Abstract
Purpose
Trastuzumab is a humanized monoclonal antibody against the human epidermal growth factor receptor 2 (HER2). The clinical benefits of adjuvant trastuzumab have been demonstrated in interim analyses of four large trials. Initial data of the combined analysis of the North Central Cancer Treatment Group (NCCTG) N9831 Intergroup trial and National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 trial were reported in 2005. Long-term follow-up results on disease-free survival (DFS) and overall survival (OS) have been awaited.
Patients and Methods
Patients with HER2-positive operable breast cancer were randomly assigned to doxorubicin plus cyclophosphamide followed by paclitaxel with or without trastuzumab in the NCCTG N9831 and NSABP B-31 trials. The similar design of both trials allowed data from the control and trastuzumab-containing arms to be combined in a joint analysis.
Results
At 3.9 years of median follow-up, there continues to be a highly statistically significant reduction in DFS event rate in favor of the trastuzumab-containing arm (P < .001). Similarly, there continues to be a statistically significant 39% reduction in death rate in favor of the trastuzumab-containing arm (P < .001).
Conclusion
These data demonstrate consistent DFS and OS advantages of adjuvant trastuzumab over time, with the longest follow-up reported to date. The clinical benefits continue to outweigh the risks of adverse effects.
INTRODUCTION
Trastuzumab1 is a humanized monoclonal antibody against the human epidermal growth factor receptor 2 (HER2), which is amplified and/or overexpressed in about 15% to 20% of invasive breast cancers.2–4 HER2-positive breast tumors are more aggressive and more susceptible to recurrence than HER2-normal tumors.4,5
In the metastatic setting, trastuzumab provides significant clinical benefit as monotherapy and in combination with chemotherapy as either first- or second-line therapy.6–11 Significant clinical benefits of trastuzumab in the treatment of early-stage breast cancer have also been observed. Four large trials (and several smaller trials) evaluating adjuvant trastuzumab demonstrated significant improvements in disease-free survival (DFS; 36% to 52% reduction in DFS events) and overall survival (OS; 33% to 37% reduction in deaths), irrespective of tumor size, nodal status, hormone receptor status, or age.12–16 On the basis of data from these trials, adjuvant trastuzumab has become the foundation of care for HER2-positive early breast cancer.
The North Central Cancer Treatment Group (NCCTG) N9831 and the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 trials assessed the efficacy and safety of adding 52 weeks of trastuzumab to standard anthracycline/taxane-based chemotherapy (doxorubicin plus cyclophosphamide [AC] followed by paclitaxel). These trials were designed similarly, enabling a joint analysis of the two studies. The interim analysis reported in 2005, with a median follow-up of 2 years, demonstrated a 52% reduction in DFS event rate with the addition of trastuzumab (P < .001) and a 33% early improvement in OS (P = .015).13 Data from a second interim analysis with a median follow-up of 2.9 years presented at the American Society of Clinical Oncology annual meeting in 2007 demonstrated a continued reduction in DFS event rate and a statistically significant 35% reduction in mortality (P < .001).12
Determining the long-term implications of adjuvant trastuzumab is of great value for patient care. The first joint analysis of N9831 arms A and C with B-31 arms 1 and 2 was based on the 3,351 patients who enrolled before a prespecified calendar date and had at least one follow-up evaluation. Here, we present the findings of the joint analysis based on all 4,045 patients enrolled onto these treatment arms before the enrollment was terminated.
PATIENTS AND METHODS
Study Design
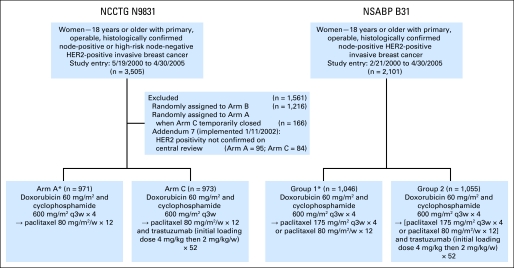
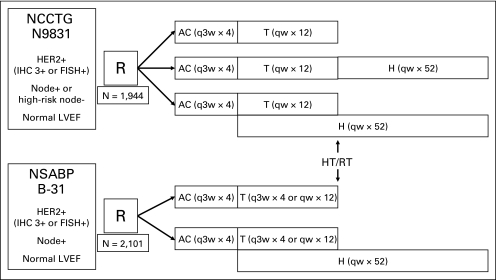
The NCCTG N9831 trial is a three-arm phase III randomized trial. Eligible patients were randomly assigned to AC followed by weekly paclitaxel (control arm, arm A); AC followed by weekly paclitaxel followed by trastuzumab (sequential arm, arm B); or AC followed by weekly paclitaxel plus trastuzumab followed by trastuzumab alone (concurrent arm, arm C). Radiation and/or hormonal therapy were administered after completion of chemotherapy, when indicated (Fig 1, Fig 2).
