Abstract
Background
Estuaries and coastal lakes receive little attention despite being heavily invaded by non-indigenous invasive species (NIS). In these situations, studies of population dynamics in invaded habitats can provide valuable insights into how NIS interact with new environments. Tarebia granifera is a prosobranch gastropod from south-east Asia which has invaded other sub-tropical parts of the world. This study addresses whether a small number of key environmental factors influences gastropod communities, and specifically how the population density and size structure of T. granifera were influenced by environmental change in estuaries and coastal lakes in southern Africa.
Methodology/Principal Findings
T. granifera's density, number of brooded juveniles and size structure were measured at the St. Lucia Estuary, Mgobozeleni Estuary, Lake Sibaya and Lake Nhlange. Size structure was classified according to shell height (SH). All dissected individuals were found to be female and free from trematode infection. Salinity, water depth, temperature, and pH were the main factors correlated with population density of gastropod communities. T. granifera often reached densities well over 1000 ind. m−2, displacing indigenous gastropods and becoming a dominant component of the benthic community. T. granifera successfully invaded estuaries despite frequent exposure to high salinity and desiccation, which could together eliminate >97% of the population. The persistence of T. granifera was ensured due to its high fecundity and the environmental tolerance of large adults (20–30 mm SH) which carried an average of 158±12.8 SD brooded juveniles. Repeat introductions were not essential for the success of this parthenogenetic NIS.
Conclusion/Significance
There is a need for a broader study on the reproductive biology of T. granifera (including the previously overlooked “brood pouch ecology”), which affects population dynamics and may be relevant to other parthenogenetic NIS, such as Melanoides tuberculata and Potamopyrgus antipodarum.
Introduction
Although asexual reproduction can be found in numerous organisms, it is rare to find obligate parthenogenetic taxa, in which single organisms can only reproduce by producing genetically identical offspring [1]. Some gastropods show patterns of geographic parthenogenesis, where asexual populations occupy different habitats to populations that reproduce sexually [2], [3]. Parthenogenetic populations may have several advantages for establishment in new and variable habitats: a single parthenogenetic organism can start a population [3]–[6]; the genotype is isolated from gene flow and adaptations to the new habitat, especially in “general purpose genotypes”, can thus not be broken by recombination (see frozen - niche variation [7]); in habitats where populations undergo frequent local extinction and recolonization events, genetic bottleneck and drift effects will have less negative fitness consequences for asexual populations [8]; also, according to the Red Queen Hypothesis, reduced biotic interactions with parasites and predators favor asexual populations (see [9], but also [10]).
The geographic patterns and rates of species' invasion are changing on an unprecedented scale due to direct and indirect anthropogenic action [11]. Non-indigenous invasive species (NIS) are a serious threat to biodiversity [12], particularly in estuarine and coastal environments [13], [14]. Fortunately, relatively few introduced species are successful in establishing populations, fewer go on to spread and fewer still become pests [15]. Chance plays a role in the invasion success of all NIS and there can be many repeated attempts before an invasion is successful [12], [16]. However, certain biological and ecological characteristics are thought to increase the probability of invasion [17]. In this context, it is not surprising that an introduced parthenogenetic species pre-adapted to colonize marginal habitats with wide physiological tolerance, high fecundity and very high population densities would also make a successful NIS [18].
Tarebia granifera is a prosobranch gastropod (Thiaridae) originally from south-east Asia. This parthenogenetic species has a brood pouch and gives birth to fully developed juveniles. T. granifera has high fecundity and has been reported to reach densities over 20 000 ind. m−2 [19]. It has invaded several sub-tropical parts of the world, including Texas, Hawaii, Caribbean islands, Mexico and Israel [20]–[23]. In South Africa, T. granifera has invaded an increasing number of estuaries and coastal lakes over the past decade [19]. The species is regarded as a freshwater dweller, but its recent invasion patterns [19], a physiological tolerance study [24] and a strontium isotope (87Sr/86Sr) study of fossils dating back 1.5 million years [25] suggest that this species is pre-adapted to brackish environments. The shallow marginal habitats of coastal lakes and estuaries can be extremely variable environments [26], [27]. Stochastic events involving changes in water level and salinity have been observed to repeatedly wipe out most of the T. granifera population and yet, this NIS not only persists but often becomes a dominant component of the shallow-water benthos (pers. obs.).
This study aimed to address two questions: 1) is gastropod community structure influenced by a small number of key environmental factors? 2) What are the longer term effects of environmental change on T. granifera within a variable estuarine setting? The size structure of T. granifera (in terms of shell height size classes) has previously only been described for freshwater bodies and in the laboratory. Differences in population density and size structure over time and under different environmental conditions revealed how T. granifera populations persisted during unfavorable periods and then recovered. T. granifera's reproductive output was tentatively assessed in terms of number of unborn juveniles in the brood pouch.
Materials and Methods
Ethics Statement
Permission for this study was granted under a Research Agreement with the iSimangaliso Wetland Park Authority for the project titled “Climate Change and the Management of KZN estuaries: St Lucia Estuary”.
