Abstract
Pain is a significant medical concern and represents a major unmet clinical need. The ability to perceive and react to tissue-damaging stimuli is essential in order to maintain bodily integrity in the face of environmental danger. To prevent damage the systems that detect noxious stimuli are therefore under strict evolutionary pressure. We developed a high-throughput behavioral method to identify genes contributing to thermal nociception in the fruit fly and have reported a large-scale screen that identified the Ca2+ channel straightjacket (stj) as a conserved regulator of thermal nociception. Here we present the minimal anatomical and neuronal requirements for Drosophila to avoid noxious heat in our novel behavioral paradigm. Bioinformatics analysis of our whole genome data set revealed 23 genes implicated in Ca2+ signaling that are required for noxious heat avoidance. One of these genes, the conserved thermoreceptor TrpA1, was confirmed as a bona fide “pain” gene in both adult and larval fly nociception paradigms. The nociceptive function of TrpA1 required expression within the Drosophila nervous system, specifically within nociceptive multi-dendritic (MD) sensory neurons. Therefore, our analysis identifies the channel TRPA1 as a conserved regulator of nociception.
Introduction
Acute and chronic pain will affect most people at some stage in their lives [1]. Chronic pain in particular represents an unmet clinical need [2]. Nociception, the neuronal sensory and processing apparatus that relays the perception of acute pain, allows an organism to avoid potential tissue damage and death, and many genes regulating this process are conserved across phyla [3]. Transient Receptor Potential (TRP) channels are a family of sensory ion channels that were first identified in Drosophila melanogaster [4], [5], and have subsequently been identified as critical mediators of nociception in mammals [6]. The TRP family channel painless was identified in Drosophila using a larval heat probe assay [7]. While painless has no mammalian orthologue [8], [9], it is possible that other components of the Drosophila nociception apparatus are indeed conserved from flies to mammals.
To interrogate Drosophila for conserved genes that regulate nociception, we developed a high-throughput screening procedure [3]. This behavioral system utilizes the robust ability of adult fruit flies to rapidly avoid noxious heat. This system has led to the identification of hundreds of candidate fly “pain” genes, for example straightjacket (stj), as regulators of nociception behavior in Drosophila [3]. Here we show this innate avoidance behavior is independent of other sensory modalities known to promote avoidance responses, such as vision, olfaction, CO2 perception, hearing, and taste and requires intact antennae and proboscis for a full response. Importantly, painless-expressing neurons, but not the mushroom body which is required for sub noxious thermo-preference [10], are a necessity for this behavior. We further provide genetic evidence that one of the candidate pain genes, TrpA1, is a bona fide mediator of thermal nociception in the fly. Tissue-specific RNAi knockdown of TrpA1 revealed that TrpA1 functions in nociceptive multi-dendritic (MD) sensory neurons. Thus, TRPA1 regulates the Drosophila behavioral response to a noxious thermal insult. Combined with TRPA1's role in chemical nociception, our results identify TRPA1 as an evolutionary conserved regulator of polymodal nociception.
Results
Set-up of a high-throughput system to screen for nociception in Drosophila
We recently developed a high-throughput assay to perform an in vivo genome-wide pain screen in Drosophila [3]. Here we report the detailed set-up and anatomical/neuronal requirements for this novel behavioral paradigm, data we believe are essential for the field and future use of this system. In preliminary pilot studies to address nociceptive responses, we found the Drosophila response to noxious heat exposure more reliable and robust compared to mechanical pain paradigms (not shown). Furthermore, while the commonly used Drosophila larval nociception paradigm has proven suitable for identifying genes required for nociceptive behavior [7], it is labor intensive and requires evaluations of larval responses not compatible with large-scale applications.
To develop a high-throughput screening system in adult Drosophila, we first defined acute noxious heat thresholds. By subjecting flies to increasing gradients of noxious heat exposure, we found that flies rapidly became incapacitated following exposure to temperatures above 40°C (Figure 1A) and continued exposure to such temperature (>2 minutes) was lethal (not shown). Temperatures below 39°C did not incapacitate flies within the 10 minute time course. This thermal tolerance profile was very similar to that reported by others, indicating a common thermal tolerance threshold across Drosophila strains and experimental paradigms [11]. Since nociception is the sense an animal employs to detect and avoid potential harm, and because exposure to temperatures about 40°C were acutely harmful to Drosophila, we exploited this rapid incapacitation as a means of large-scale screening for nociception behavior. We therefore developed an experimental test chamber where the temperature of the base surface of the chamber could be controlled and rapidly increased when required, giving flies a choice between a hot and potentially lethal surface and a surface that remained close to room temperature.
