Abstract
Background and purpose
Recent studies reported the differential effect of docosahexaenoic (DHA) and eicosapentaenoic acids (EPA). We examined the differential association of DHA and EPA with carotid intima-media thickness (IMT) in the Japanese in Japan and U.S. whites and explored whether DHA or EPA contributes to the difference in IMT between the two groups.
Methods
A population-based cross-sectional study in 608 Japanese and United States (U.S.) white men aged 40–49 was conducted to assess IMT, serum DHA, EPA, and other cardiovascular risk factors.
Results
Japanese compared to U.S. whites had significantly lower IMT (618 ± 81 and 672 ± 94 μm for Japanese and white, respectively, mean ± standard deviation, p<0.001) and had more than twofold higher levels of DHA and EPA. DHA but not EPA had an inverse association with IMT in both Japanese and U.S. whites. The inverse association remained only in Japanese after adjusting for risk and other factors. The significant difference in multivariable-adjusted IMT became non significant after further adjusting for DHA (mean difference 17 μm (95% CI, −8 to 43), p=0.177) but not EPA. In this multivariable-adjusted model, DHA but not EPA was a significant predictor of IMT (p=−0.032 vs. 0.863, respectively).
Conclusions
These data suggest that DHA may have a more potent anti-atherogenic effect than EPA, especially in levels observed in the Japanese, independent of risk factors.
Keywords: docosahexaenoic acid, eicosapentaenoic acid, carotid arteries, imaging, population study
Marine-derived n-3 fatty acids (FAs) have a cardioprotective effect. Both epidemiological and clinical trial studies have shown that marine-derived n-3 FAs reduce the risk of coronary heart disease (CHD) death.1 The reduction in CHD death by marine-derived n-3 FAs is generally attributed to their anti-arrhythmic effect.2 Marine-derived n-3 FAs are also reported to have an anti-atherogenic effect.3 Results from epidemiological and clinical trial studies in Japan4, 5 where dietary intake of marine-derived n-3 FAs is high, i.e., 1,000 mg/day, compared to 100 mg/day in a typical Western diet,6 support a hypothesis that marine-derived n-3 FAs have the anti-atherogenic effect. To further support the hypothesis, we recently reported that Japanese men had a significant inverse association of serum levels of marine-derived n-3 FAs with intima-media thickness (IMT) of the carotid artery, an independent predictor of cardiovascular events.7
Recent studies reported the differential effects of eicosapentaenoic (EPA: 20:5n-3) and docosahexaenoic acids (DHA: 22:6n-3), two major marine-derived n-3 FAs, on cardiovascular risk factors.8–10 Although EPA can theoretically be metabolized to DHA, dietary intake or supplementation of EPA does not increase serum DHA.11 Two previous studies reported the differential association of DHA and EPA with IMT, giving conflicting results.12, 13
In the current study we examined the differential association of EPA and DHA with IMT both in the Japanese in Japan and U.S. whites. Additionally we examined whether EPA or DHA contributes to the difference in IMT between the Japanese and U.S. whites. We examined these questions in the electron-beam tomography, risk factor assessment among Japanese and U.S. men in the post-World-War-II birth cohort (ERA JUMP Study): a population-based-cross-sectional study of men aged 40–49 in Japan and the U.S. whites.
Methods
Detailed descriptions of methods were published elsewhere.14 Participants were population-based samples of 623 randomly-selected men aged 40–49 examined in 2002 to 2006, without clinical cardiovascular disease: 313 Japanese men from Kusatsu, Shiga, Japan and 310 U.S. men from Allegheny County, Pennsylvania, U.S. We excluded 15 subjects with missing data, resulting in 310 Japanese and 298 U.S. whites. Informed consent was obtained from all participants. The study was approved by the Institutional Review Boards of Shiga University of Medical Science, Otsu, Japan and University of Pittsburgh, Pittsburgh, U.S.
