Abstract
MyoD inhibits cell proliferation and promotes muscle differentiation. A paradoxical feature of rhabdomyosarcoma (RMS), a tumor arising from muscle precursors, is the block of the differentiation program and the deregulated proliferation despite MyoD expression. A deficiency in RMS of a factor required for MyoD activity has been implicated by previous studies. We report here that p38 MAP kinase (MAPK) activation, which is essential for muscle differentiation, is deficient in RMS cells. Enforced induction of p38 MAPK by an activated MAPK kinase 6 (MKK6EE) restored MyoD function and enhanced MEF2 activity in RMS deficient for p38 MAPK activation, leading to growth arrest and terminal differentiation. Stress and cytokines could activate the p38 MAPK in RMS cells, however, these stimuli did not promote differentiation, possibly because they activated p38 MAPK only transiently and they also activated JNK, which could antagonize differentiation. Thus, the selective and sustained p38 MAPK activation, which is distinct from the stress-activated response, is required for differentiation and can be disrupted in human tumors.
Keywords: p38 MAPK, MKK6; muscle regulatory factors; RMS; differentiation
The capability of both escaping the differentiation program and undergoing deregulated proliferation is a hallmark of tumor cells. This may be achieved by several mechanisms, including disruption of cell growth regulatory pathways and functional inactivation of proteins that stimulate the differentiation program. For instance, rhabdomyosarcoma (RMS), one of the most common solid tumors of childhood, arises from muscle precursor cells and fails to complete the differentiation program despite the expression of the muscle regulatory protein MyoD (Dias et al. 1992; Tapscott et al. 1993). The ability of MyoD to arrest cell proliferation and to activate the myogenic program is repressed in RMS (Tapscott et al. 1993; Otten et al. 1997) but can be rescued by heterokaryon fusion with fibroblasts, suggesting that a positive regulatory pathway toward MyoD may be deficient in these tumor cells (Tapscott et al. 1993). Thus, inactivation of a pathway required for MyoD-dependent growth arrest and muscle-specific gene expression may underlie RMS formation.
Mitogen-activated protein kinase (MAPK) cascades transduce extracellular stimuli into intracellular pathways to regulate a number of cellular functions, such as proliferation, differentiation, and stress response (Hill and Treisman 1995; Marshal 1995; Minden and Karin 1997; Robinson and Cobb 1997). Three major MAPK groups have been defined, including extracellular signal-regulated kinases (ERK) and the stress-activated protein kinases, Jun N-terminal kinases (JNK), and p38 kinases (p38 MAPK). Whereas JNK and p38 MAPK are simultaneously activated by either proinflammatory cytokines, such as tumor necrosis factor α (TNFα), or stress, such as exposure to UV and osmotic agents (Minden and Karin 1997; Robinson and Cobb 1997), an independent activation of p38 occurs at the onset of differentiation in various cell types (Engelman et al. 1998; Cuenda and Cohen 1999; Zetser et al. 1999; Z. Wu, P.J. Woodring, K. Bhakta, K. Tamura, F. Wen, J. Feramisco, M. Karin, J.Y.J. Wang, and P.L. Puri, in prep.). In muscle cells p38 MAPK is activated upon serum withdrawal and is required for the expression of muscle-specific genes and myotube formation (Cuenda and Cohen 1999; Zetser et al. 1999; Z. Wu, P.J. Woodring, K. Bhakta, K. Tamura, F. Wen, J. Feramisco, M. Karin, J.Y.J. Wang, and P.L. Puri, in prep.). Although the transcriptional activity of two myogenic regulators, MEF2A and MEF2C, has been shown to be stimulated by p38 MAPK through direct phosphorylation (Han et al. 1997; Yang et al. 1999; Zhao et al. 1999), a number of observations also implicate MyoD activation under the control of p38 MAPK. For instance, MyoD-dependent myogenic conversion of fibroblasts and the expression of the cell cycle inhibitor p21, which is specifically stimulated by MyoD in muscle cells, both require the activity of p38 MAPK (Zetser et al. 1999; Z. Wu, P.J. Woodring, K. Bhakta, K. Tamura, F. Wen, J. Feramisco, M. Karin, J.Y.J. Wang, and P.L. Puri, in prep.). Thus, p38 MAPK is a potential positive regulator of MyoD.
We report here that p38 MAPK is indeed an essential activator of MyoD during myogenic differentiation and that the p38 MAPK pathway is inactivated in RMS cells induced to differentiate. Both MyoD and p38 MAPK are inhibited by serum in proliferating myoblasts. However, the enforced activation of p38 MAPK by an activated form of its upstream kinase MKK6 (MKK6EE) (Han et al. 1996; Raingeaud et al. 1996) is sufficient to activate MyoD-dependent cell cycle arrest and muscle-specific transcription in the presence of serum. Similar to proliferating myoblasts, the lack of p38 MAPK activation in a subgroup of RMS cell types correlates with the repression of MyoD function. Strikingly, the function of the latent MyoD in RMS cells deficient for p38 MAPK activation could be restored by the ectopic expression of MKK6EE, leading to suppression of tumor cell growth and induction of terminal differentiation. MKK6EE expression also enhanced MEF2-dependent transcription in RMS cells. Finally, activation of p38 MAPK by extracellular stress or cytokines fails to restore the differentiation program in RMS, suggesting that a selective and persistent activation of p38 MAPK pathway is necessary to arrest cell growth and to induce terminal differentiation in RMS.
Results
Activation of p38 MAPK pathway by constitutive active MKK6 overrides serum-dependent repression of MyoD functions
Growth factors contained in the serum (20% FBS) repress the transcriptional activity of MyoD and this allows undifferentiated myoblasts to escape the differentiation program and to proliferate (for review, see Olson 1992; Alemà and Tatò 1994; Lassar et al. 1994; Florini et al. 1996; Walsh and Perlman 1997; Arnold and Winter 1998). Serum removal stimulates p38 MAPK activity and this correlates with the induction of MyoD function (Zetser et al. 1999; Z. Wu, P.J. Woodring, K. Bhakta, K. Tamura, F. Wen, J. Feramisco, M. Karin, J.Y.J. Wang, and P.L. Puri, in prep.). To establish a functional link between p38 MAPK activation and the induction of MyoD function, we tested whether deliberate activation of p38 MAPK by the ectopic expression of the constitutive active form of MKK6EE can bypass the inhibitory effect of serum on MyoD.
The ability of MKK6EE to stimulate the activity of MyoD in the presence of serum can be demonstrated in mouse 10T1/2 fibroblasts growing in 20% FBS [growth medium (GM)] (Fig. 1). Under these culture conditions, ectopic expression of either MKK6EE or MyoD alone did not significantly activate the artificial promoter containing four MyoD-binding sites, 4RE Luc (Fig. 1A), or the MyoD-dependent p21Cip1 promoter (p21 luc) (data not shown) and failed to promote efficient myogenic conversion (Table 1) and cell cycle arrest (Table 2). Coexpressing MyoD with MKK6EE synergistically activated MyoD-dependent promoters (Fig. 1A; data not shown) and stimulated both MyoD-dependent myogenic conversion (Table 1) and cell growth arrest (Table 2). Addition of a specific inhibitor of p38 MAPK SB203580 (SB), but not the MEK1 inhibitor PD98059 (PD), eliminated the stimulatory effect of MKK6EE (Fig. 1; Tables 1 and 2), showing that p38 kinase is a necessary component in the activation of MyoD function.
Figure 1.