Study site
The St. Lucia Estuary is the largest estuarine lake in Africa, with a surface area of ≈325 km2 and average depth of 0.9 m [28]. Recently, this estuary has been experiencing unprecedented low water levels and the mouth has been closed for the most part from 2002 to present. There is a reversed salinity gradient and salinities over five times higher than seawater were recorded in the most northerly parts of the system [29]. However, areas such as the eastern shores of South Lake, receive a considerable input of freshwater from sand dune aquifers [30]. Samples were collected at Catalina Bay (28°13′S, 32°29′E), on the eastern shores of South Lake (Fig. 1). In March 2007, the St. Lucia Estuary mouth breached and seawater from the Indian Ocean entered the system [29], increasing water levels and introducing a number of marine species, including the sea hare Stylocheilus striatus (Fig. 2).
Figure 1. Map of Maputaland.
The Kosi Lakes, Lake Sibaya and the St. Lucia Estuary are Ramsar Wetlands of International Importance within the iSimangaliso Wetland Park, a UNESCO World Heritage Site in northern KwaZulu-Natal, South Africa.
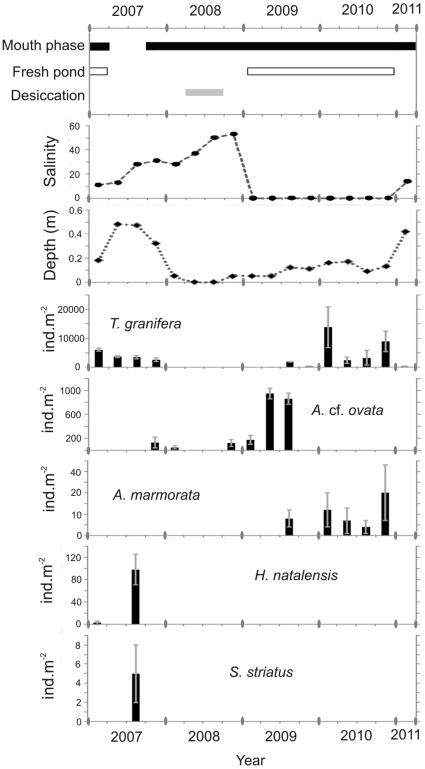
Figure 2. Gastropod population densities (± SD) under changing salinities and depths.
Data were collected at Catalina Bay, in the St. Lucia Estuary from 2007 to 2011 at quarterly intervals. The black horizontal bar represents a closed mouth phase, the white bar represents the presence of a freshwater pond, and the grey bar represents the desiccation of most of the sampling area. Species: Tarebia granifera, Assiminea cf. ovata, Aplexa marmorata, Haminoea natalensis and Stylocheilus striatus.
Mgobozeleni Estuary (27°32′S, 32°40′E; Fig. 1) has a surface area of 0.014 km2, maximum width of 25 m and average depth of 0.3 m [31]. This estuary is supplied with freshwater from Lake Mgobozeleni and is strongly influenced by tidal regimes [32]. It is a typical Temporarily Open/Closed Estuary (TOCE, Perissinotto et al. 2010) and a combination of low rainfall and spring tide may result in the periodical closure of the mouth [33].
Lake Sibaya has a surface area of 60 to 77 km2 and an average depth of 13 m [34]. This land-locked freshwater lake currently undergoes wide fluctuations in water level [34]. Samples were collected on the eastern shores (27°22′S, 32°42′E; Fig. 1).
Lake Nhlange (26°57′S, 32°49′E) is the largest of the Kosi Lakes (Fig. 1). The surface area of this lake varies from 30.7 to 37 km2 and the average depth is 7.2 m [35]. Lake Nhlange is connected to the ocean via channels and other lakes but its salinity is low [35]. Samples were collected on the western shores.
Sampling procedure
The St. Lucia Estuary was surveyed at quarterly intervals between February 2007 and March 2011; Mgobozeleni Estuary, Lake Sibaya and Lake Nhlange were surveyed during the wet seasons in 2009 and 2010 (Table S1).
Physical and chemical parameters
All samples were taken in shallow marginal habitats (<2 m depth). Salinity, dissolved oxygen, pH and temperature were measured with a YSI 6920 multiprobe. Sub-surface water samples were sieved through a GF/F filter and the supernatant was analysed for nitrates and phosphates with a Skalar SAN++ continuous flow nutrient analyzer. Sediment samples were dried and weighed before being sieved through a 2000 µm sieve. The finer sediment was analysed by a Malvern Analyser. The median sediment particle size was then calculated by taking into account the weighted sediment retained on the sieve.
Gastropods
Triplicate macrofauna samples were taken with a Zabalocki-type Ekman grab (area = 0.0236 m2). Immediately after collection, samples were washed through a 500 µm sieve and the material retained was preserved in 5% formaldehyde solution. In the laboratory, gastropods were sorted from each sample and counted in order to determine density (ind. m2). Tarebia granifera shell height (SH) was measured with a Vernier caliper to the nearest 0.01 mm. Each specimen was inspected for shell damage and if the first whorls were missing, its (damaged) shell height was multiplied by the ratio of average SH/average height of the last whorl of unaffected (undamaged) specimens belonging to that population and appropriate size class [36]. Thus, the corrected SH was used in spatial and temporal comparisons. The gastropods were sorted into one of the following 10 size classes according to (corrected) SH: <1 mm; 1–5.99 mm; 6–7.99 mm; 8–9.99 mm; 10–11.99 mm; 12–13.99 mm; 14–15.99 mm; 16–17.99 mm; 18–19.99 mm; 20–30 mm. No less than five individuals belonging to each size class were dissected. The gonads were inspected to determine sex and presence of trematode parasites. The brood pouch of adult specimens (6–30 mm SH) was carefully dissected and the shelled juveniles contained inside were counted.