Venipuncture was performed early in the clinic visit after a 12-hour fast. The samples were stored at −80C° and shipped on dry ice to University of Pittsburgh. Serum lipids were determined using CDC-standardized methods, serum glucose using an enzymatic assay, serum insulin using a radio-immuno assay, and C-reactive protein (CRP) using an immuno-sorbent assay. Serum DHA, EPA, and other FAs were determined by capillary-gas-liquid chromatography, as previously described.14 The coefficients of variation between runs for EPA and DHA were 4.5% and 7.2%, respectively.
A self-administered questionnaire was used to obtain information on demography, frequency of fish intake, and other factors as previously described.14 Pack-years of smoking were calculated as years of smoking multiplied by the number of cigarettes per day divided by 20. Those who exercised were defined as those who regularly exercised ≥one hour per week. Hypertension was defined as systolic blood pressure (BP) ≥140 mmHg, diastolic BP ≥90 mmHg, or use of anti-hypertensive medications. Diabetes mellitus was defined as fasting serum glucose level ≥ 7 mmol/L or use of anti-diabetic medications.
Carotid artery scanning
The scanning procedures were previously described.14 A Toshiba 140A scanner equipped with a 7.5 MHz-linear-array imaging probe was used for carotid scanning at both centers. The sonographers scanned the right and left common carotid arteries (CCA), the carotid bulbs, and the internal carotid arteries. The scans were recorded and sent for scoring to the Ultrasound Research Laboratory, University of Pittsburgh. Trained readers digitized the best image for scoring. Our current study used CCA.14 The readers were blinded to participant's characteristics and the study centers. Correlation coefficients of IMT between sonographers and between readers were 0.96 and 0.99, respectively.
Statistical analyses
To compare risk factors between the populations, a t-test or the Mann-Whitney U test for continuous variables or chi-square test for categorical variables was used. To examine associations of EPA, DHA, or each of other FAs with IMT, we made tertile groups of each FA for each of the Japanese and U.S. whites. Then we compared age- and multivariable-adjusted tertile-specific levels of IMT. For the multivariable adjustment we first adjusted for BP, high-density-lipoprotein cholesterol (HDL-C) and triglycerides which have significant associations with marine n-3 FAs.3 Then we further adjusted for low-density-lipoprotein cholesterol (LDL-C), pack-years of smoking, glucose, insulin, and body-mass index (BMI), which are risk factors for IMT. Finally we further adjusted for CRP and other factors. To examine the linear trend of tertile-specific levels of IMT and whether EPA or DHA was associated with lower IMT in the Japanese than U.S. whites, general-linear-model analyses were used. All P-values were two-tailed. P-value <0.05 was considered as significant. PSAW Statistics (release 18.0, IBM, NY, U.S.) was used for all statistical analyses.
Results
The Japanese as compared to U.S. whites had a less favorable or similar profile of many major cardiovascular risk factors (Table 1), including BP, LDL-C, smoking and diabetes. Meanwhile the Japanese had a more favorable profile of some risk factors including BMI, HDL-C, and CRP. More than 40% of the Japanese ate fish 4 times a week or more as opposed to 3% of U.S. whites.
Table 1.