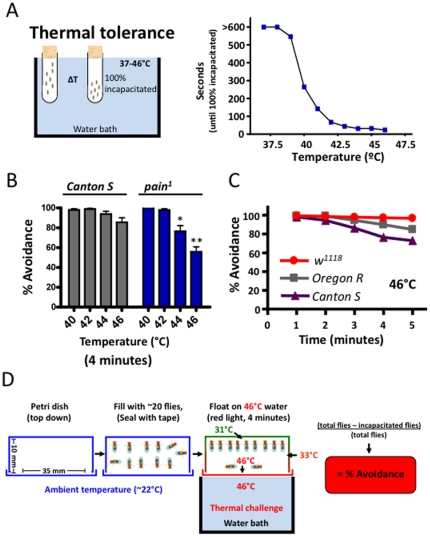
Figure 1. Development of an avoidance assay to noxious heat in adult Drosophila.
(A) Temperature-response profiles to identify acutely noxious temperatures for adult Drosophila. Experimental setup is depicted (left panel) and mean dose-response values are presented (right panel). (B) painless mutants (pain1) are impaired at avoiding noxious temperatures above 42°C compared to wild-type Canton S controls . wild type (Canton S) and pain1 avoidance responses are presented. Data are presented as mean +/− SEM. * P<0.05, ** P<0.01 by Student's t test. Data are presented as mean +/− SEM. (C) Time course of high temperature (46°C) thermal avoidance responses for 3 common Drosophila laboratory strains reveals robust avoidance responses in all strains tested. In all indicated experiments, n>20 progeny per group. (D) Schematic for high-throughput heat nociception using adult Drosophila. The final setup used for assaying heat nociception in Drosophila is depicted. Flies are placed into the experimental chamber and the chamber sealed with scotch tape. Flies are rested for at least 30 minutes in the dark, and the chambers then floated on a 46°C water bath for 4 minutes. Immobilized flies are counted as “incapacitated”. Moreover, total fly numbers are recorded to calculate the values for percent avoidance.
Using this paradigm, we found that wild-type Canton-S flies rapidly avoid all noxious temperatures tested. Flies mutant for the classical painless (pain1) gene could avoid surfaces heated up to 42°C, but failed avoiding the surface if the temperature is ≥42°C (Figure 1B). These differences between wild-type and pain1 flies were greatest at 46°C. To assess a potential influence of the genetic background, we assayed three different laboratory Drosophila melanogaster strains. Canton-S, Canton-Sw1118, and Oregon-R strains all rapidly and reproducibly avoided the heated surface during the course of the experiment (Figure 1C). Our final experimental apparatus involves an inverted petri dish with ∼20 flies, sealed with tape, and floated on a 46°C water bath (Figure 1D). The chamber is 35 mm wide with a 10 mm distance between the hot and warm surfaces. The bottom heated surface reaches 46°C within 15 seconds of the experiment, while the internal top and middle surfaces reach 31°C and 33°C by the end of the 4 minute test period. The maximum internal air temperature recorded during the experiment was 31°C. Using this system we can generate a % avoidance value for each genotype tested (Figure 1D). Thus, adult Drosophila exhibit a robust and highly reproducible innate avoidance response to noxious heat, and in the fly this response is dependent on the painless gene.
Mapping anatomical structures critical for high temperature nociception in Drosophila
Little is known about the anatomy regulating the behavioral response to an acute noxious insult in adult Drosophila [12]. We therefore assayed whether noxious heat avoidance requires sensory and higher order neurons implicated in other avoidance behaviors. Blocking synaptic transmission in neurons by driving UAS-Shits1 (Shibire ts1; a temperature sensitive dynamin mutant [13]) in subsets of neurons controlling olfaction (OR83b-Gal4), hearing and hygrosensation (nan-Gal4), and vision (GMR-GAL4) did not affect nociceptive behaviors, indicating that avoidance of noxious heat is independent of these sensory modalities (Figure 2A). Furthermore, thermal nociception did not require neurons previously implicated in other aversive behaviors such as the Gr21a-expressing CO2-sensitive olfactory neurons [14], or neurons expressing neuropeptide F (NPF-Gal4>UAS-Shits1) or its receptor (NPFR-Gal4>UAS-Shits1) which have been implicated in bitter taste avoidance [15]. Importantly, thermal nociception was dependent on synaptic transmission in painless-expressing neurons (Figure 2A). Taken together, these findings indicate that thermal nociception in adult Drosophila requires painless expressing neurons but does not rely on sensors or cells previously implicated in vision, olfaction, hearing, CO2 sensing, hygrosensation, or bitter avoidance.
