Abstract
Background and Purpose
Evaluation of infarct volumes and infiltrating immune cell populations in mice after middle cerebral artery occlusion (MCAO) strongly implicates a mixture of both pathogenic and regulatory immune cell subsets that affect stroke outcome. Our goal was to evaluate the contribution of the well-described co-inhibitory pathway, Programmed Death (PD)-1, to the development of MCAO.
Methods
Infarct volumes, functional outcomes and effects on infiltrating immune cell populations were compared in wild type C57BL/6 versus PD-1 deficient mice after 60min MCAO and 96h reperfusion.
Results
The results clearly demonstrate a previously unrecognized activity of the PD-1 pathway to limit infarct volume, recruitment of inflammatory cells from the periphery, activation of macrophages and CNS microglia and functional neurological deficits. These regulatory functions were associated with increased percentages of circulating PD-Ligand (L)-1 and PD-L2 expressing CD19+ B-cells in blood, spleen and CNS with the capacity to inhibit activation of inflammatory T-cells and CNS macrophages and microglial cells through upregulated PD-1.
Conclusions
Our novel observations are the first to implicate PD-1 signaling as a major protective pathway for limiting CNS inflammation in MCAO. This inhibitory circuit would likely be pivotal in reducing stroke-associated TLR2- and TLR4-mediated release of neurotoxic factors by activated CNS microglia.
Keywords: MCAO, inflammatory cells, Programmed Death-1, Co-inhibitory pathway
Introduction
Stroke is a devastating CNS condition marked by death of brain cells, loss of cognitive and motor functions and systemic infections that often lead to death. Mouse models of focal cerebral ischemia incurred by middle cerebral artery occlusion (MCAO) support a biphasic effect of stroke on the peripheral immune system. The initial phase is characterized by early signaling from the ischemic brain to spleen, resulting in massive production of inflammatory factors, transmigration of splenocytes to the circulation and infiltration of stroke-damaged areas of the brain by inflammatory PMN, macrophages, T-cells and B-cells1. Early activation is followed by compensatory systemic immunosuppression that occurs within days of focal stroke by profound (90%) loss of immune T- and B-cells in the spleen and thymus and reduced T-cell activation1. These changes were accompanied by an increase in TUNEL+, Annexin V+, PI+ splenocytes committed to apoptosis that suggested involvement of the ‘programmed death-1’ (PD-1) co-inhibitory pathway.
PD-1 (CD279) is an Ig-superfamily member containing an immunoreceptor tyrosine-based inhibitory motif (ITIM)2 and an immunoreceptor tyrosine-based switch motif (ITSM) that are inducibly expressed by activated T-cells, B-cells, NK-cells, monocytes and some dendritic cell (DC) subsets3,4. Binding of PD-1 to either of two ligands, PD-L1 (B7-H1/CD274) or PD-L2 (B7-DC/CD273) with overlapping expression patterns, induces inhibitory signals that control induction and maintenance of peripheral T cell tolerance and immune homeostasis5-7. Much work has focused on regulation of effector T-cell responses due to the autoimmune phenotype of PD-1-deficient (knockout/KO) mice8 and linkage of PD-1 genes with autoimmune disorders9. Moreover, other inhibitory pathways involving PD-1/PD-L have been described, including our studies on estrogen-mediated suppression via Treg-cell activation10-12, inhibition of encephalitogenic T-cell responses by PD-L-expressing myeloid APC13, ‘reverse signaling’ through PD-L that induces suppressive DC14, PD-L1 protection from CD8+ T-cell lysis of virally-infected target cells15 and PD-1-dependent immune-mediated damage of CNS oligodendroglial cells16.
Currently, there are no reports that have implicated the PD-1/PD-L co-inhibitory pathway in stroke. In this report, we found increased expression of PD-1 on brain macrophages and microglial cells and PD-L on B-cells from spleen, blood and CNS in mice after induction of MCAO. We thus compared MCAO in PD-1-KO vs. wild type (WT) male mice after 96h reperfusion. Cortical, striatal and total infarction volumes were significantly larger in PD-1-KO vs. WT C57BL/6 mice with MCAO (p<0.01). This study clearly implicates the PD-1/PD-L pathway in limiting infarct lesion volume in MCAO and provides new insights into immune regulatory interactions.