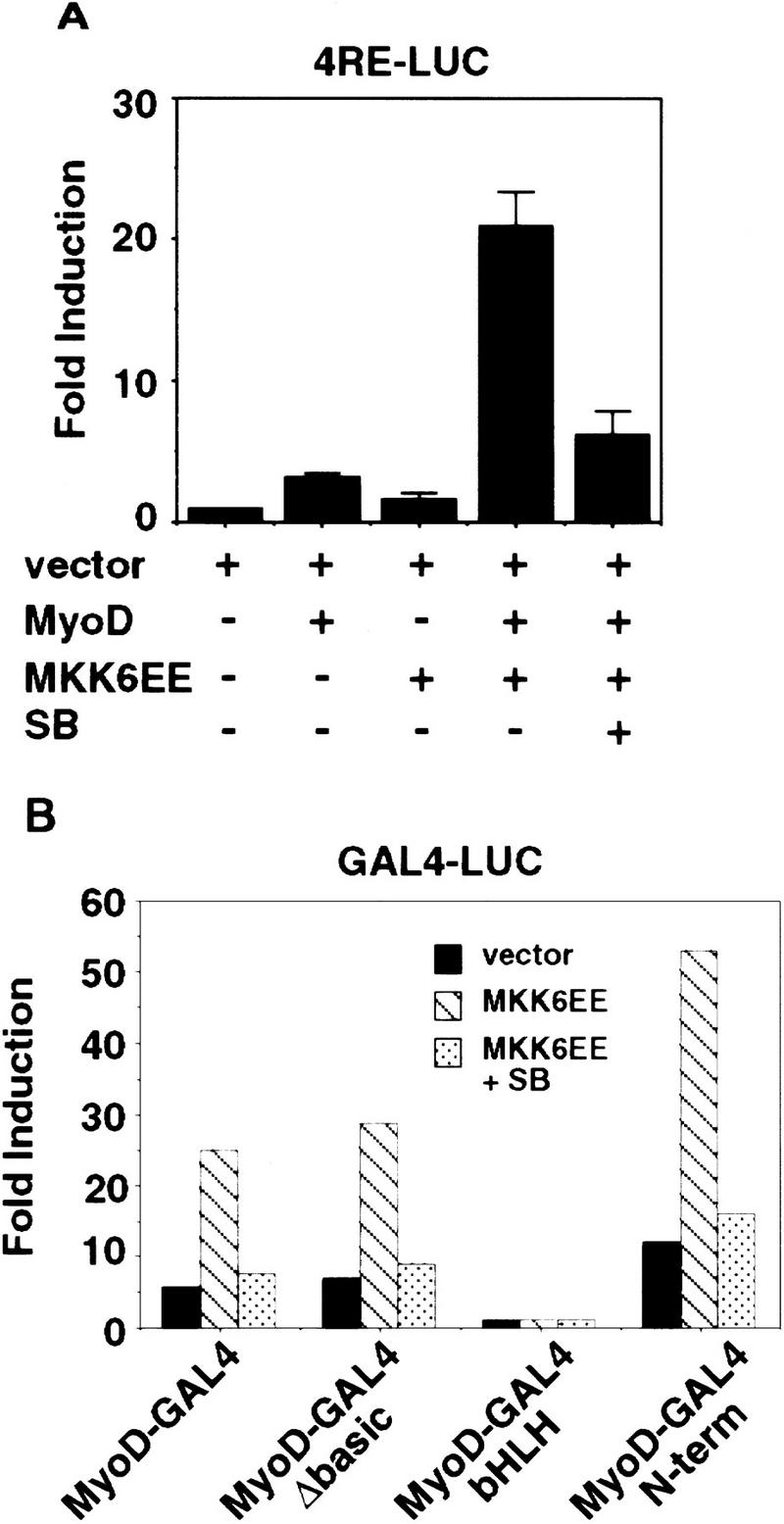
An activated mutant of MKK6EE stimulates the function of MyoD. (A) Activation of the MyoD-dependent promoter 4RE luc (four repeated MyoD-binding E-box sites) was determined by cotransfection of 10T1/2 fibroblasts with the indicated expression plasmids. Cells were transfected in GM containing 20% FBS and harvested 36 hr later for luciferase assay. Where indicated, the p38 MAPK inhibitor SB203580 (5 μm) was added to the transfected cells. The luciferase activity was normalized for the expression levels of MyoD in each sample, and the fold activation was relative to vector-transfected sample. The means and standard deviations from three independent experiments are shown. (B) MKK6EE stimulated the activity of MyoD–Gal4 fusion proteins. Plasmids expressing the indicated MyoD–Gal4 fusion proteins were cotransfected with a gal4–luc reporter gene plus a plasmid expressing MKK6EE (stippled and hatched bars) into 10T1/2 fibroblasts growing in 20% FBS. MKK6EE cotransfected cells were treated with 5 μm SB203580 (hatched bars). Cells were harvested 36 hr after transfection for LUC assay. The luciferase activity was normalized for the expression levels of the transfected Gal4 fusion proteins. The data presented are representative of four independent experiments.
Table 1.
Induction of myogenic conversion by MKK6EE in MyoD-converted fibroblasts in the presence of mitogens
| Plasmids
|
% MHC positive experiment
|
|
|---|---|---|
| 1
|
2
|
|
| Vector (GFP) | <1 | <1 |
| MKK6EE | <1 | <1 |
| MyoD | 9 | 12 |
| MyoD + SB | 5 | 6 |
| MyoD + MKK6EE | 55 | 64 |
| MyoD + MKK6EE + SB | 7 | 9 |
| MyoD + MKK6EE + PD | 64 | 75 |
Mouse 10T1/2 fibroblasts cultured in GM were transfected with the indicated plasmids. Forty-eight hours after transfection cella were fixed and the efficiency of myogenic conversion was determined by indirect immunofluorescence using anti-MHC rabbit antibody. Transfected cells were visualized either by staining with anti-MyoD (monoclonal anti-MyoD 5.8) or by the green fluorescent protein (GFP). The percentage of MHC/MyoD (or MHC/GFP) double-positive cells with more than two nuclei among 100 MyoD-positive cells was calculated.
Table 2.
Reduction of BrdU incorporation by MKK6EE in MyoD-converted fibroblasts in the presence of mitogens
| Plasmids
|
% BrdU positive experiment
|
|
|---|---|---|
| 1
|
2
|
|
| Vector (GFP) | 39 | 42 |
| MKK6EE | 32 | 34 |
| MyoD | 28 | 29 |
| MyoD + SB | 32 | 35 |
| MyoD + MKK6EE | 7 | 9 |
| MyoD + MKK6EE + SB | 33 | 32 |
| MyoD + MKK6EE + PD | 5 | 11 |
Mouse 10T1/2 fibroblasts cultured in GM were transfected with the indicated plasmids. Eighteen hours after transfection, when cells were almost completely confluent, BrdU (10 μm) was added for an additional 12 hr and the incorporation of BrdU was determined by indirect immunofluorescence after fixation, using anti-BrdU antibody. Transfected cells were visualized either by staining with anti-MyoD (monoclonal anti-MyoD 5.8) or by the green fluorescent protein (GFP). The percentage of BrdU/MyoD (or BrdU/GFP) double-positive cells among 100 MyoD-positive cells was calculated.
By coexpression of MKK6EE with Gal4–MyoD fusion mutants (Fig. 1B), we found that the basic region of MyoD was dispensable for the MKK6EE-dependent transcriptional stimulation. Because the basic region of MyoD is required for functional synergism with MEF2C (Molkentin et al. 1995; Black et al. 1998), which is directly activated by p38 MAPK (Han et al. 1997; Yang et al. 1999; Zhao et al. 1999), this result suggested that MKK6EE could stimulate MyoD function independent of MEF2C binding. In addition, MKK6EE could stimulate the transactivation function of a Gal4–MyoD chimera, in which only the amino-terminal domain of MyoD was fused to the Gal4 DNA-binding domain (Fig. 1B). As a control, a Gal4–E2F1 fusion chimera was not activated significantly (less than twofold) by MKK6EE. This observation suggests that the amino-terminal domain of MyoD is one target, either direct or indirect, of the p38 MAPK pathway. Taken together, these results show that MKK6EE can activate the function of MyoD and such activation can occur in the presence of mitogens, which are known to inhibit the differentiation function of MyoD.