Fig 1.
CONSORT diagram. HER2, human epidermal growth factor receptor 2; q3w, every 3 weeks; w, weeks.
Fig 2.
Trial schema of North Central Cancer Treatment Group (NCCTG) N9831 and National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31. Timing of chemotherapy, trastuzumab (H), radiation therapy (RT), and hormonal therapy (HT) in B-31 and N9831. AC, doxorubicin and cyclophosphamide; FISH, fluorescent in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; LVEF, left ventricular ejection fraction; q3w, every 3 weeks; qw, every week; T, paclitaxel.
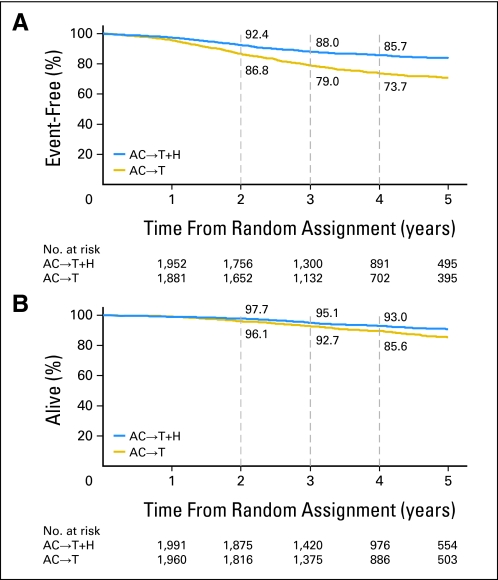
Fig 3.
Kaplan-Meier estimates of (A) event-free survival and (B) overall survival. Disease events include local, regional, or distant recurrence; contralateral breast cancer; second primary cancers; or death as a result of any cause. Overall survival is measured from the time of study enrollment to last contact or death. AC, doxorubicin and cyclophosphamide; H, trastuzumab; T, paclitaxel.
The NSABP B-31 trial is a two-arm phase III randomized trial. Eligible patients were randomly assigned to AC followed by paclitaxel every 3 weeks (arm 1) or to AC followed by paclitaxel every 3 weeks plus trastuzumab followed by trastuzumab alone (arm 2). An amendment allowing the option of administering weekly paclitaxel was activated after 39 months of accrual to B-31. When indicated, radiation therapy was given after completion of chemotherapy. Hormonal therapy was given at the start of AC before January 14, 2003, and after that, after completion of chemotherapy (Fig 1, Fig 2).
For purposes of the joint analysis, arm A from NCCTG N9831 and arm 1 from NSABP B-31 were combined for the control arm as were arm C from N9831 and arm 2 from B-31 for the trastuzumab arm; both were approved by the study teams, the National Cancer Institute (NCI), and the US Food and Durg Administration.
Eligibility
Women age ≥ 18 years with primary, operable, and histologically confirmed node-positive (both trials) or high-risk node-negative invasive breast cancer (N9831 only), with no evidence of metastases, were eligible. Tumors had to be strongly HER2 positive (immunohistochemistry [IHC] score of 3+ by reference laboratory testing or gene amplified by fluorescence in situ hybridization [FISH]) by local or reference laboratory testing (B-31) or confirmed at the study central laboratory (N9831) using the manufacturer's definition. This was done using ≥ 10% positive membrane stain as the cutoff for IHC and HER2:CEP17 ratio of ≥ 2.0 or ≥ four copies of the HER2 gene for FISH eligibility. Additional requirements included adequate hematopoietic, hepatic, and renal function and a left ventricular ejection fraction (LVEF) greater than or equal to the institution's lower limit of normal (LLN).
Contraindications to study entry included angina pectoris or arrhythmia requiring medications, severe conduction abnormality, significant valvular heart disease, cardiomegaly on chest radiography, left ventricular hypertrophy on echocardiography (B-31), poorly controlled hypertension, clinically significant pericardial effusion (N9831), or a history of myocardial infarction, congestive heart failure (CHF), or cardiomyopathy. Participating institutions obtained approval from their institutional review board and filed assurances with the Department of Health and Human Services. Written informed consent was required for enrollment.
LVEF Requirements
Initiation of trastuzumab was not permitted in patients whose LVEF had decreased by more than 15 percentage points from registration value, irrespective of the LLN, or ≤ 15 percentage points from registration level to less than the LLN, at the post-AC (3-month) LVEF evaluation. For these patients, the study did not allow initiation of trastuzumab even if a repeat LVEF assessment was ≥ the LLN. Trastuzumab was not permitted in patients who showed symptoms related to left ventricular dysfunction, cardiac ischemia, or arrhythmia while receiving AC.