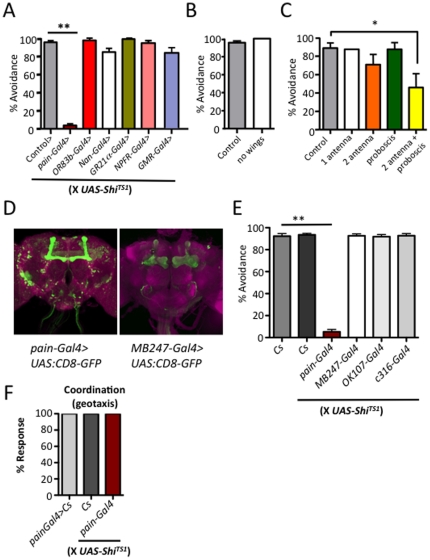
Figure 2. The Drosophila antenna, proboscis, and painless expressing neurons are required for avoidance of noxious heat.
(A) Synaptic output from painless expressing neurons (painless-Gal4>UAS-Shi ts1), but not other sensory modalities tested, is required to avoid noxious heat. Sensory nerves mediating olfaction (OR83b-Gal4>UAS-Shits1), hearing and hygrosensation (Nan-Gal4>UAS-Shits1), the CO2 sensing apparatus (GR21α-Gal4>UAS-Shits1), bitter avoidance (NPFR-Gal4>UAS-Shits1), and vision (GMR-Gal4>UAS-Shits1) are not required for thermal nociception. Control line is w1118×UAS-Shi ts1. (B) Wings are not required for noxious thermal avoidance in Drosophila. Wings were dissected off and flies were tested for the ability to avoid noxious temperature. (C) Antenna and proboscis cooperate to promote avoidance of noxious temperature. One or both antenna and the proboscis were dissected from wild type flies and then tested for avoidance of noxious temperature. (D) pain-Gal4 driving UAS-CD8:GFP labels painless expressing neurons in the mushroom body and other regions of the adult fly brain. MB247-Gal4>UAS-CD8:GFP labels the mushroom body. (E) Synaptic output from painless expressing nerves (pain-Gal4>UAS-Shits1), but not neurons from the mushroom body (MB247-Gal4>UAS-Shits1, OK107-Gal4>UAS-Shits1) or mushroom body associated DPM neurons (c316-Gal4>UAS-Shits1), is required for thermal nociception in adult Drosophila. Cs (Canton S). (F) Synaptic silencing of painless expressing nerves (pain-Gal4>UAS-Shits1) does not affect basic motor coordination as assayed by a negative geotactic assay. All experiments involving UAS-shibireTS1 were pre-incubated at 30°C for 1 hour to induce shibire-mediated synaptic silencing. Data are presented as mean values +/− SEM. ∼20 flies tested per group, in replicates of at least four cohorts. In all experiments adult flies were challenged with 46°C as outlined in Figure 1D. * P<0.05, ** P<0.01 (Student's t-test).
To map the anatomy of thermal nociception in adult fly, we surgically removed the Drosophila wings, proboscis and antennae to determine if these structures are involved in the response to avoid noxious heat. We found flies lacking wings respond normally to noxious heat, indicating that wings are not required for thermal nociception (Figure 2B). Previous studies have implicated the third-antennal segment as a component of the sensory apparatus required for rapid avoidance of elevated temperatures between 25°C and ∼33°C [16], [17]. We observed a modest requirement for antenna in avoidance of noxious heat, which appeared to cooperate with the proboscis in the observed avoidance response (Figure 2C). Thus, the antenna and proboscis are candidate organs for sensing noxious heat.
In the adult fly, it has been reported that painless is expressed in the wing, proboscis, leg, and in central brain neurons that include the mushroom bodies ([18] and Figure 2D). Mushroom bodies have been implicated in thermal preference in the more long-term (15 minutes) behavioral response to sub-noxious temperatures [10]. To silence mushroom body neurons, we used the mushroom body drivers OK107-Gal4 [19], [20] and MB247-Gal4, two of the same Gal4 driver implicated in sub-noxious thermal preference [10], both of which express broadly throughout the mushroom bodies [21]. In addition we used the mushroom body associated DPM driver c316-Gal4 [22]. Using the Gal4/UAS system to express Shi ts1, we again found that synaptic silencing of pain-Gal4 neurons was sufficient to abolish the heat avoidance response. However, silencing of the mushroom body itself, or mushroom body associated neurons, had no effect on the avoidance response to noxious heat (Figure 2E). Flies with UAS-Shits1, or pain-Gal4 alone showed wild-type avoidance, indicating the avoidance defects observed in pain-Gal4;UAS-shits1 are due to the silencing of pain-expressing neurons. Importantly, silencing of painless-expressing neurons did not result in general coordination defects, as assessed by negative geotactic response (Figure 2F). Thus, pain-Gal4 expressing cells outside of the mushroom bodies are required for adult Drosophila to sense noxious heat.
