Abstract
Background
Extracellular superoxide dismutase (SOD3), which dismutates superoxide anion to hydrogen peroxide, has been shown to reduce the free radical stress derived apoptosis in tissue injuries. Since both superoxide anion and hydrogen peroxide have a marked impact on signal transduction pathways and could potentially explain a number of apoptosis and survival -related phenomena in different pathological conditions, we clarified the impact of SOD3 on Akt and Erk1/2 cell survival pathways in rat hind limb injury model.
Methodology and Principal Findings
Based on our data, the hind limb ischemic rats treated with virally delivered sod3 have milder injury and less apoptosis than control animals that could be due to parallel activation of pro-proliferative and anti-apoptotic Erk1/2 and Akt pathways. The common downstream factor of both signaling pathways, the apoptosis related forkhead box protein O3a (FoxO3a), was phosphorylated and translocated to the cytoplasm in sod3 treated tissues and cell line. Additionally, we obtained increased mRNA production of elk-1, ets-1, and microRNA 21 (miR-21), whereas synthesis of bim mRNA was decreased in sod3 overexpressing tissues. We further showed that overexpression of sod3 modulated redox related gene expression by downregulating nox2 and inos when compared to injured control animals.
Conclusions and Significance
The study shows the complexity of SOD3-derived effects on tissue injury recovery that are not limited to the reduction of superoxide anion caused cellular stress but highlights the impact of SOD3 related signal transduction on tissue functions and suggests an important role for SOD3 in attenuating cell stress effects in different pathological conditions.
Introduction
Tissue ischemia induces rapid generation of reactive oxygen species (ROS) including superoxide (O2 −•), hydrogen peroxide (H2O2), and their derivatives, which along with acute lack of nutrient supply and disturbed cellular respiration cause severe damage to tissues. Extracellular superoxide dismutase (SOD3) is an antioxidative enzyme that converts superoxide into hydrogen peroxide thereby reducing oxidative cell stress [1], [2]. The enzyme is secreted to extracellular space where it reversibly binds to cell membrane at lipid rafts and therefore has a local impact on inactivation of phosphotyrosine phosphatases PTP1B and DEP1 [3]. In prolonged disease conditions, such as coronary artery disease, sod3 expression is decreased in a time-dependent manner [4]–[6] suggesting that the lack of the enzyme could deteriorate the condition. This is further supported by the data showing that sod3 overexpression has beneficial effects on the healing of cardiovascular injuries [7]–[10]. We have recently shown SOD3 to have a pro-proliferative effect in ischemic skeletal muscle, which is caused by SOD3-derived activation of the Ras-Erk1/2 mitogenic pathway and consequent increased growth factor expression [8] that partially elucidate the SOD3-mediated survival effect. Since the increased proliferation alone may not adequately explain the therapeutic effects caused by SOD3 and, more importantly, since previous studies have shown significantly decreased apoptosis after sod3 overexpression [7], [9], we focused in the present work on the mechanisms of SOD3-mediated reduced apoptosis in cardiovascular ischemia.
Based on our data, sod3 overexpression caused activation of Erk1/2 and Akt pathways involving cytoplasmic entry of FoxO3, increased miR-21 production, and decreased BCL-2 interacting mediator of cell death (bim) mRNA synthesis. The study suggests an important role for SOD3 in regulation of cellular signaling networks and a central impact on reduced injury development and apoptosis.
Materials and Methods
Rat hind limb ischemia model
Acute ischemic hind limb injury was induced in male Fischer 344 rats (5–6 weeks, 86–115g) by surgical ligation of distal femoral artery, lateral circumflex femoral artery and proximal femoral artery. The procedure was performed under anesthesia by single intraperitoneal injection of fentanyl fluanisone (370 µg/100 g; Janssen Pharmaceutica, Beerse, Belgium), and midazolame (180 µg/100 g; Roche, Basel, Switzerland). Adequacy of the anesthesia was determined based on the reactions of the animal during the surgery. Animals were recovering from the anesthesia in a separate pre-warmed environment. Immediately after ligation adenoviral vectors carrying rabbit sod3 or LacZ control genes, both 0.5×109 pfu in 50 µl PBS, were injected at 5 sites into the hind limb muscles before suturing the wound. Uninjured muscle was used as a control. The animals were followed for 3, 7 and 10 days (4 animals in normal control, LacZ and SOD3 groups each). All animal procedures were approved by the Southern Finland Regional Experimental Animal Committee (License STH350A), and done according to the European Commission and University of Turku guidelines.