Characteristics of participants of the Japanese in Japan and whites in the U.S. in 2002 to 2006
| Japanese (n=310) | U.S. whites (n=298) | P | |
|---|---|---|---|
| Age (years) | 45.1 ± 2.8 | 45.0 ± 2.8 | 0.723 |
| Body-mass index (kg/m2) | 23.7 ± 3.0 | 27.9 ± 4.2 | <0.001 |
| Systolic blood pressure (mmHg) | 125.2 ± 16.0 | 122.7 ± 11.3 | 0.023 |
| LDL-C (mmol/L) | 3.43 ± 0.93 | 3.48 ± 0.88 | 0.499 |
| HDL-C (mmol/L) | 1.40 ± 0.35 | 1.24 ± 0.33 | <0.001 |
| Triglycerides (mmol/L)* | 1.55 (1.17, 2.07) | 1.45 (1.05, 2.10) | 0.087 |
| Fasting glucose (mmol/L) | 5.93 ± 1.04 | 5.62 ± 0.77 | <0.001 |
| Fasting insulin (ρmol/L) | 71.2 ± 30.3 | 105.0 ± 57.8 | <0.001 |
| C-reactive protein (mg/L)* | 0.32 (0.15, 0.68) | 0.96 (0.52, 1.82) | <0.001 |
| Current smoker (%) | 49.4 | 6.7 | <0.001 |
| Pack-years of smoking* | 18.8 (0, 29.0) | 0 (0, 1.2) | <0.001 |
| Exercise (%) | 26.8 | 73.5 | <0.001 |
| Ethanol consumption (g/day) | 15.3 (2.5, 42.2) | 4.5 (1.0, 15.0) | <0.001 |
| Hypertension (%) | 26.5 | 15.1 | 0.001 |
| Diabetes (%) | 6.1 | 3.4 | 0.109 |
| Medication for hypertension | 5.2 | 8.7 | 0.083 |
| Medication for diabetes | 1.9 | 1.0 | 0.343 |
| Medication for hyperlipidemia | 3.2 | 12.8 | <0.001 |
| Frequency of fish intake (%) | |||
| Once a week or less | 16.1 | 78.5 | |
| 2 to 3 times a week | 42.3 | 18.5 | |
| 4 times a week or more | 41.6 | 3.0 | <0.001 |
| Carotid IMT (μm) | 618 ± 81 | 672 ± 94 | <0.001 |
LDL-C: low-density-lipoprotein cholesterol, HDL-C: high-density-lipoprotein cholesterol, IMT: intima-media thickness.
Values are expressed as mean ± standard deviation for continuous variables except for those variables in which are expressed as median with inter-quartile range.
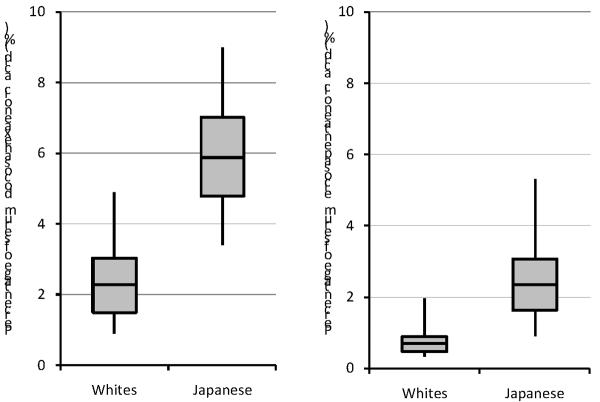
The Japanese had markedly higher serum levels of both EPA and DHA (Figure 1). The lower 5th percentiles of EPA and DHA in the Japanese were still higher than the higher 75th percentiles in U.S. whites (0.90 % versus 0.89 %, respectively for EPA, 3.34 % versus 3.03 %, respectively for DHA). The Spearman correlation of EPA and DHA was 0.349 (P<0.001) for the Japanese and 0.139 (P=0.017) for U.S. whites. Online supplement table shows means and standard deviations of other FAs.
Figure 1.
Distribution of serum docoxahexaenoic (DHA) and eicosapentaenoic acids (EPA) in Japanese and United State white men aged 40–49.
The box represents the interquartile range (25th to 75th values). The middle line across the box indicates the median. The whiskers represent the 5th and 95th values.