Methods
Animals
PD-1 deficient (PD-1-/- or PD-1-KO) mice backcrossed with C57BL/6 mice were obtained from Dr. Tasuku Honjo, Kyoto University, Japan. Age-matched 8-12 week old WT C57BL/6 mice (Jackson Laboratory, USA) were used as controls. Animals were randomized to treatment groups. All experiments were performed under approved protocols in the Animal Resource Facilities at Oregon Health & Science University and the Veterans Affairs Medical Center, Portland, OR.
MCAO model
The mice were subjected to MCAO as previously published1 by reversible right MCA occlusion (60min) under isoflurane anesthesia, followed by 96h of reperfusion. Body and head temperatures were controlled at 37±0.5°C. Occlusion and reperfusion were verified in each animal by laser Doppler flowmetry (Moor Instruments).
Quantification of Infarct
Brains were collected at 48h for standard 2,3,5-triphenyltetrazolium chloride (TTC) histology and digital image analysis of infarct volume as previously published17. To control for edema, corrected infarct volume is expressed as a percentage of contralateral structure, i.e. cortex, striatum or total hemisphere.
Neurological Deficit Score
Neurological function was evaluated in a blinded fashion using a 0-5 point neurological score: 0=no neurological dysfunctions; 1=failure to extend right forelimb fully when lifted by tail; 2=circling to the contralateral side; 3=falling to the right; 4=no spontaneous walk or in a comatose state; 5=death.
Cell isolation
Blood mononuclear cells were prepared by using red cell lysis buffer (eBioscience) following manufacturer's instructions. Splenocyte suspensions were prepared by mechanical disruption. For preparation of inflammatory cells from brain, each mouse was perfused transcardially with 30ml saline to exclude blood cells, the forebrain was dissected from the cerebellum and suspended in RPMI-1640 medium and the suspension was digested with type IV collagenase (1mg/ml, Sigma-Aldrich) and DNase I (50mg/ml, Roche) at 37°C for 45min in a shaker at 180 times per min. Inflammatory cells were isolated by 37–70% Percoll density gradient centrifugation as described18. The cells were washed twice with RPMI 1640, counted, and resuspended in stimulation medium containing 10% FBS for phenotyping.
Analysis of cell populations by FACS
Anti-mouse antibodies CD19 (1D3, BD Pharmingen), CD45 (30-F11, Invitrogen), CD11b (M1/70, eBioscience), MHCII (2G9, BD Pharmingen), Gr1 (IA8, BD Horizon), CD3 (17A2, eBioscience), IFN-γ (XMG1.2, eBioscience), TNF-α (MP6-XT22, BD Pharmingen), PD-1 (RMP1-30, eBioscience), PD-L1 (MIH5, eBioscience), and PD-L2 (TY25, eBioscience) were used for the study. Single-cell suspensions were washed with staining medium (PBS containing 0.1% NaN3 and 2% FCS). After incubation with mAb and washing, cells were acquired with LSRII (BD Biosciences) and analyzed (Flowjo software, TreeStar) using isotype control antibodies to set quadrants before calculating the percentage of positive cells.
Intracellular staining
Intracellular staining was visualized using a published immunofluorescence protocol19. Briefly, 2×106 cells/ml were resuspended in complete medium (RPMI1640 containing 10% FCS, 1mM pyruvate, 200μg/ml penicillin, 200U/ml streptomycin, 4mM L-Glutamine, and 5×10-5M 2-beta-ME with PMA (50ng/ml), ionomycin (500ng/ml) and Brefeldin A (10μg/ml)(Sigma-Aldrich) for 5h. Fc receptors were blocked with anti-FcR mAb (2.3G2; BD PharMingen) before cell-surface staining and fixed and permeabilized cells (eBioscience) were stained with anti-TNF-α or anti-IFN-γ mAb or isotype-matched mAb.