Immunohistochemistry
The thigh muscles were cut crosswise, snap frozen in 2-methylbutane and embedded in Tissue-Tek Optimal Cutting Temperature compound (Sakura Finetek, Torrance, CA, USA). Ten micrometer cryosections were fixed in acetone and stained with hematoxylin/eosin (Sigma, St. Louis, MI, USA) according to the standard protocol, and photographed digitally with Zeiss Axiovert 200 M microscope and the AxioVision program (Carl Zeiss, Oberkochen, Germany). The injured area as percentage of the whole section was determined from 7 sections per group independently by three investigators using inflammatory cell invasion, increased connective tissue formation, and fragmentation of the muscle fibers as criteria for injury analysis. The injured muscle tissue area was calculated by determining the area (%) of the tissue section containing inflammatory cells or morphological damages as compared to inflammatory cell free tissue or tissue that did not show muscle fiber fragmentation of connective tissue. The final injured region was calculated from triplicate analyses (the analysis of three investigators).
Western blot analysis
Pooled rat muscle tissue from each group was homogenized with a Retsch MM400 mixer mill using metal beads (Retsch GmbH, Haan, Germany) in lysis buffer (50 mmol/l HEPES pH 7.5, 150 mmol/l NaCl, 10% glycerol, 1% Triton X-100, 1 mmol/l MgCl, 10 mmol/l NaF, 10 mmol/l sodium pyrophosphate, 1 mmol/l Na3VO4, 10 mmol/l approtinin, 10 µg/ml leupeptin) (Sigma). Antibodies for cleaved caspase-3 (Asp175), p-Akt (Ser473), Akt, p-Erk1/2 (Trh202/Tyr204), Erk1/2, p-FoxO3a (Ser318/321), p-FoxO3a (Thr32), tubulin (Cell signaling, Danvers, MA, USA), and SP-1 (Santa Cruz, Santa Cruz CA, USA) were used to detect the designated proteins from the blotted samples.
Cell culture and cell fractionation
NIH 3T3 cells (ATCC, Teddington, UK) were grown in DMEM 10% CS (Sigma) with penicillin-streptomycin (Sigma). Empty pcDNA3 control vector or human SOD3 cDNA (a kind gift from professor Stefan L. Marklund, University of Umeå, Sweden) subcloned into the pcDNA3 plasmid, were stably transfected using Fugene 6 (Roche, Mannheim, Germany). The cells were grown in the presence of geneticin (Sigma) and prepared for cell fractionation. Nuclear and cytoplasmic fractions of the cells were isolated using NE-PER Cell Fractionation kit (Thermo Scientific, Waltham, MA, USA). The cells were lysed with the NE-PER nuclear and cytoplasmic extraction reagents. The fractionated proteins were loaded on SDS gels according to the standard procedures.
Real-time quantitative PCR
Total RNA was extracted from pooled muscle samples of each animal group with Tri-reagent (Sigma). Complementary DNA synthesis was done with Revert-Aid M-MuLV (Fermentas, Burlington, Canada) and the quantitative PCR with SYBR Green master mix reagent (Applied Biosystems, Foster City, CA, USA). Primers were: rat beta-actin forward 5′-TCGTGCGTGACATTAAGGAG-3′ and reverse 5′-GTCAGGCAGCTCGTAGCTCT-3′; endogenous rat sod3 forward 5′-GAC CTG GAG ATC TGG ATG GA-3′ and reverse 5′-GTG GTT GGA GGT GTT CTG CT-3′; exogenous rabbit sod3 forward 5′-GTT GCG TGA GCG GAA AGA TG-3′ and reverse GTG AGC GCC TGC CAG ATC TC; nox2 forward 5′-TTG TTG CAG GAG TGC TCA TC-3′ and reverse 5′-CTG CCA GCA GGT AGA TCA CA-3′; inos forward 5′-GGT GCA GAA GCA CAA AGT CA-3′ and reverse 5′-GAA CTG GGG GAA ACC ATT TT-3′; elk-1 forward 5′-AGC GGC CAG AAG TTT GTC TA-3′ and reverse 5′-CTG TCA TTC CTG CAC CCT TT-3′; ets-1 forward 5′-GAA ATG ATG TCC CAG GCA CT-3′ and reverse 5′-CTT TAC CCA GGG CAC ACA GT-3′; bim forward 5′-ATC TCA GTG CAA TGG CTT CCA-3′ and reverse 5′-GCT CCT GTG CGA TCC GTA TC-3′; miR21 and RNU5 miScript Primer Assays (Qiagen, Hilden, Germany) were used to study the amplification and to normalize the miR-21 expression.
