Abstract
Background
Coronary tortuosity (CT) is a common coronary angiography finding. The exact pathogenesis, clinical implication and long-term prognosis of CT are not fully understood. The purpose of this study is to investigate the clinical characteristics of CT in patients with suspected coronary artery disease(CAD) in a Chinese population.
Methods
A total of 1010 consecutive patients underwent coronary angiography with complaints of chest pain or related symptoms were included in the present study (544 male, mean age: 64±11 years). CT was defined by the finding of ≥3 bends (defined as ≥45° change in vessel direction) along main trunk of at least one artery in systole and in diastole. Patients with or without CAD were further divided into CT-positive and CT-negative groups, all patients were followed up for the incidence of major adverse cardiovascular events (MACE) for 2 to 4 years.
Results
The prevalence of CT was 39.1% in this patient cohort and incidence of CT was significantly higher in female patients than that in male patients (OR = 2.603, 95%CI 1.897, 3.607, P<0.001). CT was positively correlated with essential hypertension (OR = 1.533, 95%CI 1.131, 2.076, P = 0.006) and negatively correlated with CAD (OR = 0.755, 95%CI 0.574, 0.994, P = 0.045). MACE during follow up was similar between CAD patients with or without CT.
Conclusions
CT is more often seen in females and positively correlated with hypertension and negatively correlated with coronary atherosclerosis.
Introduction
Coronary artery disease (CAD) is the leading cause for mortality and morbidity severely affecting the quality of life in both developed and developing countries worldwide [1], [2]. Coronary angiography is still the golden standard for the diagnosis of CAD and coronary tortuosity (CT) is a common coronary angiography(CAG) finding. Previous studies suggested that CT might be associated with chronic pressure load and impaired left ventricular relaxation and possibly coronary ischemia [3]–[5]. However, the impact of CT on prognosis in CAD patients remains largely unknown. In this study, we observed the prevalence of CT in a large cohort patient population underwent coronary angiography due to chest pain and similar symptoms and explored the association between CT and incidence of coronary stenosis, moreover, the impact of CT on prognosis was determined in patients with and without CAD.
Methods
Subjects
A total of 1010 consecutive patients underwent coronary angiography due to chest pain and similar symptoms were included in the present study (544 male, mean age: 64±11 years).
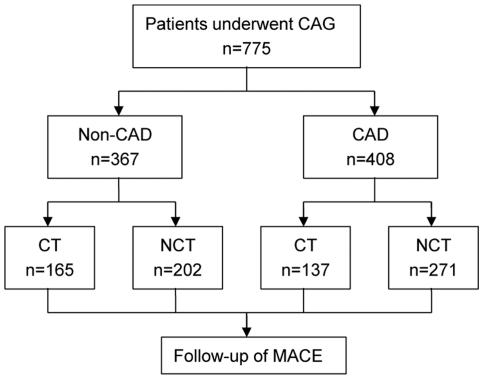
All patients were followed up for 2 to 4 years post coronary angiography. Patients were divided into 4 groups according to the presence and absence of CAD and CT (Figure 1). The study was approved by the hospital ethics committee (Zhongda Hospital, Southeast University) and in accordance with the principles embodied in the Declaration of Helsinki, written informed consent was obtained from each participating patient.
Figure 1. Flow diagram of patient follow-up.
Risk factors
Essential hypertension was defined as systolic blood pressure of ≥140 mmHg or diastolic blood pressure of ≥90 mmHg, or taking antihypertensive medication. Hyperlipidemia was diagnosed with total plasma cholesterol level of ≥200 mg/dl or low-density lipoprotein-cholesterol level of ≥130 mg/dl or triglyceride level of ≥150 mg/dl, or taking cholesterol-lowering drugs. Diabetes mellitus was defined by WHO criteria. Heart function was evaluated based on ACCF/AHA 2009 heart failure guideline [6], stage C or D was identified as chronic heart failure. Smokers were defined as those smoking regularly.