Data analysis
Analysis of covariance (ANCOVA) using size class as a covariate was used to assess differences in average number of unborn juveniles per brood pouch between sampling events, and between locations. A two-way analysis of variance (ANOVA) was used to determine if T. granifera densities differed temporally (in terms of year×season) at Catalina Bay, St. Lucia Estuary. An ANOVA and Tukey's Honestly Significant Difference (HSD) multiple comparisons were conducted to determine if T. granifera densities differed between locations. The relationship between shell height and number of juveniles in brood pouch was analysed with Pearson's correlation. All data were log-transformed to meet normality requirements. The statistical package PASW version 18 for Windows was used.
A canonical correspondence analysis was conducted between log-transformed gastropod (8 species, Fig. 3) abundance data and standardized environmental data (depth, salinity, temperature, dissolved oxygen, pH, median sediment particle size, turbidity, nitrates and phosphates) collected at the study site between 2007 and 2010. A forward selection model was used to determine the four environmental variables which best explained the variation in density data of gastropod species. The CANOCO software version 4.5 was used for this purpose.
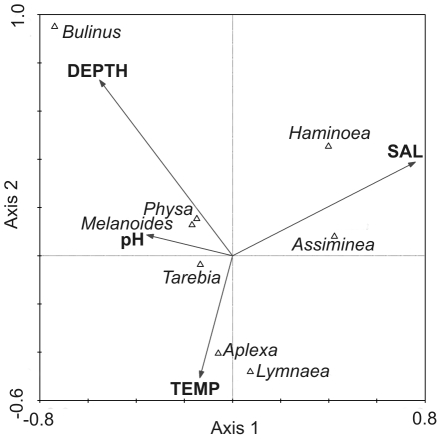
Figure 3. Maputaland gastropod population densities correlated with physical and chemical parameters.
First and second axes of a canonical correspondence analysis performed on gastropod population densities and physical and chemical parameters measured at Lake Nhlange, Lake Sibaya, Mgobozeleni Estuary and St. Lucia Estuary in northern KwaZulu-Natal, South Africa. Data were collected from 2007 to 2011at quarterly intervals. Physical and chemical parameter vectors: salinity (SAL), depth, water temperature (TEMP) and pH. Species: Tarebia granifera (Tarebia), Melanoides tuberculata (Melanoides), Physa acuta (Physa), Assiminea cf. ovata (Assiminea), Haminoea natalensis (Haminoea), Aplexa marmorata (Aplexa), Lymnaea natalensis (Lymnaea) and Bulinus natalensis (Bulinus).
Results
Canonical correspondence analysis
The canonical correspondence analysis showed that the combination of salinity, depth, temperature and pH explains 78% of the variation in the abundance data of gastropod species (Table 1). The first two axes together explain 58.3% of total variability in species abundance data (Table 1).
Table 1. Canonical correspondence analysis performed on abundances of Maputaland gastropods and physical and chemical parameters.
| Axis 1 | Axis 2 | Axis 3 | ||
| Eigenvalues | 0.392 | 0.191 | 0.146 | |
| Cumulative percentage: | of species data | 20.1 | 29.9 | 37.3 |
| species-environment relation | 50.3 | 74.8 | 93.5 | |
| Species-environment correlation | 0.82 | 0.75 | 0.656 | |
| Component loadings: | Depth | −0.628 | 0.737 | −0.223 |
| Salinity | 0.803 | 0.384 | −0.298 | |
| Temperature | −0.190 | −0.501 | −0.262 | |
| pH | −0.343 | 0.085 | 0.636 |
Population densities
In the St. Lucia Estuary, an increase in salinity was associated with increases in density of indigenous gastropods such as Haminoea natalensis and Assiminea cf. ovata and the decline of Tarebia granifera (Fig. 2). The mouth was only open for 6 months, and in 2008 water levels dropped dramatically, leaving vast areas dry. In 2009, freshwater pooled on the eastern shores of the South Lake where non-indigenous T. granifera as well as Aplexa marmorata were found (Fig. 2).
T. granifera population density was not significantly different in terms of year×season at Catalina Bay (Two-way ANOVA: F 2113 = 1.051, P>0.05). T. granifera density was however significantly different between locations (Catalina Bay, Mgobozeleni Estuary, Lake Sibaya and Lake Nhlange, 2007–2010 wet seasons only) (ANOVA: F 3,31 = 30.359, P<0.05). T. granifera densities at the freshwater Sibaya and Nhlange lakes were significantly different from densities at the brackish St. Lucia and Mgobozeleni Estuaries (Tukey's HSD: P<0.05).
Size classes and juveniles in brood pouch
All T. granifera found in this study were female. An inspection of the gonads and digestive gland also revealed no trematode infections. Specimens with shell height smaller than 6 mm had under-developed brood pouches, which did not contain shelled juveniles. Variable sizes of juveniles were found within the brood pouch.
The shells of T. granifera were most severely damaged at Catalina Bay, where in 2009 and 2010 on average 78% of the population showed signs of shell erosion and SH was reduced by an average of 12.3%. The damage was concentrated in the size classes 6–13.99 mm SH.