Statistical Analysis
Data were reported as means ± SEM. Student's t test was used for comparison of infarct volumes; ANOVA Kruskal-Wallis test followed by post hoc Dunn's Multiple Comparison test was used for comparison of neurological deficits and Fisher's exact test was used for comparing mortality rates. There was no difference in MCAO mortality between WT (5/26) and PD-1-KO (5/25) mice. For three and more groups, the ANOVA followed by post hoc Tukey's test was applied. For all tests, P-values <0.05 were considered statistically significant.
Results
Increased expression of PD-1 and PD-Ligands after MCAO suggests involvement of the co-inhibitory pathway
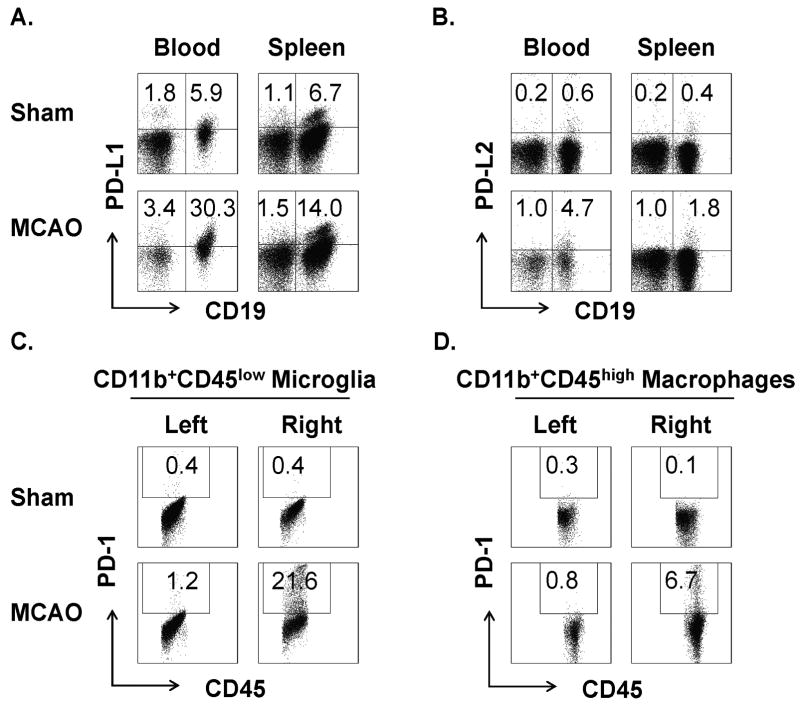
We have recently shown that peripheral B-cells have strong regulatory activity in limiting MCAO severity (Kozaburo et al., manuscript in review). B-cells are known to express the PD-1 ligands, PD-L1&2 that are important in delivering inhibitory signals through the PD-1 receptor to control induction and maintenance of peripheral T-cell tolerance and immune homeostasis and to inhibit activation of other PD-1-expressing cells. We thus evaluated the possible involvement of this important co-inhibitory pathway in limiting immune-mediated inflammation in MCAO. We found that B-cells predominantly expressed PD-L1 and PD-L2 (67-86% of total) in blood and spleen (Fig. 1A&B) of Sham-operated mice and that their levels were strongly increased (2-8 fold) after MCAO (60min occlusion, 96h reperfusion). Moreover, 77-78% of B-cells that are enriched in the ischemic hemisphere of MCAO mice expressed PD-L1 and PD-L2 (Supplementary Fig. 1, please see http://stroke.ahajournals.org). Conversely, activated resident microglia and infiltrating macrophages that are major inflammatory cell types that contribute to stroke damage in CNS, had strongly enhanced levels of PD-1 expression in the ischemic hemisphere of MCAO mice (Fig. 1C&D). The importance of these changes was further demonstrated by the impaired ability of purified B-cells to inhibit proliferation of T cells and production of TNF-α by macrophages in PD-1-deficient mice (Supplementary Figure 2, please see http://stroke.ahajournals.org). These result demonstrate the capacity of PD-L1+ and PD-L2+ B-cells to ligate and potentially to inhibit activation of PD-1+ inflammatory cells during stroke.