After release of the first joint analysis results at the American Society of Clinical Oncology annual meeting in 2005, patients previously randomly assigned to arm A of N9831 were allowed to receive trastuzumab if they had an acceptable LVEF level compared with value at registration and 6 months, at most, had passed since completion of chemotherapy. Patients randomly assigned to arm 1 of B-31 were allowed to receive trastuzumab if they were receiving AC or paclitaxel at the time when study results were disclosed, or if they had been randomly assigned on or after April 26, 2004, had completed chemotherapy, and had met the protocol requirements regarding post-AC LVEF scan results to initiate the investigational trastuzumab.
Role of the Funding Source
Both studies were conducted under a corporate research and development agreement between Genentech and the NCI. Genentech provided trastuzumab and partial funding support but did not participate in the design of the trials, collection, or data analyses. The joint analysis was developed and analyzed by the NCCTG and NSABP, with approval obtained from the NCI and US Food and Drug Administration.
Statistical Analysis
The joint analysis included all patients enrolled onto B-31 and onto N9831 from arms A and C, excluding 152 patients in arm A who were randomly assigned between January 24, 2002, and September 2, 2002 (while accrual to arm C was temporarily suspended), and 193 patients whose disease was found not to be HER2 gene amplified by FISH and/or IHC 3+ by central testing after the implementation of Addendum 7 (required HER2-positive status by central testing) in the protocol. Patients randomly assigned to arm 1 of B-31 and arm A of N9831 who received trastuzumab after release of the first joint analysis results were included in the analysis according to their original treatment assignment (Fig 1).
The primary study end point was DFS, defined as the time from random assignment to documentation of the first of the following events: local, regional, or distant recurrence of breast cancer; a contralateral breast cancer; a second primary cancer; or death as a result of any cause. Patients alive without a disease event were censored at the time of their last disease evaluation (ie, patients randomly assigned to the non–trastuzumab-containing regimens who chose to receive trastuzumab after the release of the first joint analysis of N9831 and B-31 were not censored when they began trastuzumab). Secondary end points included OS, time to recurrence, death from breast cancer, contralateral breast cancer, and other second primary cancers. OS was defined as the time from random assignment to death as a result of any cause.
The overall distributions of DFS and OS were estimated using the Kaplan-Meier method. Stratified proportional hazards modeling was used to assess whether DFS or OS differed with respect to treatment. The strata were study (B-31 v N9831), intended paclitaxel schedule (every 3 weeks v weekly), number of positive nodes (zero to three v four to nine v ≥ 10 nodes), and hormone receptor status (estrogen receptor and/or progesterone receptor positive v estrogen receptor and progesterone receptor negative). Age, tumor size, and tumor grade were assessed for their impact on DFS and OS. Proportional hazards modeling was then used to assess the impact of trastuzumab on DFS and OS after adjusting for the stratification factors and other significant patient or disease characteristics.
RESULTS
The study cohort consisted of the 2,101 women enrolled onto B-31 and the 1,944 women enrolled onto arm A or arm C of N9831 during the period both arms were open to enrollment and who, after January 11, 2002, were found to have HER2 gene amplification or IHC 3+ disease by central or reference laboratory testing. Pretreatment characteristics of these 4,045 women are listed in Table 1. The study populations differ because women with high-risk, node-negative disease were eligible for N9831 but not for B-31; 14.5% of patients enrolled onto N9831 had node-negative disease.
Table 1.
Patient Demographics and Clinical Characteristics
| Demographic or Characteristic | B-31 |
N9831 |
||||||
|---|---|---|---|---|---|---|---|---|
| Control Arm (n = 1,046) |
Trastuzumab Arm (n = 1,055) |
Control Arm (n = 971) |
