Abstract
Purpose
Adjuvant chemotherapy is typically considered for patients with stage II colon cancer characterized by poor prognostic features, including obstruction, perforation, emergent admission, T4 stage, resection of fewer than 12 lymph nodes, and poor histology. Despite frequent use, the survival advantage conferred on patients with stage II disease by chemotherapy is yet unproven. We sought to determine the overall survival benefit of chemotherapy among patients with stage II colon cancer having poor prognostic features.
Patients and Methods
A total of 43,032 Medicare beneficiaries who underwent colectomy for stage II and III primary colon adenocarcinoma diagnosed from 1992 to 2005 were identified from the Surveillance, Epidemiology, and End Results (SEER) –Medicare database. χ2 and two-way analysis of variance were used to assess differences in patient- and disease-related characteristics. Five-year overall survival was examined using Kaplan-Meier survival analysis and Cox proportional hazards regression with propensity score weighting.
Results
Of the 24,847 patients with stage II cancer, 75% had one or more poor prognostic features. Adjuvant chemotherapy was received by 20% of patients with stage II disease and 57% of patients with stage III disease. After adjustment, 5-year survival benefit from chemotherapy was observed only for patients with stage III disease (hazard ratio[HR], 0.64; 95% CI, 0.60 to 0.67). No survival benefit was observed for patients with stage II cancer with no poor prognostic features (HR, 1.02; 95% CI, 0.84 to 1.25) or stage II cancer with any poor prognostic features (HR, 1.03; 95% CI, 0.94 to 1.13).
Conclusion
Among Medicare patients identified with stage II colon cancer, either with or without poor prognostic features, adjuvant chemotherapy did not substantially improve overall survival. This lack of benefit must be considered in treatment decisions for similar older adults with colon cancer.
INTRODUCTION
Complete surgical resection is the primary treatment for patients with locoregional colon cancer (stage I, II, and III). Additional survival benefit from chemotherapy seems to be stage-specific: although patients with stage III (lymph-node positive) colon cancer enjoy a 10% absolute increase in 5-year survival after 6 months of fluorouracil-based chemotherapy, this same benefit has not been convincingly demonstrated in patients with stage II (node-negative) disease.1–10 The commonly cited Quick and Simple and Reliable (QUASAR) prospective trial reported a pooled relative risk (RR) of death of 0.82 (95% CI, 0.70 to 0.95) for patients with stage I to III colon and rectal cancer receiving chemotherapy compared with surgery alone. However, it importantly failed to demonstrate a significant chemotherapy-related survival benefit for the stage II colon cancer subgroup (RR, 0.86; 95% CI, 0.66 to 1.12).8 In contrast, a more recent article that does report a survival advantage of chemotherapy for this population (hazard ratio [HR], 0.58; 95% CI, 0.48 to 0.71) required a meta-analysis combining data from five National Surgical Bowel and Breast Project trials reports to reach significance.11 A larger meta-analysis of 12 clinical trials, including two of those cited in the Wilkinson study, was conducted as part of developing the consensus recommendations from the American Society of Clinical Oncology. These recommendations conclude that existing trial data do not support routine use of chemotherapy in patients with stage II disease, citing a possible 2% to 4% increase in absolute survival, a statistically nonsignificant improvement.12
Despite its uncertain impact on survival, adjuvant chemotherapy administration is common, with one study identifying chemotherapy receipt among 27% of younger Medicare beneficiaries with stage II colon cancer.13 This practice is due to concerns that any potential benefit has been obscured by other factors, including insufficient patient numbers in clinical trials, good baseline prognosis, competing non–cancer-related deaths in the elderly population, and understaging of some patients with inadequate nodal resection.8,12,14,15 The American Society of Clinical Oncology guidelines suggest that certain poor prognostic features might reasonably prompt practitioners to consider therapy: elevated preoperative carcinoembryonic antigen (CEA) more than 5 ng/mL, diagnosis in the setting of bowel obstruction or perforation, need for emergent operation, T4 stage (extension to adjacent organs), inadequate nodal resection (< 12 nodes), or peritumoral lymphatic/venous invasion.12,14,16,17 However, there is no evidence that these characteristics, although associated with worse outcomes, are predictive of a successful response to adjuvant chemotherapy.12,17
We hypothesized that patients with stage II colon cancer with poor prognostic features may demonstrate improved outcomes from adjuvant chemotherapy that were not observed in previous clinical trials due to insufficient power to detect clinically relevant change. To address this question, we examined the relationship between receipt of chemotherapy and 5-year overall survival among three groups of Medicare beneficiaries with colon cancer: stage II with no poor prognostic features, stage II with any poor prognostic features, and stage III. The purpose of the present study is to describe the effectiveness of chemotherapy in improving survival after surgical resection for this generalizable population of older adults.