Statistical analyses
The experiments were repeated at least three times. All results are expressed as mean ±SD. The p-values (* = p<0.05, ** = p<0.01, *** = p<0.001) were determined by one-way Anova with Tukey-Kramer multiple comparison post-analysis test.
Results
SOD3 attenuates ischemic injury
Previously SOD3 has been shown to have growth regulatory [8], anti-inflammatory [11], anti-oxidative [2], and anti-apoptotic [7], [9] characteristics. Since the latter is not well characterized, in the present study we focused on sod3 overexpression-mediated cellular signaling events leading to decreased apoptotic signaling in ischemic tissue injury.
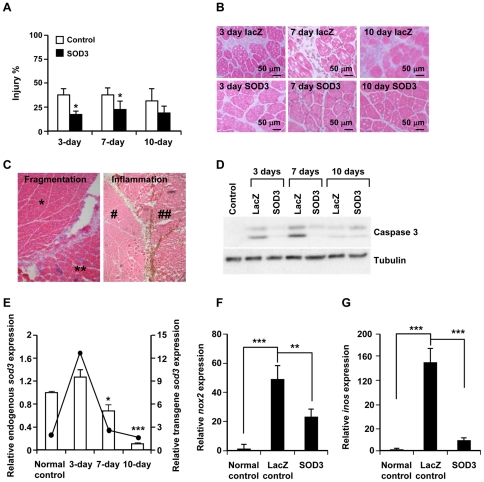
To justify that the in vivo model used in the study is applicable for the present aims we first determined the relative sizes of injured tissue areas from the histological cryo sections. We observed significantly smaller injury regions in sod3-treated animals as compared to control rats on 3-day (53% lower value, p<0.05) and on 7-day (40% lower value, p<0.05) time points (Figure 1A-C). To further characterize the effect of SOD3 and to verify whether the enzyme has an impact on modulating apoptosis in our model, we performed a cleaved caspase-3 Western blot that showed increased apoptotic activity in lacz control animals as compared to sod3-treated rats at 3-day and at 7-day time points (Figure 1D).
Figure 1. SOD3 overexpression affects tissue injury development and modifies redox enzyme expression.
(A) Sod3 transduced ischemic muscle tissues had significantly lower injury score at 3-day (p<0.05 (*)) and at 7-day (p<0.05 (*)) time points. White bars refer to lacz control animals and black bars to sod3 transfected rats. (B) Corresponding hematoxylin/eosin staining showed increased fibrosis in LacZ control animals in different time points as compared to SOD3 treated muscles. (C) The edges of the injured region were clearly visible. The left panel shows hematoxylin/eosin staining of normal uninjured (*) tissue region and injured fragmented (**) muscle section. In the right panel there is an inflammatory cell free region (#) and heavily positive CD68 inflammation muscle area (##). Since the fragmented and inflammatory cell positive regions were partially different the injured muscle tissue area was analyzed using both parameters separately. (D) Western blot with cleaved caspase-3 (17 kDa and 19 kDa) antibody indicated increased apoptosis at 3-day and 7-day time points in LacZ control animals as compared to sod3 treated animals. (E) Rat endogenous sod3 mRNA expression (open bars) was downregulated at 7-day and 10-day time points (p>0.05 (*) and p<0.001 (***), respectively). The overexpression of transgene sod3 (line) was highest 3 days after the gene transfer and reduced to background levels by the day 10. The left Y-axis refers to relative endogenous sod3 and the right Y-axis relative transgene sod3 expression. (F) Ischemic injury caused significantly (p<0.001) increased nox2 mRNA expression as compared to normal uninjured control animals. Sod3 overexpression attenuated the increase causing a significant (p<0.01 (**)) reduction of nox2 expression as compared to 3-day LacZ control animals. (G) Injury-related increased (p<0.001 (***)) inos expression was significantly (p<0.001 (***) decreased by sod3 expressing tissues.
Since it has been reported that the cardiovascular damages are characterized by reduced sod3 expression [4]–[6] we analyzed the expression of endogenous rat sod3 mRNA in ischemic skeletal muscle from the control animals at different time points. At 3-day time point there was an initial increase in sod3 mRNA synthesis that was followed by 2-fold (p<0.05) reduction at 7-day time point and 10-fold reduction (p<0.001) at 10-day time point as compared to normal uninjured muscle (Figure 1E). The down-regulation of endogenous sod3 expression in injured tissues was paralleled by increased levels of pro-inflammatory molecules, such as NADPH oxidase 2 (nox2) and inducible nitric oxide synthase (inos) that, as previously shown [9], [12], [13] can be modulated by sod3 overexpression. The injury-related marked increase in nox2 and inos mRNA production (Figure 1F–G) could be due to macrophage infiltration into the ischemic injury region [14]. However, the expression of both enzymes was significantly (p<0.01 and p<0.001, respectively) attenuated by sod3 overexpression as compared to lacz control muscles. These observations from animal tissues indicate that SOD3 overexpression has a significant impact on tissue injury related gene expression.