Genes implicated in calcium signaling are over-represented among candidate heat nociception genes
We have previously reported 580 candidate genes involved in nociception in Drosophila [3]. Based on these primary hits, we performed hypergeometric enrichment analysis using KEGG pathways and Broad Institute C2 gene sets to identify groups of genes that were over-represented in our genome wide screen for nociception. One prominent gene category identified by this analysis was calcium signaling (Figure 3A, Table S1 for full data set). For instance, Calphotin (Cpn; CG4795) is a calcium binding molecule implicated in rhabdomere development. Moreover, we hit the calcium channel subunit straightjacket as we have reported in detail elsewhere [3]. We also found the fly ortholog of DREAM (Calsenilin, KChip3), a calcium-regulated central pain gene in mice [23], the calcium regulated cell adhesion molecule CG7100 (CadN), a calcium-dependent EF hand protein serine/threonine phosphatase (CG6571, rdgC), CAMKII, a gene that has also been linked to the modulation of TRPV1 channel function [24], as well as the CAMKII activator Caki (CG6703, Camguk), a member of the MAGUK family of proteins that contains a CaMKII-like domain and participates in regulation of calcium channel function in other species [25]. Finally, our approach identified dTrpA1 (TrpA1), the Drosophila ortholog of the chemical and cold sensing human TRPA1, a Ca2+-permeable non-selective cation channel implicated in acute chemical pain and cold hypersensitivity in rodents [26], [27] and infrared sensation in snakes [28].
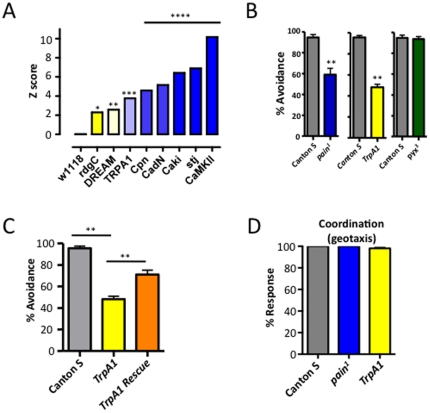
Figure 3. TrpA1 is a novel Drosophila nociception gene.
(A) Thermal avoidance of a select list of elav-Gal4×UAS-IR lines targeting genes involved in Ca2+ signaling are depicted with Z-score and calculated significance. (B) TrpA1 and painless (pain1), but not pyrexia (pyx3) mutant flies exhibit defects in thermal nociception in adult Drosophila. (C) Re-introduction of TrpA1 on the TrpA1 mutant background is sufficient to rescue the defective adult thermal nociception response, establishing that the observed thermal nociception defect is specific to TrpA1 expression and not the result of secondary effects. (D) Loss of painless or TRPA1 does not affect basic motor coordination as assayed by a negative geotactic assay. Data are presented as mean values +/− SEM. ∼20 flies tested per group, in replicates of at least four cohorts. In all experiments ∼20 flies were tested per group in replicates of at least four. In all experiments adult flies were challenged with 46°C as outlined in Figure 1D. * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0005 (based on (A) Z score and (B–C) Students t-test).
TRPA1 is required for thermal nociception in both larvae and adult Drosophila
TrpA1 is a member of the TRPA subfamily of TRP channels. Drosophila TRPA1 is the fly ortholog of human TRPA1 and the two proteins share ∼33% overall sequence identity, whereas other insect TRPA family members such as painless and pyrexia belong to a distinct subclass of TRPAs lost during vertebrate evolution [8]. Drosophila TRPA1 has been found to act as a warmth-activated ion channel required for thermotaxis at non-noxious temperatures and to act as a receptor mediating avoidance of reactive electrophilic chemicals [8], [29], [30]. To definitively demonstrate that fly TrpA1 is essential for noxious heat avoidance, we tested whether classical mutants for TrpA1 exhibit impaired avoidance of noxious heat. Consistent with the RNAi knockdown results, animals homozygous for a loss-of-function mutation in TrpA1 (TrpA1ins) [30] failed to avoid noxious temperature to a level similar to painless mutants (Figure 3B). To determine if the observed TrpA1 adult pain phenotype was the result of increased temperature-induced paralysis at 46°C, we exposed TrpA1 and control flies to a chamber set to 46°C and recorded the kinetics of temperature-induced paralysis. In support of an adult pain phenotype, we observed no difference in temperature-induced paralysis between these lines (36.6+/−1.4 seconds control, 33.2+/−1.3 seconds TrpA1 flies, n = 12, not significant by t test). The avoidance defect was rescued by reintroduction of a TrpA1 minigene into the TrpA1 mutant background, confirming that the observed defect in thermal nociception was due to the disruption of TrpA1 (Figure 3C). In contrast, we did not observe a noxious heat avoidance phenotype in a mutant for another Drosophila TRPA, pyrexia (pyx3) (Figure 3B), previously implicated in high temperature (40°C) thermal tolerance [31]. Importantly, painless and TRPA1 mutant flies exhibit normal coordination as assessed by a negative geotactic response (Figure 3D). Thus, both painless and TrpA1 are required for avoidance of noxious heat in adult Drosophila.