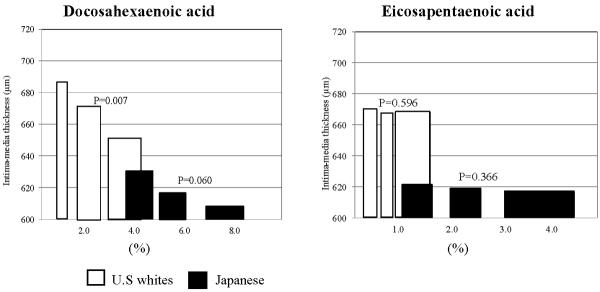
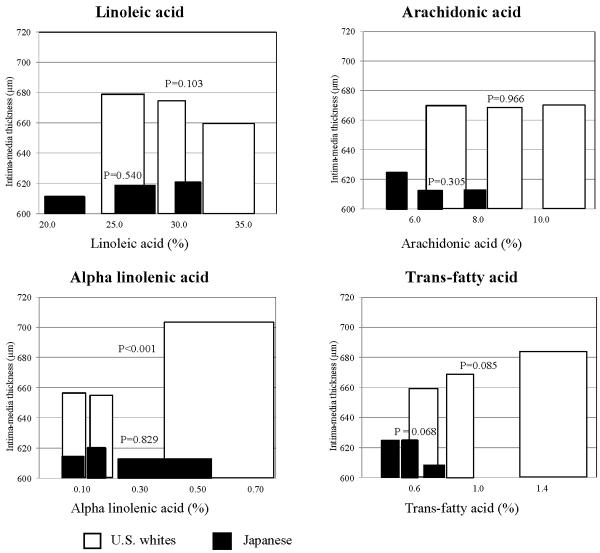
In the age-adjusted analysis, DHA had an inverse association with IMT both in the Japanese and U.S. whites (Figure 2). The inverse association was marginally significant in the Japanese (P = 0.060) and significant (P=0.007) in U.S. whites. In contrast, EPA had no significant association with IMT in either group. Among other FAs, alpha-linolenic and trans-fatty acids had significant and marginally significant positive association with IMT, respectively, only in U.S. whites (Figure 3). Linoleic acid had a non-significant inverse association with IMT in U.S. whites. Other serum FAs shown in online supplement table did not have significant inverse associations with IMT in either the Japanese or U.S. whites (data not shown).
Figure 2.
Age-adjusted associations of serum docoxahexaenoic (DHA) and eicosapentaenoic acids (EPA) with carotid intima-media thickness in Japanese and United States white men aged 40–49
We created tertile groups of each of DHA and EPA acid for each population. We then plotted tertile-specific carotid intima-media thickness using bar. To represent the tertile-specific value of fatty acids, we used a median value. Width of each bar represents inter-quartile range of the corresponding tertile. Data were age-adjusted.
Figure 3.
Age-adjusted associations of linolenic, arachidonic, alpha-linolenic, and trans fatty acids with carotid intima-media thickness in Japanese and United States white men aged 40–49
We created tertile groups of each of linolenic, arachidonic, alpha-linolenic, and trans fatty acids for each population. We then plotted tertile-specific carotid intima-media thickness using bar. To represent the tertile-specific value of fatty acids, we used a median value. Width of each bar represents inter-quartile range of the corresponding tertile. Data were age-adjusted.
After adjusting for BP, HDL-C, and triglycerides, DHA in the Japanese but not in U.S. whites had a significant inverse association with IMT (model I in Table 2). The significant inverse association remained after further adjusting for other factors (models II and III in Table 2). In contrast, EPA had no significant association with IMT in either group (Table 2).
Table 2.
Associations of docosahexaenoic (DHA) and eicosapentaenoic acids (EPA) with multivariable-adjusted intima-media thickness (μm) in the Japanese in Japan and U.S. whites
| Japanese in Japan | ||||||
|---|---|---|---|---|---|---|
| DHA | Median (%) (IQR) | Low 4.43 (3.77,4.77) | Middle 5.77 (5.36,6.11) | High 7.47 (7.01,8.44) | P for trend | |
| Model I | 629 (7) | 618 (7) | 607 (7) | 0.037 | ||
| Model II | 630 (7) | 619 (7) | 605 (7) | 0.016 | ||
| Model III | 631 (7) | 619 (7) | 604 (7) | 0.014 | ||
|
|
||||||
| EPA | Median (%) (IQR) | Low 1.24 (1.01,1.64) | Middle 2.22 (2.02,2.42) | High 3.59 (3.01,4.51) | P for trend | |
|---|---|---|---|---|---|---|
| Model I | 621 (8) | 622 (8) | 611 (7) | 0.341 | ||
| Model II | 623 (7) | 624 (7) | 606 (7) | 0.104 | ||
| Model III | 626 (7) | 623 (7) | 606 (8) | 0.064 | ||
| U.S. Whites | ||||||
|---|---|---|---|---|---|---|
| DHA | Median (%) (IQR) | Low 1.27 (1.01,1.49) | Middle 2.07 (1.81,2.38) | High 3.54 (3.02,4.27) | P for trend | |
| Model I | 680 (9) | 677 (9) | 658 (9) | 0.107 | ||
| Model II | 677 (9) | 678 (9) | 658 (9) | 0.129 | ||
| Model III | 678 (9) | 678 (9) | 658 (9) | 0.129 | ||
|
|
||||||
| EPA | Low | Middle | High | |||
|---|---|---|---|---|---|---|
| Median (%) (IQR) | 0.41 (0.36, 0.46) | 0.64 (0.58,0.71) | 1.12 (0.90,1.55) | P for trend | ||
| Model I | 669 (9) | 667 (9) | 678 (9) | 0.486 | ||
| Model II | 668 (9) | 673 (9) | 673 (9) | 0.702 | ||
| Model III | 668 (9) | 672 (9) | 674 (9) | 0.651 | ||
Model I: Adjusted for blood pressure, HDL-C, and triglycerides
Model II: Further adjusted for LDL-C, pack-years of smoking, glucose, insulin, and BMI
Model III: Further adjusted for CRP, ethanol consumption, exercise, and medications for diabetes, hypertension, and hyperlipidemia
Median and inter-quartile range are expressed within each tertile group of DHA or EPA in the Japanese in Japan or U.S. whites.
