Abstract
Oral mucositis (OM) is a devasting toxicity associated with cytotoxic cancer therapy. Antrum mucosal protein (AMP)-18 and a synthetic peptide surrogate, exhibit cell protective and mitogenic properties in in vitro and in vivo models of gastrointestinal epithelial cell injury. The mucosal barrier-protective effects may be mediated by AMP-18 's capacity to increase accumulation of specific tight junction (TJ) and adherens junction proteins, and also protect against their loss after injury. Here we asked if AMP peptide could protect the oral mucosa and speed healing from radiation-induced injury. We found AMP peptide prevented radiation-induced OM in a murine model. The peptide also stimulated HaCaT cell growth used to model the oral mucosa. Binding of recombinant human (rh) AMP-18 to the plasma membrane of keratinocytes in normal human oral mucosal tissue suggested that its effects may be receptor mediated. Using an immobilized His-tagged rhAMP-18 fusion protein the receptor was identified as the cholecystokinin-B/gastrin receptor (CCKBR) by affinity purification and mass spectrometry analysis. CCKBR was expressed and co-immunoprecipitated with exogenous rhAMP-18 in diverse epithelial cell lines. Immunofluorescence staining revealed that rhAMP-18 colocalized with CCKBR on the surface of CCKBR-transfected cells. Furthermore, rhAMP-18-stimulated signaling pathways were blocked by a CCKBR-specific antagonist, YM022. RhAMP-18 enhanced viability and growth of CCKBR-transfected, but not empty vector-transfected cells. These results suggest the importance of epithelial junctional integrity in the pathogenesis of OM and demonstrate that AMP-18, by targeting TJ proteins through the activation of CCKBR, could provide a novel strategy for the prevention and treatment of OM.
Keywords: Head and neck cancer, Oral mucositis, Tight junction, Antrum mucosal protein -18, Gastrokine-1, Cholecystokinin-B/gastrin receptor
Introduction
OM is a frequent and severe side effect associated with radio/chemotherapy for head and neck malignancies. It also occurs with clinically significant frequency and severity in patients who receive conditioning therapy for hematopoietic stem cell transplantation (HSCT), and in those with solid tumors given chemotherapy. 1 The syndrome, characterized by disruption of mucosal epithelial barrier structure and function, results in pain, erythema, edema, pseudomembrane (fibrinous/bacteria-laden exudate) formation, reduced saliva, bleeding, and ulcerations, and has a significant impact on the patients’ quality of life. 2 Moreover, severe OM is one of the leading causes for unplanned treatment interruptions, dose reductions of drugs used in chemotherapy and/or alteration in the selection of antineoplastic agents. Treatment options for OM are limitied. Only Palifermin (Kepivance®, Amgen/Biovitrum; human recombinant keratinocyte growth factor-1) is approved for an OM indication and its use is limited to patients with hematological malignancies undergoing HSCT. 3 Current standard therapy is predominantly palliative with a goal of providing symptomatic relief.
Much has been learned about the complexities of the pathogenesis of OM in the past decade. 1 A potential role of the oral epithelium, particularly its tight junctions, has been hypothesized as a target for therapeutic intervention in OM. 4, 5 Consequently, we reasoned that the barrier-protective function of AMP-18 seen in our previous studies might offer a novel strategy to treat OM.
We previously identified and characterized an 18-kDa antrum mucosa protein (AMP-18), also called Gastrokine-1 (GKN1), whose expression at high levels is restricted to cells of the gastric epithelium. 6 Subsequent study has demonstrated that full-length AMP-18, and a synthetic 21-amino acid peptide fragment corresponding to a central domain of the protein, exhibit cell protective, mitogenic, and motogenic (enhanced motility) properties in several tissue culture models of gastrointestinal (GI) mucosal injury. 7 This 21-mer peptide stimulated growth of epithelial cells, but not fibroblasts, and increased restitution of scrape-wounded gastric epithelial monolayers, suggesting an important role for AMP-18 in maintaining integrity of the mucosal barrier. 7 AMP-18 appears to protect and seal the barrier by acting on tight junctions (TJ) which connect adjacent epithelial cells. 5 Administration of AMP peptide to normal mice stimulated accumulation of TJ proteins such as occludin and ZO-1 in colonic mucosal epithelial cells, and in monolayer cultures of human colonic epithelial Caco2/bbe (C2) cells. Administration of the 21-mer AMP peptide to mice with experimental colonic injury induced by the polyanion dextran sulfate sodium (DSS), delayed the onset of bloody diarrhea, and reduced weight loss. 5 Treatment with AMP-18 peptide also protected cell monolayers against decreases in transepithelial electrical resistance (TER) induced by the oxidant monochloramine, indomethacin, or DSS which disrupt TJs. Laser scanning confocal microscopy (LSCM) showed that AMP peptide increased accumulation of occludin and ZO-1 in TJ domains of epithelial cell monolayers, and immunoblotting analysis of detergent-insoluble fractions (which contain TJ proteins) indicated that the peptide protected against loss of these TJ proteins following oxidant injury. AMP peptide also protected against a fall in TER during disruption of actin filaments by cytochalasin D, and stabilized perijunctional actin during oxidant injury when assessed by LSCM. AMP-18 stimulates growth of diverse types of GI epithelial cells (AGS, IEC-6 and IEC-18). 6, 7
Expression of AMP-18 in the gastric antrum mucosa can be reduced in situations that cause mucosal injury, although the mechanisms are not known. Low-dose aspirin decreases expression of AMP-18 in the antrum mucosa of healthy human subjects, 8 whereas indomethacin gavaged into the stomach of mice reduced AMP-18 content in antrum mucosal tissue before histological injury was detected, 7 The expression level of AMP-18 is downregulated or absent in gastric cancer, 9, 10 and Helicobacter pylori-positive gastritis. 11, 12 In addition, AMP-18 shows a progressive decrease from chronic gastritis to atrophy and intestinal metaplasia, and its expression is upregulated considerably in gastric epithelial cells following eradication of H. pylori. 13
Our previous observations have suggested that the pleiotropic actions of AMP-18 are receptor-mediated. 7 Several signaling pathways are activated in cells exposed to AMP peptide (unpublished results). To better understand the function of AMP-18, and provide a compelling rationale for using AMP peptide as a therapeutic agent for diseases of the GI tract and oral mucosa, we set out to identify and characterize the AMP-18 receptor(s) and signaling pathways that mediate its function and ability to target specific TJ proteins. In the present study CCKBR was identified as a putative receptor for AMP-18 based on binding, colocalization and functional studies.
Experimental Procedures
Recombinant human AMP-18
Full-length recombinant human (rh) AMP-18 with a His6 tag at the N-terminus, or His6 and Flag tags at N- and C-termini respectively were prepared by GenScript (Piscataway, NJ) as instructed. Briefly, the coding sequence was cloned into an E. coli expression vector, pGSE3, and the expressed protein was purified from 5 liters of culture medium by affinity column chromatography. Purity of the protein was estimated to be >80% using Coomassie blue staining of a gel after SDS-polyacrylamide gel electrophoresis (PAGE). RhAMP-18 selectively bound to cobalt agarose beads was used in a pull-down assay as described below.
Murine model of oral mucositis
Anesthetized (ketamine, xylazine) female BDF-1 mice (~ 18 gm) received a single dose of radiation of 30 Gy to the snout on day 0 to induce oral mucositis. This strain of mice was chosen because it had been used by Farrell et al. to demonstrate that keratinocyte growth factor-1 induced epithelial thickening of the oral mucosa. 14 AMP peptide (25 mg/kg) was administered subcutaneously (s.c.) once daily four days before radiation and continued until day 10 afterwards. Mucositis was evaluated by histological evaluation of the tongue on day 10. This dose was chosen because previous studies demonstrated that it induced thickening (hyperplasia and hyperkeratosis) of the oral epithelium (but not duodenum) in a dose-dependent manner. This protocol was approved by the Animal Care and Use Committee and performed at Biomodels (Watertown, MA).
Cell culture, protein extraction, immunoprecipitation and immunoblotting
Various epithelial cell lines, either normal or transformed, were used, including HaCaT, human oral squamous cell carcinoma (OSCC)-3 cells, and IEC-18 cells. HaCaT cells are a spontaneously transformed keratinocyte cell line from histologically normal skin. 15 IEC-18 is a nontransformed epithelial cell line derived from normal rat ileum. 16 Human embryonic kidney 293T cells were used in transfection experiments.
Cells were maintained in DMEM (Invitrogen) containing 10% fetal calf serum (FCS) (Invitrogen) supplemented with penicillin and streptomycin, and were grown in a humidified incubator with 5% CO2 at 37°C. To prepare cell lysates, monolayer cultures were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed on ice for 30 min in lysis buffer (50 mM Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 100 mM NaF, 10% glycerol, 10 mM EDTA) containing protease and phosphatase inhibitors (2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 50 µg/ml antipain, 1 µg/ml aprotinin, 1 µg/ml leupeptin, and 1 µg/ml pepstatin). Cell lysates were clarified by centrifugation at 14000 × g for 15 min at 4°C. Protein concentration was determined by BCA assay (Pierce). For immunoblotting assays, 30 to 50 µg protein/lane was resolved by SDS-PAGE and transferred onto Immobilon membranes (Millipore, Bedford, MA) followed by incubation with designated antibodies. Immunoreactive bands were visualized using chemiluminescence (ECL, Amersham Biosciences). When reprobed, blots were first stripped with a buffer containing 50 mM Tris-HCl, pH 6.8, 2% SDS, and 0.1 M 2-mercaptoethanol. The following primary antibodies were used for immunoblotting: anti-CCKBR and anti-His antibodies (Santa Cruz Biotechnology), anti-phosphorylated ERK and p38 MAPK antibodies (Cell Signaling), and anti-actin antibody (Sigma).