Figure 1. Increased expression of PD-L on peripheral B-cells and PD-1 on CNS microglia and macrophages after MCAO.
PD-L1 (A) and PD-L2 (B) levels were strongly up-regulated on CD19+ B-cells from blood and spleen, and PD-1 expression was strongly up-regulated on CD11bhighCD45low microglia (C) and Gr1-CD11bhighCD45high macrophages (D) from the right ipsilateral brain hemisphere after 60min MCAO treatment and 96h reperfusion in WT mice. Results represent at least two independent experiments with three mice per group.
PD-1 deficiency exacerbates stroke outcomes and alters cerebral inflammatory cell infiltration
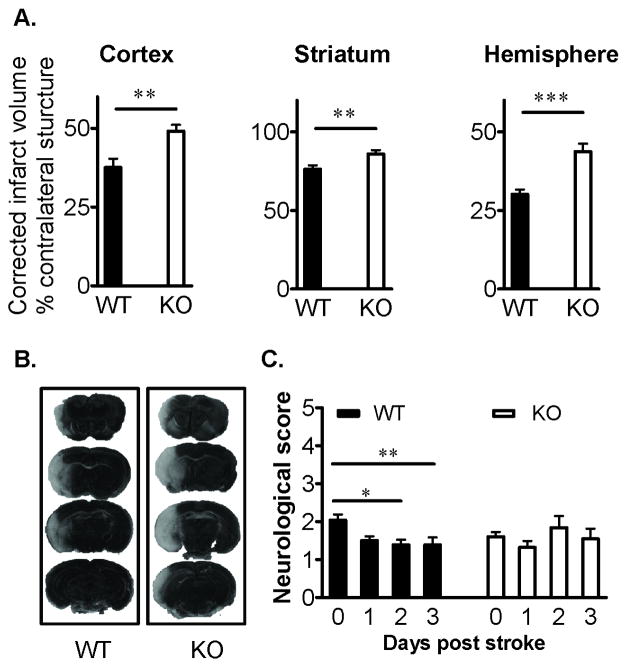
To assess the role of PD-1 in stroke development, infarct volume, neurological outcome and infiltration of inflammatory cells into brain were evaluated in cohorts of PD-1 deficient vs. C57BL/6 WT mice treated with 60min of focal cerebral ischemia and 96h of reperfusion. Loss of PD-1 resulted in significantly larger hemispheric infarct volumes in cortex (p=0.01), striatum (p=0.01) and total hemisphere (p=0.0001) relative to WT mice (Figure 2A). Representative histological staining of injured brain is shown Figure 2B. These data clearly implicate the role of PD-1 in limiting histological damage after MCAO. In a further cohort, there was significant recovery of neurological scores in WT mice that did not occur in PD-1KO mice (Fig. 2C). To confirm that the ischemic insult was equivalent among all animals, relevant physiological parameters were assessed before and during MCAO. As is shown in Supplementary Table 1 (please see http://stroke.ahajournals.org), rectal temperature, mean arterial blood pressure, arterial blood gases and pH were comparable between groups. Similarly, intra-ischemic cortical blood flow as estimated by laser Doppler flowmetry was not different between PD-1 deficient and WT mice.
Figure 2. Deficiency of PD-1 exacerbates ischemic infarct volume and worsens behavioral recovery after MCAO.
(A) Infarct volumes in Cortex, Striatum and Hemisphere, corrected for the presence of edema, were significantly increased (**, P<0.01; ***, P<0.0001) in PD-1-KO (n=9) versus WT (n=9) mice after 60min MCAO and 96h reperfusion. Values represent Mean ± SEM. (B) Representative TTC-stained cerebral sections of the MCAO modeled to analyze infarct volume. (C) Neurological dysfunction scores (Mean ± SEM) after reperfusion were significantly worsened (*, P<0.05; **, P<0.01) in PD-1 KO (n=25) versus WT (n=26) mice.