Coronary angiography
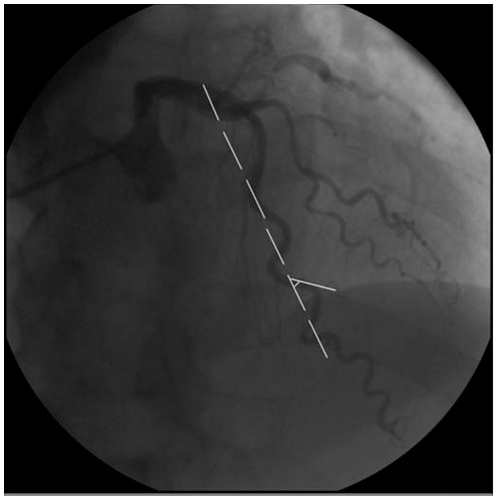
All patients underwent elective coronary angiography according to the Judkins technique using a Philip FD-10 X-ray system. The left anterior descending coronary artery (LAD), the left circumflex coronary artery (LCX) and the right coronary artery (RCA) were observed by various angulations. Images were recorded on CD-R. CAD was verified as ≥50% luminal narrowing in at least one main coronary artery. CT was evaluated on special angulations, LAD was assessed in right anterior oblique with cranial angulations and LCX in left anterior oblique with caudal angulations, while RCA in right anterior oblique. CT was identified by ≥3 bends (defined as ≥45° change in vessel direction) along main trunk of at least one artery, present both in systole and in diastole [4] (Figure 2).
Figure 2. Diagnosis of coronary tortuosity.
Major adverse cardiovascular events (MACE)
Cardiac death was defined as death of cardiac causes or any death without another known cause. Acute myocardial infarction was defined in accordance with the universal definition proposed [7]. Coronary revascularization was defined as angioplasty or stenting or coronary artery bypass grafting. Stroke was defined as loss of neurologic function due to an ischemic or hemorrhagic event. Composite end point included all cause mortality, myocardial infarction, coronary revascularization, non-fatal angina needing hospital admission and stroke. MACE was verified by hospital medical records and telephone.
Statistical Analysis
The data were analyzed using the statistical software package of SPSS (SPSS Inc., Chicago, IL, USA, Version 13). Continuous variables were expressed as mean ± standard deviation and categorical variables as percentages. Continuous variables between groups were compared by unpaired Student's t test. Categorical variables were compared by Chi-square test. Multivariate logistic regression analysis was performed, odds ratio (OR) and 95% confidence intervals (CI) were calculated. Two-tailed P values<0. 05 were considered significant.
Results
Prevalence of coronary tortuosity
The prevalence of CT was 39.1% in this cohort. Incidence of CT was significantly higher in the females than in males (62.8 vs. 37.2%, P<0.001) and in non CAD patients than in CAD patients (45.1% vs. 34.4%, P = 0. 001). Among the three coronary arteries, incidence of CT was 26. 9% in LCX, 21. 1% in LAD and 1.4% in RCA. CT was identified in both LAD & LCX in 9.9% of patients.
Clinical characteristics of patients with or without coronary tortuosity
Of the 1010 patients, there were 723 patients with essential hypertension (71.6%, 381 male), 212 patients with diabetes mellitus (21%, 109 male), 198 patients with hyperlipidemia (19.6%, 87 male), 297 smokers (29.4%, 292 male), 170 patients with heart failure (16.8%, 99 male) and 569 patients with CAD (56.3%, 344 male).
Table 1 summarized the clinical characteristics of patients with suspected CAD with or without CT. CT incidence was significantly higher in females, hypertensive patients, cigarette smoker and patients without CAD.
Table 1. Clinical characteristics of coronary tortuosity.
| Characteristic | CT group(n = 395) | NCT group(n = 615) | p value |
| age(year) | 64±10. 3 | 64±10. 7 | 0. 690 |
| female gender | 247 (62. 8) | 218 (35. 4) | <0. 001 |
| hypertension | 300 (75. 9) | 423 (68. 8) | 0. 014 |
| diabetes mellitus | 71 (18. 0) | 141 (22. 9) | 0. 059 |
| hyperlipidemia | 68 (17. 2) | 130 (21. 1) | 0. 125 |
| cigarette smoking | 72 (18. 2) | 225 (36. 6) | <0. 001 |
| chronic heart failure | 64 (16. 2) | 106 (17. 2) | 0. 668 |
| CAD | 196 (49. 6) | 373 (60. 7) | 0. 001 |
Table 2 showed female gender and hypertension were associated higher incidence of CT while diabetes, hyperlipidemia and CAD were linked with reduced incidence of CT.