To immunoprecipitate CCKBR, total cell lysates were pre-cleared with protein G beads and then incubated with anti-CCKBR antibody and protein G beads. For co-immunoprecipitation, 2 µg/ml His-tagged rhAMP-18 was added. After incubation overnight at 4°C, the immunocomplexes bound to the protein G beads were washed 5 times with lysis buffer. The bound proteins were then subjected to immunoblotting with an anti-His antibody. Fifty ng rhAMP-18 was included in a separate lane on the immunoblot as a control. Alternatively, a reciprocal co-immunoprecipitation was performed under the same conditions by incubating rhAMP-18 with pre-cleared total cell lysate from HaCaT followed by incubation with agarose-conjugated anti-His antibody.
Protein pull-down assay
Proteins bound to rhAMP-18 protein were pulled-down using the ProFound Pull-Down Protein Interaction Kit (Thermo Fisher Scientific Inc., Rockford, IL) according to the manufacturer’s instructions. His-tagged rhAMP-18 protein was immobilized on cobalt agarose beads by incubation at 4°C for 1 h. The beads were washed 6 times at the end of the incubation to remove unbound rhAMP-18 protein. After pre-clearing, cell membrane extracts prepared from cultured HaCaT cells by differential centrifugation were incubated with the immobilized rhAMP-18 overnight at 4°C. After washing 6 times, the binding proteins were selectively eluted with imidazole elution buffer, resolved by SDS-PAGE, and subjected to silver staining (SilverSNAP Stain for Mass Spectrometry kit, Thermo Scientific). Bands of interest were then excised and identified by mass spectrometric analysis in the University of Chicago Proteomics Core Laboratory, as described below.
Mass spectrometric analysis
Bands excised from SDS-PAGE gels were cut into approximately 1-mm3 pieces, washed in water, and completely destained with 100 mM ammonium bicarbonate (pH 7.5) in 50% acetonitrile. A reduction step was performed by addition of 100 µl of 50 mM ammonium bicarbonate (pH 7.5) and 10 µl of 20 mM Tris(2-carboxyethyl)phosphine (TCEP), and allowed to reduce at 37°C for 30 min. An alkylation step was performed on the gel bands by addition of 100 µl of 50 mM iodoacetamide and allowed to react in the dark for 30 min. Bands were washed in water, dried with acetonitrile, and then in a SpeedVac for 10 min. Digestion was carried out using sequencing grade modified trypsin (40 ng/µl) (Promega) in 50 mM ammonium bicarbonate (pH 7.5). Sufficient trypsin solution was added to swell the gel pieces which were kept at 4°C for 45 min and then incubated at 37°C overnight. Peptides were extracted from the gel pieces with 5% formic acid and analyzed by LC-MS/MS on a Thermo Electron linear ion trap mass spectrometer (LTQ) (Thermo Electron Corp., San Jose, CA), equipped with a nano-electrospray ion source (New Objective, Inc., Woburn, MA) and operated in positive ion mode. A Dionex U-3000 Ultimate (Bannockburn, IL) nano LC system was used to provide the gradient for elution of the peptides. The sample was injected onto a Dionex Acclaim PepMap100 C18 column (5 µm, 100 Å, and 300 µm×5 mm) at 50 µl/min. Chromatographic separation was performed on an Agilent Zorbax 300SB-C18 column (3.5 µm, 75 µm×150 mm) by eluting with 0.1% formic acid in 95% water, 5% acetronitrile (mobile phase A) and 0.1% formic acid in 95% acetonitrile, 5% water (mobile phase B). A linear gradient from 15 to 45% mobile phase B for 30 min at a flow rate of 250 nL/min was applied. The LTQ spray voltage was 2.0 kV and the capillary temperature was set at 200°C. A survey full scan (m/z = 400–2000) was acquired in which the four most intense ions were selected for a zoom scan to determine the charge state. If the charge state was determined to be greater than +1, MS/MS was triggered with minimum signal required (1000), isolation width of 2.0, normalized collision energy of 35 eV, activation Q 0.25 and activation time of 30 ms. The dynamic exclusion list was restricted to 250 entries with duration of 60s. Mass spectrometry data was acquired with Xcalibur software.
Rho activation assay
Cells were cultured to approximately 80% confluence and treated with rhAMP-18 in the presence or absence of a CCKBR inhibitor, YM022 (Tocris Bioscience, Ellisville, Missouri), or left untreated. After treatment, cells were rinsed twice with ice-cold PBS and lysed in Mg2+ lysis buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% Igepal CA-630, 10 mM MgCl2, 1 mM EDTA and 2% glycerol) on ice. Cell lysates were then cleared by centrifugation (5 min, 14000 ×g, 4°C). Rho protein was pulled down by adding Rhotekin RBD conjugated to agarose beads. An aliquot of untreated cell lysates was loaded with GTPγS as a positive control. The reaction was incubated for 1 h at 4°C with gentle agitation. The agarose beads were then washed 3 times with Mg2+ lysis buffer and bound proteins were dissolved in Laemmli reducing sample buffer and boiled for 5 min. Proteins were resolved using SDS-PAGE and transferred onto Immobilon membranes followed by immunoblotting assays with anti-Rho antibodies. Aliquots of original cell lysates were subjected to immunoblotting assays with an anti-actin antibody to confirm equal loading.
Cell transfection, treatment and immunofluorescence microscopy
CCKBR was expressed in 293T cells using pBabe-CCKBR (generously provide by Dr. Thomas V. O. Hansen, Rigshospitalet, Denmark). An empty pBabe vector was introduced into 293T cells as a control. For immunofluorescence staining, cells were grown on poly-lysine-coated coverslips and fixed with 4% paraformaldehyde at room temperature for 10 min followed by permeabilization with cold methanol for 10 min at −20°C. Cells were incubated with rhAMP-18 overnight at 4°C and unbound rhAMP-18 was thoroughly washed away. Cells were stained with anti-His or anti-Flag (Sigma), or anti-CCKBR antibodies, which were then detected with Alexa 594-conjugated anti-rabbit, Alexa 488-conjugated anti-goat or mouse IgG, or Alexa 647-conjugated anti-mouse IgG (Invitrogen). Sections were observed and imaged using a fluorescence-equipped Olympus microscope.
Cell viability and growth were assessed by using the WST-1 reagent (BioVision, Mountain View, CA), which measures the cleavage of the tetrazolium salt WST-1 to formazan by mitochondrial dehydrogenases. 17
Results
AMP peptide prevents development of oral mucositis induced by acute radiation injury
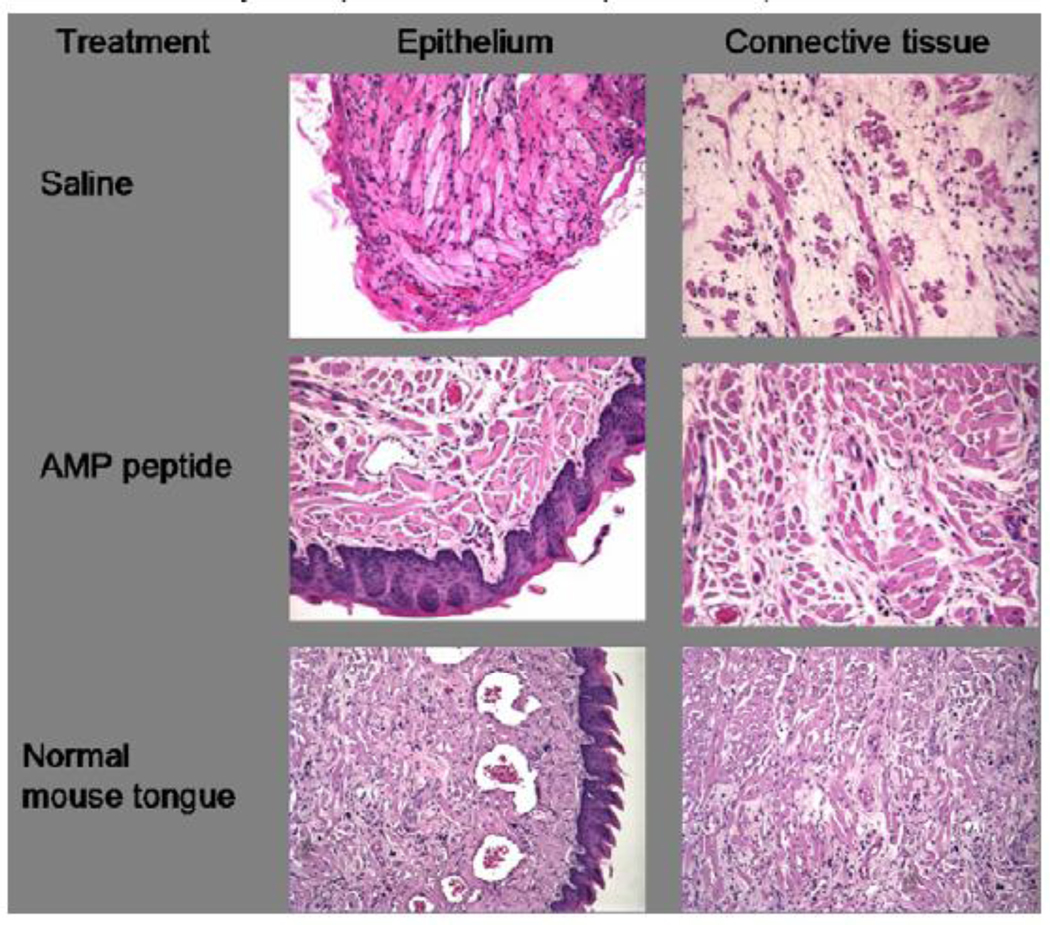
Murine oral mucositis in the snout was induced by a single dose of radiation. AMP peptide (25 mg/kg) was administered once daily four days before radiation and continued until day 10. Mucositis was evaluated by histological evaluation of the tongue on day 10. The majority of saline-treated control mice (n=8) had complete loss of tongue epithelium in addition to significant connective tissue pathology (e.g., muscle necrosis) compared to normal mouse tongue (Fig. 1, top panels vs. bottom panels), and the effect was more pronounced on the dorsal than the ventral surface of the tongue. In contrast, mice treated with AMP peptide (n=8) showed more intact epithelial and connective tissue histology (Fig. 1, middle panels); 3 of 8 specimens appeared normal. In addition, there was no evidence of any toxicity associated with administration of the peptide.
