vascular variability and the fMRI-BOLD response in normal aging. Magn Reson Imaging. doi: 10.1016/j.mri.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Sanz J, van Erp TG, Bearden CE, Nuechterlein KH, Cannon TD. Re-evaluating dorsolateral prefrontal cortex activation during working memory in schizophrenia. Schizophr Res. 2009;108:143–150. doi: 10.1016/j.schres.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Keshavan M, Montrose DM, Rajarethinam R, Diwadkar V, Prasad K, Sweeney JA. Psychopathology among offspring of parents with schizophrenia: relationship to premorbid impairments. Schizophr Res. 2008;103:114–120. doi: 10.1016/j.schres.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Dick E, Mankowski I, Harenski K, Montrose DM, Diwadkar V, DeBellis M. Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophr Res. 2002a;58:173–183. doi: 10.1016/s0920-9964(01)00404-2. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Spencer SM, Harenski KA, Luna B, Sweeney JA. A preliminary functional magnetic resonance imaging study in offspring of schizophrenic parents. Prog Neuropsychopharmacol Biol Psychiatry. 2002b;26:1143–1149. doi: 10.1016/s0278-5846(02)00249-x. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, Kendler KS, Evans AC, Giedd JN. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum Brain Mapp. 2009;30:163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos Trans R Soc Lond B Biol Sci. 2002;357:1003–1037. doi: 10.1098/rstb.2002.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A Users Guide. Lawrence Erlbaum Associates; Mahwah, NJ: 2005. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- MathWorks I. Matlab. The MathWorks, Inc; Natick, MA: 2007. [Google Scholar]
- Meunier D, Achard S, Morcom A, Bullmore E. Age-related changes in modular organization of human brain functional networks. Neuroimage. 2009;44:715–723. doi: 10.1016/j.neuroimage.2008.09.062. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, McFarlane W, Perkins DO, Pearlson GD, Woods SW. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, Woods SW. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Pommert A, Tiede U, Hohne KR. Volume visualization. In: Toga AW, Mazziotta JC, editors. Brain Mapping: The Methods. Academic Press; New York: 2002. pp. 707–723. [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JD. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Reske M, Kellermann T, Stocker T, Shah NJ, Zilles K, Braus DF, Schmitt A, Schlosser R, Wagner M, Frommann I, Kircher T, Rapp A, Meisenzahl E, Ufer S, Ruhrmann S, Thienel R, Sauer H, Henn FA, Gaebel W. Neural correlates of working memory dysfunction in first-episode schizophrenia patients: an fMRI multi-center study. Schizophr Res. 2007;89:198–210. doi: 10.1016/j.schres.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, Toga AW, Rapoport JL. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snellenberg JX, Torres IJ, Thornton AE. Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychology. 2006;20:497–510. doi: 10.1037/0894-4105.20.5.497. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. Medical College of Wisconsin; Milwaukee, WI: 2000. [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Simonotto E, Flett S, Marshall I, Ebmeier KP, Owens DG, Goddard NH, Johnstone EC, Lawrie SM. fMRI correlates of state and trait effects in subjects at genetically enhanced risk of schizophrenia. Brain. 2004;127:478–490. doi: 10.1093/brain/awh070. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Waldie B, Rogers B, Tibbo P, Seres P, Purdon SE. Abnormal prefrontal cortical activity and connectivity during response selection in first episode psychosis, chronic schizophrenia, and unaffected siblings of individuals with schizophrenia. Schizophr Res. 2009;109:182–190. doi: 10.1016/j.schres.2008.11.028. [DOI] [PubMed] [Google Scholar]