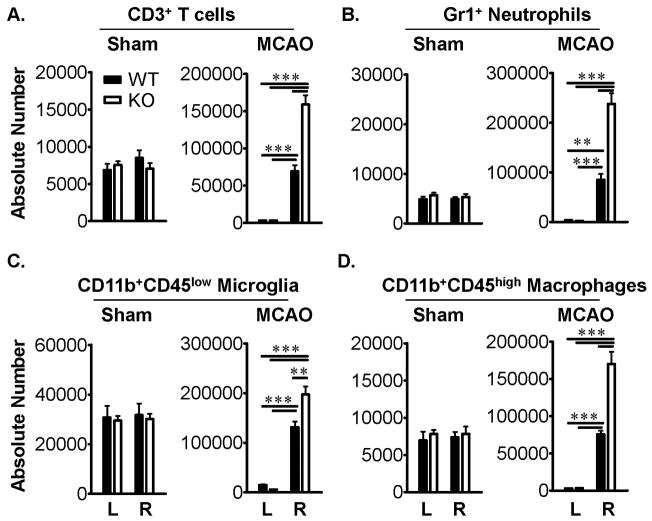
Leukocytes are major effectors of inflammatory damage after experimental brain ischemia. To determine if the loss of PD-1 altered leukocyte composition in brain after MCAO, numbers of infiltrating CD3+ T-cells, Gr1+ neutrophils, CD11b+CD45low microglia and CD11b+CD45high macrophages were evaluated by flow cytometry. After 96h reperfusion, accumulation of all of these leukocyte subtypes was significantly greater in the ischemic (Right) vs. non-ischemic (Left) hemisphere of MCAO-treated PD-1-KO mice as compared to MCAO-treated WT mice (Figure 3A-D).
Figure 3. PD-1 reduces the infiltration of inflammatory cells into the ischemic brain after MCAO.
Absolute numbers of CD3+ T cells (A), Gr1+ neutrophils (B), CD11b+CD45low microglia (C), and CD11b+CD45high macrophages (D) were significantly increased (**, P<0.001; ***, P<0.0001) in ipsilateral right (R) but not contralateral left (L) brain hemispheres of PD-1-KO versus WT mice after 60min MCAO and 96h reperfusion but not Sham-treatment. Values represent Mean ± SEM from five mice per group.
PD-1 limits inflammatory T-cell responses in the periphery and CNS of MCAO mice
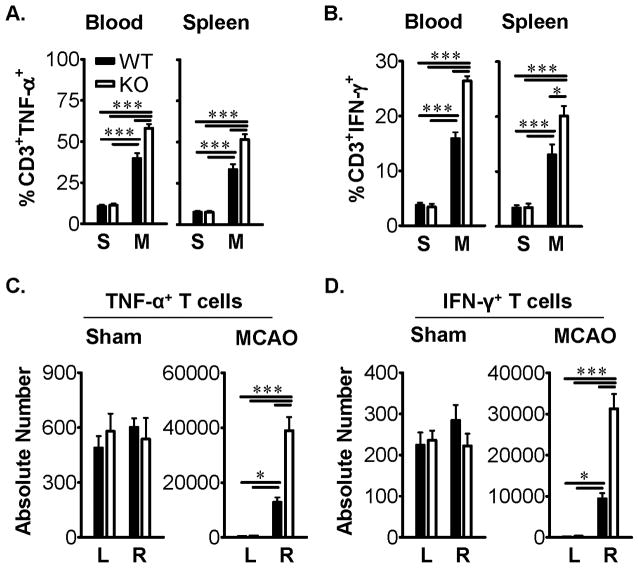
To further evaluate possible regulatory effects of PD-1 on T-cell cytokine production during MCAO, inflammatory factors were quantified in ex vivo activated cells from blood, spleen and CNS after 96h reperfusion in PD-1-KO vs. WT mice. As is shown in Figure 4A&B, the percentages of CD3+ T-cells secreting TNF-α and IFN-γ were significantly increased in both blood and spleen in PD-1 deficient vs. WT mice after MCAO. Loss of PD-1 further permitted significant 3-5-fold increases in the absolute numbers of IFN-γ- and TNF-α-secreting CD3+ T-cells in the ischemic hemisphere of MCAO mice (Figure 4C&D).