Differentiation-programmed activation of p38 MAPK pathway is deficient in RMS cells
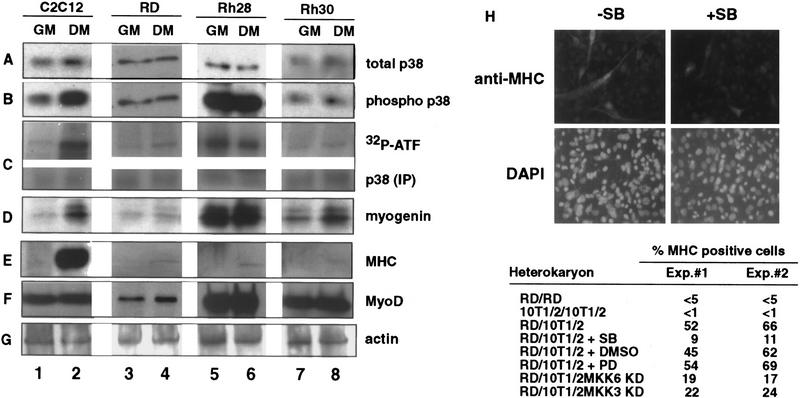
Given its ability to stimulate MyoD in the presence of mitogenic cues, we reasoned that MKK6EE might be able to activate the latent MyoD in RMS cells. Because p38 MAPK is an essential component in the MKK6EE effect, we first examined p38 MAPK activity in RMS cells, in comparison with that of C2C12 myoblasts (Fig. 2). The p38 MAPK was expressed in C2C12 myoblasts, as well as the RMS cell lines RD, Rh28, and Rh30 (Fig. 2A). With the C2C12 myoblasts, which undergo terminal differentiation upon transfer from GM to differentiation media (DM) (Fig. 2A–F, lanes 1,2), the levels of the phosphorylated (and thereby activated) p38 kinase (phospho-p38) increased in DM (Fig. 2B, cf. lanes 2 and 1). The activation of p38 MAPK in DM was also demonstrated by in vitro kinase assays using ATF-2 as a substrate (Fig. 2C, lanes 1,2). p38 MAPK function was required for the induction of myogenic differentiation, because treatment of muscle cells with SB prevents myotube formation (Cuenda and Cohen 1999; Zetser et al. 1999; Z. Wu, P.J. Woodring, K. Bhakta, K. Tamura, F. Wen, J. Feramisco, M. Karin, J.Y.J. Wang, and P.L. Puri, in prep.). With RMS cells, neither the levels of phospho-p38 nor the activity of p38 kinase were affected by the culture conditions (Fig. 2B,C, lanes 3–8). The RMS cell lines could be further distinguished by their p38 MAPK activity. In RD and Rh30 cells, a low level of phospho-p38 and kinase activity was detected in GM and DM (Fig. 2B,C, lanes 3,4,7,8). In contrast, high levels of phospho-p38 and kinase activity were detected in Rh28 cells regardless the culture conditions (Fig. 2B,C, lanes 5,6). The inability of RMS cells to modulate their p38 MAPK activity correlated with the lack of induction of MHC (Fig. 2E, lanes 3–8), despite the expression of MyoD and myogenin in these cells (Fig. 2 D,F, lanes 3–8). To verify the existence of two distinct subgroups of RMS also in vivo, we analyzed a panel of RMS tumor samples from human patients for the expression of phosphorylated p38 MAPK. Of the 15 tumor samples we examined, only 3 were stained positive for phospho-p38, despite the normal expression of total p38 in all the samples (P. Zhang and P.L. Puri, unpubl.), supporting the existence also in vivo of two distinct classes of RMS: either with or without activated p38 MAPK.
Figure 2.
Human RMS cell lines express p38 MAPK but do not modulate its activity under differentiation conditions. The levels of p38 MAPK (A) and phosphorylated p38 MAPK (B) were determined by immunoblotting with the appropriate antibodies (see Materials and Methods) in total lysates of mouse C2C12 (lanes 1,2) and three human RMS cell lines: RD (lanes 3,4), Rh28 (lanes 5,6), and Rh30 (lanes 7,8). (GM) Growth medium; (DM) differentiation medium. (C) p38 MAPK activity was measured by an immunocomplex kinase assay using GST-ATF2 fusion protein as a substrate. The levels of myogenin (D), MHC (E), MyoD (F), and actin (G) were also determined by immunoblotting with the appropriate antibodies. The formation of heterokaryons of RD and 10T/12 fibroblasts (H) was performed as described previously (Tapscott et al. 1993). The heterokaryons were transferred into DM with (+SB) or without (−SB) SB203580 (5 μm) to inhibit p38 MAPK. DMSO and PD98059 were also used as negative controls. After 36 hr in DM, heterokaryons were fixed and stained for the expression of MHC (top) and for the visualization of nuclei with DAPI (bottom). The different pattern of nuclear staining–punctate for mouse nuclei in 10T1/2 cells and uniform staining for human nuclei in RD cells—indicates the formation of heterokaryons. The number of MHC-positive heterokaryons/field was scored and the results of two experiments are summarized. In the case of MKK3KD and MKK6KD overexpression, these dominant-negative forms were expressed by transient transfection in 10T1/2 prior the fusion with RD. A GFP-encoding plasmid was cotransfected to localize successfully transfected cells. The number of GFP/MHC-positive heterokaryons/field was scored and the results of two experiments are summarized.
Previous studies have demonstrated that fusion of RD cells with 10T1/2 fibroblasts can rescue MyoD function (Tapscott et al. 1993). To determine whether the activation of p38 MAPK is important for that rescue, heterokaryons were prepared in the presence of SB (Fig. 2H). Similar to normal muscle cells (Cuenda and Cohen 1999; Zetser et al. 1999; Z. Wu, P.J. Woodring, K. Bhakta, K. Tamura, F. Wen, J. Feramisco, M. Karin, J.Y.J. Wang, and P.L. Puri, in prep.), the inactivation of p38 by SB reduced the number of MHC-positive heterokarions. This inhibitory effect was not observed with vehicle (DMSO) or PD98059, which inhibits MEK1 (Fig. 2H). Thus, the p38 MAPK activity was required for the rescue of MyoD function. A reduction in MHC-positive heterokaryons was also observed by the ectopic expression of kinase-defective forms of MKK6 or MKK3 (another upstream activator of p38 MAPK) in 10T1/2 fibroblasts prior the fusion with RD cells (Fig. 2H). These results further supported the notion that the provision of a functional MKK3/6 to p38 MAPK pathway by fibroblasts is a critical event in the reactivation of the myogenic program in RMS on heterokaryon fusion.
Enforced activation of p38 MAPK pathway by MKK6 overrides the differentiation block in RMS cells
To activate p38 MAPK in RMS cells, we infected them with an adenovirus expressing a HA-tagged MKK6EE protein (Fig. 3A,B) (Huang et al. 1997). After infection, cells were cultured in DM and then examined for the induction of MHC. Infection of Rh28 cells with MKK6EE adenovirus did not lead to an induction of MHC expression (data not shown). Because the Rh28 cells already contained a high level of phospho-p38 (Fig. 2B, lanes 5,6), its differentiation defect was likely to be either downstream to p38 MAPK activation or in another pathway. Hence, the resistance of Rh28 cells to MKK6EE was not unreasonable. In contrast, infection of either RD or Rh30 cells with MKK6EE adenovirus caused several changes that were indicative of terminal differentiation (Fig. 3A,B). With Rh30 cells, infection with MKK6EE adenovirus followed by culture in DM altered the morphology of the cells, which became larger and flatter, and induced the formation of multinucleated cells, which expressed MHC (Fig. 3A). After four days of culture in DM, >70% of the infected cells became MHC-positive multinucleated myotubes. Treating the infected cells with SB abrogated the effect of MKK6EE in Rh30 cells (Fig. 3A). A similar induction of MHC was observed when RD cells were infected with the MKK6EE adenovirus and then cultured in DM (Fig. 3B). The morphological alteration and the formation of multinucleated cells were less evident with the infected RD cells (Fig. 3B), suggesting that the morphological changes could be unlinked from the induction of myogenic-specific gene expression in this cell line. The p38 MAPK inhibitor SB also abolished the induction of MHC by MKK6EE in RD cells (Fig. 3B).
Figure 3.