Table 2. Multivariate logistic regression analysis of selected variables for coronary tortuosity.
| Variable | B | SE | X 2 value | P value | OR | 95%CI |
| female | 0. 957 | 0. 166 | 33. 083 | <0. 001 | 2. 603 | 1. 897–3. 607 |
| hypertension | 0. 427 | 0. 155 | 7. 598 | 0. 006 | 1. 533 | 1. 131–2. 076 |
| diabetes | −0. 353 | 0. 175 | 4. 158 | 0. 041 | 0. 702 | 0. 500–0. 986 |
| hyperlipidemia | −0. 421 | 5. 787 | 5. 787 | 0. 016 | 0. 657 | 0. 466–0. 925 |
| CAD | −0. 48 | 0. 140 | 4. 023 | 0. 045 | 0. 755 | 0. 574–0. 994 |
As shown in Table 3, female and hypertension were also related to higher incidence of LAD tortuosity while LAD atherosclerosis was negatively associated with LAD tortuosity. There were no significant difference between LCX(RCA) tortuosity and LCX(RCA) artherosclerosis.
Table 3. Multivariate logistic regression analysis of selected variables for LAD tortuosity.
| Variable | B | SE | X 2 value | P value | OR | 95%CI |
| female | 1. 040 | 0. 165 | 39. 929 | <0. 001 | 2. 830 | 2. 049–3. 907 |
| hypertension | 0. 612 | 0. 194 | 9. 963 | 0. 002 | 1. 844 | 1. 261–2. 697 |
| LAD≥50% stenosis | −0. 192 | 0. 210 | 97. 095 | <0. 001 | 0. 755 | 0. 574–0. 994 |
Clinical characteristics and impact on prognosis of CT in patients with or without CAD
Table 4 showed the baseline characteristics of CAD patients with or without CT. There were significantly more females and hypertensive patients while less smokers and patients with three vessel disease in CT group than in NCT(no coronary tortuosity) group. There were more patients with three coronary vessel lesions in NCT group than in CT group.
Table 4. Baseline characteristics of the patients with CAD followed up.
| Characteristic | CT group(n = 137) | NCT group(n = 271) | p value |
| age(year) | 68±8.9 | 66±10.6 | 0. 163 |
| female gender | 74(54.0) | 85(31.4) | <0. 001 |
| medical history | |||
| hypertention | 112(81.8) | 194(71.6) | 0. 025 |
| diabetes mellitus | 32(23.4) | 82(30.3) | 0. 142 |
| hyperlipemia | 21(15.3) | 59(21.8) | 0. 122 |
| cigarette smoking | 33(24.1) | 112(41.3) | 0. 001 |
| heart failure | 29(21.2) | 52(19.2) | 0. 636 |
| previous myocardial infarction | 33(24.1) | 68(25.1) | 0. 824 |
| previous coronary revascularization | |||
| PCI | 86(62. 8) | 177(65.3) | 0. 613 |
| Coronary-artery bypass grafting | 2(1.5) | 3(1.1) | 0. 760 |
| No. of vessel (≥50% stenosis) | |||
| One vessel | 71(51.8) | 119(43.9) | 0.130 |
| Two vessel | 37(27.0) | 64(23.6) | 0.454 |
| Three vessel | 29(21.2) | 88(32.5) | 0.017 |
| asprin therapy | 115(83.9) | 228(84.1) | 0. 960 |
| clopidogrel therapy | 135(98.5) | 267(98.5) | 0. 990 |
| statin therapy | 75(54.7) | 138(50.9) | 0. 465 |
| beta-blockers therapy | 66(48.2) | 127(46.9) | 0. 802 |
| calcium channel blocker | 72(52.6) | 136(50.2) | 0.651 |
| ACEI/ARB therapy | 78(56.9) | 142(52.4) | 0. 385 |
Table 5 showed baseline characteristics of CT and NCT patients without CAD. There were significantly more females while less smokers in CT group than in NCT group.