PATIENTS AND METHODS
Data Sources
After the study protocol approval was obtained from the University of Wisconsin institutional review board with a waiver of consent, we used data from the Surveillance, Epidemiology, and End Results (SEER) cancer registries linked to Medicare claims files to identify patients with a diagnosis of colon cancer. The SEER-Medicare data resource has been described previously by our group and others.18–21
Patient Selection
All Medicare-enrolled patients aged 66 years and older diagnosed with primary American Joint Committee on Cancer (AJCC) stage II or III colon adenocarcinoma within a SEER region during the years 1992 to 2005 were considered for study inclusion. Colon adenocarcinoma was identified by site and histology codes (codes for all variables, Appendix Table A1, online only). Those who underwent primary tumor resection with likely curative intent within 6 months of diagnosis were selected, excluding presumably palliative operations (eg, ostomy formation without colectomy). Continuous enrollment in Medicare Part A and Part B was required from 12 months preceding diagnosis through 5 years after discharge, death, or December 31, 2005 (whichever came first) to allow ascertainment of comorbidities, postoperative chemotherapy administration, and survival.
Exclusion criteria included health maintenance organization enrollment, unknown month of colon cancer diagnosis, diagnosis noted only on death certificate or autopsy report, or diagnosis of other malignancy within 1 year before the date of colon cancer diagnosis. From an initial cohort of 51,182 patients, the following patients were sequentially excluded: missing information (AJCC stage [n = 2,784], nodal assessment [n = 1,335], tumor stage or grade [n = 1,236]); initial cancer treatment other than surgery (preoperative radiation therapy [n = 44] or chemotherapy [n = 144]); or death before discharge from the surgical hospitalization (n = 146) or within 30 days of surgery (n = 2,461).
Variables
Outcome variable.
The primary outcome measure for our study was 5-year overall mortality, defined as death within 5 years of primary surgery for colon cancer, based on dates of death recorded in the SEER Patient Entitlement and Diagnosis Summary File (PEDSF) according to Social Security Administration data.
Explanatory variable.
The primary explanatory variable was receipt of adjuvant chemotherapy, based on previous work by Bradley et al22 and Dobie et al23 defining postoperative chemotherapy in Medicare claims data. To avoid misclassification of chemotherapy for treatment of cancer recurrence as adjuvant chemotherapy, we considered only those claims within a designated treatment window, beginning with the date of primary surgery and ending with (1) claim date after which there were 3 months without colon cancer treatment, including chemotherapy or surgery; (2) evidence of distant cancer recurrence; or (3) 9 months after primary surgery, whichever came first.22,23 Patients with at least one claim in the treatment window were classified as having received chemotherapy.
Stratification variables.
AJCC stage was identified from SEER data. Poor prognostic features identified from Medicare data included diagnosis in the setting of intestinal obstruction or perforation, emergent admission for surgery, T4 stage, poor/undifferentiated histology, and fewer than 12 lymph nodes examined.12,14,16,17 Other reported poor prognostic features (preoperative CEA level and peritumoral lymphatic/venous invasion) were not available in the SEER-Medicare data set.
Control variables.