Figure 4. PD-1 inhibits cytokine production by T-cells in blood, spleen and ischemic brain hemisphere after MCAO.
Percentages and absolute numbers of CD3+ T-cells expressing TNF-α (A&C) and IFN-γ (B&D) were significantly increased (*, P<0.05, ***, P<0.0001) in blood and spleen (A&B) and ipsilateral right (R) but not contralateral left (L) brain hemispheres (C&D) after 60min MCAO (M) treatment and 96h reperfusion but not Sham (S) treatment of PD-1-KO versus wild-type (WT) mice. Values represent mean ± SEM from five mice per group.
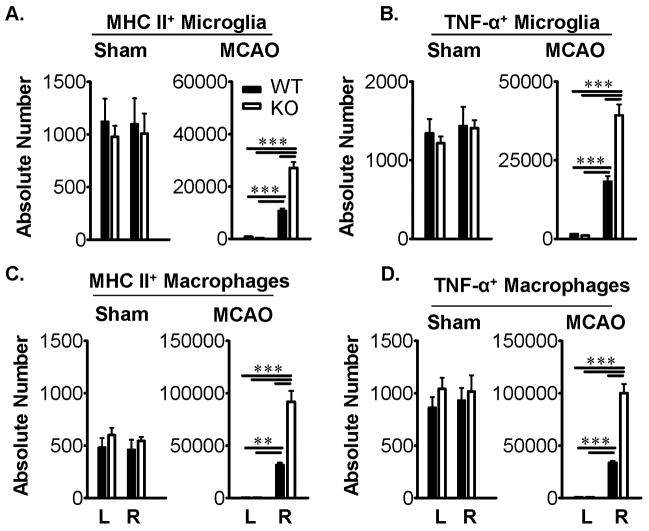
Increased PD-1 expression inhibits activation of microglia and macrophages in ischemic brain after MCAO
As is shown above (Fig. 1C&D), PD-1 expression was strongly up-regulated on both microglia and macrophages within the ischemic CNS lesion after MCAO in WT mice, and obviously could not be expressed similarly in PD-1-KO mice. The effect of the loss of PD-1 was to permit significant 2-3-fold increases in the absolute numbers of MHC class II+ and TNF-α-secreting microglia (Figure 5A&B) and macrophages (Figure 5C&D) in the ischemic hemisphere after activation ex vivo. Of note, there was approximately 2-3-fold more infiltrating macrophages present in the ischemic hemisphere than T-cells or microglia after MCAO. These results clearly demonstrate enhanced infiltration of inflammatory cells into the affected CNS in PD-1 deficient mice after MCAO.
Figure 5. PD-1 inhibits the activation of microglia and infiltrating macrophages in ischemic brain after MCAO.
FACS analysis of counted MHCII+ microglia (A), TNF-α+ microglia (B), MHCII+ macrophages (C) and TNF-α+ macrophages (D) in the contralateral left (L) and ipsilateral right (R) hemispheres of Sham and MCAO treated wild-type (WT) and PD-1 knockout (KO) mice. CD11bhighCD45low and Gr1-CD11bhighCD45high populations identified microglia and macrophages, respectively. Significant differences between sample means (± SEM) from five mice per group are indicated: **, P < 0.01, ***, P < 0.0001.