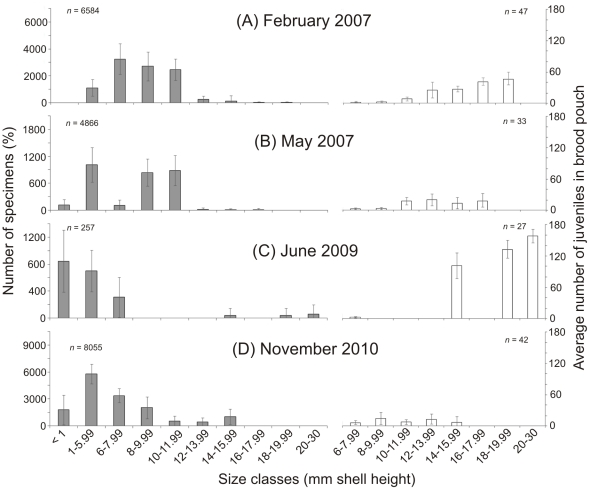
At Catalina Bay in February 2007, the distribution of the T. granifera population was unimodal, with size class 6–7.99 mm SH being the most common and contributing 32.7%±11 SD of the total (Fig. 4A). Each adult of 6–7.99 mm SH carried on average 1.3±0.6 SD juveniles, whereas each adult of 18–19.99 mm SH carried 48.6±12.1 SD juveniles in its brood pouch (Fig. 4a). In May 2007, after mouth breach and under salinity≈28 (Fig. 2, Table S1), the T. granifera population appeared to have a bimodal distribution and the size class 6–7.99 mm SH was poorly represented (Fig. 4B). The largest size class represented was 16–17.9 mm SH and each snail in this class carried an average of 18±13.4 SD juveniles in its brood pouch. In 2008 there were no T. granifera recorded at the seepage area of Catalina Bay, which dried out completely resulting in the T. granifera population being reduced by >97%. In June 2009, seepage water accumulated to form a freshwater pond independent of the South Lake. Large T. granifera (14–30 mm SH) were found making conspicuous trails on the sediment surface and the largest (20–30 mm SH) carried on average 158±12.8 SD juveniles in the brood pouch (Fig. 4C). 77.3%±22.8 SD of the total population was composed of juveniles (Fig. 4C, Table S1). In November 2010, 50.5%±18.1 SD of the total population was composed of juveniles at Catalina Bay (Fig. 4D).
Figure 4. Tarebia granifera size structure over time.
Size class (mm shell height) distribution of T. granifera specimens (percentages ± SD) and average number of unborn juveniles (± SD) per adult size classes (6–30 mm shell height) collected by triplicate Ekman grab at Catalina Bay, in the St. Lucia Estuary during (A) February 2007, (B) May 2007, (C) June 2009 and (D) November 2010.
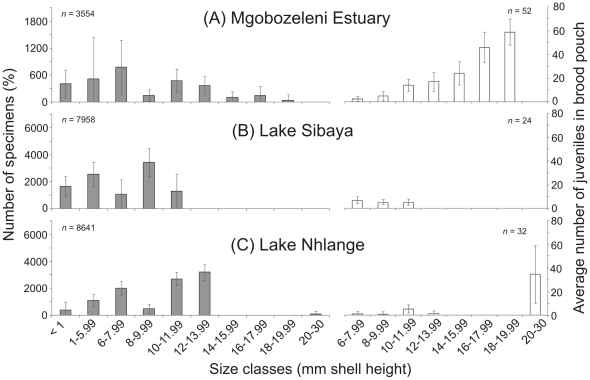
At the Mgobozeleni Estuary, juveniles contributed 30.9%±40.3 SD and adults ranged from 6 to 19.99 mm SH (Fig. 5A). At Lake Sibaya, the largest adults found on the open terraces of the eastern shores were 10–11.9 mm SH and carried on average 4±1.3 SD juveniles in the brood pouch (Fig. 5B). At Lake Nhlange, 59%±10.3 SD of the population was composed of size classes 10–13.99 mm SH in November 2010 (Fig. 5C). The largest adults (20–30 mm SH) carried 35±24 SD juveniles in the brood pouch (Fig. 5C).
Figure 5. Tarebia granifera size structure at different locations.
Size class (mm shell height) distribution of T. granifera specimens (percentages ± SD) and average numbers of unborn shelled juveniles (± SD) per adult size class (6–30 mm shell height) collected by triplicate Ekman grab in November 2010 at (A), Mgobozeleni Estuary (B) Lake Sibaya and (C) Lake Nhlange.
T. granifera shell height and number of unborn juveniles in brood pouch were positively correlated (Pearson's correlation coefficient: r = 0.806, P<0.01, n = 186). There was no significant difference in average number of unborn juveniles per brood pouch between sampling events at Catalina Bay (ANCOVA: F 11,59 = 1.626, P = 0.115). There was also no significant difference in average number of unborn juveniles per brood pouch between locations (ANCOVA: F 3,57 = 1.174, P = 0.328).