Erk1/2 pathway is the principal pathway in SOD3-mediated tissue recovery
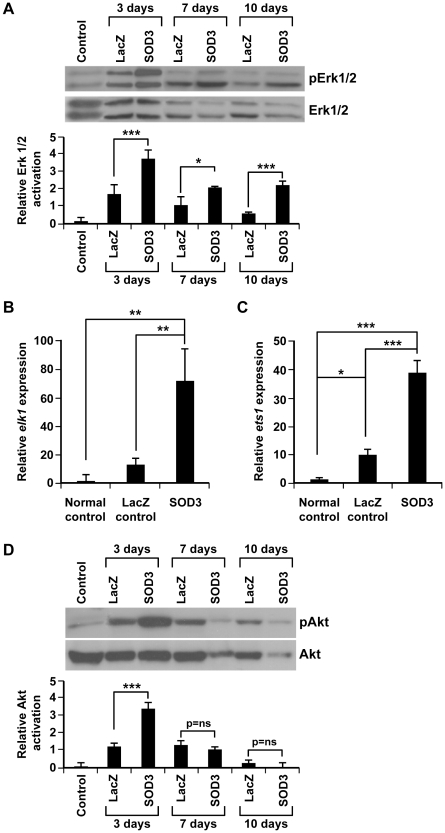
To explain the reduced injury development and reduced apoptosis in our hind limb injury model we next studied the cell survival signaling pathways that become activated in sod3-treated tissues. Since we have previously proved that sod3 is able to modulate the activation of Ras-Erk1/2 signaling pathway [8], which is a major regulator of cell growth and survival, we verified the level of Erk1/2 phosphorylation in injured control and sod3 treated animals as compared to uninjured tissues. Figure 2A shows that the injury itself is causing an increase of phospho-Erk signaling, and that this effect is significantly up-regulated by Sod3 treatment at 3-day (p<0.001), 7-day (p<0.05), and 10-day (p<0.001) time points. We further investigated the effect of SOD3 in modulating the activity of Erk1/2 pathway by monitoring its downstream target transcription factors Elk-1 and Ets-1 [15], [16]. Both elk-1 and ets-1 expressions were significantly (p<0.01 and p<0.001, respectively) increased in sod3 animals as compared to LacZ controls (Figure 2B–C). Interestingly, the injury itself had a minor effect on the synthesis of these two transcription factors, suggesting that sod3 overexpression plays an important role in the prolonged activation of mitogenic signal transduction routes controlling cell proliferation and tissue recovery. We then focused on the effect of SOD3 on the regulation of PI3K/Akt anti-apoptotic pathway to determine the reduced caspase-3 cleavage observed in sod3 treated animals by investigating phosphorylation of Akt in injured tissues. Our results showed that sod3 stimulated phosphorylation of Akt was visible only at early time point (3 days after the injury) (Figure 2D), whereas the simultaneous effect on Erk1/2 phosphorylation was long-lasting (up to 10 days) (Figure 2A).
Figure 2. The effect of SOD3 on Erk1/2 signaling pathway.
(A) Western blot analysis showed increased Erk1/2 phosphorylation in sod3 transduced muscles at 3-day, 7-day, and 10-day time points (p<0.001 (***), p<0.05 (*), and p<0.001 (***), respectively) as compared to corresponding lacz controls. The samples were normalized with total Erk1/2 antibody. Erk1/2 activation was supported by significant up-regulation of its downstream transcription factors (B) elk-1 (p<0.01 (**)) and (C) ets-1 (p<0.001 (***)), measured by mRNA synthesis in sod3 animals as compared to LacZ control tissues. The injury itself moderately increased production of both molecules as compared to normal uninjured tissue control. However, the significant difference between normal control tissue and LacZ control (p<0.05 (*)) was achieved only in ets-1 expression. (D) Sod3 transduced muscles have increased levels of Akt phosphorylation only at 3-day time point (p<0.001 (***)). The samples were normalized with total Akt antibody.