As TrpA1 participates in thermal nociception in adult Drosophila, we also assayed whether TrpA1 is involved in thermal pain behavior in larvae (Figure 4). Larvae were gently touched with a 46°C probe and avoidance time was measured. While control larvae showed a rapid response to noxious heat, the TrpA1 mutants showed a significantly diminished capacity for thermal nociception (Figure 4). In contrast to adult flies, pyx3 mutant larvae also showed an impairment in this larval nociception assay. Thus, we report a key role for Drosophila TrpA1 in larval and adult thermal nociception, i.e. TrpA1 is a bona fide pain gene in Drosophila.
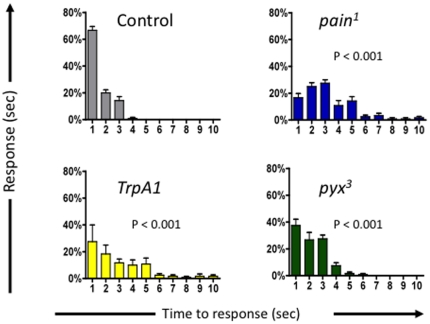
Figure 4. Both Painless and TrpA1 are required for thermal nociception in the Drosophila larvae.
TrpA1 (TrpA1ins), painless (pain1), and pyrexia (pyx3), mutant larvae were tested for their response to high temperature using a 46°C probe. % Response for each genotype is presented at each second within a ten second test period. A Kruskal-wallis non-parametric test for median comparison followed by the Dunn's post-hoc test was used for statistical analysis. P values are indicted in the panels. Data are presented as mean values +/− SEM. In all experiments ∼20 larvae were tested per group in replicates of at least four.
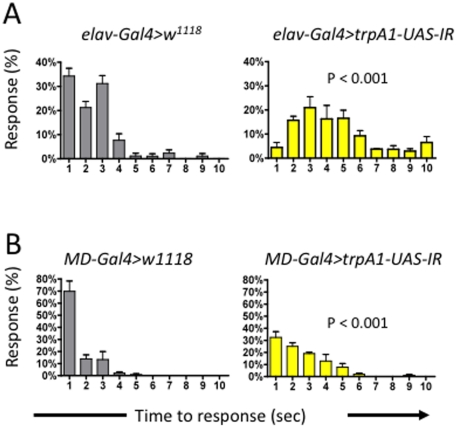
Blocking neurotransmission from multi-dendritic (MD) sensory neurons reduces thermal nociception responses in Drosophila larvae [7]. To localize the site of TrpA1 function in thermal nociception we therefore employed UAS-TrpA1-IR flies. Driving TrpA1-RNAi in all neurons using elav-Gal4 (elav-Gal4>TrpA1-UAS-IR) resulted in significant impairment of the larval thermal nociception response (Figure 5A). Driving TrpA1 RNAi in MD-sensory neurons alone (MD-Gal4>TrpA1-UAS-IR) also impaired the larval thermal nociception response (Figure 5B). These results indicate that TRPA1 is, at least in part, acting in multi-dendritic sensory neurons.
Figure 5. TrpA1 functions in multi-dendritic sensory neurons in the larval pain response.
Larvae pain response profiles in (A) elav-Gal4>TrpA1-UAS-IR lines to target TrpA1 in all neurons and (B) MD-Gal4>TrpA1-UAS-IR to target TrpA1 in sensory neurons. % Response to a 46°C heat probe is presented for each genotype at each second within a ten second test. A Kruskal-wallis non-parametric test for median comparison followed by the Dunn's post-hoc test was used for statistical analysis. P values are indicted in the panels. Data are presented as mean values +/− SEM. In all experiments ∼20 flies were tested per group.
Discussion
Our novel high-throughput system for assessing nociception behavior in adult Drosophila has allowed us to screen the entire Drosophila genome to examine the neural basis of thermal nociceptive behavior. Here we describe neuronal requirements and anatomical structures involved in this innate behavioral paradigm of thermal nociception. We also provide first experimental proof that TrpA1 functions in sensory neurons as a novel component of the Drosophila thermal nociception apparatus.
TRPA1 is recognized as a key component of the nociception apparatus in mammals. Not only do mice mutant for TRPA1 show defects in nociception, a mutation in TRPA1 underlies a human episodic pain syndrome [32]. At the molecular level, the role of the mammalian TRPA1 as a receptor for electrophilic chemicals and other irritants is well established [27], and the mechanism of how TRPA1 functions in chemical nociception is highly conserved between humans and flies [8]. Mammalian TRPA1 is also implicated in cold nociception, at least under pathological conditions [26], suggesting it contributes to multiple nociceptive sensory modalities. In Drosophila, TrpA1 has been shown to act as an internal thermosensor regulating temperature preference at non-noxious temperatures [30] and as a chemoreceptor for noxious electrophilic irritants [8]. That TRPA1 participates in both subnoxious thermal preference [30] and avoidance of noxious heat is an interesting observation. This could reflect distinctions between the mechanisms that control responses to steep versus shallow gradients, with thermal preference behavior involving long-term (>15 minute) exposure to shallow (∼0.5°C/mm) temperature gradients [30], while the current assay assesses behavioral short-term (<4 minutes) responses to much steeper gradients (∼4.5°C/mm by assay endpoint). These potential distinctions are currently being explored in our laboratories.