Trastuzumab Arm (n = 973) |
|||||
| No. of Patients | %* | No. of Patients | %* | No. of Patients | %* | No. of Patients | %* | |
| Age at random assignment, years | ||||||||
| 18-39 | 170 | 16.3 | 172 | 16.3 | 163 | 16.8 | 150 | 15.4 |
| 40-49 | 351 | 33.6 | 367 | 34.8 | 324 | 33.4 | 329 | 33.8 |
| 50-59 | 355 | 33.9 | 343 | 32.5 | 328 | 33.8 | 310 | 31.9 |
| ≥ 60 | 170 | 16.3 | 173 | 16.4 | 156 | 16.1 | 184 | 18.9 |
| Extent of surgery | ||||||||
| Mastectomy | 628 | 60.0 | 640 | 60.7 | 587 | 60.5 | 603 | 62.0 |
| Breast sparing | 407 | 38.9 | 408 | 38.7 | 383 | 39.4 | 370 | 38.0 |
| Unknown | 11 | 1.1 | 7 | 0.7 | 1 | 0.1 | 0 | 0 |
| Tumor size, cm | ||||||||
| ≤ 2.0 | 422 | 40.3 | 401 | 38.0 | 392 | 40.4 | 370 | 38.0 |
| 2.1-5.0 | 527 | 50.4 | 528 | 50.1 | 509 | 52.4 | 521 | 53.6 |
| > 5.0 | 79 | 7.6 | 112 | 10.6 | 69 | 7.1 | 82 | 8.4 |
| Unknown | 18 | 1.7 | 14 | 1.3 | 1 | 0.1 | 0 | 0 |
| Histologically positive nodes | ||||||||
| 0 | 0 | 0 | 0 | 0 | 149 | 15.4 | 133 | 13.7 |
| 1-3 | 601 | 57.5 | 611 | 57.9 | 456 | 47.0 | 476 | 48.9 |
| 4-9 | 304 | 29.1 | 302 | 28.6 | 236 | 24.3 | 240 | 24.7 |
| ≥ 10 | 141 | 13.5 | 142 | 13.5 | 129 | 13.3 | 124 | 12.7 |
| Unknown | 0 | 0 | 0 | 0 | 1 | 0.1 | 0 | 0 |
| Tumor grade | ||||||||
| 1 | 29 | 2.8 | 23 | 2.2 | 10 | 1.0 | 14 | 1.4 |
| 2 | 311 | 29.7 | 289 | 27.4 | 256 | 26.4 | 259 | 26.6 |
| 3 | 683 | 65.3 | 724 | 68.6 | 693 | 71.4 | 684 | 70.3 |
| Unknown | 23 | 2.2 | 19 | 1.8 | 12 | 1.2 | 16 | 1.6 |
| Estrogen receptor status | ||||||||
| Positive | 547 | 52.3 | 549 | 52.0 | 499 | 51.4 | 484 | 49.7 |
| Negative | 488 | 46.7 | 499 | 47.3 | 471 | 48.5 | 489 | 50.3 |
| Unknown | 11 | 1.0 | 7 | 0.7 | 1 | 0.1 | 0 | 0 |
| Progesterone receptor status | ||||||||
| Positive | 422 | 40.3 | 410 | 38.9 | 383 | 39.4 | 369 | 37.9 |
| Negative | 611 | 58.4 | 637 | 60.4 | 587 | 60.5 | 601 | 61.8 |
| Unknown | 13 | 1.2 | 8 | 0.8 | 1 | 0.1 | 3 | 0.3 |
| Intended paclitaxel schedule | ||||||||
| Every 3 weeks | 878 | 83.9 | 885 | 83.9 | 0 | 0 | 0 | 0 |
| Weekly | 168 | 16.1 | 170 | 16.1 | 971 | 100 | 973 | 100 |
| Adjuvant radiation therapy | ||||||||
| Yes | 800 | 76.5 | 811 | 76.9 | 631 | 65.0 | 655 | 67.3 |
| No | 246 | 24.5 | 244 | 24.1 | 246 | 25.3 | 256 | 26.3 |
| Unknown | 0 | 0 | 0 | 0 | 94 | 9.7 | 62 | 6.4 |
| Hormonal therapy | ||||||||
| Yes | 585 | 55.9 | 591 | 56.0 | 498 | 51.3 | 496 | 51.0 |
| No | 461 | 44.1 | 464 | 44.0 | 461 | 47.5 | 470 | 48.3 |
| Unknown | 0 | 0 | 0 | 0 | 12 | 1.2 | 7 | 0.7 |
Percentages have been rounded.
AC Chemotherapy
Thirty-nine women (29 on the control arm and 10 on the trastuzumab arm) did not begin study treatment because they were found to be ineligible or refused their assigned treatment. Of the 4,006 women who began AC chemotherapy, 101 women (2.5%) did not complete all four treatment cycles; 174 women (4.3%) completed AC chemotherapy but met the criteria for preclusion of trastuzumab because of cardiac symptoms or a post-AC LVEF that declined by ≥ 16 percentage points from pretreatment levels or declined to less than the institutional LLN; 3,646 women (91.0%) completed AC chemotherapy with a satisfactory post-AC cardiac evaluation; and 85 women (2.1%) completed AC without undergoing a post-AC cardiac evaluation.
Paclitaxel ± Trastuzumab
After initial results of the joint analysis released in April 2005, 339 patients randomly assigned to the control arm received trastuzumab (concurrently with paclitaxel or up to 6 months after the completion of AC). The durations of trastuzumab among the 1,845 patients randomly assigned to a trastuzumab-containing regimen who began post-AC treatment were as follows: none (4.7%); 0.1 to 3 months (7.9%); 3.1 to 6 months (7.9%); 6.1 to 9 months (5.1%); and 9.1 to 12 months (74.5%). Cardiac adverse events including asymptomatic decreases in LVEF (n = 259), confirmed CHF (n = 41), and other severe (NCI Common Toxicity Criteria version 2.0 grade ≥ 3) cardiac toxicities (n = 20) led to the early discontinuation of trastuzumab in the patients who experienced these events. The overall CHF rates (based on assessment by the patient's primary oncology team) were 1.3% and 0.9% in the control arms of B-31 and N9831, respectively, and 3.8% and 2.3% in the concurrent trastuzumab arms of B-31 and N9831, respectively.