SOD3-mediated anti-apoptotic signaling
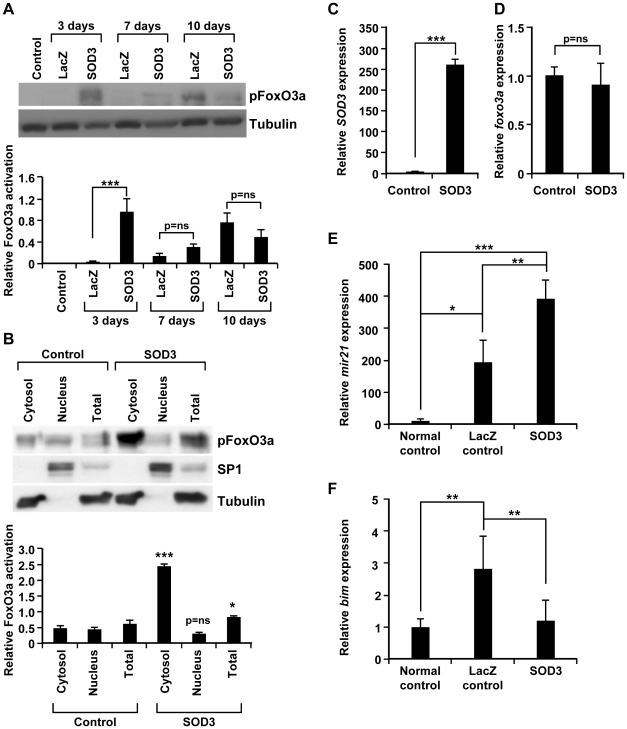
It has been shown that co-operative action of Akt and Ras-Erk1/2 signaling cascades mediate anti-apoptotic and pro-proliferative signals by e.g. causing FoxO3a phosphorylation, inactivation and consequent cytoplasmic entry [17], [18] that in ischemic tissues has been reported to result in reduction of transcriptional effect on FoxO3a target genes [19]. We therefore investigated whether SOD3, by modulating Erk1/2 and Akt activation, could also affect FoxO3a phoshorylation status. Based on our current data SOD3 causes increased FoxO3a phosphorylation at 3-day time point (Figure 3A). Since phosphorylation determines the nuclear/cytoplasmic location of FoxO3a activity, we further characterized the SOD3-derived effect on FoxO3a localization in NIH 3T3 cells. The cells were stably transfected with SOD3 or control eukaryotic expression vector, fractionated to separate nuclear and cytoplasmic compartments, and analyzed by Western blotting. According to our results sod3 transfected cells had markedly (p<0.05) more phosphorylated total FoxO3a, and interestingly the amount of phosphorylated protein was significantly (p<0.001) enriched in the cytosolic compartment as compared to control vector transfected cells (Figure 3B). To determine the effect of SOD3 on the total amount of FoxO3a, we then performed a real time PCR analysis and found that SOD3 expressing cells (Figure 3C) had similar levels of foxo3a mRNA as compared to control cells (Figure 3D), suggesting that SOD3 is mainly influencing the phosphorylation levels and consequent cytoplasmic entry of FoxO3a, but not the total protein amount.
Figure 3. SOD3-mediated anti-apoptotic signaling.
(A) The phosphorylation of FoxO3a (Thr32) in sod3 treated muscle tissues was strongly up-regulated at 3-day time point, had non-significant tendency at 7-day time point and showed no difference to LacZ control tissues at 10-day time point. The blot was normalized with tubulin antibody. (B) In vitro cell fractionation assay showed increased phosphorylation of FoxO3a in SOD3 stably transfected NIH 3T3 cells. The amount of phosphorylated protein was significantly (p<0.05 (*)) higher in the total cell extract and was enriched in the cytoplasmic fractions (p<0.001 (***)) of cells transfected with SOD3 when compared to control cells. Normalization was performed by using α-SP1 antibody for the nuclear fractions and α-tubulin antibody for the cytoplasmic fraction. For statistical analysis cytosol, nucleus, and total protein compartments were compared between control and SOD3 samples. (C–D) To determine the effect of SOD3 on the amount of total FoxO3a, we measured in NIH3T3 cells stably transfected with SOD3 (panel C) the mRNA synthesis of foxo3a (panel D) that showed no difference to control cells. (E) To verify whether FoxO3a targets were also regulated by SOD3 we then performed expression analysis for mir-21 in rat tissues showing significantly (p<0.05 (*)) increased miRNA synthesis caused by the ischemic injury that was further induced by sod3 overexpression (p<0.01 (**)) as compared to LacZ control animals). (F) Similarly, also the expression of bim, which is another target of FoxO3a, was found to be modulated by SOD3, as its expression was significantly stimulated (p<0.01 (**)) due to ischemia and then decreased by sod3 overexpression (p<0.01 (**)).