Our study expands the role of Drosophila TrpA1 signaling to responses to noxious heat. Combined with recent work demonstrating its role in chemical nociception [8], TrpA1 mediates both chemical and thermal nociception in Drosophila. Thus, while the temperature-responsiveness of TRPA1 has undergone significant diversification within the animal lineage, from being heat-activated in flies [33] and snakes [28] to potentially cold-responsiveness in mammals [26], [34], a role for TRPA1 in polymodal nociception has been retained from flies to mammals.
In addition to TrpA1, painless and stj (and to a lesser extend pyrexia in larvae) are required for the pain response in Drosophila. Thus it appears that, similar to mammals, multiple cation channels are involved in pain-responses in flies. While painless and pyrexia encode insect-specific TRPA-channels, TrpA1 and stj are conserved in humans. Both TRPA1 and stj (CACNA2D3 in humans) have been implicated in human nociception suggesting core genetic regulators of nociception are strongly conserved from flies to humans [3].
The ability to perform high-throughput screening for mediators of nociception has until now been limited to in vitro models and not intact behaving animals. Our in vivo system expands the toolbox available for pain researchers. Coordinated use of this fly system could accelerate the identification of new compounds to be short listed for validation in mammalian models of pain. Using this novel paradigm, we have now described hundreds of new candidate “pain” genes, opening the field up to multiple new candidate analgesic targets. Among these genes, our RNAi-screen identified multiple genes that are predicted to play a role in Ca2+ signaling such as TRPA1. Future analysis of these genes should provide valuable insight into the neural basis of nociceptive behavior and the role of calcium signaling in pain.
Materials and Methods
Fly stocks
UAS-IR transgenic fly lines and Canton S, Oregon R, and w1118 flies were obtained from the VDRC RNAi library [35]. elav with UAS-Dicer 2 was a gift from B. Dickson (Institute for Molecular Pathology) [35]. painless (EP(2)2451), pain-Gal4 and MD-Gal4 were gifts from D. Tracey (Duke Medical School). Pyrexia3 was a gift from J. Kim (Korea Advanced Institute of Science & Technology). Nan-Gal4 was a gift from C. Kim (Chonnam National University). GR21a-Gal4 was a gift from D. Anderson (Cal Tech). NPFR-Gal4 was obtained from P. Shen (University of Georgia). OR83b-Gal4 was a gift from L. Vosshall (Rockefeller University). MB247-Gal4 was generated by Robert Schulz [21], OK107-Gal4 was described in [19] and characterized further in [20], and c316-Gal4 was described in ref. [22]; all three lines were provided by Scott Waddell (University of Massachusetts Medical School). Eyeless-Gal4, gmr-Gal4, UAS-Shibirets1, and UAS-CD8-GFP were obtained from Bloomington. dTrpA1ins and TrpA1 rescue lines have been previously described [30].
Behavior experiments
For adult avoidance of noxious heat, ∼20 four day old flies were placed into a behavioral chamber (35 mm×10 mm Petri dish; Nunclon) and the chamber was sealed with scotch tape. Flies were rested for at least 30 minutes in the dark. The chambers were then floated on a 46°C water bath for 4 minutes. The bottom of the chamber was heated to 46°C over 15 seconds by floating on a water bath while the sub-noxious zone was measured to be 31°C (inner top of chamber) and 33°C (middle edge of the chamber) at the end of the 4 minute experiment. Chamber temperature was monitored using an electronic thermometer (Testo 925, Germany) coated in heat sink gel (RS components, UK). Chambers were then removed from the water and immobilized flies were counted as “incapacitated”. In addition, total fly number was recorded. Percentage Avoidance was calculated by determining the percentage of flies that avoid the noxious temperature compared to the total number of flies in the chamber. All tests were performed under low red light. Of note, this assay is an absolute measurement where flies that avoid the bottom heated surface were considered capable of noxious thermal avoidance, independent of how far they move away from the 46°C surface, though the vast majority of flies avoiding the hot surface were found on the top of the chamber. For experiments involving UAS-Shibirets1, flies were transferred to the experimental chambers followed by a temperature shift to 30°C for 60 minutes. Larval pain assays were performed as described [7]. For assessing noxious temperature-induced paralysis, wild type flies were placed in 5 ml polystyrene round bottom tubes (BD Falcon, Germany) and exposed to temperatures ranging from 37–46°C with 1° increments) or only 46°C (for control vs TrpA1 flies) and the temperature at which 100% of flies were paralyzed was recorded. General coordination was assessed by tapping the test chamber on the bench and observing activity as flies move away from the site of impact [35]. This response was quantified using a geotactic repulsion assay where flies were knocked to the bottom of a 15 ml polystyrene tube and the geotactic response (number of flies climbing up the tube / total number of flies) was recorded.