Fig. 1. Effect of AMP peptide on tongue histology in mice with oral mucositis induced by acute radiation injury to the snout.
Tongues from mice with oral mucositis are shown in the top and middle panels, treated with saline (vehicle) or AMP peptide, respectively. Bottom panels show epithelial and connective tissue histology of normal mouse tongue. Formalin fixed, paraffin-embedded tissue was sectioned and stained with hematoxylin and eosin. X240.
AMP peptide is a mitogen for the HaCaT cells
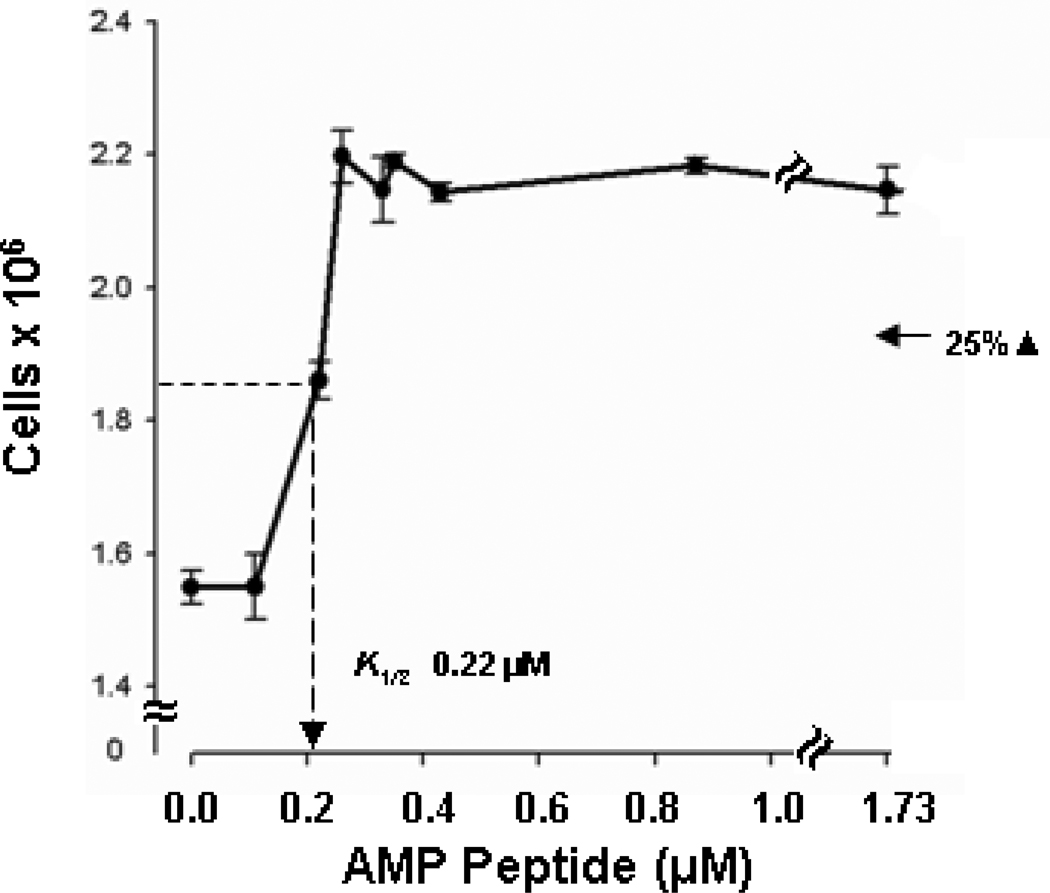
To determine if cells used to model the stratified squamous epithelium of the oral mucosa could respond directly to AMP peptide in culture to permit future mechanistic studies, we asked if the peptide was mitogenic for HaCaT cells. 15, 18, 19 These Human, adult, (low concentration of) Calcium, elevated Temperature keratinocytes (HaCaT) are an immortalized but nontumorigenic epithelial cell line derive from histologically normal human skin. Fig. 2 shows that AMP peptide stimulated growth of HaCaT cells in a dose-dependent manner at approximately the same half-maximal concentration we reported for epithelial cells of the intestine and stomach. 7
Fig. 2. AMP peptide is a mitogen for HaCaT cells.
Sub-confluent cultures were prepared in DMEM with 5% fetal calf serum (FCS). Medium was replaced 2 days later with fresh DMEM to which different amounts of AMP peptide were added. The number of cells in each culture was counted 3 days later. Growth was stimulated about 40% (P<0.001) by AMP peptide with a half-maximal concentration (K1/2) of 0.22 µM.
AMP-18 binds to the surface of oral mucosal cells in normal human tissue
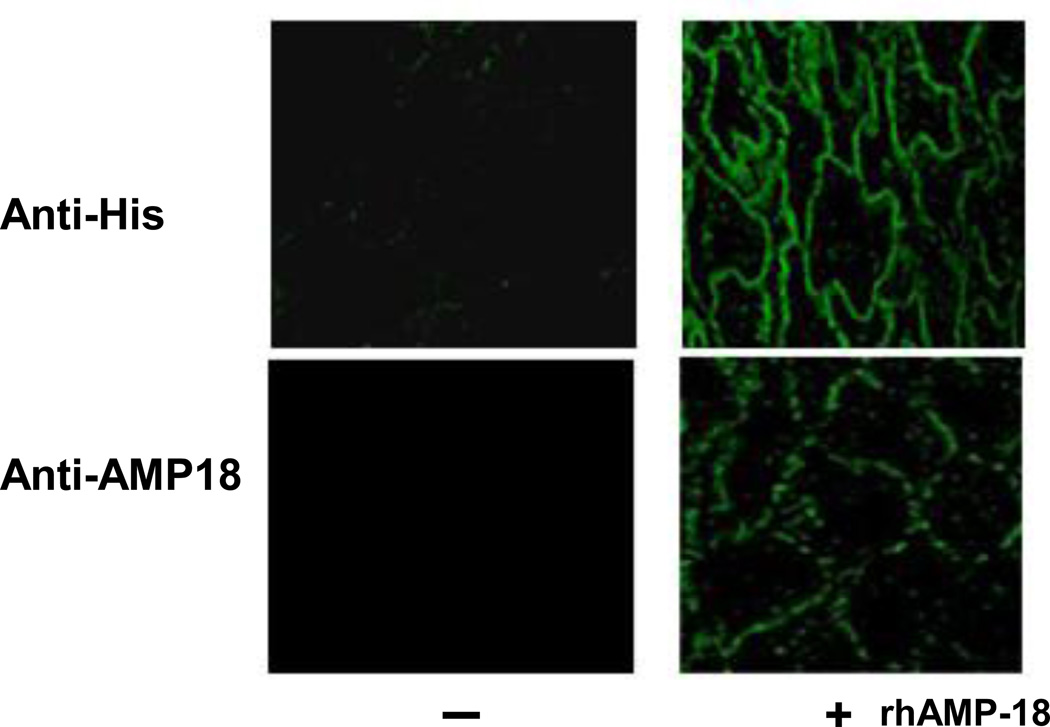
To investigate whether an AMP-18 receptor is expressed in human oral mucosal tissue and localize the receptor sites, 5-µm sections of archived normal human oral mucosal tissue were incubated with His6-tagged rhAMP-18. As shown in Fig. 3, both a commercial anti-His antibody and our anti-rhAMP-18 rabbit monospecific antibody immunolocalized to putative AMP-18 receptors on the plasma membranes of oral keratinocytes.
Fig. 3. Distribution of putative AMP-18 receptors in human oral tissue.
Sections (5 µm) of normal human oral mucosal tissue were deparaffinized, subjected to antigen retrieval, washed with Tris-buffered saline Tween-20 (TBST), blocked using 1% BSA, and then incubated with His6-tagged rhAMP-18 (+) or vehicle (−) overnight at 4°C. Then sections were rinsed with TBST, blocked with 0.25% soluble casein, and incubated with a rabbit anti-His antibody (top panels) or a rabbit anti-rhAMP-18 (bottom panels) for 1 hr at room temperature. The sections were rinsed, and Cy2 donkey anti-rabbit IgG was used to localize the AMP-18 ligand-receptor complex (green color). Images obtained with a Fluoview 200 laser scanning confocal microscope (60X) localize rhAMP-18 along the plasma membranes of keratinocytes (right panels). No signal was detected in tissue sections incubated in the absence of rhAMP-18 (left panels).