Since it was recently shown that in the nucleus FoxO3a suppresses the anti-apoptotic miR-21 transcription [20], [21] we next analyzed the microRNA (miRNA) expression. Based on the quantitative real time-PCR data, the expression of mir-21 in sod3 overexpressing ischemic tissues at 3-day time point was significantly (p<0.001) increased as compared to uninjured control muscles. Even though the injury itself had an activating effect on miRNA expression (p<0.05) sod3 was able to significantly (p<0.01) further increase it as compared to LacZ injured control tissues (Figure 3E). To characterize another FoxO3a downstream target we then checked the mRNA expression of pro-apoptotic bim, which is known to be downregulated by coordinated action of Akt and Erk1/2 [22]–[24]. As shown in figure 3F, the injury-related increased (p<0.01) bim mRNA expression was significantly (p<0.01) decreased in sod3 tissues thereby indicating that the SOD3-derived activation of Akt in coordination with Erk1/2 signal transduction routes may represent a key mechanism to reduce the apoptotic response in tissues.
Discussion
The skeletal muscle ischemia induces production of ROS by the disrupted metabolism and by the infiltrating inflammatory cells, such as macrophages. The physiological function of SOD3 is to convert superoxide anion to hydrogen peroxide on the extracellular side of the cell membrane [1], [2] relieving the free radical superoxide anion derived damages in the tissue environment. However, this reaction leading to reduced oxidative stress does not explain per se the decreased apoptosis seen in SOD3 overexpressing tissues. Therefore, our purpose in the current work was to clarify the PI3K-Akt and Erk1/2 response caused by SOD3 in apoptosis model.
Based on the current data the significantly increased nox2 and inos mRNA expressions together with decreased sod3 expression at later time points (Figure 1E–G) suggest a marked imbalance in redox enzyme expression levels in developing tissue injury. Previously, we have shown that sod3 gene transfer to ischemic hind limb injury is able to increase the active SOD3 concentration in the tissue by 2-fold [8], which together with the remarkably long half-life of the enzyme in the muscle tissue, up to 100 hours [25], would be able correct the decrease of the endogenous enzyme expression causing the therapeutic response. The current analysis of the effect of sod3 overexpression on the apoptosis (Figure 1D) was in line with previous reports demonstrating the anti-apoptotic role of the enzyme [7], [9] further suggesting SOD3-derived response on cell survival signaling.
Among the anti-apoptotic and pro-survival pathways that are activated after ischemic injuries, the PI3K-Akt and Erk1/2 routes are considered to be the most important. The numerous substrates of these kinases include several apoptosis-related factors such as Foxo3a and caspases, which are affected by Akt and/or Erk phosphorylation [17], [18], [26]–[28]. The interplay between SOD3 and Akt has been confirmed previously in an in vitro experiment in which sod3 transfection increased phospho-Akt levels in cells but not at late time point in rat tissues [8]. In the present work, we found notably increased level of sod3 promoted Akt phosphorylation at early phase of the injury that, however, was lost at later phase unlike Erk1/2, suggesting milder SOD3-related stimulation to PI3K-Akt than Erk1/2 signaling. To further strengthen the role of Erk1/2 in cell survival we showed upregulation of ets-1 and elk-1 transcription factors in sod3 treated animals as compared to controls (Figure 2B–C), which let us to speculate that the earlier robust Erk1/2 activation induced by SOD3 continuously boosts the ets-1 and elk-1 expression, lifting it to a significantly higher level than in the control animals.
One of the direct Erk1/2 and Akt target proteins, the ROS responsive transcription factor FoxO3a, regulates the expression of many cell cycle arrest- and apoptosis-related genes [29]–[32] and is also known to protect normal quiescent cells from oxidative stress by regulating several antioxidant genes e.g. peroxiredoxins, glutathione peroxidases, and superoxide dismutases including SOD3 [33]–[35]. We discovered increased FoxO3a phosphorylation in sod3 treated animals at day 3 after the ischemic injury, which was then decreased to the level of the control animals (Figure 3A). The effect of sod3 overexpression on FoxO3a phosphorylation and cytoplasmic entry was confirmed in sod3 stable NIH3T3 cell line that demonstrated increased phosphorylation of the transcription factor while the total amount of foxo3a remained unaffected (Figure 3B–D).
Recently, FoxO3a was found to negatively regulate an anti-apoptotic microRNA, mir-21 by binding the mir-21 promoter to suppress its expression [21]. In line with this, we demonstrated increased mir-21 expression in vivo in sod3-transduced tissues (Figure 3E). Next, to confirm that sod3 is affecting FoxO3a function we monitored the inhibition of bim, another common downstream target of Akt and Erk1/2 pathways. BH3-only proteins, such as BIM, are cell death initiators that are activated in cellular stress conditions including response to DNA damages, decreased metabolism, growth factor withdrawal, and hypoxia. Based on our results the significantly (p<0.01) decreased expression of bim mRNA production correlated with sod3 overexpression in muscles suggesting that SOD3-derived increased FoxO3a phosphorylation at 3-day time point might be able to attenuate the initiation of the apoptotic process by BH3-only protein BIM (Figure 3F).