IRQ: inter-quartile range. Please see the footnote for table 1 for other abbreviations.
The significant difference in IMT between the Japanese and U.S. whites remained after further adjusting for traditional and other risk factors (models I to III in Table 3). However, further adjusting for DHA but not EPA made the significant difference in multivariable-adjusted IMT non-significant. It is noted that DHA itself was a significant predictor of IMT in this model (P=0.032). The effect of DHA was independent of EPA (Table 3) or other FAs (data not shown).
Table 3.
Multivariable-adjusted mean differences in intima-media thickness (IMT) between the Japanese in Japan and U.S. whites
| Intima-media thickness of the carotid artery (μm) | |||||
|---|---|---|---|---|---|
| Japanese in Japan | U.S. Whites | Mean difference in IMT (95% CI) | P for the difference in IMT | P for DHA or EPA in final model | |
| Model I | 619 (7) | 670 (5) | 52 (38, 65) | <0.001 | - |
| Model II | 625 (5) | 664 (5) | 40 (22, 57) | <0.001 | - |
| Model III | 625 (6) | 664 (6) | 39 (20, 58) | <0.001 | - |
| Added to model III | |||||
| DHA | 636 (7) | 653 (7) | 17 (−8, 43) | 0.177 | 0.009 |
| EPA | 629 (6) | 660 (7) | 31 (9,53) | 0.007 | 0.134 |
| DHA + EPA | 636 (7) | 653 (7) | 17 (−8, 43) | 0.175 | 0.032 for DHA 0.863 for EPA |
Values of carotid IMT are expressed as mean and standard error.
Model I Adjusted for age, blood pressure, HDL-C and triglycerides
Model II Further adjusted for LDL-C, pack-years of smoking, glucose, insulin, smoking and BMI
Model III Further adjusted for CRP, ethanol consumption, exercise, and medications for diabetes, hypertension, and hyerlipidemia
CI: Confidence interval. Please see the footnote for table 1 for other abbreviations.
Discussion
This study shows that DHA but not EPA in the Japanese had an inverse association with IMT and that the inverse association was significant after adjusting for various risk and other factors. This study also shows that DHA contributed to the lower IMT in the Japanese than in U.S. whites. Our results suggest that DHA may have an anti-atherogenic effect.
Our finding that DHA but not EPA has a significant inverse association with IMT is consistent with the result from a previous study among subjects with primary hyperlipidemia in Spain12 where fish intake is high compared to other Western countries. This study reported a significant inverse association of DHA but not EPA with IMT after adjusting for risk and other factors. Although a population-based study of 487 Swedish men reported a significant inverse correlation of EPA but not DHA with IMT,13 the association does not remain significant after adjusting for covariates.
Result from a clinical trial and a recent review on the association of tissue n-3 FAs with non-fatal CHD support the hypothesis that DHA has the anti-atherogenic effect. The Estrogen Replacement and Atherosclerosis Trial reported that DHA but not EPA is associated with reduced progression of coronary atherosclerosis.15 The recent review found that tissue DHA had a significant inverse association with non-fatal CHD.