Table 5. Baseline characteristics of the patients without CAD followed up.
| Characteristic | CT group(n = 165) | NCT group(n = 202) | p value |
| age(year) | 61±10.8 | 60±10.8 | 0.787 |
| female gender | 111(67.3) | 89(44.1) | <0.001 |
| medical history | |||
| hypertention | 115(69.7) | 131(64.9) | 0.326 |
| diabetes mellitus | 17(10.3) | 33(16.3) | 0.094 |
| hyperlipemia | 23(13.9) | 38(18.8) | 0.212 |
| cigarette smoking | 23(13.9) | 60(29.7) | <0.001 |
| heart failure | 14(8.5) | 30(14.9) | 0.062 |
| aspirin therapy | 74(44.8) | 103(51.0) | 0.241 |
| statin therapy | 22(13.3) | 30(14.9) | 0.678 |
| beta-blockers therapy | 25(15.2) | 28(13.9) | 0.727 |
| calcium channel blocker | 48(29.1) | 75(37.1) | 0.105 |
| ACEI/ARB therapy | 42(25.5) | 65(32.2) | 0.159 |
A total of 408 patients with CAD and 367 patients without CAD were followed up for 2 to 4 years, the average follow-up time was 2.4±0.5 years. All hypertensives received regular antihypertensive drug treatment. In patients with CAD, incidence of cardiac death was 1. 5% in patients with CT and 2. 2% in patients without CT (P = 0.604). Incidence of coronary death (0.7% vs. 2.2%, P = 0.276), myocardial infarction (3.6% vs. 4. 8%, P = 0.594), as well as incidence of all cause mortality, coronary revascularization, non-fatal angina needing hospital admission and stroke were also similar between CT group and NCT group. The incidence of composite end points was also similar between the two groups (Table 6). There were no significant differences in the primary, second end point and composite end point between the CT and NCT group in fame or female with CAD (Table 7).
Table 6. Major adverse cardiovascular events of the patients followed-up.
| MACE | CAD Patients(n = 408) | Non CAD Patients(n = 367) | ||||
| CT group(n = 137) | NCT group(n = 271) | p value | CT group(n = 165) | NCT group(n = 202) | p value | |
| the primary end point | ||||||
| cardiac death | 2(1.5) | 6(2.2) | 0.604 | 1(0.6 ) | 1(0.5 ) | 0.886 |
| cardiac death due to CAD | 1(0.7) | 6(2.2) | 0.276 | 0(0) | 1(0.5) | 0.365 |
| acute myocardial infarction | 5(3.6) | 13(4.8) | 0.594 | 0(0 ) | 2(1.0) | 0.200 |
| the secondary end point | ||||||
| all cause mortality | 4(2.9) | 10(3.7) | 0.686 | 2(1.2) | 3(1.5) | 0.822 |
| coronary revascularization | 13(9.5) | 39(14.4) | 0.161 | 1(0.6 ) | 1(0.5) | 0.886 |
| non-fatal angina needing hospital admission | 50(36.5) | 103(38.0) | 0.766 | 1(0.6 ) | 1(0.5) | 0.886 |
| stroke | 12(8.8) | 33(12.7) | 0.298 | 7(4.2) | 9(4. 5) | 0.921 |
| composite end point | 55(40.1) | 130(48.0) | 0.134 | 11(6.7) | 13(6.4) | 0.929 |
Table 7. Major adverse cardiovascular events of the patients with CAD followed-up.
| MACE | Male (n = 249) | Female(n = 159) | ||||
| CT group(n = 63) | NCT group(n = 186) | p value | CT group(n = 74) | NCT group(n = 85) | p value | |
| the primary end point | ||||||
| cardiac death | 0(0) | 3(1.6) | 0.311 | 2(2.7 ) | 3(3.5 ) | 0.766 |
| cardiac death due to CAD | 0(0) | 3(1.6) | 0.311 | 1(1.4) | 3(3.5) | 0.382 |
| acute myocardial infarction | 2(3.2) | 11(5.9) | 0.398 | 3(4.1 ) | 2(2.4) | 0.540 |
| the secondary end point | ||||||
| all cause mortality | 1(1.6) | 6(3.2) | 0.496 | 3(4.1) | 4(4.7) | 0.842 |
| coronary revascularization | 6(9.5) | 24(12.9) | 0.476 | 7(9.5 ) | 15(17.6) | 0.136 |
| non-fatal angina needing hospital admission | 23(36.5) | 74(39.8) | 0.645 | 27(36.5 ) | 29(34.1) | 0.755 |
| stroke | 6(9.5) | 23(12.4) | 0.543 | 6(8.1) | 10(11. 8) | 0.445 |
| composite end point | 26(41.3) | 89(47.8) | 0.365 | 31( 41.9) | 36(42.4) | 0.953 |
In patients without CAD, there were no significant differences in the primary, second end point and composite end point between the two groups (Table 6).