Cholecystokinin-B/Gastrin receptor is identified as a putative receptor for AMP-18
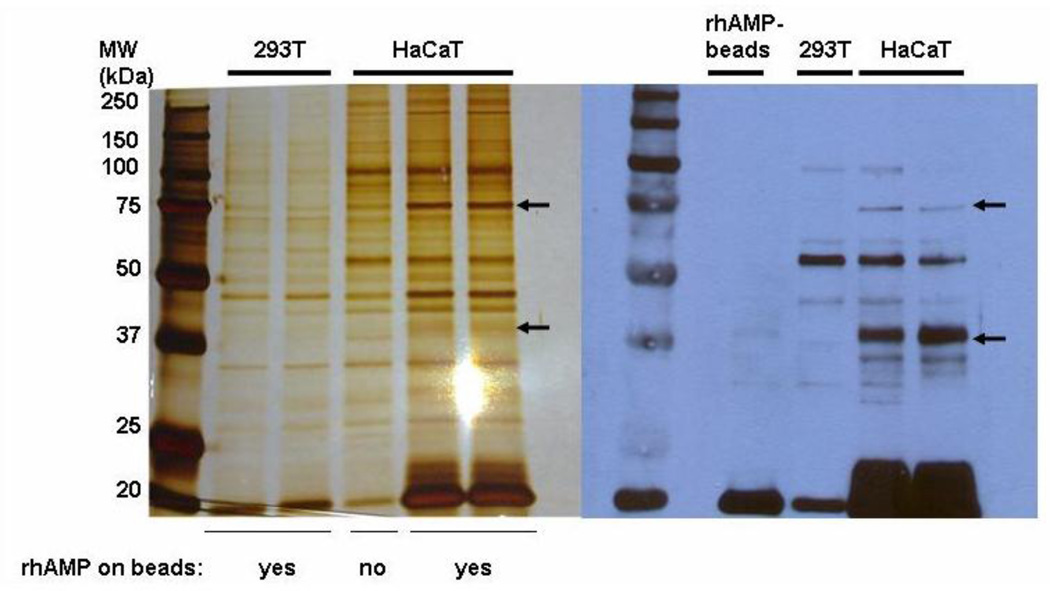
To identify and characterize the cell surface receptor for AMP-18 we set out with an affinity purification strategy. In a pilot immunofluorescence staining study, significant binding of rhAMP-18 was detected on the surface of HaCaT cells, whereas no remarkable binding was detected on 293T cells (data not shown). Membrane extracts were prepared and pre-cleared from cultured HaCaT cells, and then incubated with His-tagged rhAMP-18 protein immobilized on cobalt agarose beads to capture a putative receptor while membrane extracts prepared in parallel from 293T cells served as a negative control. After extensive washing, the captured proteins were resolved by SDS-PAGE followed by silver staining (Fig. 4). As shown on the silver-stained gel, (Fig. 4, left panel) two bands (75 and 37 kDa in size) were observed in HaCaT cells, but were not readily detected in 293T cells, or a HaCaT cell control sample containing only membrane proteins and agarose beads but no His-tagged rhAMP-18 protein.
Fig. 4. Cell membrane proteins that bind to AMP-18.
Left panel: Membrane proteins were prepared from HaCaT or 293T cells and then incubated overnight at 4°C with His-tagged rhAMP-18 protein immobilized on cobalt agarose beads (labeled as “yes”). Unconjugated cobalt agarose beads (without rhAMP-18) (labeled as “no”) were also incubated with HaCaT cell membrane extracts as a control. After extensive washing, interacting membrane proteins were eluted from the beads, resolved by SDS-PAGE and subjected to silver staining. Two bands were identified in HaCaT cells that were not readily apparent in HaCaT control or 293T cells. One band was ~75 kDa (←) and a second faint band was ~37 kDa (←). These two bands were each excised from the silver-stained gel and analyzed by mass spectrometry. Right Panel: Aliquots of original membrane protein extracts were resolved on a duplicate SDS-PAGE gel and proteins were transferred onto Immobilon membranes. The blot was incubated overnight with His-tagged rhAMP-18 and analyzed by immunoblotting with an anti-His antibody. Bands of ~75 kDa and ~37 kDa (←) were visualized in HaCaT cells on the blot, but not in HaCaT control or 293T cells. Duplicate lanes for HaCaT cells were included in both panels.
In a second experimental strategy, aliquots of original membrane protein extracts were resolved on an SDS-PAGE gel and transferred onto an Immobilon membrane. The blot was then incubated with His-tagged rhAMP-18 (2 µg/ml) overnight at 4°C, washed, and analyzed by immunoblotting with an anti-His antibody (Fig. 4, right panel). Consistent with the silver-stained gel, two bands at 75 and 37 kDa were observed in HaCaT cells but not in 293T cells. These two bands were then excised from the silver-stained gel, digested with trypsin, and the resultant peptides were subjected to mass spectrometric analysis to identify a potential receptor.
Mass spectrometry analysis of the 75 kDa band identified CCKBR. CCKBR is a G-protein coupled receptor (GPCR) for cholecystokinin (CCK) and gastrin 20. It is primarily found in cells of the GI tract and central nervous system. Another protein identified in the 75 kDa band was a Rho guanine nucleotide exchange factor (GEF), GEF7. Rho GTPases are important components of GPCR-mediated signaling pathways that control many aspects of cell function such as cytoskeletal organization, cell migration, cell-cell and cell matrix adhesion, cell cycle progression, gene expression, and cell polarity. 21 The involvement and importance of GEF7 and Rho GTPases in AMP-18 activated CCKBR signaling is under investigation. Analysis of the 37 kDa band identified several proteins, including Heat-Shock Protein (HSP) 40 (DnaJ homolog). This role of this protein in CCKBR regulation and function is not known at present, however, it has been shown to regulate trafficking of other GPCRs. 22
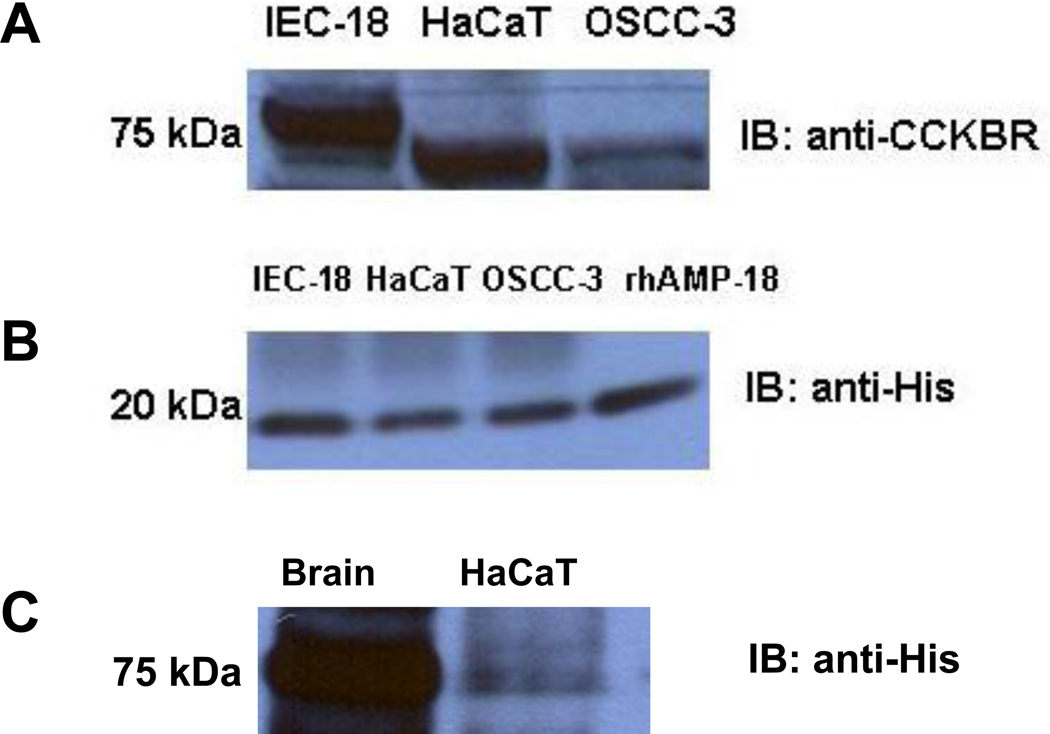
Expression of CCKBR was subsequently confirmed by immunoblotting assays of HaCaT, IEC-18, and OSCC-3 cells (Fig. 5, panel A), although the molecular size of the receptor varied slightly. This may be due to different degrees of glycosylation and/or phosphorylation since CCKBR has multiple sites (3–4 potential N-linked glycosylation sites in the N-terminus) that can undergo these modifications, and it has been suggested that the extent of CCKBR glycosylation differs in different cell types. 23–25 For example, CCKBR isolated from canine parietal cells displays an apparent size of 76 kDa, while CCKBR in canine pancreatic membranes is approximately 47 kDa. The bovine CCKBR appears as a 42 – 47 kDa protein in pancreatic membranes, but is expressed as an 82 kDa protein in African green monkey kidney COS-7 cells. 24 Alternatively, splice variants have been reported for CCKBR, although their functional significance remains to be thoroughly defined. 26
Fig. 5. Expression of CCKBR in IEC-18, HaCaT and OSCC-3 cells, and coimmunoprecipitation with rhAMP-18.
Panel A. Total cell lysates from IEC-18, HaCaT and OSCC-3 cells were each analyzed using an immunoblotting assay with an anti-CCKBR antibody. An expected band of 75 kDa was detected in HaCaT and OSCC-3 cells while a band of slightly higher molecular weight was observed in IEC-18 cells (see text). Panel B. Total cell lysates from IEC-18, HaCaT or OSCC-3 cells were each immunoprecipitated with anti-CCKBR antibody and protein G beads in the presence of 2 µg/ml His-tagged rhAMP-18. After incubation and extensive washing, the immunocomplexes bound to the beads were resolved by SDS-PAGE and then subjected to immunoblotting with an anti-His antibody to detect the presence of rhAMP-18 in the ligand-receptor complex. Fifty ng rhAMP-18 was included in a separate lane on the SDS-PAGE gel as a control (rhAMP-18). Panel C. Reciprocal co-immunoprecipitation was performed under the same conditions by incubating rhAMP-18 with pre-cleared lysate of HaCaT cells followed by incubation with an agarose-conjugated anti-His antibody. Since CCKBR is abundantly expressed in the CNS, mouse brain homogenate (Brain) was used a positive control.
The interaction between CCKBR and AMP-18 was confirmed using a co-immunoprecipitation assay. A total cell lysate from IEC-18, HaCaT or OSCC-3 cells was immunoprecipitated with an anti-CCKBR antibody and protein G beads in the presence of 2 µg/ml His-tagged rhAMP-18. After incubation and extensive washing, rhAMP-18 in the immunocomplexes was detected by immunoblotting with an anti-His antibody. As shown in Fig. 5, panel B, rhAMP-18 was pulled-down by CCKBR in all three cell lines, indicating that AMP-18 could be a ligand for CCKBR. The interaction between CCKBR and rhAMP-18 was also seen in a reciprocal co-immunoprecipitation in which rhAMP-18 was incubated with a pre-cleared HaCaT total cell lysate followed by incubation with agarose-conjugated anti-His antibody (Fig. 5, panel C).