Results from the Japan Eicosapentaenoic acid Lipid Interventions Study (JELIS) shows EPA reduces non-fatal CHD. JELIS shows 900 mg of highly purified EPA per day is effective in reducing the risk of non-fatal CHD.5 Dietary intake or supplementation of EPA increases serum EPA but not DHA11 although JELIS did not report the changes in serum DHA during the trial. Thus, the reduction of non-fatal CHD in JELIS is likely to be mediated through EPA not DHA.
Our results, therefore, may suggest that DHA has a more potent anti-atherogenic effect than EPA. Despite extensive research on the effects of marine-derived n-3 FAs on cardiovascular risk factors, only a small number of study examined the differential effects of DHA and EPA.16 DHA but not EPA reduces platelet aggregatory responses ex-vivo and platelet-derived thromboxane B2.9 DHA but not EPA improves dilator and constrictor responses in the forearm microcirculation.8in-vitro studies show that DHA but not EPA decreases the expression of pro-inflammatory cytokines10 and cell-adhesion molecules,17 which contribute to the development of atheroslerosis.18 DHA and EPA are equally effective in reducing urinary F2-isoprostane.19
We observed higher prevalence of diabetes in the Japanese than in U.S. whites, although not statistically significant. This is in accordance with the fact that the Japanese are more susceptible to developing diabetes than whites.20 We observed significantly lower rates of lipid-lowering medications in the Japanese than in the U.S. whites. This is partly due to the difference in clinical practice between the two countries.21
Although the significant difference in multivariable-adjusted IMT between the Japanese and U.S. whites became non-significant after further adjusting for DHA, our intent was not to explain the difference in IMT, but rather to demonstrate that DHA substantially attenuated the difference. Many factors besides traditional risk factors account for the difference in IMT, including genetics, age-ethnicity interaction, and cohort effect. However, among these factors cohort effect is unlikely to account for the difference. This is because in this birth cohort, levels of serum total cholesterol and BP have been similar throughout their lifetime and prevalence of diabetes is similarly high between the two groups.14
Several limitations of the study warrant discussion. Serum EPA and DHA reflect short-term dietary intake and may not reflect long-term dietary intake. However in populations where fish intake is high and stable like the Japanese in Japan, a single measurement of serum EPA and DHA reflects the ranking of habitual intake of EPA and DHA, respectively.22 Additionally, because the variation in serum DHA occurs randomly, the actual association of DHA with carotid IMT would be stronger than was observed in our study. Our cross-sectional study does not allow us to infer whether a lifelong exposure to high DHA is necessary to observe its effect. The sample size is relatively small. Our study included men and only those aged 40–49 and the results may not be generalizable to women or other age groups. The study is observational and we cannot exclude the possibility of residual or unmeasured confounding.
Our results suggest that DHA may have a more potent anti-atherogenic effect than EPA, especially in levels observed in the Japanese. Further research on the differential effects of DHA and EPA is needed.
Acknowledgements
None
Funding sources This research was supported by HL68200 from the National Institutes of Health, B 16790335 and A 13307016 from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Footnotes
Disclosures We have no conflict to disclose.