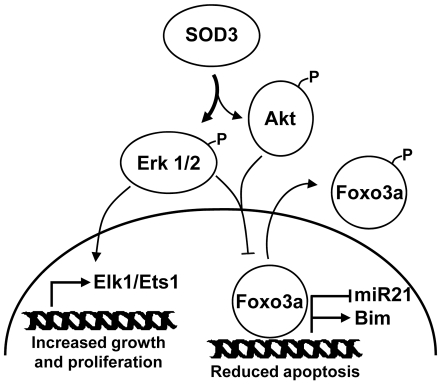
In summary, we have elucidated the signaling events by which the extracellular sod3 promotes cell survival and tissue recovery in skeletal muscle ischemia model (Figure 4). The overexpression of sod3 correlated with simultaneous activation of Akt and Erk1/2, consequent FoxO3a cytoplasmic entry, anti-apoptotic mir-21 upregulation and pro-apoptotic bim mRNA downregulation. On the tissue level, sod3 overexpression attenuated the injury development and decreased apoptosis. Therefore, the present data support our previous findings connecting the Erk1/2 mitogenic signaling cascade and downstream effectors to sod3 expression and cell survival effects.
Figure 4. Schematic representation of the SOD3 action in promoting the cell survival in injured tissues.
Temporal simultaneous activation of Erk1/2 and Akt leads to phosphorylation and consequent cytoplasmic translocation of FoxO3a, which then increases miR-21 production and downregulates the bim mRNA expression. Erk1/2 activation further stimulates the cell survival signaling by increasing cell proliferation related transcription factor production.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by Fondazione SDN (decision number RC2010-M-0001, http://www.sdn-napoli.it/) and Academy of Finland (decision number 141136, http://www.aka.fi/en-GB/A/Centres-of-Excellence-/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- 2.Marklund SL. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci U S A. 1982;79:7634–7638. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oshikawa J, Urao N, Kim HW, Kaplan N, Razvi M, et al. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS One. 2010;5:e10189. doi: 10.1371/journal.pone.0010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landmesser U, Merten R, Spiekermann S, Buttner K, Drexler H, et al. Vascular extracellular superoxide dismutase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation. 2000;101:2264–2270. doi: 10.1161/01.cir.101.19.2264. [DOI] [PubMed] [Google Scholar]
- 5.Laukkanen MO, Kivela A, Rissanen T, Rutanen J, Karkkainen MK, et al. Adenovirus-mediated extracellular superoxide dismutase gene therapy reduces neointima formation in balloon-denuded rabbit aorta. Circulation. 2002;106:1999–2003. doi: 10.1161/01.cir.0000031331.05368.9d. [DOI] [PubMed] [Google Scholar]
- 6.Leite PF, Danilovic A, Moriel P, Dantas K, Marklund S, et al. Sustained decrease in superoxide dismutase activity underlies constrictive remodeling after balloon injury in rabbits. Arterioscler Thromb Vasc Biol. 2003;23:2197–2202. doi: 10.1161/01.ATV.0000093980.46838.41. [DOI] [PubMed] [Google Scholar]
- 7.Laukkanen MO, Leppanen P, Turunen P, Tuomisto T, Naarala J, et al. EC-SOD gene therapy reduces paracetamol-induced liver damage in mice. J Gene Med. 2001;3:321–325. doi: 10.1002/jgm.194. [DOI] [PubMed] [Google Scholar]
- 8.Laurila JP, Castellone MD, Curcio A, Laatikainen LE, Haaparanta-Solin M, et al. Extracellular superoxide dismutase is a growth regulatory mediator of tissue injury recovery. Mol Ther. 2009;17:448–454. doi: 10.1038/mt.2008.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozumi K, Tasaki H, Takatsu H, Nakata S, Morishita T, et al. Extracellular superoxide dismutase overexpression reduces cuff-induced arterial neointimal formation. Atherosclerosis. 2005;181:55–62. doi: 10.1016/j.atherosclerosis.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 10.Qin Z, Reszka KJ, Fukai T, Weintraub NL. Extracellular superoxide dismutase (ecSOD) in vascular biology: an update on exogenous gene transfer and endogenous regulators of ecSOD. Transl Res. 2008;151:68–78. doi: 10.1016/j.trsl.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folz RJ, Abushamaa AM, Suliman HB. Extracellular superoxide dismutase in the airways of transgenic mice reduces inflammation and attenuates lung toxicity following hyperoxia. J Clin Invest. 1999;103:1055–1066. doi: 10.1172/JCI3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandes RP, Weissmann N, Schroder K. NADPH oxidases in cardiovascular disease. Free Radic Biol Med. 2010;49:687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 13.Younes M, Schoenberg MH, Jung H, Fredholm BB, Haglund U, et al. Oxidative tissue damage following regional intestinal ischemia and reperfusion in the cat. Res Exp Med (Berl) 1984;184:259–264. doi: 10.1007/BF01852385. [DOI] [PubMed] [Google Scholar]