Colocalization of AMP-18 and CCKBR
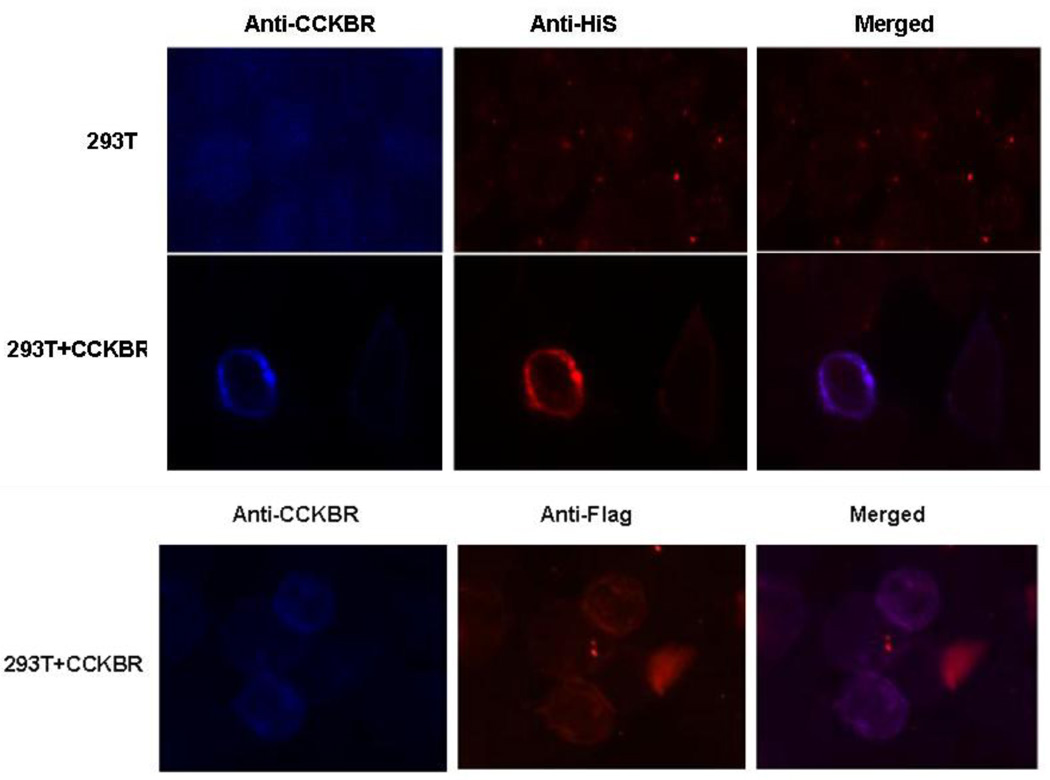
To determine if the interaction between AMP-18 and CCKBR occurred on the cell surface, CCKBR was expressed in 293T cells. Cells were fixed, incubated with rhAMP-18 overnight at 4°C, and then stained for AMP-18 with an anti-His or anti-Flag antibody, and for CCKBR with an anti-CCKBR antibody. As shown in Fig. 6, CCKBR was expressed on the surface of 293T cells (left panels, blue). When cells were incubated with rhAMP-18, binding of AMP-18 on the surface of CCKBR-expressing cells was observed (middle panels, red). In contrast, control 293T cells did not demonstrate significant and specific staining of CCKBR and rhAMP-18. Furthermore, the staining of CCKBR, and rhAMP-18 with either an anti-His or anti-Flag antibody, co-localized on the surface of transfected cells (right, merged, purple). These observations, together with the co-immunoprecipitation results shown in Fig. 5, strongly suggest that CCKBR can serve as a cell surface receptor for AMP-18.
Fig. 6. Binding of rhAMP-18 to CCKBR expressed in 293T cells.
CCKBR was expressed in 293T cells (293T+CCKBR). Control 293T cells (293T) were transfected with empty pBaBe vectors (top 3 panels). Cells were grown on poly-lysine-coated coverslips. Forty-eight hours later the cells were fixed and incubated with His-tagged or His-and Flag-tagged rhAMP-18 overnight. Expressed CCKBR and bound AMP-18 were detected and co-localized by double immunofluorescence using an anti-CCKBR antibody (blue) and an anti-His antibody (red, middle 3 panels), or anti-Flag antibody (red, bottom 3 panels) followed by staining with appropriate Alexa-conjugated secondary antibodies (purple, merged). The purple merged images suggest colocalization of rhAMP-18 and CCKBR. Representative images are shown from 3 experiments.
AMP-18 stimulation of CCKBR function
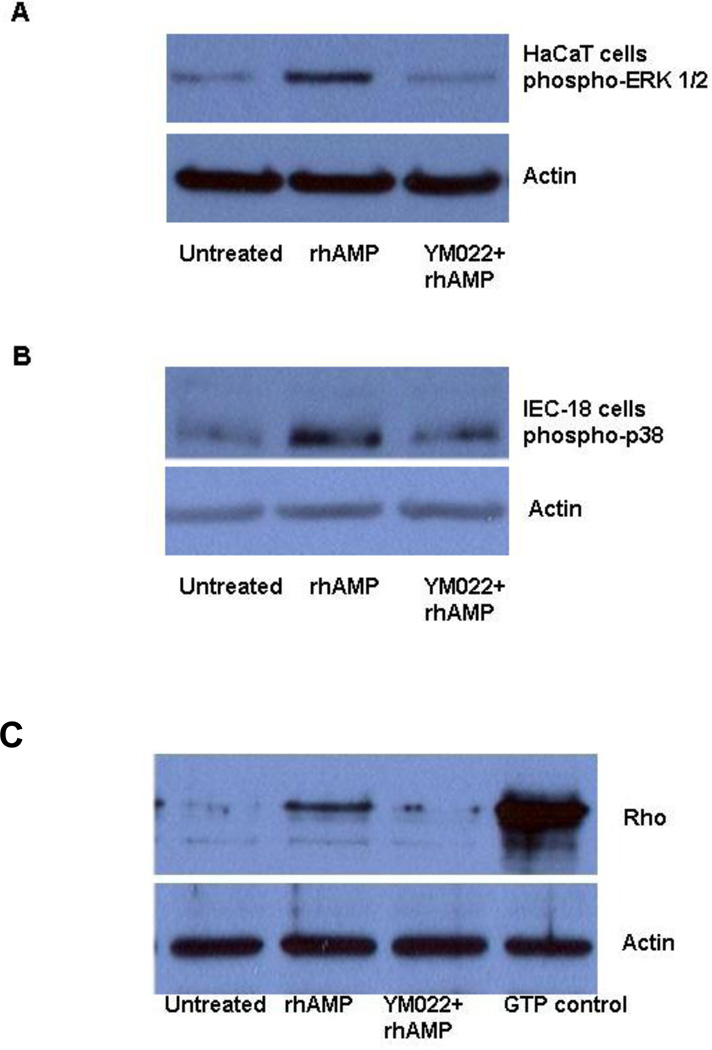
To find out if AMP-18 exerts its effects through CCKBR activation of signaling pathways we studied the cell response to AMP-18 in the presence or absence of a potent and selective CCKBR antagonist, YM022. 27 HaCaT or IEC-18 cells were pretreated or untreated with 50 nM YM022 prior to exposure to 2 µg/ml rhAMP-18. As shown in Fig. 7, treatment of HaCaT cells with rhAMP-18 stimulated ERK activation (Fig. 7, panel A), but not p38 MAPK (data not shown). In contrast, treatment with rhAMP-18 stimulated p38 MAPK phosphorylation in IEC-18 cells (Fig. 7, panel B), while ERK was not significantly affected (data not shown). In both cell types, pretreatment with YM022 completely inhibited the activation response. These data suggest that AMP-18 can activate cell signaling via CCKBR, but may utilize diverse pathways in different types of cells.
Fig. 7. Activation of ERK and p38 MAPK signaling pathways and Rho by rhAMP-18 is inhibited by the CCKBR inhibitor YM022.
Cells were grown to approximately 80% confluence, exposed to 50 nM YM022 for 5 min, or not exposed, and then treated with 2 µg/ml rhAMP-18 for 1 h, or left untreated. After treatment, total cell lysates were prepared. Thirty to fifty micrograms protein/lane were resolved by SDS-PAGE and analyzed by immunoblotting assays for the phosphorylation of ERK and p38 MAPK (A and B). For Rho activation assays, IEC-18 cell lysates prepared in Mg2+ lysis buffer were used (C). An aliquot of untreated IEC-18 cell lysate was loaded with GTPγS to serve as a positive control (GTP control). Immunoblotting assays with anti-actin antibodies were included to demonstrate equal loading. Representative results from 2–3 experiments are shown.
Rho GTPases are known to be an important component in CCKBR-mediated signaling. They can regulate cytoskeletal organization, cell migration, cell-cell and cell matrix adhesion, and could thereby contribute to AMP-18 mediated protection of the GI or oral mucosal barrier by targeting TJs. 5 Rho activation was studied in IEC-18 cells after treatment with rhAMP-18 in the presence or absence of YM022 (Fig. 7, panel C). Treatment with rhAMP-18 activated Rho, while pretreatment with YM022 significantly inhibited the response.