Clinical Course
The median follow-up time of patients alive at last contact was 3.9 years (for the combined trials). Among the 2,028 women on the trastuzumab arm at last contact, 1,739 women (85.8%) are alive without evidence of disease; 143 women (7.1%) are alive with disease recurrence, a second primary cancer, and/or contralateral breast disease; 131 women (6.5%) are dead with disease recurrence, second primary cancer, and/or contralateral breast disease; three women (< 0.1%) are dead as a result of treatment-related causes; and 12 women (0.6%) are dead without disease recurrence as a result of other or unknown causes.
Among the 2,017 women on the control arm at last contact, 1,528 women (75.8%) are alive without evidence of disease; 261 women (12.9%) are alive with disease recurrence, a second primary cancer, and/or contralateral breast disease; 214 women (10.6%) are dead with disease recurrence, second primary cancer, and/or contralateral breast disease; two women (< 0.1%) are dead as a result of treatment-related causes; and 12 women (0.6%) are dead without disease recurrence as a result of other or unknown causes.
Impact of Trastuzumab on DFS
The types of first disease event for each treatment group are listed in Table 2. Women randomly assigned to the trastuzumab arm had a significantly increased DFS (P < .001; stratified hazard ratio [HR], 0.52; 95% CI, 0.45 to 0.60; Fig 2A) and OS (P < .001; stratified HR, 0.61; 95% CI, 0.50 to 0.75; Fig 2B) compared with women randomly assigned to the control arm.
Table 2.
First DFS Events by Trial Arms
| Event | B-31 |
N9831 |
||||||
|---|---|---|---|---|---|---|---|---|
| Control Arm (n = 1,046) |
Trastuzumab Arm (n = 1,055) |
Control Arm (n = 971) |
Trastuzumab Arm (n = 973) |
|||||
| No. of Patients | %* | No. of Patients | %* | No. of Patients | %* | No. of Patients | %* | |
| Patients with a DFS event | 290 | 27.7 | 162 | 15.3 | 199 | 20.5 | 128 | 13.2 |
| DFS events | ||||||||
| Local, regional, or distant recurrence | 243 | 23.2 | 137 | 13.0 | 174 | 17.9 | 100 | 10.3 |
| Contralateral breast cancer | 10 | 1.0 | 7 | 0.7 | 5 | 0.5 | 9 | 0.9 |
| Other second primary cancer | 28 | 2.7 | 11 | 1.0 | 15 | 1.5 | 12 | 1.2 |
| Death without evidence of disease | 9 | 0.9 | 8 | 0.8 | 5 | 0.5 | 7 | 0.7 |
| No. of DFS events with brain involvement | 17 | 32 | 19 | 29 | ||||
Abbreviation: DFS, disease-free survival.
Percentages have been rounded.
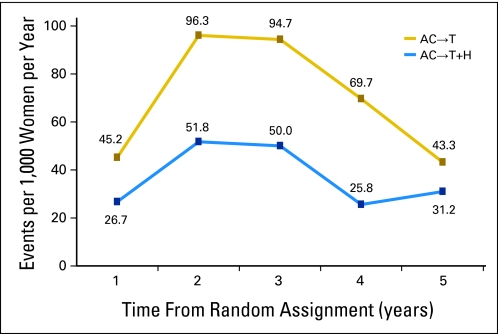
The incidence of a DFS event per 1,000 women per year for each year of follow-up is shown in Appendix Figure A1 (online only). There is a marked increase in this incidence in both treatment arms 12 to 24 months after registration (ie, for the trastuzumab-containing regimens, the last 6 months of trastuzumab and the first 6 months of no adjuvant systematic therapy). Notably, there is a continued dampening of the incidence of a DFS event after the discontinuation of trastuzumab compared with the control group.
Table 3 lists the results of multivariate proportional hazards modeling. OS was significantly decreased for women with larger tumors who were older than age 60 years at study entry after accounting for the stratification factors. Moreover, women randomly assigned to the trastuzumab arm had a significantly increased OS (adjusted HR, 0.59; 95% CI, 0.48 to 0.73) after adjusting for all these factors (Table 3).
Table 3.