Discussion
The major finding of present study is that CT is positively related with essential hypertension and female gender while negatively linked with CAD in this cohort. However, prognosis may be not affected by CT in patients with CAD. CT is a common coronary angiography finding. There are several possible mechanisms responsible for the formation of CT. Artery toutuosity may be associated with age, hypertention, atherosclerosis and genetic syndrome [8]–[11]. Hemodynamic forces are vital modulators of vascular structure. Arteries may become tortuous due to reduced axial strain and hypertensive pressure in an elastic cylindrical arterial model [12]. A reduction in axial strain results in arterial tortuosity attributable to aberrant MMP activity [13]. It has been demonstrated in animal model that enlargement, elongation, and tortuosity of artery is a adaptive change to high flow and high shear stress due to smooth muscle cell proliferation and endothelial cell proliferation, and distal migration [14]. Coronary tortuosity is more pronounced in patients with chronic pressure and decreases with volume overload [5]. The close association between hypertension and CT is expected in that CT might be one of the forms of artery remodeling induced by hypertension due to increased coronary pressure and blood flow. CT could thus be recognized as an adaptive change of hypertension. Our study also found female gender is associated with higher incidence of CT compared to males. It may be due to smaller cardiac chamber of female's for coronary tortuosity decreases with cardiac enlargement [15]. The difference of CT prevalence could not be explained by the incidence of hypertension since hypertension prevalence was similar between female and male patients in this cohort.
Interestingly, our study demonstrates that CT was negatively correlated with CAD. The relationship between arterial tortuosity and atherosclerosis are customarily considered to be co-enhanced. Groves et al [16] showed that severe coronary tortuosity was associated with a significant lower incidence of significant CAD. However, tortuosity of femoral arterial was shown to enhance the development of atherosclerosis [10]. Local hemodynamic factors, especially low shear stress plays an important role in influencing atherosclerosis and plaque stability through modulating endothelial cell function and gene expression, while high shear stress is associated with protection from atherosclerosis [17]–[19]. So the negative relatiopnship between CT and CAD may due to the effect on shear stress by CT. This may be a cause for lower incidence of CAD in female.
To the best of our knowledge, this is the first study to investigate the impact of CT on prognosis of patients with CAD. Our results showed that prognosis was not affected by CT in patients with CAD. Wang et al. [20] found that the elongation and tortuosity of internal carotid artery could result in decreased blood pressure in the distal segment of tortuous internal carotid artery, kinking of internal carotid artery may be one of the factors related to attack of cerebral ischemia and there was a significant association between carotid artery kinking and transient ischemic attacks [8].
Study limitation: It is to note that the effect of artery tortuosity could be affected by hemodynamic changes such as flow pattern and shear stress, these factors were not investigated in this study and further studies are warranted to clarify related issues. Moreover, tortuous coronary arteries were not evaluated with intravascular unltrasound in our study to determine whether arterial remodeling was present in the tortuous arterial segment [16]. The relative small patient number and short follow up time might be one of the reasons for the observed neutral impact of CT on prognosis in patients with CAD. A larger cohort and longer follow up time are needed to clarify the impact of CT on prognosis of patients with CAD.