Date of birth, sex, race/ethnicity, and marital status were obtained from SEER data. Census tract-level median household income and level of education were obtained from the SEER PEDSF (Census 1990 data used for diagnosis years 1992 through 1999; Census 2000 data used for years 2000+) and used as proxies for patient socioeconomic status. Geographic region was represented by SEER registry and rural/urban county of residence on the basis of 2003 Rural/Urban Continuum Codes identified from the PEDSF. For risk adjustment, we used Centers for Medicare and Medicaid Services Hierarchical Condition Categories (HCC) based on outpatient and inpatient diagnoses from the 12 months before colon cancer diagnosis. The resulting score can be interpreted as a patient's predicted level of “future health care need,” relative to the average Medicare beneficiary (HCC = 1.0).24 We also identified Medicare claims for hospitalizations in the year before diagnosis, in-hospital complications, rehospitalizations, and oncologist visits within 30 days of discharge from the surgical stay. Year of diagnosis and tumor location were obtained using SEER data.
Statistical Analysis
We described the patient- and disease-related characteristics for each stage group by chemotherapy administration status and evaluated univariate statistical differences using χ2 tests for categorical variables and two-way analysis of variance tests for continuous variables. Kaplan-Meier survival analysis techniques were used to compare 5-year overall survival between stage groups and by chemotherapy status within each stage group. Cox proportional hazards regression-based methods were used to determine adjusted HRs and 95% CIs of 5-year overall mortality in patients who did or did not receive chemotherapy within each stage group, controlling for patient- and disease-related covariates.
In clinical practice, significant differences exist between patients who are and are not treated with chemotherapy, particularly with regard to age, comorbidities, and provider/patient preferences. Propensity score techniques were used, as has been previously described, to address selection bias owing to nonrandom treatment assignment.25–29 Logistic regression models were built for each stage group to estimate each patient's probability of receiving adjuvant chemotherapy conditional on sociodemographic and clinical characteristics. These models included main effects and interaction terms as needed to produce balance of covariates across treated and nontreated strata with equivalent mean propensity scores, ρ (variables included in the final models, Table 1). The Cox regression models for each stage group were then weighted by (1/ρ) for individuals receiving chemotherapy, and (1/[1 − ρ]) for nontreated individuals. For propensity score-based analyses, only subjects in the common support region were included.
Table 1.
Propensity Score Models
| Stage II With No Poor Prognostic Features | Stage II With Any Poor Prognostic Features | Stage III | |
|---|---|---|---|
| Main effect variables | Age | Age | Age |
| Male | Marital status | Marital status | |
| Marital status | Residence location | Year of diagnosis | |
| Year of diagnosis | Year of diagnosis | Visit to oncologist within 30 days | |
| Visit to oncologist within 30 days | Visit to oncologist within 30 days | HCC score | |
| HCC score | Census-tract median household income | Hospitalizations in prior year | |
| Hospitalizations in prior year | Census-tract education | Rehospitalizations within 30 days | |
| In-hospital complications | Hospitalizations in prior year | In-hospital complications | |
| In-hospital complications | |||
| Interactions | Age*complication | Age*oncologist visit | Age*oncologist visit |
| Marital status*male | Age*complication | Age*complication | |
| Age*oncologist visit | Complication*oncologist visit | ||
| Rehospitalization*oncologist visit |
Abbreviation: HCC, Hierarchical Condition Categories.
Analyses were performed by the lead author using SAS 8.02 (SAS Institute, Cary, NC) and STATA 10.0 software (STATA, College Station, TX). All tests of significance used two-sided P values at the .05 level. Robust estimates of the SE were used in all regression analyses.
RESULTS
Receipt of Adjuvant Chemotherapy
We identified 43,032 individuals for inclusion in our study and stratified them into three analysis groups: stage II with no poor prognostic features (n = 6,234), stage II with any poor prognostic features (n = 18,613), and stage III (n = 18,185). Among the stage II with no poor prognostic features group, 19% received any adjuvant chemotherapy, compared with 21% of patients in the stage II with any poor prognostic features group and 57% of patients with stage III disease (P < .001). Among patients receiving chemotherapy, the mean number of months was 5.41 (95% CI, 5.37 to 5.45) for all patients, 5.17 (95% CI, 5.03 to 5.31) for stage II with no poor prognostic features, 5.12 (95% CI, 5.04 to 5.20) for stage II with any poor prognostic features, and 5.54 (95% CI, 5.49 to 5.59) for stage III.