Discussion
Tarebia granifera population densities were often well over 1000 ind. m−2 regardless of location, making this NIS a dominant component of the local invertebrate macrofauna. However, at the St. Lucia and Mgobozeleni estuaries, population densities were variable, whereas at the Sibaya and Nhlange coastal lakes the T. granifera populations appeared relatively stable (Table S1). South African estuaries are particularly variable environments in terms of salinity and water depth and these factors can change unpredictably and directly influence macrofauna [27]. As expected for any benthic invertebrate, the spatial distribution of T. granifera was heterogeneous [37], [38], [39]. In general, shallow water (depth<2 m) freshwater sources and sheltered bays with organic deposits seemed to be favored. Extreme changes in salinity and water depth, which affected the populations of all gastropod species, were particularly evident at the St. Lucia Estuary during the study period (Fig. 2). Yet the highest T. granifera population densities were recorded at Catalina Bay (Table S1). Indeed, it has been suggested that the high densities of T. granifera may minimize the risk of extirpation under harsh conditions [10].
Salinity, water depth, temperature and pH were identified as the four main factors associated with the population density of the dominant gastropod species currently found in the estuaries and coastal lakes of Maputaland (Fig. 3, Table 1). Most gastropods appear to be associated with a specific set of environmental conditions. For instance, Bulinus natalensis was associated with deeper water, whereas Aplexa marmorata and Lymnaea natalensis were associated with very shallow and warm waters (Fig. 3). Haminoea natalensis and Assiminea cf. ovata were the only species associated with high salinity (Fig. 3). However, in comparison to other gastropods, T. granifera tended to be least associated with any one environmental factor (Fig. 3) and was the most widespread and abundant gastropod in all study areas.
The T. granifera population density did not appear to undergo seasonal patterns and year-round births were recorded. The average number of juveniles per brood pouch, which was assumed to indicate reproductive output, did appear to increase when the salinity suddenly increased in the St. Lucia Estuary in 2007 and also when the population was recovering in 2009 (Table S1). An increase in average number of juveniles per brood pouch was also measured during T. granifera's recovery at the Mgobozeleni Estuary. T. granifera may increase its reproductive output in response to disturbances, such as sudden salinity increases that negatively affects the population, therefore accelerating its recovery. However, size classes were not taken into account in this interpretation.
The structure of the T. granifera population was defined in terms of its proportional contribution to different shell height size classes. T. granifera's shell height (SH) ranged from >1 mm to 28.75 mm. The largest specimens were recorded at the St. Lucia Estuary. T. granifera adults collected in Lake Nhlange and particularly at Lake Sibaya tended to be small (Fig. 5B), although larger specimens were found in sheltered and eutrophic bays. The size of specimens may have been influenced by food availability and quality [36], [40]. Both Lake Nhlange and Sibaya are nutrient poor and other species have been reported to have unusually smaller sizes [41]. In contrast, the St. Lucia Estuary has been reported to have very high levels of accumulated microphytobenthos that T. granifera can feed on [42], [43]. T. granifera maturity in this study was reached between 6 and 7.99 mm SH [36]. The erosion of T. granifera shells at Catalina Bay was most likely caused by low pH and abrasive effect of sand particles. Interstitial pore water at Catalina Bay, which seeped from sand dune aquifers, had a pH≈6.3. T. granifera populations are sensitive to low pH [36], [44]. Lake Bhangazi South [45] (Fig. 1) and surrounding small streams tend to have low pH and this may partially explain why the T. granifera invasion has not taken hold in those habitats (pers. obs.).
T. granifera size classes were clearly affected by stochastic events in different ways. Size classes between >1 and 7.99 mm SH seemed particularly vulnerable to the sudden increase in salinity during the 2007 mouth breach of St. Lucia (Fig. 4A–B). Yet a higher proportion of juveniles was recorded during that period (Fig. 4A–B) and the juvenile to adult ratio also increased (Table S1), indicating that birth rate did not slow down. The ensuing desiccation in 2008 killed most of the population, with only the largest adults able to survive such extreme conditions. This was at least in part due to their tolerance to desiccation [46], ability to burrow and undergo periods of quiescence [20], but also because large T. granifera probably took shelter in freshwater seeps. In comparison, smaller and juvenile snails are less tolerant to desiccation [46] and also move over smaller spatial ranges [37], therefore they were not able to reach freshwater seeps and survive. Once favourable conditions were re-established in 2009, adults returned and gave birth to large numbers of juveniles, thus quickly increasing the juvenile to adult ratio. These larger adults carried a great number of juveniles in their brood pouch, thus contributing to the perceived high reproductive output during the recovery period of 2009 (Fig. 4C, Table S1). By the end of 2010, the Catalina Bay population had recovered and its structure and density was comparable to those of other T. granifera populations in Maputaland.
The evolutionary significance of brooding may be unclear [47]. However, in T. granifera, brooding is associated with increased parental care, which minimizes mortality of vulnerable early life stages. The anatomy of the brood pouch of T. granifera is similar to that of M. tuberculata [36], [48] and the larger the animal, the greater the number of juveniles it can carry. The current study also found a variety of sizes of juveniles in the brood pouch. This suggests that juveniles may be retained during adverse conditions and raises the question of whether the number of juveniles present in a brood pouch is an adequate indicator of fecundity. Under salinities higher than 20, an adult can shift its energy from reproduction to survival [49], [50], which involves entering a quiescent state [24]. Therefore, an increase in number of juveniles in brood immediately after an increase in salinity may be due to retention, rather than increase in reproduction rate. The increase in the ratio of juveniles to adults during adverse conditions could then be explained by the release of brooded juveniles after the death of adults and/or births by large and more tolerant adults. An empirical study is needed to address this hypothesis since many factors affect birth [20].