In conclusion, CT is a common angiographic finding in Chinese population with suspected CAD. CT is positively related with essential hypertension and female gender but negatively linked with CAD. However, there is no significant difference in MACE among CAD patients with or without CT. Future studies with larger patient number and longer follow up time are warranted to clarify the impact of CT on prognosis of patients with CAD. Moreover, hemodynamic changes such as coronary pressur and shear stress in CT segment should be investigated in vivo and in vitro conditions.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported in part by the Open Project Program of the Jiangsu Key Laboratory of Medicine-high technology platform for molecular biological diagnosis and treatment of severe diseases, Jiangsu Provincial People's Hospital (no. KF200940). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Zhang XH, Lu ZL, Liu L. Coronary heart disease in China. Heart. 2008;94:1126–1131. doi: 10.1136/hrt.2007.132423. [DOI] [PubMed] [Google Scholar]
- 3.Zegers ES, Meursing BT, Zegers EB, Oude Ophuis AJ. Coronary tortuosity: a long and winding road. Neth Heart J. 2007;15:191–195. doi: 10.1007/BF03085979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turgut O, Yilmaz A, Yalta K, Yilmaz BM, Ozyol A, et al. Tortuosity of coronary arteries: an indicator for impaired left ventricular relaxation? Int J Cardiovasc Imaging. 2007;23:671–677. doi: 10.1007/s10554-006-9186-4. [DOI] [PubMed] [Google Scholar]
- 5.Jakob M, Spasojevic D, Krogmann ON, Wiher H, Hug R, et al. Tortuosity of coronary arteries in chronic pressure and volume overload. Cathet Cardiovasc Diagn. 1996;38:25–31. doi: 10.1002/(SICI)1097-0304(199605)38:1<25::AID-CCD7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 7.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 8.Pancera P, Ribul M, Presciuttini B, Lechi A. Prevalence of carotid artery kinking in 590 consecutive subjects evaluated by Echocolordoppler. Is there a correlation with arterial hypertension? J Intern Med. 2000;248:7–12. doi: 10.1046/j.1365-2796.2000.00611.x. [DOI] [PubMed] [Google Scholar]
- 9.Del Corso L, Moruzzo D, Conte B, Agelli M, Romanelli AM, et al. Tortuosity, kinking, and coiling of the carotid artery: expression of atherosclerosis or aging? Angiology. 1998;49:361–371. doi: 10.1177/000331979804900505. [DOI] [PubMed] [Google Scholar]
- 10.Smedby O, Bergstrand L. Tortuosity and atherosclerosis in the femoral artery: what is cause and what is effect? Ann Biomed Eng. 1996;24:474–480. doi: 10.1007/BF02648109. [DOI] [PubMed] [Google Scholar]
- 11.Satish G, Nampoothiri S, Kappanayil M. Images in cardiovascular medicine. Arterial tortuosity syndrome: phenotypic features and cardiovascular manifestations. Circulation. 2008;117:e477–478. doi: 10.1161/CIRCULATIONAHA.107.739839. [DOI] [PubMed] [Google Scholar]
- 12.Han HC. A biomechanical model of artery buckling. J Biomech. 2007;40:3672–3678. doi: 10.1016/j.jbiomech.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson ZS, Dajnowiec D, Gotlieb AI, Langille BL. Partial off-loading of longitudinal tension induces arterial tortuosity. Arterioscler Thromb Vasc Biol. 2005;25:957–962. doi: 10.1161/01.ATV.0000161277.46464.11. [DOI] [PubMed] [Google Scholar]
- 14.Sho E, Nanjo H, Sho M, Kobayashi M, Komatsu M, et al. Arterial enlargement, tortuosity, and intimal thickening in response to sequential exposure to high and low wall shear stress⋆. Journal of Vascular Surgery. 2004;39:601–612. doi: 10.1016/j.jvs.2003.10.058. [DOI] [PubMed] [Google Scholar]
- 15.Hutchins GM, Bulkley BH, Miner MM, Boitnott JK. Correlation of age and heart weight with tortuosity and caliber of normal human coronary arteries. Am Heart J. 1977;94:196–202. doi: 10.1016/s0002-8703(77)80280-9. [DOI] [PubMed] [Google Scholar]
- 16.Groves SS, Jain AC, Warden BE, Gharib W, Beto RJ., 2nd Severe coronary tortuosity and the relationship to significant coronary artery disease. W V Med J. 2009;105:14–17. [PubMed] [Google Scholar]
- 17.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85:9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 18.Gertz SD, Roberts WC. Hemodynamic shear force in rupture of coronary arterial atherosclerotic plaques. Am J Cardiol. 1990;66:1368–1372. doi: 10.1016/0002-9149(90)91170-b. [DOI] [PubMed] [Google Scholar]
- 19.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, et al. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 20.Wang LJ, Wang DM, Zhao F, Liu JC, Lu J, et al. [Clinical study and numerical simulation of hemodynamics in the tortuosity of internal carotid artery]. Zhonghua Wai Ke Za Zhi. 2008;46:1658–1661. [PubMed] [Google Scholar]