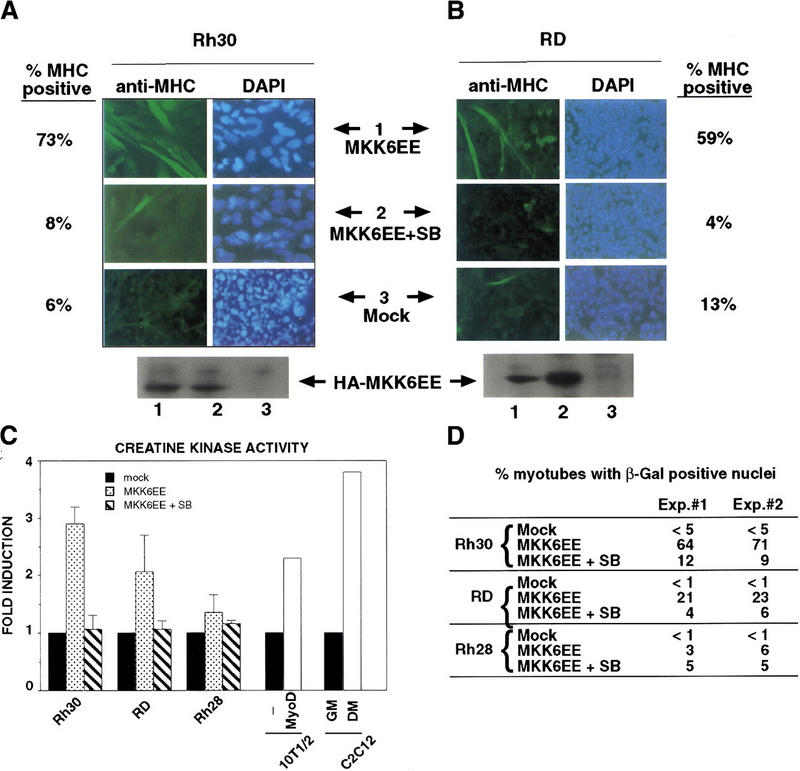
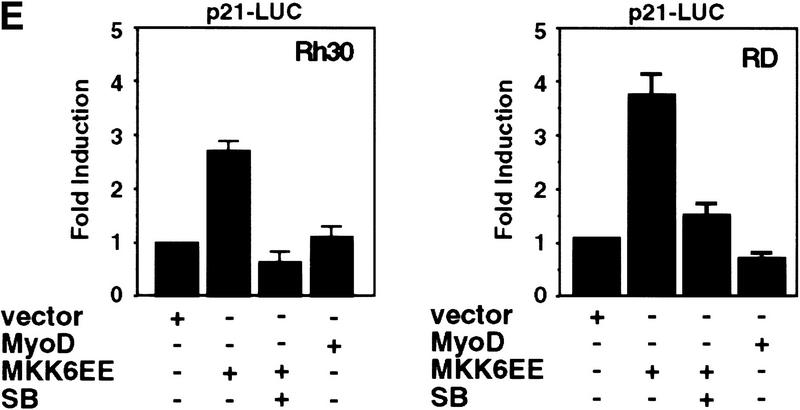
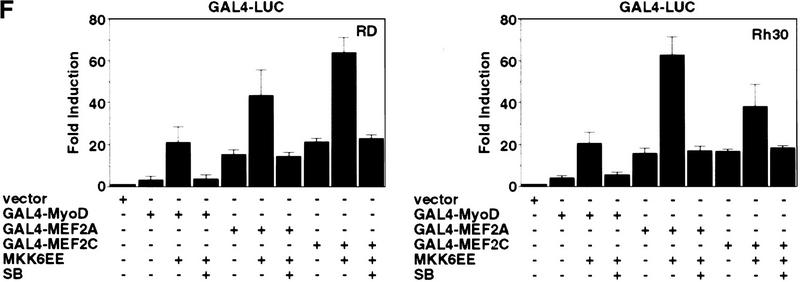
Introduction of MKK6EE reactivates myogenic differentiation in RMS cells. Two RMS cell lines, Rh30 (A) and RD (B), were infected with adenovirus expressing either the HA–MKK6EE gene or the β-gal gene (Mock) as described previously (Huang et al. 1997). Cells were transferred (12 hr postinfection) into DM with and without SB203580 (5 μm) for 96 hr and then fixed and stained for MHC or for DNA (DAPI). The percentage of MHC-positive multinucleated cells per field was counted and a representative value is reported. Expression of the exogenous MKK6EE proteins in the infected cells was shown by immunoblotting with anti-HA antibody. (C) Induction of CK activity in the indicated cell lines was assayed using a commercial kit (Sigma). (Solid bars) Mock; (stippled bars) MKK6EE; (hatched bars) MKK6EE + SB. The CK activity was calculated as micromoles of creatine formed/min per mg of protein extract. (D) RMS cells were first transfected with a reporter expressing β-gal fused to the nuclear localization signal (nls) and under the control of the MLC promoter (MLC–β-gal). After transfection (24 hr), the cells were infected with MKK6EE-expressing virus in the presence or absence of the p38 inhibitor SB. The percentage of myotubes with β-gal-positive nuclei is a measurement of multinucleation in myogenic cells. As a negative control cells were infected with an adenovirus expressing the β-gal gene (Mock) without the nls. Multinucleation was defined as a single cell with more than two β-gal-positive nuclei. Mock-infected cells were distinguished by the MKK6EE-infected cells as mononucleated cells with exclusively cytoplasmic blue staining. (E) The p21 luc reporter was transfected with the indicated plasmids in Rh30 (left) and RD (right) cells with or without SB (5 μm). At 18 hr post transfection, cells were transferred into DM and they were harvested after 24 hr. Luciferase activity was normalized to the expression levels of the transfected proteins (MyoD and MKK6EE were tagged with Flag and HA epitopes, respectively). The values shown are means and standard deviations from three independent experiments. (F) Plasmids expressing the indicated Gal4 fusion proteins were cotransfected with a gal4–luc reporter gene plus a plasmid expressing MKK6EE into RD and Rh30 cells growing in 20% FBS. After 12 hr, cells were placed in DM. MKK6EE cotransfected cells were also treated with 5 μm SB203580. Cells were harvested after 36 hr of culture in DM for LUC assay. The luciferase activity was normalized for the expression levels of the transfected Gal4 fusion proteins.
In addition to the induction of MHC, we examined several other markers of myogenic differentiation in RMS cells infected with MKK6EE virus. We measured the activity of creatine kinase (CK), which is up-regulated during MyoD-induced myogenic conversion of 10T1/2 fibroblasts and in C2C12 myotubes (Fig. 3C). A similar increase in CK activity was observed in MKK6EE-infected Rh30 and RD cells but not in Rh28 cells (Fig. 3C). We also determined the extent of multinucleation in RMS cells infected with MKK6EE virus. RMS cells were transfected with a reporter expressing a nuclearly localized β-galactosidase under the control of the myosin light chain promoter (MLC–β-gal), which is activated by MyoD. These RMS cells were subsequently infected with MKK6EE virus. Nuclei would become positive for β-galactosidase activity only after MyoD was activated. Multinucleation was defined as a single cell with more than two β-galactosidase-positive nuclei. Therefore, the measurement of blue nuclei into multinucleated cells was simultaneously scoring the extent of fusion and the activity of the reporter. As shown in Figure 3D, an increase in multinucleation was observed in Rh30 cells and, to a lesser extent, in RD cells infected with the MKK6EE virus. The induction of multinucleation was abolished by SB treatment. In similar experiments, we found that the ectopic expression of MKK3EE, which also activates p38 MAPK (Raingeaud et al. 1996), was equally effective in stimulating differentiation in RD and Rh30 cells (data not shown).