Propagule pressure can be described in terms of quantity of released propagules, the quality of the propagules and the quantity of release events [51]. T. granifera reaches high population densities and a single adult with several brooded juveniles may start a new population. The brood pouch plays an important role in the internal cycle of propagule production and dispersal [11], which ensures T. granifera persistence and spread even if there is no further input from an external propagule pool. Juveniles grow within the protection of the brood pouch, thus increasing their chance of survival and reducing the time to maturity after birth. This may ensure year-round continuity in T. granifera reproductive output, despite unfavorable and unpredictable events.
T. granifera is known to displace indigenous gastropods in freshwater [19], [22], [23] but its ecological impacts in brackish water are largely unknown. However, ecological impact on estuaries and coastal lakes is difficult to assess because of unprecedented overlaps and interactions between NIS and other stressors [52] such as drought intensification. It would have been very useful to make a comparison of T. granifera size structure with that of indigenous Melanoides tuberculata and Bellamya capillata at Lake Sibaya, since all three species reproduce via parthenogenesis [53]. Unfortunately though, the historically abundant native snails were not found during this study, having possibly been displaced by T. granifera. However, it is likely that they persist in deeper water or in parts of Lake Sibaya that were not surveyed. T. granifera has been reported to displace M. tuberculata under natural settings [23], [54]. However, at Lake Nhlange, T. granifera and M. tuberculata have been found together in a small sheltered and eutrophic bay, but T. granifera was more numerous.
An understanding of the population dynamics of NIS is important for predicting their interaction with the environment and determining the best control strategy [55]. Already invaded habitats with reduced biotic influences can present an opportunity to gain insights into how NIS interact with the environment. This study has revealed how T. granifera's population density and structure can change in variable estuarine and coastal environments. High densities, fecundity, and particularly the environmental tolerance of adults with brooded juveniles, ensure the persistence of T. granifera despite frequent mouth breaching events and desiccation. The mode of reproduction and the type of embryo development affect population dynamics. However, the ecology of the post-larval and pre-birth stage in the life history of T. granifera, which takes place within its brood pouch, needs further consideration. It is suggested that this brood pouch ecology plays a very significant role in the establishment and spread of T. granifera, thus also affecting population dynamics. These findings are also relevant to invasions by other parthenogenetic NIS, such as M. tuberculata and P. antipodarum, across the world.
Supporting Information
Summary of physical and chemical parameters, and Tarebia granifera populations in Maputaland estuaries and coastal lakes.
(DOC)
Acknowledgments
We are grateful to the iSimangaliso Park Authority and Ezemvelo KZN Wildlife for supporting this project. Special thanks go to R. Taylor, C. Fox and R. Kyle for their invaluable assistance and logistical support. We are also grateful to two anonymous reviewers for their constructive suggestions and contribution to the final manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This project was funded by the National Research Foundation, South Africa (www.nrf.ac.za); Marine and Coastal Management (DEAT-MCM, www.mcm-deat.gov.za); and the World Wide Fund (WWF, www.wwf.org.za). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Vrijenhoek RC. Animal clones and diversity. Bio Science. 1998;48:617–628. [Google Scholar]
- 2.Jacobsen R, Forbes VE. Clonal variation in life-history traits and feeding rates in the gastropod, Potamopyrgus antipodarum: performance across a salinity gradient. Funct Ecol. 1997;11:260–267. [Google Scholar]
- 3.Ben-Ami F, Heller J. Temporal patterns of geographic parthenogenesis in a freshwater snail. Biol J Linn Soc. 2007;91:711–718. [Google Scholar]
- 4.Maynard-Smith J. What use is sex? J Theor Biol. 1971;30:319–335. doi: 10.1016/0022-5193(71)90058-0. [DOI] [PubMed] [Google Scholar]
- 5.Gerritsen J. Sex and parthenogenesis in sparse populations. Amer Nat. 1980;115:718–742. [Google Scholar]
- 6.Jokela J, Lively CM, Dybdahl MF, Fox JA. Evidence for a cost of sex in the freshwater snail Potamopyrgus antipodarum. Ecology. 1997;78:452–460. [Google Scholar]
- 7.Vrijenhoek RC. Factors affecting clonal diversity and coexistence. Amer Zool. 1979;19:787–797. [Google Scholar]