Confocal microscopy
Drosophila brains were dissected in PBS, fixed in 4% paraformaldehyde in PBS for 30 min at room temperature (RT), washed three times for 10 min in PBS containing 0.3%Triton X-100, blocked for 1 hr at RT in PBT containing 5% normal goat serum, and incubated with primary anti-GFP (Sigma) and NC82 (Iowa Hybridoma Bank) counterstain antibodies in blocking solution overnight at 4 C. Samples were washed three times for 10 min in PBT at RT, and secondary antibodies were applied in blocking solution for 2 hr at RT. After washing three times for 10 min in PBS, samples were mounted in Vectashield (Vector Labs). Confocal images were captured on a Zeiss LSM510 Meta, Axiovert 200 M, and processed with LSM510 Image Examiner.
Hypergeometric enrichment test
A hypergeometric test, similar to the test used for GO enrichment analysis, was used to identify over-represented gene lists (C2 from Msigdb, BROAD Institute) and pathways (KEGG) amongst the pain hits. The hypergeometric test considers only the percentage representation of genes corresponding to a biological pathway in the pre-computed heart function gene list. This analysis was performed on the gene list identified as adult pain hits (Z-score>1.65) in Drosophila and their corresponding mouse or human orthologs.
Statistics
For analysis of adult Drosophila avoidance responses a Student's t test was performed. For analysis of larval pain behavior we have performed the Kruskal-wallis non-parametric test for median comparison followed by the Dunn's post-hoc test. For presentation of screening data, a Z-score was generated from (mean control avoidance − mean test avoidance)/standard deviation control) and P values were generated from total Z-score distributions. Unless otherwise indicated, data are represented as mean values ± SEM.
Supporting Information
Ca2+ signalling involved in thermal nociception responses. Ca2+ signaling components (elav-Gal4>UAS-IR fly lines) that exhibit defects in thermal nociception are listed from the strongest to weakest phenotype for noxious heat avoidance. An avoidance Z-score +/− SEM and repetitions are included. Lethality was scored for each cross (0 = lethal, 0.5 = semi-lethal, 1 = viable). A mean score of ≤0.6666 was considered lethal. A qualitative coordination score (0 = uncoordinated, 1 = coordinated) +/− SEM and number of repetitions are indicated for the re-screened lines. The CG numbers according to flybase annotation version 4.3 (FB4.3) and flybase annotation 5.7 (FB5.7) are included, as is the S19 score for RNAi off targeting effects (OTEs) and number of can repeats. A p-value is also included. Drosophila gene symbols and predicted human and mouse orthologs are shown.
(XLSX)
Acknowledgments
We thank all members of our laboratories and the VDRC for helpful discussions and excellent technical support. We thank Ricardo de Matos Simoes for support with statistical analysis. We especially thank B.J. Dickson for kindly supplying elav/UAS Dicer 2 stocks.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: JP is supported by grants from Institute of Molecular Biotechnology, the Austrian Ministry of Sciences, the Austrian Academy of Sciences, Genome Research in Austria (AustroMouse), and European Union European Research Council Advanced Grant. AK is supported by National Institutes of Health (NIH) National Research Service Award 1 F32GM086207-01. CW is supported by NIH NS039518 and NS038253. GN was supported by a Marie Curie Incoming International Fellowship and EuroThymaide. PG is supported by NIH NS044232. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001;17:417–431, v. doi: 10.1016/s0749-0690(05)70078-1. [DOI] [PubMed] [Google Scholar]
- 2.Woolf CJ. Overcoming obstacles to developing new analgesics. Nat Med. 2010;16:1241–1247. doi: 10.1038/nm.2230. [DOI] [PubMed] [Google Scholar]
- 3.Neely GG, Hess A, Costigan M, Keene AC, Goulas S, et al. A genome-wide Drosophila screen for heat nociception identifies alpha2delta3 as an evolutionarily conserved pain gene. Cell. 2010;143:628–638. doi: 10.1016/j.cell.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- 5.Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Woolf CJ. Pain TRPs. Neuron. 2005;46:9–12. doi: 10.1016/j.neuron.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 8.Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, et al. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glauser DA, Chen WC, Agin R, Macinnis BL, Hellman AB, et al. Heat Avoidance Is Regulated by Transient Receptor Potential (TRP) Channels and a Neuropeptide Signaling Pathway in Caenorhabditis elegans. Genetics. 2011;188:91–103. doi: 10.1534/genetics.111.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong ST, Bang S, Hyun S, Kang J, Jeong K, et al. cAMP signalling in mushroom bodies modulates temperature preference behaviour in Drosophila. Nature. 2008;454:771–775. doi: 10.1038/nature07090. [DOI] [PubMed] [Google Scholar]