We then examined the activation of MyoD transcriptional function by the MKK6/p38 MAPK pathway by transfecting an MKK6EE expression vector with the MyoD-regulated p21 promoter (p21 luc) into RD or Rh30 cells (Fig. 3E). We observed a three- to fourfold activation of the p21 promoter by MKK6EE in these RMS cells and this activation was sensitive to SB (Fig. 3E). Similar results were also obtained using 4RE luc as a reporter (data not shown). Because activation of p38 MAPK pathway has been reported to stimulate the transcription factors MEF2A and MEF2C (Han et al. 1997; Ornatansky et al. 1999; Yang et al 1999; Zhao et al. 1999; Zetser et al. 1999), we also investigated the activity of these two factors in response to MKK6EE. The transcriptional activity of Gal4–MEF2A and Gal4–MEF2C chimeras in RMS cells was increased by the coexpression of MKK6EE (Fig. 3F). The activation of Gal4–MEF2 by MKK6EE was similar to the activation of Gal4–MyoD both in the extent of activation and sensitivity to SB (Fig. 3F). A similar enhancement by MKK6EE was also detected using an artificial construct containing multimerized MEF2-binding sites (MEF2–luc) as a reporter for the activity of the endogenous MEF2 proteins (data not shown). Notably, the basal activity of MEF2A or MEF2C in RD and Rh30 cells (Fig. 3F) was comparable to that found in C2C12 cells or 10T1/2 fibroblasts (P.L. Puri, unpubl.). In contrast, the basal activity of MyoD is virtually undetectable in RMS cells (Tapscott et al. 1993; Fig. 3F). This basal activity of MEF2 factors in RMS deficient in p38 MAPK activation suggested that, unlike MyoD, MEF2 factors could also be activated by a p38 MAPK-independent pathway in RMS cells. This is also supported by the fact the MEF2A and MEF2C mutants, impaired in receiving p38 MAPK activation by the substitution of threonine residues phosphorylated by p38 MAPK, display a basal activity similar to that of the wild-type constructs (data not shown).
The establishment of stably transfected Rh30–MKK6EE clones allowed further analysis of the effect of MKK6EE (Fig. 4). Control Rh30 clones stably transfected with vector alone were established in parallel. The expression of the HA-tagged MKK6EE in these cells was confirmed by immunoblotting with anti-HA antibody (Fig. 4C). Rh30 vector clones incorporated BrdU with similar efficiency in GM and DM (Fig. 4A). The Rh30–MKK6EE cells showed a marked reduction in BrdU incorporation even when cultured in GM (Fig. 4B) as compared with vector control (Fig. 4A). Upon transfer into DM, Rh30–MKK6EE cells formed multinucleated myotubes, which did not incorporate BrdU (Fig. 4B). Rh30–MKK6EE cells underwent a permanent growth arrest in DM because they did not incorporate BrdU after restimulation with serum (Fig. 4B). The inhibition of DNA synthesis could be correlated with the accumulation of p21Cip1 in these Rh30–MKK6EE cells (Fig. 4D). The level of p21Cip1 was higher in Rh30–MKK6EE cells cultured in GM, when compared with that of vector-transfected Rh30 cells (Fig. 4D, cf. lanes 5 and 1). A low level of p21Cip1 was induced in Rh30 vector cells when cultured in DM (Fig. 4D, cf. lanes 6,7 and 5); however, this level of p21Cip1 was unable to stop DNA synthesis (Fig. 4A). Treatment with SB inhibited the induction of p21Cip1 in Rh30–MKK6EE cells, consistent with the negative effect of SB on the p21Cip1 promoter in RMS cells (Fig. 3C). Rh30–MKK6EE clones also displayed increased levels of muscle-specific structural and contractile proteins, such as MHC and the fast and the slow isoforms of troponin T (Fig. 4D). A similar up-regulation of differentiation-specific markers was detected in RD–MKK6EE stable clones, whereas Rh28–MKK6EE clones continued to escape the differentiation program (data not shown).
Figure 4.
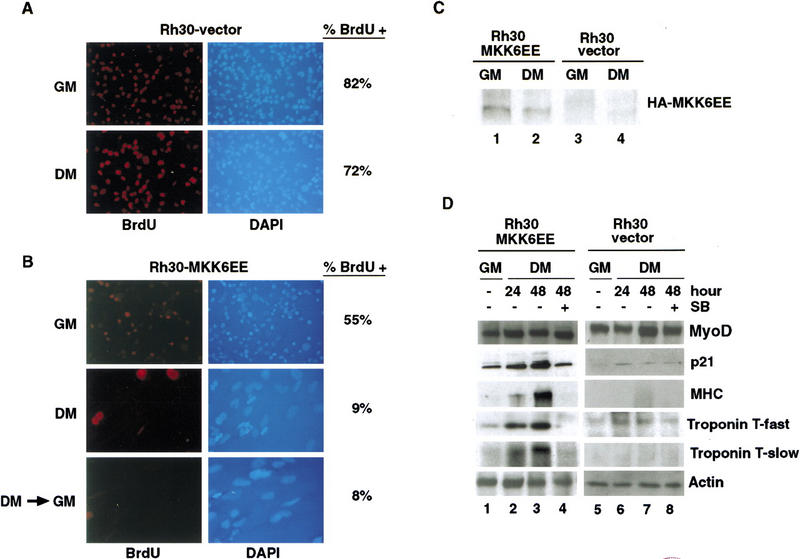
Stable expression of MKK6EE restores terminal differentiation in RMS cells. Polyclonal populations of Rh30 cells stably expressing HA–MKK6EE or vector alone were prepared (see Materials and Methods). (A,B) DNA synthesis was determined following the incubation with BrdU for 12 hr. Nuclei were visualized by staining the DNA with DAPI. Images are presented at the same magnification and show the increased size of nuclei belonging to MKK6EE-expressing Rh30 cells cultured in DM. (GM) Growth media; (DM) differentiation media; (DM > GM) serum restimulation. (C) The expression of HA–MKK6EE was demonstrated by immunoblotting of total cell lysates with anti-HA antibody. (D) Rh30 cells stably expressing MKK6EE (lanes 1,2) or vector control (lanes 3,4). The levels of MyoD, p21Cip1, MHC, troponin T (fast and slow), and actin were determined in total cell lysates by immunoblotting with the appropriate antibodies. The indicated cells were harvested after the indicated hours of culturing in DM. As indicated, SB (5 μm) was added for 48 hr in DM.
Taken together, results shown in Figures 3 and 4 demonstrated that activation of p38 MAPK by MKK6EE could restore MyoD activity and override the differentiation block in RMS cells, which do not contain an activated p38 MAPK. The p38 MAPK-mediated enhancement of MEF2 activity could also contribute to the rescue of the myogenic program in these RMS cells.