Discussion
The results presented above demonstrate two important and novel findings which have potentially high impact in our understanding of immunological mechanisms of ischemic brain injury. First, a previously unrecognized activity of the PD-1/PD-L co-inhibitory pathway contributes to limit infarct volume and functional neurological deficits, as well as to inhibit activation and recruitment of inflammatory T-cells, granulocytes, macrophages and microglia into the growing infarct. These regulatory activities were significantly decreased in MCAO-treated PD-1 deficient mice, thus implicating unequivocally the protective activity of this regulatory pathway. Second, PD-L1&2 expression was increased on peripheral and CNS B-cells and PD-1 expression was up-regulated on CNS microglia and infiltrating macrophages within the lesioned brain hemisphere 96h after MCAO. Since peripheral B-cells, T-cells and macrophages migrate across the blood-brain barrier to contribute to the ischemic injury, our novel results suggest a previously undescribed regulatory circuit in which PD-L1/2+, IL-10-secreting B cells (Kozoburo, et al., manuscript submitted) may directly inhibit T-cells and regulate activation and release of neurotoxic factors by PD-1+ MG and macrophages. These putative interactions are illustrated in Supplementary Figure 3 (please see http://stroke.ahajournals.org). These findings implicate the PD-1/PD-L immunoregulatory pathway as a novel target for protection from CNS damage in experimental stroke.
It is of potential importance that PD-1 has been reported to be constitutively expressed on neurons20, and it is conceivable that interaction of PD-L+ APC with PD-1 expressed by neurons could be directly neurotoxic. This event would contribute to the MCAO lesion only in WT but not PD-1-KO mice, thus producing larger lesions in WT mice (the opposite of what was observed in our study). In the absence of PD-1, there would be less neuronal death due to PD-L/PD-1 interactions but more neuronal damage from neurotoxic factors released by unregulated microglial cells and macrophages.
Although our results unequivocally implicate PD-1 as a contributor to CNS lesion development during 96h reperfusion after MCAO, a prior report did not observe a change in lesion volumes in PD-1 deficient mice 24h after MCAO21. This result suggested a lack of involvement of PD-1 as a co-stimulator for adaptive T-cell responses in early stroke, and the 24h observation time in PD-1 deficient mice was apparently too early after occlusion to allow for the production of larger infarct volumes as was observed in our study. Congruent with this interpretation, our study implicates the PD-1 effect at a later time point, involving reduced recruitment and cytokine release by activated T-cells, macrophages and brain microglial cells.
As mentioned above, the cytoplasmic domain of PD-1 contains an immunoreceptor tyrosine-based switch motif (ITSM) sequence, and it was later demonstrated that the tyrosine within the ITSM motif is essential for binding the protein tyrosine phosphatases SHP-1 and SHP-222.23 that mediate inhibitory PD-1 function. In T-cells, inhibition of activation requires co-ligation of the TCR23. In macrophages (and presumably MG), PD-1 can be up-regulated and cell activation inhibited by an interferon-sensitive response element (ISRE)24, Toll-like receptor (TLR)-2, TLR3 & TLR4 and other agents25. This is of particular importance in stroke, where MG activation and release of CNS neurotoxic factors is known to occur through ligation of TLR2 and TLR426-28, possibly by heat-shock protein 60 released from CNS cells undergoing necrotic or apoptotic cell death29. Thus, ligation of PD-1 expressed on macrophages and MG by PD-L expressed by regulatory B-cells could result in the inhibition of a key neurodestructive process in stroke.
In conclusion, our study provides new insights into the contribution of the PD-1/PD-L co-inhibitory pathway that limits stroke-induced endogenous inflammatory responses.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Suz Dziennis, Dr. Sushmita Sinha, Dr. Sheetal Bodhankar and Ms. Sandhya Subramanian for helpful discussions and Ms. Eva Niehaus for assistance in preparing the manuscript.
Funding Sources: This work was supported by NIH grants NR03521, NS49210 and the Biomedical Laboratory R&D Service, Department of Veterans Affairs.
Footnotes
Disclosures: The authors have no conflicts of interest to report.