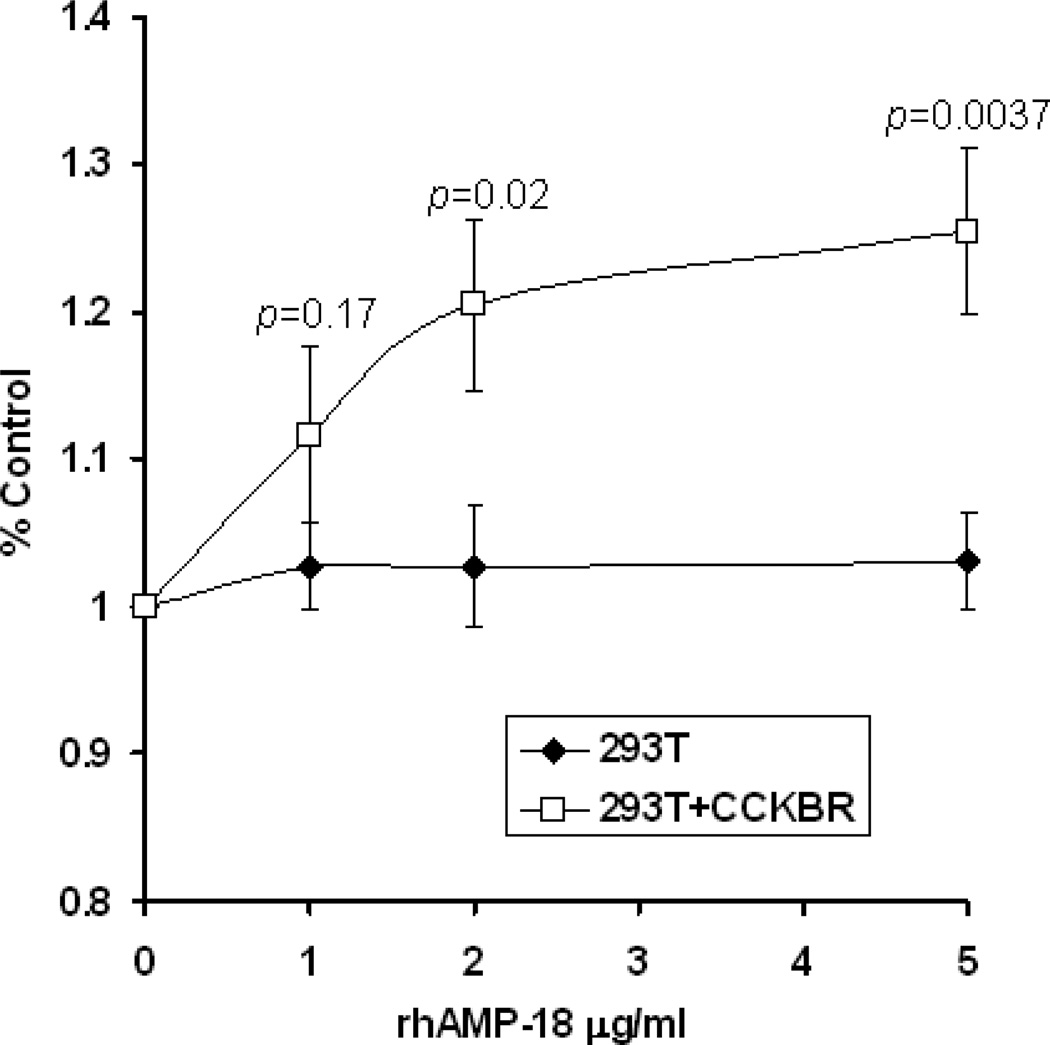
Confirmation of AMP-18 as a physiological ligand that activates cell growth and viability via CCKBR was sought by comparing its effects on 293T cells that did or did not express the receptor. CCKBR-expressing 293T cells and control 293T cells (transfected with an empty vector) were grown in medium with 0.5% FBS in the presence of increasing concentrations of rhAMP-18. Cell viability was evaluated with WST-1 cell proliferation reagents. AMP-18 enhanced viability and growth in CCKBR-expressing cells compared to cells that did not express the receptor (Fig. 8).
Fig. 8. AMP-18 enhances viability and growth of CCKBR-expressing 293T cells.
CCKBR-expressing 293T cells (293T+CCKBR) or control empty vector transfected 293T cells (293T) were grown in medium with 0.5% FBS in the presence of different concentrations of rhAMP-18 for 48 h. Cell viability and growth was measured and compared using WST-1 cell proliferation reagents. Values shown are means ± SE (n = 6 cultures). Treatment of CCKBR-expressing cells with rhAMP-18 promoted viability and growth by 20 – 25%.
Discussion
OM associated with cytotoxic cancer therapy remains a common, devasting, unmet clinical problem. However, current agents used to treat OM provide only symptomatic relief, so new agents are needed for this condition. Therefore we set out to investigate the therapeutic effects of AMP peptide on OM based on its capacity to maintain epithelial barrier function and structure.
AMP-18 is a protein constitutively expressed in epithelial cells of the gastric antrum that is cell protective, mitogenic and motogenic in cell culture and in vivo. Treatment with AMP peptide protected the surface epithelium of the mouse oral mucosa (Fig. 1). AMP-18 peptide stimulates growth of diverse types of epithelial cells including HaCaT cells (Fig. 2). It appears to exert its pleiotropic effects via a cell surface receptor (Fig. 3) 7, shown here to be CCKBR. CCKBR was identified by mass spectrometry of HaCaT membrane proteins that selectively bound to an immobilized His-tagged rhAMP-18 fusion protein following affinity purification (Fig. 4). AMP-18 also interacted with CCKBR to form complexes that were pulled–down by CCKBR immunoprecipitation (Fig. 5). Immumofluorescence staining revealed that AMP-18 colocalized with CCKBR on the surface of transfected cells (Fig. 6). Functional assays performed in GI epithelial cell lines and/or CCKBR-transfected 293T cells showed that rhAMP-18 activated Rho and MAPK signaling pathways that were blocked by a CCKBR-specific antagonist, YM022, and also enhanced cell viability and growth (Figs. 7, 8).
The mucosal barrier function is mainly regulated by epithelial cells and TJs at the apical-most domains of the plasma membrane that seal the epithelial cells, which have been shown to regulate paracellular permeability across the epithelium in monolayer cell cultures and in vivo. 28 TJs consist of integral membrane proteins including occludin, claudins, and junctional adhesion molecule (JAM), and multiple cytoplasmic proteins such as zonula occludens (ZO)-1, ZO-2, cingulin, and others that form the terminal plaque. 29–34 The TJ transmembrane protein occludin is linked via ZO-1 to the apical perijunctional F-actin ring enabling TJ and cytoskeletal proteins to regulate paracellular permeability in both physiological and pathological states. 35–37 In addition, the TJs are also important mediators of cell adhesion and “outside –in” and “inside-out” signaling pathways that regulate epithelial proliferation and differentiation. AMP peptide stimulated accumulation of the TJ proteins occludin and ZO-1 and enhanced the structure and function of TJs. 5 These effects protected cell monolayers against decreases in TER induced by oxidant injury, and disruption of actin filaments by cytochalasin D. In addition, AMP peptide stimulated the growth of HaCaT cells, which may contribute to the barrier protective function seen in Fig. 1.
In the efforts to better understand the signaling mechanism by which AMP-18 exert its barrier protective function, CCKBR was identified as a putative receptor for AMP-18. CCKBR is one of the two known CCK/gastrin receptors: CCKAR and CCKBR. 20 Both CCK receptors exhibit a three-dimensional structure that is similar to the prototypical GPCR, rhodopsin. However, the two receptors exhibit a relatively low degree of sequence homology to each other (50%), demonstrate different tissue distributions and different affinities towards their endogenous ligands, i.e., CCK and gastrin. 38–41 CCKAR binds and responds to sulfated CCK more favorably, whereas CCKBR demonstrates a promiscuous property, binding and responding to gastrin or CCK with nearly the same affinity regardless of the extent of sulfation. 42, 43 In addition, the binding sites of CCK on CCKAR and CCKBR are slightly different, and the downstream signaling pathways triggered by receptor activation are distinct. The sulfated tyrosine residue in CCK plays an important role in positioning the N-terminus of CCK in the proximity of specific amino acid residues in the second extracellular loop (Met and Arg residues) and the third extracellular loop, while the C-terminal appears to be embedded among transmembrane (TM) domains III, V, VI, and VII in CCKAR. In contrast, a His residue in the second extracellular loop interacts with the penultimate Asp of CCK, while the C-terminal amidated Phe residue interacts with TM domains IV and VI in CCKBR. These subtle differences may contribute to the different signaling and functions of these two receptors. Identifying CCKBR rather than CCKAR as a receptor for AMP-18 is consistent with the receptor’s relatively low ligand specificity that could allow AMP-18 to bind. In addition, different splice variants have been reported for CCKBR, 26, 44 and it is unknown if these variants bind their ligands through different mechanisms.
The functions and effects of CCK and gastrin binding to CCKBR have been extensively studied. 20, 45, 46 In response to the engagement of gastrin or CCK to CCKBR, a variety of signaling pathways are activated. 47–52 Similar to other GPCRs, activation of CCKBR induces rapid hydrolysis of phosphatidylinositol bisphosphate by phospholipase C (PLC) family members to generate inositol trisphosphate (IP3) and diacylglycerol (DAG), which respectively induce calcium mobilization and stimulate several protein kinases C (PKCs). While CCKAR and CCKBR primarily activate PLCβ, PLCγ is also implicated in CCKBR-mediated signaling pathways. 52, 53 Activation of PKCs leads to subsequent activation of MAPK and other signaling pathways.