Differentiation-programmed and stress-induced p38 MAPK activation are two distinct responses
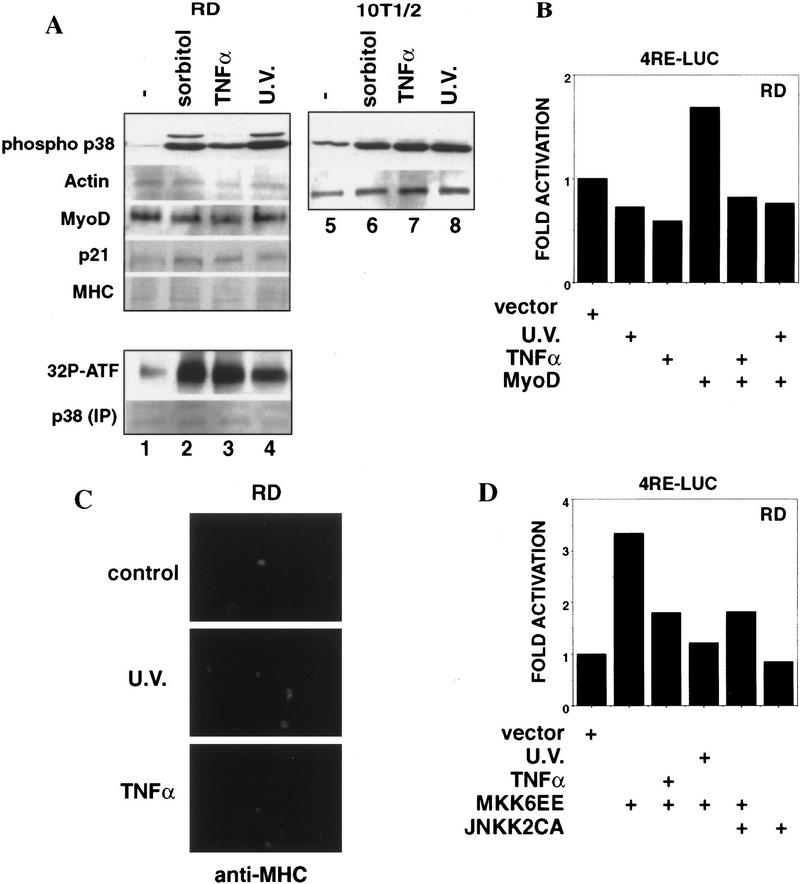
We tested whether the activity of p38 MAPK could be stimulated in RMS cells by extracellular stress and proinflammatory cytokines, which are known to trigger the stress-activated JNK and p38 pathways (Minden and Karin 1997; Robinson and Cobb 1997). Exposure to Sorbitol, UV, or TNFα could stimulate p38 MAPK (Fig. 5A, lanes 2,3,4) to a similar extent as in normal fibroblasts (Fig. 5A, cf. lanes 2, 3, and 4 with 6, 7, and 8). However, stress or cytokine stimulation did not activate MyoD (Fig. 5B), nor did they relieve the differentiation block in RD cells (Fig. 5A,C). In other experiments, we have found that UV and TNFα actually repressed myogenic differentiation in normal myoblasts, despite a transient activation of p38 MAPK (P.L. Puri, unpubl.). The inability of stress or cytokines to activate MyoD could be due to two reasons. First, these extracellular stimuli led only to a transient p38 MAPK activation (data not shown), which might not be sufficient to activate MyoD and hence the myogenic program (Marshall 1995). Second, these extracellular stimuli also activated parallel pathways, which might exert an anti-differentiation effect. Beside p38 MAPK, JNK is also activated by stress and cytokines (Minden and Karin 1997). JNK stimulation leads to the activation of the Jun family of transcription factors, which have been reported both to antagonize MyoD function and to repress myogenic differentiation (Bengal et al. 1992; Li et al. 1992). We found that TNFα and UV could indeed antagonize the positive effect of MKK6EE on myogenic transcription (Fig. 5D), supporting the notion that they can activate an inhibitory pathway. Furthermore, the ectopic expression of an activated form of JNKK2 (JNKK2CA) (Wu et al. 1997), which induces JNK activity, partially abrogated the stimulatory effect of MKK6EE on MyoD function in RD cells (Fig. 5D). The anti-myogenic effect of JNK can be also observed in muscle cell lines stably expressing JNKK2CA (Z. Wu. and P.L. Puri, unpubl.) We cannot be certain that JNK is the only inhibitory kinase responsible for the anti-myogenic effect of TNFα and UV, because we have not been able to completely inactivate the endogenous JNK in RMS cells by using JNK and JNKK dominant negatives (data not shown). Nevertheless, our data demonstrate that the constitutive activation of p38 MAPK by MKK6EE (or MKK3EE) was able to restore differentiation and that the activation of JNK pathway by stress or an inflammatory cytokine is inhibitory to the positive effect of MKK6EE.
Figure 5.
Activation of p38 MAPK in RD cells by stress and cytokines fails to induce myogenic differentiation. (A) The levels of phosphorylated (activated) p38 MAPK were determined by immunoblotting with the appropriate antibodies (see Materials and Methods) in total lysate from RD and 10T1/2 cell lines after exposure to several stress (0.4 m Sorbitol for 30 min; 10 ng/ml TNFα for 15 min; 40 J/m2 UV, for 20 min). The levels of actin, MyoD, p21Cip1, and MHC were also determined by immunoblotting with the appropriate antibodies (see Materials and Methods). The p38 MAPK assay activity was measured by an immunocomplex kinase assay using GST–ATF2 fusion protein as a substrate. (B) Activation of the MyoD-dependent promoter 4RE luc in RD cells exposed or not to UV (40 J/m2, 20 min) and TNFα (10 ng/ml for 15 min) was determined by luciferase assay. Cells were transfected in GM and, at 18 hr post-transfection, transferred into DM, and then exposed to TNFα or UV. After an additional 24 hr, cells were harvested and the luciferase activity calculated. The luciferase activity was normalized to the expression of cotransfected β-gal-encoding plasmid. The data presented is representative of four independent experiments. (C) RD cells were exposed or not to UV (40 J/m2, 20 min) and TNFα (10 ng/ml for 15 min) and kept 36 hr in DM. After fixation, cells were stained for MHC expression (visualized by using a fluorescein-conjugated secondary antibody). (D) Activation of the MyoD-dependent promoter 4RE luc in RD cells exposed or not to UV (40 J/m2, 20 min) and TNFα (10 ng/ml for 15 min) was determined after cotransfection with the indicated plasmids (0.2 μg MKK6EE, 0.4 μg JNKK2CA). Cells were transfected in GM and, at 18 hr post-transfection, transferred into DM. After additional 24 hr cells were harvested and the luciferase activity calculated. The luciferase activity was normalized to the expression of cotransfected β-gal-encoding plasmid. The data presented are representative of four independent experiments.
Discussion
We have shown that the ability of MyoD to stimulate muscle-specific gene expression (Lassar et al. 1994) and to suppress cell growth (Crescenzi et al. 1990; Sorrentino et al. 1990), requires the activity of p38 MAPK. The differentiation-programmed pathway leading to p38 MAPK activation is repressed in RMS cells, in which MyoD is inactive. Our results indicate that stimulation of the latent MyoD in RMS can be achieved by the enforced activation of p38 MAPK through the expression of an activated form of its upstream activator (either MKK6 or MKK3). The ectopic expression of MKK6EE in RMS cell lines that do not contain activated p38 MAPK led to the induction of morphological differentiation (formation of multinucleated myotubes), biochemical differentiation (increased activity of muscle creatine kinase), and in the up-regulation of contractile proteins, such as myosin heavy chain (MHC) and troponin T (fast and slow isoforms). The activation of such a broad spectrum of muscle markers distinguished the effect of MKK6EE from the previously reported partial activation of myogenic markers in some RMS cell lines by certain compounds, like TPA and INFα2a (Bouchè et al. 1992; Thulasi et al. 1996). MKK6EE activates MyoD as well as MEF2 factors in RMS cells. Thus, the p38-dependent enhancement of MEF2 activity could also contribute to the rescue of the myogenic program in these RMS cells. Notably, we noticed that, unlike MyoD, the basal activity of MEF2A and MEF2C in RD and Rh30 cells was not reduced as compared with that observed in normal fibroblasts or myocytes. This implicates a p38-independent basal activation of MEF2 factors in RMS that may explain why these cells display normal levels of certain myogenic markers, like myogenin, whose promoter is mainly regulated by MEF2-binding sites (Yee and Rigby 1993).
In contrast to MKK6EE and MKK3EE, overexpression of the p38 isoforms α, β, γ, and δ, either alone or in combination, failed to restore the differentiation program in RMS (data not shown), indicating that the pathway leading to MyoD activation is silenced upstream of p38 MAPK. In addition, the ectopic expression of the wild-type MKK6 also failed to rescue the RMS defects (data not shown), indicating a block that is also upstream of MKK6. However, because MKK6EE can override the differentiation block in RD and Rh30 RMS cells, there are no defects downstream of MKK6 in these two RMS cells. The differentiation defect with the Rh28 cells, on the other hand, appears to lie either downstream of p38 MAPK or in parallel to this pathway. Based on the failure of RD/Rh28 heterkaryons to rescue their latent MyoD, Tapscott et al. (1993) have proposed previously that these two tumor cell lines may share a common genetic defect. Our data show that the defect in RD cells is recessive to the constitutive activation of p38 MAPK, whereas the genetic defect of Rh28 cells is dominant over the effect of MKK6EE. Because Rh28 cells, which have constitutively high p38 MAPK activity, could not complement the defect of RD cells in heterokaryon experiments, it is possible that additional anti-myogenic pathways, such as those activated by stress or cytokines (i.e., JNK pathway), might also be constitutively activated in Rh28 cells. Alternatively, the p38 MAPK of Rh28 cells might be activated by an MKK3/6-independent pathway, which does not exert the same myogenic effect. Furthermore, inhibitory mechanism(s) downstream to p38 MAPK could also operate in Rh28 cells to block differentiation. The presence of any of these inhibitory mechanisms, either alone or in combination, in Rh28 cells might explain the failure to achieve complementation between Rh28/RD heterokaryon fusions.