- 11.Gioia A, Zars T. Thermotolerance and place memory in adult Drosophila are independent of natural variation at the foraging locus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195:777–782. doi: 10.1007/s00359-009-0455-2. [DOI] [PubMed] [Google Scholar]
- 12.Xu SY, Cang CL, Liu XF, Peng YQ, Ye YZ, et al. Thermal nociception in adult Drosophila: behavioral characterization and the role of the painless gene. Genes Brain Behav. 2006;5:602–613. doi: 10.1111/j.1601-183X.2006.00213.x. [DOI] [PubMed] [Google Scholar]
- 13.Dubnau J, Grady L, Kitamoto T, Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411:476–480. doi: 10.1038/35078077. [DOI] [PubMed] [Google Scholar]
- 14.Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q, Zhao Z, Shen P. Regulation of aversion to noxious food by Drosophila neuropeptide Y- and insulin-like systems. Nat Neurosci. 2005;8:1350–1355. doi: 10.1038/nn1540. [DOI] [PubMed] [Google Scholar]
- 16.Sayeed O, Benzer S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc Natl Acad Sci U S A. 1996;93:6079–6084. doi: 10.1073/pnas.93.12.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zars T. Two thermosensors in Drosophila have different behavioral functions. J Comp Physiol A. 2001;187:235–242. doi: 10.1007/s003590100194. [DOI] [PubMed] [Google Scholar]
- 18.Al-Anzi B, Tracey WD, Jr, Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol. 2006;16:1034–1040. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Connolly JB, Roberts IJ, Armstrong JD, Kaiser K, Forte M, et al. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science. 1996;274:2104–2107. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- 20.Aso Y, Grubel K, Busch S, Friedrich AB, Siwanowicz I, et al. The mushroom body of adult Drosophila characterized by GAL4 drivers. J Neurogenet. 2009;23:156–172. doi: 10.1080/01677060802471718. [DOI] [PubMed] [Google Scholar]
- 21.Zars T, Fischer M, Schulz R, Heisenberg M. Localization of a short-term memory in Drosophila. Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]
- 22.Waddell S, Armstrong JD, Kitamoto T, Kaiser K, Quinn WG. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell. 2000;103:805–813. doi: 10.1016/s0092-8674(00)00183-5. [DOI] [PubMed] [Google Scholar]
- 23.Cheng HY, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, et al. DREAM is a critical transcriptional repressor for pain modulation. Cell. 2002;108:31–43. doi: 10.1016/s0092-8674(01)00629-8. [DOI] [PubMed] [Google Scholar]
- 24.Jung J, Shin JS, Lee SY, Hwang SW, Koo J, et al. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- 25.Hsueh YP. The role of the MAGUK protein CASK in neural development and synaptic function. Curr Med Chem. 2006;13:1915–1927. doi: 10.2174/092986706777585040. [DOI] [PubMed] [Google Scholar]
- 26.del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, et al. TRPA1 contributes to cold hypersensitivity. J Neurosci. 2010;30:15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 28.Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, et al. Molecular basis of infrared detection by snakes. Nature. 2010;464:1006–1011. doi: 10.1038/nature08943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, et al. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee Y, Lee Y, Lee J, Bang S, Hyun S, et al. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat Genet. 2005;37:305–310. doi: 10.1038/ng1513. [DOI] [PubMed] [Google Scholar]
- 32.Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, et al. A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron. 2010;66:671–680. doi: 10.1016/j.neuron.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, et al. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- 34.Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ca2+ signalling involved in thermal nociception responses. Ca2+ signaling components (elav-Gal4>UAS-IR fly lines) that exhibit defects in thermal nociception are listed from the strongest to weakest phenotype for noxious heat avoidance. An avoidance Z-score +/− SEM and repetitions are included. Lethality was scored for each cross (0 = lethal, 0.5 = semi-lethal, 1 = viable). A mean score of ≤0.6666 was considered lethal. A qualitative coordination score (0 = uncoordinated, 1 = coordinated) +/− SEM and number of repetitions are indicated for the re-screened lines. The CG numbers according to flybase annotation version 4.3 (FB4.3) and flybase annotation 5.7 (FB5.7) are included, as is the S19 score for RNAi off targeting effects (OTEs) and number of can repeats. A p-value is also included. Drosophila gene symbols and predicted human and mouse orthologs are shown.
(XLSX)