- 8.Haag CR, Ebert D. A new hypothesis to explain geographic parthenogenesis. Ann Zool Fenn. 2004;41:539–544. [Google Scholar]
- 9.Lively CM. Trematode infection and the distribution and dynamics of parthenogenetic snail populations. Parasitology. 2001;123:19–26. doi: 10.1017/s0031182001008113. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Ami F, Heller J. Spatial and temporal patterns of parthenogenesis and parasitism in the freshwater snail Melanoides tuberculata. J Evolution Biol. 2005;18:138–146. doi: 10.1111/j.1420-9101.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- 11.Davis MA. Invasion Biology. New York Oxford University Press; 2009. [Google Scholar]
- 12.Williamson M. Biological Invasions. London: Chapman & Hall; 1996. [Google Scholar]
- 13.Grosholz E. Ecological and evolutionary consequences of coastal invasions. Trends Ecol Evol. 2002;17:22–27. [Google Scholar]
- 14.Williams S, Grosholz E. The invasive species challenge in estuarine and coastal environments: marrying management and science. Estuar Coast. 2008;31:3–20. [Google Scholar]
- 15.Miller AW, Ruiz GM, Minton MS, Ambrose RF. Differentiating successful and failed molluscan invaders in estuarine ecosystems. Mar Ecol Prog Ser. 2007;332:41–51. [Google Scholar]
- 16.Caley P, Lonsdale WM, Pheloung PC. Quantifying uncertainty in predictions of invasiveness, with emphasis on weed risk assessment. Biol Invasions. 2006;8:1595–1604. [Google Scholar]
- 17.Kolar CS, Lodge DM. Progress in invasion biology: predicting invaders. Trends Ecol Evol. 2001;16:199–204. doi: 10.1016/s0169-5347(01)02101-2. [DOI] [PubMed] [Google Scholar]
- 18.Jones JPG, Rasamy JR, Harvey A, Toon A, Oidtmann B, et al. The perfect invader: a parthenogenic crayfish poses a new threat to Madagascar's freshwater biodiversity. Biol Invasions. 2009;11:1475–1482. [Google Scholar]
- 19.Appleton CC, Forbes AT, Demetriades NT. The occurrence, bionomics and potential impacts of the invasive freshwater snail Tarebia granifera (Lamarck, 1822) (Gastropoda: Thiaridae) in South Africa. Zool Med Leiden. 2009;83:525–536. [Google Scholar]
- 20.Chaniotis BN, Butler JM, Ferguson F, Jobin WR. Bionomics of Tarebia granifera (Gastropoda:Thiaridae) in Puerto Rico, an Asian vector of Paragonimiasis westermani. Caribbean J Sci. 1980;16:81–89. [Google Scholar]
- 21.Pointier JP, Facon B, Jarne P, David P. Thiarid snails, invading gastropods of tropical freshwater habitats. Xenophora. 2003;104:14–20. [Google Scholar]
- 22.Karatayev AY, Burlakova LE, Karatayev VA, Padilla DK. Introduction, distribution, spread, and impacts of exotic freshwater gastropods in Texas. Hydrobiologia. 2009;619:181–194. [Google Scholar]
- 23.López-López E, Sedeño-Díaz JE, Vega PT, Oliveros E. Invasive mollusks Tarebia granifera Lamarck, 1822 and Corbicula fluminea Müller, 1774 in the Tuxpam and Tecolutla rivers, Mexico: spatial and seasonal distribution patterns. Aquat Invasions. 2009;4:435–450. [Google Scholar]
- 24.Miranda NAF, Perissinotto R, Appleton CC. Salinity and temperature tolerance of the invasive freshwater gastropod Tarebia granifera. S Afr J Sci. 2010;106:55–61. [Google Scholar]
- 25.Joordens JCA, Wesselingh FP, de Vos J, Vonhof HB, Kroon D. Relevance of aquatic environments for hominins: a case study from Trinil (Java, Indonesia). J Hum Evol. 2009;57:656–671. doi: 10.1016/j.jhevol.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann H, Lorke A, Peeters F. Temporal scales of water-level fluctuations in lakes and their ecological implications. Hydrobiologia. 2008;613:85–96. [Google Scholar]
- 27.Perissinotto R, Stretch DD, Whitfield AK, Adams JB, Forbes AT, et al. Temporarily Open/Closed Estuaries in South Africa. New York: Nova Publishers; 2010. [Google Scholar]
- 28.Taylor R, Adams JB, Haldorsen S. Primary habitats of the St Lucia Estuarine System, South Africa, and their responses to mouth management. Afr J Aquat Sci. 2006;31:31–41. [Google Scholar]
- 29.Bate GC, Taylor RH. Sediment salt-load in the St Lucia Estuary during the severe drought of 2002–2006. Environ Geol. 2007;55:1089–1098. [Google Scholar]
- 30.Været L, Kelbe B, Haldorsen S, Taylor R. A modelling study of the effects of land management and climatic variations on groundwater inflow to Lake St Lucia, South Africa. Hydrogeol J. 2009;17:1949–1967. [Google Scholar]
- 31.Begg G. The Estuaries of Natal. Natal Town and Regional Planning report. 1978;41 [Google Scholar]
- 32.Bruton MN, Appleton CC. Survey of Mgobezeleni Lake-System in Zululand, with a Note on the Effect of a Bridge on the Mangrove Swamp. T Roy Soc S Afr. 1975;41:283–293. [Google Scholar]
- 33.Bruton MN. An outline of the ecology of the Mgobozeleni Lake System at Sodwana, with emphasis on the mangrove community. In: Bruton MN, Cooper KH, editors. Studies on the ecology of Maputaland. Grahamstown: Rhodes University; 1980. [Google Scholar]