Interestingly, the activity of p38 MAPK can be stimulated in RMS cells by extracellular stress and cytokines (Sorbitol, TNFα, UV), which activate a number of MAPK pathways in addition to p38 MAPK. However, stress or cytokines did not induce RMS differentiation. This observation implies the existence of two distinct programs leading to the activation of the MKK6–p38 MAPK pathway. The first is the prototypical stress-activated program, which is transient and is still functional in RMS. An alternative program that is triggered in myogenic cells by a differentiation cue yet to be identified, stimulates persistent p38 MAPK activation, and is blocked in a majority of RMS. The expression of MKK6EE would mimic the differentiation-programmed activation of p38 MAPK. By contrast, activation of p38 MAPK by stress and proinflammatory cytokines is part of a stress-activated response that does not lead to induction of differentiation. The failure of these extracellular stimuli to restore myogenic differentiation in RMS therefore could be due to two factors: the transient activation of p38 MAPK, and the activation of a parallel pathway that exerts anti-myogenic activity. Thus, the sustained and selective activation of the MKK6–p38 pathway, either by pharmacological agents or gene therapy approach, might be useful to induce the differentiation and thus inhibit the proliferation of RMS.
Although the induction of terminal differentiation by MKK6EE in RMS correlates with the MyoD-dependent increase of p21Cip1 (Figs. 3C and 4D), the accumulation of p21Cip1 cannot entirely account for the effect induced by MKK6EE in these cells. Overexpression of p21Cip1 in RMS cells placed in DM could only cause a serum-reversible cell cycle arrest (P.L. Puri, unpubl.), without inducing the expression of differentiation (Knudsen et al. 1998). Therefore, transient cell cycle arrest and acquisition of a differentiated phenotype are two separable processes not only in normal myocytes (Puri et al. 1997a) but also in RMS cells. Activation of p38 MAPK by MKK6/3 might stimulate terminal differentiation in RMS cells by coupling these two processes. Consistent with our finding, Ellinger-Ziegelbauer et al. (1999) have reported that a conditionally activated form of another upstream activator of p38 MAPK (MEKK3) can induce cell cycle arrest and reverse Ras-induced transformation, further implying p38 MAPK pathway as a negative regulator of cell growth in transformed cells. Although in RMS cells p38 MAPK is likely to exert its growth-suppressive effect by restoring MyoD activity, the molecular basis underlying such a effect are still unknown. Our data (Fig. 1B) indicate that p38 MAPK stimulates MyoD activity by a mechanism that does not involves the interaction with the p38 MAPK substrate MEF2. Moreover, we failed to detect a p38 MAPK-dependent activation of MyoD through direct phosphorylation (data not shown). Thus, p38 MAPK could indirectly activate MyoD by targeting bHLH-interacting proteins or cofactors like p300 and PCAF (Puri et al. 1997b).
Our results may also have interesting implications in diagnostic typing of RMS. The conventional histological typing of RMS distinguishes between embryonal, alveolar, and botryoid forms, based on morphological features (Arndt and Crist 1999). Consistent with the results obtained in RMS cell lines, we have found, by examining the p38 MAPK status in RMS tumor samples, that a majority of the RMS tumors did not contain the activated form of p38 MAPK regardless their histotype (P. Zhang and P.L. Puri, unpubl.). We propose that analysis of p38 MAPK might be a tool to identify RMS that are sensitive to therapeutic strategy based on the enforced activation of p38 MAPK.
Materials and methods
Cell culture conditions, transfections, and luciferase assay
Mouse C2C12 and 10T1/2 cells were cultured in DMEM supplemented with either 20% FBS (GM) or 2% horse serum (DM). Human RMS cells (RD, Rh30, and Rh28), kindly provided by K. Arden (Ludwig Cancer Research Institute, University of California, San Diego), were cultured in RPMI 1640 DMEM supplemented with either 20% FBS (GM) or 2% horse serum (DM). Polyclonal population of Rh30, RD, and Rh28 cells stably expressing MKK6EE was prepared by transfecting these cells with pcDNA3–HA–MKK6EE, followed by selection in G418 (0.5 mg/ml) for 3 weeks. Ten clones were expanded and each tested for the expression of HA–MKK6EE and for p38 kinase activity and the positive clones were pooled. The experiments were done at the first three passages of these clones (at later passages the expression of HA–MKK6EE and the p38 MAPK activity decrease). RD-10T1/2 heterokaryons were prepared as described (Tapscott et al. 1993). Transfections were performed using lipofectamine reagent (GIBCO) and luciferase assays were performed as described previously (Puri et al. 1997b). The kinase inhibitors SB203580 and PD98059 were purchased from Calbiochem.
BrdU incorporation
BrdU incorporation was determined by indirect immunofluorescence using anti-BrdU antibodies, as described previously (Puri et al. 1997b).
Muscle creatine kinase assay
The activity of the enzyme creatine phosphokinase (CPK) was measured following the procedures of the commercial kit from Sigma. Cells were washed in TBS and the cell extracts used for the assay were prepared in TBS-containing buffer with 1% Tween, phosphatase, and protease inhibitors.
Immunoblot analysis
Endogenous myogenin, MHC, MyoD, and actin of C2C12 and RMS cell lines were detected by immunoblotting using monoclonal anti-myogenin (F5D), anti-MyoD (5.8), anti-MHC (MF20), and anti-actin (Ab-1, Oncogene). Levels of either total or phosphorylated p38 in the same cell lines were detected by immunoblotting using anti-p38 antibodies from New England Biolabs. Endogenous p21Cip1 levels in Rh30 cell clones were detected by using polyclonal anti-p21 (Ab-5, Oncogene). Anti- troponin T fast (C-18) and slow (C-19) antibodies were purchased from Santa Cruz.
Kinase assay
In vitro kinase assays were performed as described previously (Wu et al. 1997). Briefly, 50- to 100-μg cell extracts were incubated with antibodies against p38α in the presence of 30 μl of protein A–Sepharose beads for 2 hr at 4°C. After extensive washing, the immunocomplexes were incubated with 1 μg of GST–ATF in the presence of 10 μCi of [γ-32P] ATP in 25 μl of kinase buffer for 20 min at 30°C, analyzed by SDS-PAGE, and visualized by autoradiography.
Immunofluorescence
Cells were fixed in 3.3% formaldehyde for 10 min and permeabilized with 0.25% Triton X-100 for additional 10 min. Indirect immunofluorescence was carried out using polyclonal rabbit anti-MHC as described previously (Puri et al. 1997b)
Adenoviral infections
Adenoviral infection was performed in confluent RMS cells as described previously (Huang et al. 1997).
Acknowledgments
We thank Dr. Karen Arden, Dr. Vittorio Sartorelli, Dr. Stefano Alema, Dr. Peter Houghton and Dr. Woodring E. Wright for generously providing reagents. Dr. Vittorio Sartorelli and Dr. Gioacchino Natoli are also acknowledged for their comments and helpful suggestions during the preparation of the manuscript. This work was supported by fellowships from Medical Research Council of Canada (Z.W.), from American Cancer Society (L.D.W.), and by grants from Telethon (P.L.P.), from NIH State of California Cancer Research Program (M.K.), and from NCI (J.Y.J.W.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL jywang@ucsd.edu; FAX (858) 534-2821.