References
- 1.Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–6531. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 2.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimura H, Minato N, Nakano T, Honjo T. Immunological studies on PD-1-deficient mice: Implication of PD-1 as a negative regulator for B cell responses. Intl Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 4.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Current Opinion in Immunology. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Magnus T, Schreiner B, Korn T. Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: Implications for immune responses and autoimmunity in the CNS. Journal of Neuroscience. 2005;25:2537–2546. doi: 10.1523/JNEUROSCI.4794-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nature Immunology. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 7.Dinesh RK, Hahn BH, Singh RP. PD-1, gender, and autoimmunity. Autoimmunity Reviews. 2010;9:583–587. doi: 10.1016/j.autrev.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Treg suppressive activity involves estrogen dependent expression of programmed death-1 (PD-1) Intl Immunol. 2007;19:337–343. doi: 10.1093/intimm/dxl151. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Dehghani B, Li Y, Kaler LJ, Proctor T, Vandenbark AA, et al. Membrane estrogen receptor regulates experimental autoimmune encephalomyelitis through up-regulation of programmed death 1. J Immunol. 2008;182:3294–3303. doi: 10.4049/jimmunol.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Dehghani B, Li Y, Kaler LJ, Vandenbark AA, Offner H. Oestrogen modulates experimental autoimmune encephalomyelitis and interleukin-17 production via programmed death 1. Immunology. 2009;126:329–335. doi: 10.1111/j.1365-2567.2008.03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schreiner B, Bailey SL, Shin T, Chen L, Miller SD. PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T cell responses in EAE. Eur J Immunol. 2008;38:2706–2717. doi: 10.1002/eji.200838137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuipers H, Muskens F, Willart M, Hijdra D, vanAssema FBJ, Coyle AJ et al. Contribution of the PD-1 ligands/PD-1 signaling pathway to dendritic cell-mediated CD4+ T cell activation. European Journal of Neuroimmunology. 2006;36 doi: 10.1002/eji.200635978. [DOI] [PubMed] [Google Scholar]
- 15.Phares TW, Ramakrishna C, Parra GI, Epstein A, Chen L, Atkinson R, et al. Target-dependent B7-H1 regulation contributes to clearance of central nervous system infection and dampens morbidity. J Immunol. 2009;182:5430–5438. doi: 10.4049/jimmunol.0803557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroner A, Schwab N, Ip CW, Leder C, Nave KA, Maurer M, et al. PD-1 regulates neural damage in oligodendroglia-induced inflammation. PLoS One. 2009;4:e4405. doi: 10.1371/journal.pone.0004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, et al. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cerebral Blood Flow & Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;22:586–592. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- 19.Yanaba K, Bouazis JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotye controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Pai V, Levinson R, Sharpe AH, Freeman GJ, Braun J, et al. Constitutive neuronal expression of the immune regulator, programmed death 1 (PD-1) identified during experimental autoimmune uveitis. Ocul Immunol Inflamm. 2009;17:47–55. doi: 10.1080/09273940802491884. [DOI] [PubMed] [Google Scholar]
- 21.Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, et al. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. 2010;115:3835–3842. doi: 10.1182/blood-2009-10-249078. [DOI] [PubMed] [Google Scholar]
- 22.Okazaki T, Maeda A, Nishimura H, Kurosake T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci USA. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;172:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 24.Cho HY, Lee SW, Seo SK, Choi JW, Choi I. Interferon-sensitive response element (ISRE) is mainly responsible for IFN-alpha-induced upregulation of programmed death-1 (PD-1) in macrophages. Biochim Biophys Acta. 2008;1779:811–819. doi: 10.1016/j.bbagrm.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Yao S, Wang S, Zhu Y, Luo L, Zhu G, Flies S, et al. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood. 2009;113:5811–5818. doi: 10.1182/blood-2009-02-203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- 27.Urra X, Cervera A, Obach V, Climent N, Planas AM, Chamorro A. Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke. 2009;40:1262–1268. doi: 10.1161/STROKEAHA.108.532085. [DOI] [PubMed] [Google Scholar]
- 28.Arslan F, Keogh B, McGuirk P, Parker AE. TLR2 and TLR4 in ischemia reperfusion injury. Mediators Inflamm. 2010;2010:704202. doi: 10.1155/2010/704202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehnardt S, Schott E, Trimbuch T, Laubisch D, Krueger C, Wulczyn G, et al. A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci. 2008;28:2320–2331. doi: 10.1523/JNEUROSCI.4760-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.