- 34.Bruton MN. An outline of the ecology of Lake Sibaya, with emphasis on the vertebrate community. In: bruton MN, Cooper KH, editors. Studies on the ecology of Maputaland. Grahamstown: Rhodes University; 1980. [Google Scholar]
- 35.Begg GW. The Kosi system: aspects of its biology, management and research. In: Bruton MN, Cooper KH, editors. Studies on the ecology of Maputaland. Grahamstown: Rhodes University; 1980. [Google Scholar]
- 36.Abbott RT. A study of an intermediate snail host (Thiara granifera) of the oriental lung fluke (Paragonimus). Proc U S Nat Mus. 1952;102:72–116. [Google Scholar]
- 37.De la Vega RAR, Fernández LD, Espinosa AQ, García AMH. Change of the weight/foot area coefficient in relation to aggregation in Tarebia granifera. Rev Saude Publica. 2003;37:297–302. [PubMed] [Google Scholar]
- 38.Facon B, David P. Metapopulation dynamics and biological invasions: A spatially explicit model applied to a freshwater snail. Amer Nat. 2006;168:769–783. doi: 10.1086/508669. [DOI] [PubMed] [Google Scholar]
- 39.Snider SB, Gilliam JF. Movement ecology: Size-specific behavioral response of an invasive snail to food availability. Ecology. 2008;89:1961–1971. doi: 10.1890/07-0715.1. [DOI] [PubMed] [Google Scholar]
- 40.Yong M, Sanchez R, Perera G. Seasonal Studies of Two Populations of Tarebia granifera. Walkerana Trans POETS Soc. 1987;2:159–163. [Google Scholar]
- 41.Bruton MN. The fishes of Lake Sibaya. In: Allanson BR, editor. Lake Sibaya. Hague: Dr. W. Junk Publishers; 1979. pp. 162–245. [Google Scholar]
- 42.Miranda NAF, Perissinotto R, Appleton CC. Feeding dynamics of the invasive gastropod Tarebia granifera in coastal and estuarine lakes of northern KwaZulu Natal, South Africa. Estuar Coast Shelf Sci e. 2011;91:442–449. [Google Scholar]
- 43.Van der Mollen J, Perissinotto R. Microalgal productivity in an estuarine lake during a drought cycle: The St. Lucia Estuary, South Africa. Estuar Coast Shelf Sci. 2011;92:1–9. [Google Scholar]
- 44.Perera G, Yong M. The influence of some abiotic factors on the distribution of freshwater mollusks on the Isle of Youth (Isle of Pines), Cuba. Walkerana Trans POETS Soc. 1984;2:131–139. [Google Scholar]
- 45.Hart RC, Appleton CC. A limnological synopsis of. Bhangazi South, a dystrophic coastal lake in the Greater St Lucia. Wetland Park (KwaZulu/Natal), with comments on its conserva- tion value. South Afr J Aquat Sci. 1997;18:20–41. [Google Scholar]
- 46.Facon B, Machline E, Pointier JP, David P. Variation in desiccation tolerance in freshwater snails and its consequences for invasion ability. Biol Invasions. 2004;6:283–293. [Google Scholar]
- 47.Strong EE, Glaubrecht M. The morphology and independent origin of ovoviviparity in Tiphobia and Lavigeria (Caenogastropoda: Cerithioidea: Paludomidae) from Lake Tanganyika. Org Divers Evol. 2007;7:81–105. [Google Scholar]
- 48.Ben-Ami F, Hodgson AN. Ovoviviparity and the structure of the brood pouch in Melanoides tuberculata (Gastropoda: Prosobranchia: Thiaridae). J Morphol. 2005;263:322–329. doi: 10.1002/jmor.10307. [DOI] [PubMed] [Google Scholar]
- 49.Arking R, Buck S, Hwangbo D, Lane M. Metabolic alterations and shifts in energy allocations are corequisites for the expression of extended longevity genes in Drosophila. Ann NY Acad Sci. 2002;959:251–262. doi: 10.1111/j.1749-6632.2002.tb02097.x. [DOI] [PubMed] [Google Scholar]
- 50.Núñez V. Differences on allocation of available resources, in growth, reproduction, and survival, in an exotic gastropod of Physidae compared to an endemic one. Iheringia, Sér Zool, Porto Alegre. 2010;100:275–279. [Google Scholar]
- 51.Lockwood JL, Hoopes MF, Marchetti MP. Invasion Ecology. Malden, U.S.A.: Blackwell Publishing; 2007. [Google Scholar]
- 52.Ruiz GM, Fofonoff P, Hines AH, Grosholz ED. Non-indigenous species as stressors in estuarine and marine communities : Assessing invasion impacts and interactions. Lymnol Oceanogr. 1999;44:950–972. [Google Scholar]
- 53.Appleton CC. Non-marine molluscs and shistosomiasis in Maputaland. In: Bruton MN, Cooper KH, editors. Studies on the ecology of Maputaland. Grahamstown: Rhodes University; 1980. [Google Scholar]
- 54.Ben-Ami F. First report of the invasive freshwater snail Tarebia granifera (Lamarck, 1816) (Gastropoda : Thiaridae) from Israel. Nautilus. 2006;120:156–161. [Google Scholar]
- 55.Burlakova L, Padilla D, Karatayev A, Hollas D, Cartwright L, et al. Differences in population dynamics and potential impacts of a freshwater invader driven by temporal habitat stability. Biol Invasions. 2010;12:927–941. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of physical and chemical parameters, and Tarebia granifera populations in Maputaland estuaries and coastal lakes.
(DOC)