- 14.Laurila JP, Laatikainen LE, Castellone MD, Laukkanen MO. SOD3 reduces inflammatory cell migration by regulating adhesion molecule and cytokine expression. PLoS One. 2009;4:e5786. doi: 10.1371/journal.pone.0005786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yordy JS, Muise-Helmericks RC. Signal transduction and the Ets family of transcription factors. Oncogene. 2000;19:6503–6513. doi: 10.1038/sj.onc.1204036. [DOI] [PubMed] [Google Scholar]
- 16.Wasylyk B, Hagman J, Gutierrez-Hartmann A. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci. 1998;23:213–216. doi: 10.1016/s0968-0004(98)01211-0. [DOI] [PubMed] [Google Scholar]
- 17.Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10:138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 19.Hu Y, Wang X, Zeng L, Cai DY, Sabapathy K, et al. ERK phosphorylates p66shcA on Ser36 and subsequently regulates p27kip1 expression via the Akt-FOXO3a pathway: implication of p27kip1 in cell response to oxidative stress. Mol Biol Cell. 2005;16:3705–3718. doi: 10.1091/mbc.E05-04-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 21.Wang K, Li PF. Foxo3a regulates apoptosis by negatively targeting miR-21. J Biol Chem. 2010;285:16958–16966. doi: 10.1074/jbc.M109.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Essafi A, Fernandez de Mattos S, Hassen YA, Soeiro I, Mufti GJ, et al. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24:2317–2329. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- 23.Balmanno K, Cook SJ. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009;16:368–377. doi: 10.1038/cdd.2008.148. [DOI] [PubMed] [Google Scholar]
- 24.Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem. 2006;281:813–823. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- 25.Karlsson K, Sandstrom J, Edlund A, Marklund SL. Turnover of extracellular-superoxide dismutase in tissues. Lab Invest. 1994;70:705–710. [PubMed] [Google Scholar]
- 26.Gao Y, Ordas R, Klein JD, Price SR. Regulation of caspase-3 activity by insulin in skeletal muscle cells involves both PI3-kinase and MEK-1/2. J Appl Physiol. 2008;105:1772–1778. doi: 10.1152/japplphysiol.90636.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allan LA, Morrice N, Brady S, Magee G, Pathak S, et al. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol. 2003;5:647–654. doi: 10.1038/ncb1005. [DOI] [PubMed] [Google Scholar]
- 28.Biswas SC, Greene LA. Nerve growth factor (NGF) down-regulates the Bcl-2 homology 3 (BH3) domain-only protein Bim and suppresses its proapoptotic activity by phosphorylation. J Biol Chem. 2002;277:49511–49516. doi: 10.1074/jbc.M208086200. [DOI] [PubMed] [Google Scholar]
- 29.Liu JW, Chandra D, Rudd MD, Butler AP, Pallotta V, et al. Induction of prosurvival molecules by apoptotic stimuli: involvement of FOXO3a and ROS. Oncogene. 2005;24:2020–2031. doi: 10.1038/sj.onc.1208385. [DOI] [PubMed] [Google Scholar]
- 30.Lee HY, You HJ, Won JY, Youn SW, Cho HJ, et al. Forkhead factor, FOXO3a, induces apoptosis of endothelial cells through activation of matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2008;28:302–308. doi: 10.1161/ATVBAHA.107.150664. [DOI] [PubMed] [Google Scholar]
- 31.Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid Redox Signal. 2005;7:752–760. doi: 10.1089/ars.2005.7.752. [DOI] [PubMed] [Google Scholar]
- 32.Lei H, Quelle FW. FOXO transcription factors enforce cell cycle checkpoints and promote survival of hematopoietic cells after DNA damage. Mol Cancer Res. 2009;7:1294–1303. doi: 10.1158/1541-7786.MCR-08-0531. [DOI] [PubMed] [Google Scholar]
- 33.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 34.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]