References
- 1.Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, et al. N-3 fatty acids from fish or fish-oil supplements, but not {alpha}-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: A systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 3.Balk E, Chung M, Lichtenstein A, Chew P, Kupelnick B, Lawrence A, et al. Effects of omega-3 fatty acids on cardiovascular risk factors and intermediate markers of cardiovascular disease. Evidence report/technology assessment no. 93. Agency for Healthcare Research and Quality; Rockville, MD: 2004. [PMC free article] [PubMed] [Google Scholar]
- 4.Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, Kita Y, et al. Intake of fish and n3 fatty acids and risk of coronary heart disease among japanese: The japan public health center-based (jphc) study cohort i. Circulation. 2006;113:195–202. doi: 10.1161/CIRCULATIONAHA.105.581355. [DOI] [PubMed] [Google Scholar]
- 5.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (jelis): A randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 6.Stamler J, Elliott P, Chan Q, for the INTERMAP Research Group Intermap appendix tables. Journal of Human Hypertension. 2003;17:665–775. doi: 10.1038/sj.jhh.1001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorenz MW, von Kegler S, Steinmetz H, Markus HS, Sitzer M. Carotid intima-media thickening indicates a higher vascular risk across a wide age range: Prospective data from the carotid atherosclerosis progression study (caps) Stroke. 2006;37:87–92. doi: 10.1161/01.STR.0000196964.24024.ea. [DOI] [PubMed] [Google Scholar]
- 8.Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation. 2000;102:1264–1269. doi: 10.1161/01.cir.102.11.1264. [DOI] [PubMed] [Google Scholar]
- 9.Woodman RJ, Mori TA, Burke V, Puddey IB, Barden A, Watts GF, et al. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis. 2003;166:85–93. doi: 10.1016/s0021-9150(02)00307-6. [DOI] [PubMed] [Google Scholar]
- 10.Weldon SM, Mullen AC, Loscher CE, Hurley LA, Roche HM. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human thp-1 macrophages more effectively than eicosapentaenoic acid. The Journal of Nutritional Biochemistry. 2007;18:250–258. doi: 10.1016/j.jnutbio.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83:S1467–1476. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 12.Sala-Vila A, Cofan M, Perez-Heras A, Nunez I, Gilabert R, Junyent M, et al. Fatty acids in serum phospholipids and carotid intima-media thickness in spanish subjects with primary dyslipidemia. Am J Clin Nutr. 2010;92:186–193. doi: 10.3945/ajcn.2009.28807. [DOI] [PubMed] [Google Scholar]
- 13.Lindqvist HM, Sandberg A-S, Fagerberg B, Hulthe J. Plasma phospholipid epa and dha in relation to atherosclerosis in 61-year-old men. Atherosclerosis. 2009;205:574–578. doi: 10.1016/j.atherosclerosis.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 14.Sekikawa A, Curb JD, Ueshima H, El-Saed A, Kadowaki T, Abbott RD, et al. Marine-derived n-3 fatty acids and atherosclerosis in japanese, japanese-american, and white men: A cross-sectional study. J Am Coll Cardiol. 2008;52:417–424. doi: 10.1016/j.jacc.2008.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erkkila AT, Matthan NR, Herrington DM, Lichtenstein AH. Higher plasma docosahexaenoic acid is associated with reduced progression of coronary atherosclerosis in women with cad. J Lipid Res. 2006;47:2814–2819. doi: 10.1194/jlr.P600005-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006;9:95–104. doi: 10.1097/01.mco.0000214566.67439.58. [DOI] [PubMed] [Google Scholar]
- 17.De Caterina R, Liao JK, Libby P. Fatty acid modulation of endothelial activation. Am J Clin Nutr. 2000;71:213S–223S. doi: 10.1093/ajcn/71.1.213S. [DOI] [PubMed] [Google Scholar]
- 18.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 19.Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ. Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med. 2003;35:772–781. doi: 10.1016/s0891-5849(03)00407-6. [DOI] [PubMed] [Google Scholar]
- 20.Fujimoto WY. The growing prevalence of non-insulin-dependent diabetes in migrant asian populations and its implications for asia. Diabetes Res Clin Pract. 1992;15:167–183. doi: 10.1016/0168-8227(92)90022-j. [DOI] [PubMed] [Google Scholar]
- 21.Roth GA, Fihn SD, Hokdad AH, Aekplakorn W, Hasegawa T, Lim SS. High total serum cholesterol, medication coverage and therapeutic control: An analysis of national health examination survey data from eight countries. Bulletin of the World Health Organization. 2011;89:92–101. doi: 10.2471/BLT.10.079947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi M, Sasaki S, Kawabata T, Hasegawa K, Akabane M, Tsugane S. Single measurement of serum phospholipid fatty acid as a biomarker of specific fatty acid intake in middle-aged japanese men. European Journal of Clinical Nutrition. 2001;55:643–650. doi: 10.1038/sj.ejcn.1601194. [DOI] [PubMed] [Google Scholar]