Estimated HRs From Multivariate Proportional Hazards Modeling
| Factor | DFS |
Overall Survival |
||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| No. of positive nodes | ||||
| 0-3 | 1.00 | 1.00 | ||
| 4-9 | 1.50 | 1.27 to 1.77 | 1.77 | 1.39 to 2.28 |
| 10+ | 2.79 | 2.34 to 3.34 | 3.43 | 2.66 to 4.42 |
| Intended paclitaxel schedule | ||||
| Weekly | 1.00 | 1.00 | ||
| Every 3 weeks | 1.43 | 1.01 to 2.03 | 1.23 | 0.71 to 2.13 |
| Receptor status | ||||
| ER and PR negative | 1.00 | — | ||
| ER or PR positive | 0.66 | 0.57 to 0.76 | 0.57 | 0.46 to 0.69 |
| Trial | ||||
| B-31 | 1.00 | 1.00 | ||
| N9831 | 1.12 | 0.79 to 1.60 | 0.96 | 0.55 to 1.66 |
| Tumor size, cm | ||||
| ≤ 2 | 1.00 | 1.00 | ||
| 2.01-5.0 | 1.62 | 1.38 to 1.91 | 1.51 | 1.19 to 1.90 |
| > 5.0 | 2.20 | 1.73 to 2.79 | 1.64 | 1.16 to 2.32 |
| Age, years | ||||
| < 60 | — | 1.00 | ||
| ≥ 60 | — | 1.38 | 1.08 to 1.76 | |
| Treatment arm | ||||
| Control arm | 1.00 | 1.00 | ||
| Trastuzumab arm | 0.51 | 0.44 to 0.59 | 0.59 | 0.48 to 0.73 |
Abbreviations: DFS, disease-free survival; ER, estrogen receptor; HR, hazard ratio; PR, progesterone receptor.
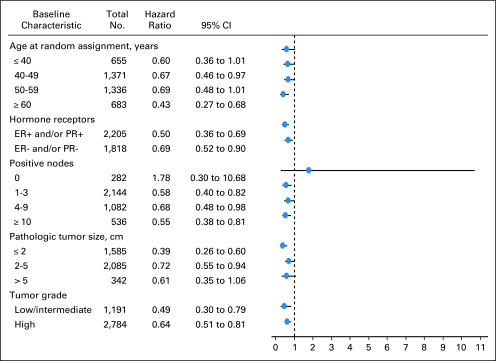
DFS was also found to be significantly decreased for women with larger tumors after accounting for the stratification factors. Moreover, women randomly assigned to the trastuzumab arm had a significantly increased DFS (adjusted HR, 0.51; 95% CI, 0.44 to 0.59) after adjusting for these factors (Table 3). Appendix Figure A2 (online only) depicts the hazard of a DFS event among women randomly assigned to trastuzumab-containing regimen relative to women randomly assigned to nontrastuzumab regimen in a variety of patient subpopulations. In addition, Table 4 lists the 4-year DFS rates in these subpopulations by regimen.
Table 4.
N9831/B-31 Joint Analysis
| Factor | 4-Year DFS Rate by Regimen (%) |
|
|---|---|---|
| Control Arm (n = 2,017) | Trastuzumab Arm (n = 2,028) | |
| Age, years | ||
| < 40 | 69.2 | 84.2 |
| 40-49 | 75.7 | 87.4 |
| 50-59 | 75.8 | 84.6 |
| ≥ 60 | 70.0 | 86.1 |
| No. of positive nodes | ||
| 0 | 89.6 | 86.9 |
| 1-3 | 80.6 | 89.7 |
| 4-9 | 71.1 | 83.5 |
| ≥ 10 | 46.5 | 73.7 |
| Hormone receptors | ||
| ER and PR negative | 69.4 | 81.6 |
| ER and/or PR positive | 77.2 | 89.4 |
| Tumor size, cm | ||
| 0-2 | 81.6 | 90.9 |
| 2.1-5.0 | 70.3 | 83.2 |
| > 5.0 | 52.0 | 78.2 |
| Tumor grade | ||
| Low/intermediate | 77.0 | 88.5 |
| High | 72.0 | 84.4 |
Abbreviations: DFS, disease-free survival; ER, estrogen receptor; PR, progesterone receptor.
DISCUSSION
These updated joint analysis data indicate that after 4-years of follow-up, the addition of adjuvant trastuzumab to chemotherapy maintains both a significant DFS and OS benefit compared with chemotherapy alone. At the time of the initial report (with median follow-up of 2 years),13 the relative reduction in DFS event rate was 52% (HR, 0.48; P < .001), and at 2.9 years, the relative reduction in death rate was 35% (HR, 0.65; P < .001).12 With the additional follow-up, the relative reduction in DFS event rate was 48% (P < .001), and the relative reduction in death rate was 39% (P < .001). Thus, our data demonstrate long-term continued benefit with trastuzumab administered concurrently with chemotherapy (anthracycline/cyclophosphamide followed by paclitaxel + trastuzumab, with 1 year of trastuzumab treatment). Absolute differences in DFS increased by number of nodes; it was most pronounced for patients with ≥ 10 involved nodes, who had an unprecedented 27% absolute improvement.