After cells were treated with rhAMP-18 in the presence or absence of a CCKBR-specific inhibitor, YM022, the activation of two MAPK signaling pathways, ERK and p38 were analyzed. ERK activation was induced in HaCaT cells whereas p38 was induced in IEC-18 cells. Both activations were significantly inhibited by YM022, suggesting that AMP-18-induced signaling pathways may be cell-type specific. Several mechanisms could be involved in this phenomenon. First, a different modification (e.g., glycosylation) of CCKBR presented as a different apparent molecular weight may contribute to this cell-type specific response. Another mechanism might be activation via different PKC isoforms, which has been documented in physiological and pathological processes of not only the GI tract 54–57 but other tissues. 58, 59 Analogically, AMP-18 may be able to activate distinct signaling pathways through CCKBR in a manner similar to gastrin. In gastric epithelial cells, binding of gastrin to CCKBR induces the expression and release of heparin-binding epidermal-like growth factor (HB-EGF), which subsequently transactivates the EGF receptor and downstream signaling pathways. 60 In contrast, in intestinal epithelial cells gastrin induces EGF transactivation via SRC kinase. 61 Activation of the ERK signaling pathway by CCKBR in these cells controls proliferation and migration, and participates in transcriptional regulation of several gastrin-sensitive genes. 46
Regarding the relatively low specificity CCKBR demonstrates towards its ligands, identification of AMP-18 as a new putative ligand for CCKBR, in addition to gastrin and CCK, introduces another level of complexity in the regulation and function of CCKBR. The significance of AMP-18 stimulated signaling relative to that of gastin and CCK is not known. Major questions that remain to be addressed include how AMP-18 competes with other ligands in vivo, and whether binding of AMP-18 to CCKBR would stimulate the same signaling events in a specific cell or tissue. Clearly, small GTPase-mediated pathways appear worthy of further pursuit. Rho, Rac, and cdc42 have been reported to be activated by Gα12/13 coupled to CCKBR, 51 which appears to regulate the formation of stress fibers and modulate cell morphology in response to CCK stimulation. In our study, Rho activation was induced by AMP-18 treatment in IEC-18 cells and inhibited by the CCKBR specific inhibitor YM022. This Rho-mediated pathway may be responsible for the capacity of AMP-18 to re-organize TJs and protect intestinal mucosal barrier function and structure in monolayer cell cultures and in vivo. 5 Furthermore, activation of Rho proteins is regulated by Rho GEFs and other regulatory proteins, particularly GTPase activating proteins (GAPs) and guanine nucleotide dissociation inhibitors (GDIs). In our mass spectrometry study of rhAMP-18 bound proteins we identified Rho GEF 7 (Fig. 4), whose involvement in AMP-18-activated CCKBR signaling warrants further investigation.
Taken together, our observations suggest that AMP peptide, by activating CCKBR, targets TJs to maintain mucosal integrity, and sets in motion protective and cell regenerative mechanisms for the prevention and treatment of OM.
Acknowledgements
This work was supported by NIH grant R21 DE018811 to F.G. Toback. The authors thank Thomas V. O. Hansen, Rigshospitalet, Denmark for providing the pBabe-CCKBR expression vector, and Donald F. Steiner, Department of Biochemistry and Molecular Biology, University of Chicago, for his critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004 Apr;4(4):277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 2.Sonis ST. Mucositis: The impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol. 2009 Dec;45(12):1015–1020. doi: 10.1016/j.oraloncology.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T, et al. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N Engl J Med. 2004 Dec 16;351(25):2590–2598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- 4.Sonis ST. Pathobiology of oral mucositis: novel insights and opportunities. J Support Oncol. 2007 Oct;5(9 Suppl 4):3–11. [PubMed] [Google Scholar]
- 5.Walsh-Reitz MM, Huang EF, Musch MW, Chang EB, Martin TE, Kartha S, et al. AMP-18 protects barrier function of colonic epithelial cells: role of tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2005 Jul;289(1):G163–G171. doi: 10.1152/ajpgi.00013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin TE, Powell CT, Wang Z, Bhattacharyya S, Walsh-Reitz MM, Agarwal K, et al. A novel mitogenic protein that is highly expressed in cells of the gastric antrum mucosa. Am J Physiol Gastrointest Liver Physiol. 2003 Aug;285(2):G332–G343. doi: 10.1152/ajpgi.00453.2002. [DOI] [PubMed] [Google Scholar]
- 7.Toback FG, Walsh-Reitz MM, Musch MW, Chang EB, Del Valle J, Ren H, et al. Peptide fragments of AMP-18, a novel secreted gastric antrum mucosal protein, are mitogenic and motogenic. Am J Physiol Gastrointest Liver Physiol. 2003 Aug;285(2):G344–G353. doi: 10.1152/ajpgi.00455.2002. [DOI] [PubMed] [Google Scholar]
- 8.Martin G, Wex T, Treiber G, Malfertheiner P, Nardone G. Low-dose aspirin reduces the gene expression of gastrokine-1 in the antral mucosa of healthy subjects. Aliment Pharmacol Ther. 2008 Sep 15;28(6):782–788. doi: 10.1111/j.1365-2036.2008.03793.x. [DOI] [PubMed] [Google Scholar]
- 9.Oien KA, McGregor F, Butler S, Ferrier RK, Downie I, Bryce S, et al. Gastrokine 1 is abundantly and specifically expressed in superficial gastric epithelium, down-regulated in gastric carcinoma, and shows high evolutionary conservation. J Pathol. 2004 Jul;203(3):789–797. doi: 10.1002/path.1583. [DOI] [PubMed] [Google Scholar]
- 10.Moss SF, Lee JW, Sabo E, Rubin AK, Rommel J, Westley BR, et al. Decreased expression of gastrokine 1 and the trefoil factor interacting protein TFIZ1/GKN2 in gastric cancer: influence of tumor histology and relationship to prognosis. Clin Cancer Res. 2008 Jul 1;14(13):4161–4167. doi: 10.1158/1078-0432.CCR-07-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nardone G, Rippa E, Martin G, Rocco A, Siciliano RA, Fiengo A, et al. Gastrokine 1 expression in patients with and without Helicobacter pylori infection. Dig Liver Dis. 2007 Feb;39(2):122–129. doi: 10.1016/j.dld.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Nardone G, Martin G, Rocco A, Rippa E, La Monica G, Caruso F, et al. Molecular expression of Gastrokine 1 in normal mucosa and in Helicobacter pylori-related preneoplastic and neoplastic gastric lesions. Cancer Biol Ther. 2008 Dec;7(12):1890–1895. doi: 10.4161/cbt.7.12.6936. [DOI] [PubMed] [Google Scholar]
- 13.Resnick MB, Sabo E, Meitner PA, Kim SS, Cho Y, Kim HK, et al. Global analysis of the human gastric epithelial transcriptome altered by Helicobacter pylori eradication in vivo. Gut. 2006 Dec;55(12):1717–1724. doi: 10.1136/gut.2006.095646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrell CL, Rex KL, Kaufman SA, Dipalma CR, Chen JN, Scully S, et al. Effects of keratinocyte growth factor in the squamous epithelium of the upper aerodigestive tract of normal and irradiated mice. Int J Radiat Biol. 1999 May;75(5):609–620. doi: 10.1080/095530099140258. [DOI] [PubMed] [Google Scholar]
- 15.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988 Mar;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma TY, Hollander D, Bhalla D, Nguyen H, Krugliak P. IEC-18, a nontransformed small intestinal cell line for studying epithelial permeability. J Lab Clin Med. 1992 Aug;120(2):329–341. [PubMed] [Google Scholar]
- 17.Huhtala A, Alajuuma P, Burgalassi S, Chetoni P, Diehl H, Engelke M, et al. A collaborative evaluation of the cytotoxicity of two surfactants by using the human corneal epithelial cell line and the WST-1 test. J Ocul Pharmacol Ther. 2003 Feb;19(1):11–21. doi: 10.1089/108076803762718079. [DOI] [PubMed] [Google Scholar]
- 18.Hasina R, Hulett K, Bicciato S, Di Bello C, Petruzzelli GJ, Lingen MW. Plasminogen activator inhibitor-2: a molecular biomarker for head and neck cancer progression. Cancer Res. 2003 Feb 1;63(3):555–559. [PubMed] [Google Scholar]
- 19.Merne M, Heikinheimo K, Saloniemi I, Syrjanen S. Effects of snuff extract on epithelial growth and differentiation in vitro. Oral Oncol. 2004 Jan;40(1):6–12. doi: 10.1016/s1368-8375(03)00109-x. [DOI] [PubMed] [Google Scholar]
- 20.Wank SA. G protein-coupled receptors in gastrointestinal physiology. I. CCK receptors: an exemplary family. Am J Physiol. 1998 Apr;274(4 Pt 1):G607–G613. doi: 10.1152/ajpgi.1998.274.4.g607. [DOI] [PubMed] [Google Scholar]
- 21.Samarin S, Nusrat A. Regulation of epithelial apical junctional complex by Rho family GTPases. Front Biosci. 2009;14:1129–1142. doi: 10.2741/3298. [DOI] [PubMed] [Google Scholar]
- 22.Chapple JP, Cheetham ME. The chaperone environment at the cytoplasmic face of the endoplasmic reticulum can modulate rhodopsin processing and inclusion formation. J Biol Chem. 2003 May 23;278(21):19087–19094. doi: 10.1074/jbc.M212349200. [DOI] [PubMed] [Google Scholar]
- 23.Caplin ME, Clarke P, Grimes S, Dhillon AP, Khan K, Savage K, et al. Demonstration of new sites of expression of the CCK-B/gastrin receptor in pancreatic acinar AR42J cells using immunoelectron microscopy. Regul Pept. 1999 Oct 22;84(1–3):81–89. doi: 10.1016/s0167-0115(99)00071-3. [DOI] [PubMed] [Google Scholar]
- 24.Dufresne M, Escrieut C, Clerc P, Le Huerou-Luron I, Prats H, Bertrand V, et al. Molecular cloning, developmental expression and pharmacological characterization of the CCKB/gastrin receptor in the calf pancreas. Eur J Pharmacol. 1996 Feb 15;297(1–2):165–179. doi: 10.1016/0014-2999(95)00737-7. [DOI] [PubMed] [Google Scholar]