References
- Alemà S, Tatò F. Oncogenes and muscle differentiation: Multiple mechanism of interference. Semin Cancer Biol. 1994;5:147–156. [PubMed] [Google Scholar]
- Arndt CAS, Crist WM. Medical progress: Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999;341:342–353. doi: 10.1056/NEJM199907293410507. [DOI] [PubMed] [Google Scholar]
- Arnold H-H, Winter B. Muscle differentiation: More complexity to the network of myogenic regulators. Curr Opin Genet Dev. 1998;8:539–544. doi: 10.1016/s0959-437x(98)80008-7. [DOI] [PubMed] [Google Scholar]
- Bengal E, Ransone L, Scharfmann R, Dwarki VJ, Tapscott SJ, Weintraub H, Verma IM. Functional antagonism between cJun and MyoD proteins: a direct physical association. Cell. 1992;68:507–519. doi: 10.1016/0092-8674(92)90187-h. [DOI] [PubMed] [Google Scholar]
- Black BL, Molkentin JD, Olson E. Multiples roles for the MyoD basic region in transmission of transcriptional activation signals and interaction with Mef2. Mol Cell Biol. 1998;18:69–77. doi: 10.1128/mcb.18.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchè M, Zappelli F, Polimenu M, Adamo S, Wetsel WC, Senni MI, Molinaro M. Rapid activation and downregulation of protein kinase C in 12-O-tetradecanoylphorbol-13-acetate-induced differentiation of human rhabdomyosarcoma cells. Cell Growth Differ. 1992;6:845–852. [PubMed] [Google Scholar]
- Crescenzi M, Fleming TP, Lassar AB, Weintraub H, Aaronson AS. MyoD induces growth arrest independent of differentiation in normal and transformed cells. Proc Natl Acad Sci. 1990;87:8442–8446. doi: 10.1073/pnas.87.21.8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenda A, Cohen P. Stress activated protein kinase-2 /p38 and a rapamycin-sensitive pathway are required for C2C12 myogenesis. J Biol Chem. 1999;274:4341–4346. doi: 10.1074/jbc.274.7.4341. [DOI] [PubMed] [Google Scholar]
- Dias P, Parham DM, Shapiro DN, Tapscot SJ, Houghton PJ. Monoclonal antibodies to the myogenic regulatory protein MyoD1: Epitope mapping and diagnostic utility. Cancer Res. 1992;52:6431–6439. [PubMed] [Google Scholar]
- Ellinger-Ziegelbauer H, Kelly K, Siebenlist U. Cell cycle arrest and reversion of Ras-induced transformation by a conditionaly activated form of mitogen-activated protein kinase kinase kinase 3. Mol Cell Biol. 1999;19:3857–3868. doi: 10.1128/mcb.19.5.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Lisanti MP, Scherer PE. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J Biol Chem. 1998;27:32111–32120. doi: 10.1074/jbc.273.48.32111. [DOI] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JD, Jiang Y, Li Z, Feng L, Ulevitch RJ. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- Han J, Jiang Y, Li Z, Kravchenko VV, Ulevitch RJ. Activation of the transcription factor Mef2c by the MAP kinase p38 in inflammation. Nature. 1997;368:296–299. doi: 10.1038/386296a0. [DOI] [PubMed] [Google Scholar]
- Hill CS, Treisman R. Transcriptional regulation by extracellular signals: Mechanism and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- Huang S, Jang Y, Li Z, Nishida E, Mathias P, Lin S, Ulevitch RJ, Nemerow GR, Han J. Apoptosis signalling pathway in T cells is composed of ICE/CED-3 family proteases and MAP kinase kinase 6b. Immunity. 1997;6:739–749. doi: 10.1016/s1074-7613(00)80449-5. [DOI] [PubMed] [Google Scholar]
- Knudsen ES, Pazzagli C, Born TL, Bertolaet BL, Knudsen KE, Arden KC, Henry RR, Feramisco JR. Elevated cyclins and cyclin dependent kinase activity in rhabdomyosarcoma cell line RD. Cancer Res. 1998;58:2042–2049. [PubMed] [Google Scholar]
- Lassar AB, Skapek SX, Novich B. Regulatory mechanisms that coordinate skeletal muscle differentiaion and cell cycle withdrawal. Curr Opin Cell Biol. 1994;6:788–794. doi: 10.1016/0955-0674(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Li L, Chambard JC, Karin M, Olson E. Fos and Jun repress transcriptional activation by myogenin and MyoD: the amino terminus of Jun can mediate repression. Genes & Dev. 1992;6:676–689. doi: 10.1101/gad.6.4.676. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signalling: Transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Minden A, Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochim Biophys Acta. 1997;1333:F85–F104. doi: 10.1016/s0304-419x(97)00018-8. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Black BL, Martin JF, Olson EN. Cooperative activation of muscle gene expression by Mef2 and myogenic bHLH proteins. Cell. 1995;83:1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
- Olson EN. Interplay between proliferation and differentiaiton within the myogenic lineage. Dev Biol. 1992;154:261–272. doi: 10.1016/0012-1606(92)90066-p. [DOI] [PubMed] [Google Scholar]
- Ornatansky OI, Cox DM, Tangirala P, Andreucci JJ, Quinn ZA, Wrana JL, Prywes R, Yu Y-T, McDermott J. Posttranscriptional control of the MEF2A transcriptional regulatory protein. Nucleic Acids Res. 1999;21:2646–2656. doi: 10.1093/nar/27.13.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten AD, Firpo EJ, Gerber AN, Brody LL, Roberts JM, Tapscott SJ. Inactivation of MyoD mediated expression of p21 in tumor cell lines. Cell Growth & Diff. 1997;8:1151–1160. [PubMed] [Google Scholar]
- Puri PL, Balsano C, Burgio VL, Chirillo P, Natoli G, Ricci L, Mattei E, Graessmann A, Levrero M. MyoD prevents cyclinA/cdk2 containing E2F complex formation in terminally differentiated myocytes. Oncogene. 1997a;14:1171–1184. doi: 10.1038/sj.onc.1200941. [DOI] [PubMed] [Google Scholar]
- Puri PL, Sartorelli V, Yang X-J, Hamamori Y, Ogryzko VV, Howard BH, Kedes L, Wang JYJ, Graessmann A, Nakatani Y, Levrero M. Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol Cell. 1997b;1:35–45. doi: 10.1016/s1097-2765(00)80005-2. , [DOI] [PubMed] [Google Scholar]
- Raingeaud J, Whitmarsh AJ, Barret T, Derijard B, Davis R. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- Sorrentino V, Peppekok R, Davis RL, Ansorge W, Philipson L. Cell proliferation inhibited by MyoD independent of differentiation. Nature. 1990;345:813–815. doi: 10.1038/345813a0. [DOI] [PubMed] [Google Scholar]
- Tapscott SJ, Thayer MJ, Weintraub H. Deficiency in Rhabdomyosarcoma of a factor required for MyoD activity and myogenesis. Science. 1993;259:1450–1453. doi: 10.1126/science.8383879. [DOI] [PubMed] [Google Scholar]
- Thulasi R, Dias P, Houghton PJ, Houghton JA. A2a-Interferon-induced differentiation of human alveolar rhabdomyosarcoma cells: Correlation with down-regulation of the Insulin-like growth factor type I receptor. Cell Growth & Differ. 1996;7:531–541. [PubMed] [Google Scholar]
- Walsh K, Perlman H. Cell cycle exit upon myogenic differentiation. Curr Opin Genet Dev. 1997;7:597–602. doi: 10.1016/s0959-437x(97)80005-6. [DOI] [PubMed] [Google Scholar]
- Wu Z, Wu J, Jacinto E, Karin M. Molecular cloning and characterization of human JNKK2, a novel Jun NH2-terminal kinase-specific kinase. Mol Cell Biol. 1997;17:7407–7416. doi: 10.1128/mcb.17.12.7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Galanis A, Sharrocks AD. Targeting of p38 mitogen activated protein kinases to MEF2 transcription factors. Mol Cell Biol. 1999;19:4028–4038. doi: 10.1128/mcb.19.6.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee SP, Rigby PW. The regulation of the myogenin gene expression during embryonic development of the mouse. Genes & Dev. 1993;7:1277–1289. doi: 10.1101/gad.7.7a.1277. [DOI] [PubMed] [Google Scholar]
- Zhao M, New L, Kravchenko VV, Kato Y, Gram H, di Padova F, Olson EN, Ulevitch RJ, Han J. Regulation of the Mef2 family of transcription factors by p38. Mol Cell Biol. 1999;19:21–30. doi: 10.1128/mcb.19.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetser A, Gredinger E, Bengal E. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. J Biol Chem. 1999;274:5193–5200. doi: 10.1074/jbc.274.8.5193. [DOI] [PubMed] [Google Scholar]