Cardiac safety in these trials has been studied extensively.17–20 In N9831, at a median follow-up of 3.75 years, the 3-year cumulative incidence of cardiac events (ie, symptomatic CHF or probable or definite cardiac death) was 0.3% without trastuzumab, 2.8% with sequential paclitaxel and trastuzumab, and 3.3% with concurrent paclitaxel and trastuzumab.18 There was no evidence of a significant increase in cardiac events over time.18 In B-31, the 5-year cumulative incidence of cardiac events was 0.9% without trastuzumab and 3.8% with concurrent paclitaxel and trastuzumab.19 There was no evidence of an increase in cardiac events over time.19 The findings of an independent board of cardiologists reviewing the data from patients reported to have had cardiac events (n = 173) in N9831 and B-31 have recently been reported.20 The panel concluded that the rate of symptomatic heart failure was 0.5% with chemotherapy alone and 2.0% with AC followed by paclitaxel and trastuzumab. Moreover, the panel reported that 86% of patients (31 of 36 patients) in the trastuzumab arms had either complete or partial resolution of the cardiac event in follow-up. Analyses of risk factors for cardiac events in N9831 have shown that patients ≥ 60 years old (P = .003), patients with prior or current use of antihypertensive medication (P = .005), and patients with LVEF near the LLN at registration (P = .033) were at increased risk for such events.18 On the basis of our own data, we are reassured of the favorable therapeutic ratio (benefit over toxicity) of the AC followed by paclitaxel/trastuzumab adjuvant regimen as administered in NCCTG N9831 and NSABP B-31.12,13,17–20
A marked increase in the incidence of a DFS event was noted 1 year after random assignment in both treatment arms. This is an expected pattern in the clinical course of these patients, but the trastuzumab-containing arm continued to report a lower disease incidence after the discontinuation of trastuzumab compared with controls.
A number of phase III clinical trials have examined the impact of combining trastuzumab with chemotherapy on DFS, varying the type of chemotherapy, duration of trastuzumab, and timing of introduction of trastuzumab in relationship to chemotherapy.12,15,16,21 The Breast Cancer International Research Group 006 trial, with a median follow-up of more than 5 years, demonstrated significant improvements in DFS and OS with a nonanthracycline regimen (docetaxel, carboplatin, and trastuzumab [TCH]) and with AC followed by trastuzumab and concurrent docetaxel (AC-TH) compared with AC followed by docetaxel.12 The study was not powered to compare the two trastuzumab-containing arms. Notably, the number of deaths from any cause, number of deaths from breast cancer, and number of progression events were greater in the TCH arm relative to the AC-TH arm. The incidence of symptomatic CHF was greater in the AC-TH arm than in the TCH arm (2.0% v 0.4%, respectively; P < .001). There were no reported cardiac deaths. The Herceptin Adjuvant (HERA) trial assessed the impact of 1 year of trastuzumab after standard chemotherapy in the neoadjuvant or adjuvant setting.15,16 At 2 years of follow-up, there was a significant DFS and OS benefit in patients receiving 1 year of trastuzumab compared with observation only.16 At 3.6 years of median follow-up, rates of cardiotoxicity were relatively low, but patients in the trastuzumab group, compared with the observation group, had a greater incidence of severe CHF (0.8% v 0%, respectively) and symptomatic CHF (1.9% v 0.1%, respectively).21 The smaller Neoadjuvant Herceptin (NOAH) study evaluated the clinical benefit of chemotherapy alone (ie, doxorubicin plus paclitaxel followed by paclitaxel followed by cyclophosphamide, methotrexate, and fluorouracil) or with trastuzumab starting concurrently with doxorubicin in patients with HER2-positive locally advanced or inflammatory breast cancer.22 Patients in the trastuzumab arm received trastuzumab for a total of 1 year, starting in the neoadjuvant portion. At 3 years of follow-up, the addition of trastuzumab to chemotherapy significantly improved rates of pathologic complete response and event-free survival and reduced risks of recurrence, progression, or death compared with patients who did not receive trastuzumab. Trastuzumab therapy was well tolerated, with treatment-responsive symptomatic CHF in 2% of patients (two of 115 patients) being the most notable adverse event.
In conclusion, longer term analysis of N9831 and B-31 demonstrates continued benefit of adding 1 year of trastuzumab to standard anthracycline-based chemotherapy. A number of adjuvant treatment combinations of chemotherapy with trastuzumab are now available, allowing physicians to select a regimen they consider most appropriate for patients with early-stage invasive HER2-positive breast cancer. Longer-term follow-up and identification of predictive biomarkers will provide further insight into optimal trastuzumab adjuvant therapies. For patients with eligibility consistent with our adjuvant trastuzumab trials, the AC-TH regimen remains an excellent choice of treatment.