- 25.Fourmy D, Zahidi A, Fabre R, Guidet M, Pradayrol L, Ribet A. Receptors for cholecystokinin and gastrin peptides display specific binding properties and are structurally different in guinea-pig and dog pancreas. Eur J Biochem. 1987 Jun 15;165(3):683–692. doi: 10.1111/j.1432-1033.1987.tb11495.x. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz F, Schrader H, Otte J, Schmitz H, Stuber E, Herzig K, et al. Identification of CCK-B/gastrin receptor splice variants in human peripheral blood mononuclear cells. Regul Pept. 2001 Sep 15;101(1–3):25–33. doi: 10.1016/s0167-0115(01)00281-6. [DOI] [PubMed] [Google Scholar]
- 27.Murayama T, Matsumori Y, Iwata N, Ito M, Taniguchi T, Chihara K, et al. Antiproliferative effect of a novel cholecystokinin-B/gastrin receptor antagonist, YM022. Jpn J Cancer Res. 1996 Jul;87(7):743–750. doi: 10.1111/j.1349-7006.1996.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madara JL. Regulation of the movement of solutes across tight junctions. Annu Rev Physiol. 1998;60:143–159. doi: 10.1146/annurev.physiol.60.1.143. [DOI] [PubMed] [Google Scholar]
- 29.Byers SW, Citi S, Anderson JM, Hoxter B. Polarized functions and permeability properties of rat epididymal epithelial cells in vitro. J Reprod Fertil. 1992 Jul;95(2):385–396. doi: 10.1530/jrf.0.0950385. [DOI] [PubMed] [Google Scholar]
- 30.Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998 Jul 13;142(1):117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998 Jun 29;141(7):1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jesaitis LA, Goodenough DA. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol. 1994 Mar;124(6):949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keon BH, Schafer S, Kuhn C, Grund C, Franke WW. Symplekin, a novel type of tight junction plaque protein. J Cell Biol. 1996 Aug;134(4):1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byers S, Graham R, Dai HN, Hoxter B. Development of Sertoli cell junctional specializations and the distribution of the tight-junction-associated protein ZO-1 in the mouse testis. Am J Anat. 1991 May;191(1):35–47. doi: 10.1002/aja.1001910104. [DOI] [PubMed] [Google Scholar]
- 35.McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, et al. Occludin is a functional component of the tight junction. J Cell Sci. 1996 Sep;109(Pt 9):2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- 36.Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol. 1997 Jan 27;136(2):399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000 Aug;279(2):G250–G254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 38.Jensen RT, Lemp GF, Gardner JD. Interaction of cholecystokinin with specific membrane receptors on pancreatic acinar cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2079–2083. doi: 10.1073/pnas.77.4.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silvente-Poirot S, Escrieut C, Wank SA. Role of the extracellular domains of the cholecystokinin receptor in agonist binding. Mol Pharmacol. 1998 Aug;54(2):364–371. doi: 10.1124/mol.54.2.364. [DOI] [PubMed] [Google Scholar]
- 40.Langer I, Tikhonova IG, Travers MA, Archer-Lahlou E, Escrieut C, Maigret B, et al. Evidence that interspecies polymorphism in the human and rat cholecystokinin receptor-2 affects structure of the binding site for the endogenous agonist cholecystokinin. J Biol Chem. 2005 Jun 10;280(23):22198–22204. doi: 10.1074/jbc.M501786200. [DOI] [PubMed] [Google Scholar]
- 41.Zhou W, Povoski SP, Rosen NA, Longnecker DS, Bell RH., Jr Characterization of cholecystokinin receptors in rat pancreas. Evidence for expression of CCK-A receptors but not CCK-B (gastrin) receptors. Ann N Y Acad Sci. 1994 Mar 23;713:331–333. doi: 10.1111/j.1749-6632.1994.tb44084.x. [DOI] [PubMed] [Google Scholar]
- 42.Silvente-Poirot S, Escrieut C, Gales C, Fehrentz JA, Escherich A, Wank SA, et al. Evidence for a direct interaction between the penultimate aspartic acid of cholecystokinin and histidine 207, located in the second extracellular loop of the cholecystokinin B receptor. J Biol Chem. 1999 Aug 13;274(33):23191–23197. doi: 10.1074/jbc.274.33.23191. [DOI] [PubMed] [Google Scholar]
- 43.Silvente-Poirot S, Wank SA. A segment of five amino acids in the second extracellular loop of the cholecystokinin-B receptor is essential for selectivity of the peptide agonist gastrin. J Biol Chem. 1996 Jun 21;271(25):14698–14706. doi: 10.1074/jbc.271.25.14698. [DOI] [PubMed] [Google Scholar]
- 44.Chao C, Ives KL, Goluszko E, Kolokoltsov AA, Davey RA, Townsend CM, Jr, et al. SRC regulates constitutive internalization and rapid resensitization of a cholecystokinin 2 receptor splice variant. J Biol Chem. 2005 Sep 30;280(39):33368–33373. doi: 10.1074/jbc.M506337200. [DOI] [PubMed] [Google Scholar]
- 45.Nagata A, Ito M, Iwata N, Kuno J, Takano H, Minowa O, et al. G protein-coupled cholecystokinin-B/gastrin receptors are responsible for physiological cell growth of the stomach mucosa in vivo. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11825–11830. doi: 10.1073/pnas.93.21.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hocker M. Molecular mechanisms of gastrin-dependent gene regulation. Ann N Y Acad Sci. 2004 Apr;1014:97–109. doi: 10.1196/annals.1294.010. [DOI] [PubMed] [Google Scholar]
- 47.Daulhac L, Kowalski-Chauvel A, Pradayrol L, Vaysse N, Seva C. Src-family tyrosine kinases in activation of ERK-1 and p85/p110-phosphatidylinositol 3-kinase by G/CCKB receptors. J Biol Chem. 1999 Jul 16;274(29):20657–20663. doi: 10.1074/jbc.274.29.20657. [DOI] [PubMed] [Google Scholar]
- 48.Dehez S, Bierkamp C, Kowalski-Chauvel A, Daulhac L, Escrieut C, Susini C, et al. c-Jun NH(2)-terminal kinase pathway in growth-promoting effect of the G protein-coupled receptor cholecystokinin B receptor: a protein kinase C/Src-dependent-mechanism. Cell Growth Differ. 2002 Aug;13(8):375–385. [PubMed] [Google Scholar]
- 49.Noble PJ, Wilde G, White MR, Pennington SR, Dockray GJ, Varro A. Stimulation of gastrin-CCKB receptor promotes migration of gastric AGS cells via multiple paracrine pathways. Am J Physiol Gastrointest Liver Physiol. 2003 Jan;284(1):G75–G84. doi: 10.1152/ajpgi.00300.2002. [DOI] [PubMed] [Google Scholar]
- 50.Paulssen RH, Fraeyman N, Florholmen J. Activation of phospholipase C by cholecystokinin receptor subtypes with different G-protein-coupling specificities in hormone-secreting pancreatic cell lines. Biochem Pharmacol. 2000 Sep 15;60(6):865–875. doi: 10.1016/s0006-2952(00)00383-x. [DOI] [PubMed] [Google Scholar]
- 51.Stepan V, Ramamoorthy S, Pausawasdi N, Logsdon CD, Askari FK, Todisco A. Role of small GTP binding proteins in the growth-promoting and antiapoptotic actions of gastrin. Am J Physiol Gastrointest Liver Physiol. 2004 Sep;287(3):G715–G725. doi: 10.1152/ajpgi.00169.2003. [DOI] [PubMed] [Google Scholar]
- 52.Yassin RR, Abrams JT. Gastrin induces IP3 formation through phospholipase C gamma 1 and pp60c-src kinase. Peptides. 1998;19(1):47–55. doi: 10.1016/s0196-9781(97)00276-3. [DOI] [PubMed] [Google Scholar]
- 53.Arnould M, Tassa A, Ferrand A, Archer E, Esteve JP, Penalba V, et al. The G-protein-coupled CCK2 receptor associates with phospholipase Cgamma1. FEBS Lett. 2004 Jun 18;568(1–3):89–93. doi: 10.1016/j.febslet.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 54.Farhadi A, Keshavarzian A, Ranjbaran Z, Fields JZ, Banan A. The role of protein kinase C isoforms in modulating injury and repair of the intestinal barrier. J Pharmacol Exp Ther. 2006 Jan;316(1):1–7. doi: 10.1124/jpet.105.085449. [DOI] [PubMed] [Google Scholar]
- 55.McGarrity TJ, Peiffer LP, Neely EB, Palavarapu RG, Koltun WA, Parker P, et al. Localization of protein kinase C alpha isoform expression in the human gastrointestinal tract. Cell Growth Differ. 1996 Jul;7(7):953–959. [PubMed] [Google Scholar]
- 56.Osada S, Hashimoto Y, Nomura S, Kohno Y, Chida K, Tajima O, et al. Predominant expression of nPKC eta, a Ca(2+)-independent isoform of protein kinase C in epithelial tissues, in association with epithelial differentiation. Cell Growth Differ. 1993 Mar;4(3):167–175. [PubMed] [Google Scholar]
- 57.Perletti GP, Marras E, Concari P, Piccinini F, Tashjian AH., Jr PKCdelta acts as a growth and tumor suppressor in rat colonic epithelial cells. Oncogene. 1999 Feb 4;18(5):1251–1256. doi: 10.1038/sj.onc.1202408. [DOI] [PubMed] [Google Scholar]
- 58.Chandrasekher G, Bazan NG, Bazan HE. Selective changes in protein kinase C (PKC) isoform expression in rabbit corneal epithelium during wound healing. Inhibition of corneal epithelial repair by PKCalpha antisense. Exp Eye Res. 1998 Nov;67(5):603–610. doi: 10.1006/exer.1998.0555. [DOI] [PubMed] [Google Scholar]
- 59.Villanueva-Garcia D, Wang KT, Nielsen HC, Ramadurai SM. Expression of specific protein kinase C (PKC) isoforms and ligand-specific activation of PKCalpha in late gestation fetal lung. Exp Lung Res. 2007 Apr–May;33(3–4):185–196. doi: 10.1080/01902140701385073. [DOI] [PubMed] [Google Scholar]
- 60.Sinclair NF, Ai W, Raychowdhury R, Bi M, Wang TC, Koh TJ, et al. Gastrin regulates the heparin-binding epidermal-like growth factor promoter via a PKC/EGFR-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2004 Jun;286(6):G992–G999. doi: 10.1152/ajpgi.00206.2002. [DOI] [PubMed] [Google Scholar]
- 61.Guo YS, Cheng JZ, Jin GF, Gutkind JS, Hellmich MR, Townsend CM., Jr Gastrin stimulates cyclooxygenase-2 expression in intestinal epithelial cells through multiple signaling pathways. Evidence for involvement of ERK5 kinase and transactivation of the epidermal growth factor receptor. J Biol Chem. 2002 Dec 13;277(50):48755–48763. doi: 10.1074/jbc.M209016200. [DOI] [PubMed] [Google Scholar]