Acknowledgment
We thank all patient participants and research coordinators and nurses who assisted with follow-up and data collection. Support for third-party copyediting assistance was provided by Genentech. We also wish to acknowledge support from the Breast Cancer Research Foundation.
Appendix
Fig A1.
Disease event incidence rate. AC, doxorubicin and cyclophosphamide; H, trastuzumab; T, paclitaxel.
Fig A2.
Forest plots. ER, estrogen receptor; PR, progesterone receptor.
Footnotes
Written on behalf of the North Central Cancer Treatment Group N9831 and the National Surgical Adjuvant Breast and Bowel Project B-31 trial teams.
Supported by National Institutes of Health Grants No. U10-CA25224 and RO1-CA129949; National Surgical Adjuvant Breast and Bowel Project Grants No. U10-CA12027, U10-CA69651, U10-CA37377, and U10-CA69974; the Breast Cancer Research Foundation; and Genentech (35-03). P.A.K. received research funding from Cancer and Leukemia Group B.
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00005970.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Charles E. Geyer Jr, Genentech (C); Eleftherios P. Mamounas, Genentech (C); Peter A. Kaufman, Genentech (C); Norman Wolmark, Genentech/Roche (U) Stock Ownership: None Honoraria: Charles E. Geyer Jr, Genentech; Eleftherios P. Mamounas, Genentech Research Funding: Edith A. Perez, Genentech, GlaxoSmithKline, sanofi-aventis; Peter A. Kaufman, Genentech Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Edith A. Perez, Edward H. Romond, Vera J. Suman, Nancy E. Davidson, Silvana Martino, Eleftherios P. Mamounas, Norman Wolmark
Financial support: Edith A. Perez
Administrative support: Edith A. Perez, Charles E. Geyer Jr, Silvana Martino, Norman Wolmark
Provision of study materials or patients: Edith A. Perez, Edward H. Romond, Nancy E. Davidson, Charles E. Geyer Jr, Silvana Martino, Eleftherios P. Mamounas, Peter A. Kaufman
Collection and assembly of data: Edith A. Perez, Vera J. Suman, Jong-Hyeon Jeong, Charles E. Geyer Jr, Eleftherios P. Mamounas, Peter A. Kaufman
Data analysis and interpretation: Edith A. Perez, Edward H. Romond, Vera J. Suman, Jong-Hyeon Jeong, Nancy E. Davidson, Charles E. Geyer Jr, Silvana Martino, Eleftherios P. Mamounas, Peter A. Kaufman
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Genentech. Herceptin (trastuzumab). Package insert. 2009. Mar, http://www.gene.com/gene/products/information/pdf/herceptin-prescribing.pdf.
- 2.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–69. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 3.Sjögren S, Inganas M, Lindgren A, et al. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998;16:462–469. doi: 10.1200/JCO.1998.16.2.462. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 6.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 7.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 8.Perez EA. Impact, mechanisms, and novel chemotherapy strategies for overcoming resistance to anthracyclines and taxanes in metastatic breast cancer. Breast Cancer Res Treat. 2009;114:195–201. doi: 10.1007/s10549-008-0005-6. [DOI] [PubMed] [Google Scholar]
- 9.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 10.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: A German Breast Group 26/Breast International Group 03-05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 11.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 12.Perez EA, Romond EH, Suman VJ, et al. Updated results of the combined analysis of NCCTG N9831 and NSABP B-31 adjuvant chemotherapy with/without trastuzumab in patients with HER2-positive breast cancer. J Clin Oncol. 2007;25(suppl 18S):6s. abstr 512. [Google Scholar]
- 13.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 14.Slamon DJ, Eiermann W, Robert NJ, et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC-T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC-TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2neu positive early breast cancer patients: BCIRG006 study. Cancer Res. 2009;69:500s. abstr 62. [Google Scholar]
- 15.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 16.Smith I, Procter M, Gelber RD, et al. 2-Year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 17.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2–overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 18.Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rastogi P, Jeong J, Geyer CE, et al. Five-year update of cardiac dysfunction on NSABP B-31, a randomized trial of sequential doxorubicin/cyclophosphamide (AC) → paclitaxel (T) compared to AC→T with trastuzumab (H) J Clin Oncol. 2007;25(suppl 18S):6s. abstr LBA513. [Google Scholar]
- 20.Russell SD, Blackwell KL, Lawrence J, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: A combined review of cardiac data from the National Surgical Adjuvant Breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010;28:3416–3421. doi: 10.1200/JCO.2009.23.6950. [DOI] [PubMed] [Google Scholar]
- 21.Procter M, Suter TM, de Azambuja E, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol. 2010;28:3422–3428. doi: 10.1200/JCO.2009.26.0463. [DOI] [PubMed] [Google Scholar]
- 22.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): A randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]