Characteristics of Patients Receiving Chemotherapy
Table 2 compares the patient- and disease-related characteristics of individuals who did or did not receive chemotherapy within each stage group. Those who received chemotherapy were younger, more likely to be male or married, and had lower comorbidity, as measured by HCC, prior-year hospitalization, and 30-day rehospitalization after surgery. Although the majority of tumors were located in the right colon, as noted previously,30,31 a larger proportion of left-sided tumors were present among those receiving adjuvant chemotherapy. In all three stage groups, the proportion of individuals who had an oncologist visit within 30 days of surgery was significantly higher among those receiving chemotherapy.
Table 2.
Characteristics of Medicare Beneficiaries Undergoing Resection for Stage II/III Colon Cancer
| Characteristic | Stage II With No Poor Prognostic Features (n = 6,234) |
Stage II With Any Poor Prognostic Features (n = 18,613) |
Stage III (n = 18,185) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No Chemo (n = 5,057) | Chemo (n = 1,177) | P | No Chemo (n = 14,779) | Chemo (n = 3,834) | P | No Chemo (n = 7,817) | Chemo (n = 10,368) | P | |
| Patient characteristics | |||||||||
| Age, years, % | < .001 | < .001 | < .001 | ||||||
| 65-69 | 10.2 | 25.4 | 7.7 | 21.6 | 5.0 | 19.4 | |||
| 70-74 | 18.5 | 30.1 | 15.9 | 31.5 | 11.2 | 29.6 | |||
| 75-79 | 24.7 | 28.8 | 23.1 | 28.0 | 20.2 | 29.0 | |||
| 80-84 | 25.7 | 11.7 | 25.7 | 13.7 | 28.5 | 16.7 | |||
| 85+ | 20.9 | 4.0 | 27.7 | 5.2 | 35.0 | 5.3 | |||
| Male sex, % | 41.6 | 49.5 | < .001 | 38.3 | 47.0 | < .001 | 36.3 | 45.2 | < .001 |
| Race/ethnicity, % | .005 | .09 | < .001 | ||||||
| White | 86.8 | 86.2 | 87.0 | 86.7 | 83.9 | 84.8 | |||
| Black | 5.8 | 4.1 | 6.2 | 5.4 | 8.0 | 6.3 | |||
| Asian or Pacific Islander | 3.9 | 5.8 | 3.3 | 3.7 | 4.4 | 5.0 | |||
| Hispanic or other | 3.5 | 4.0 | 3.6 | 4.2 | 3.7 | 4.0 | |||
| SEER registry, % | < .001 | < .001 | .02 | ||||||
| California | 27.2 | 29.1 | 29.1 | 27.6 | 28.0 | 26.9 | |||
| Connecticut | 12.1 | 6.9 | 10.5 | 8.3 | 10.3 | 9.8 | |||
| Detroit | 8.6 | 10.8 | 10.3 | 12.7 | 10.6 | 11.1 | |||
| Hawaii | 2.4 | 2.6 | 1.2 | 1.0 | 1.9 | 2.0 | |||
| Iowa | 12.6 | 13.0 | 14.7 | 13.6 | 13.0 | 12.2 | |||
| New Mexico | 2.7 | 2.5 | 2.3 | 2.3 | 2.8 | 2.7 | |||
| Seattle | 7.4 | 6.7 | 7.6 | 6.8 | 7.2 | 6.8 | |||
| Utah | 3.4 | 3.1 | 2.7 | 2.4 | 3.4 | 3.4 | |||
| Atlanta and rural Georgia | 4.0 | 4.8 | 3.8 | 4.1 | 3.9 | 3.9 | |||
| Kentucky | 5.4 | 5.4 | 4.2 | 5.4 | 5.0 | 5.0 | |||
| Louisiana | 4.0 | 4.3 | 4.1 | 5.0 | 3.9 | 4.6 | |||
| New Jersey | 10.3 | 10.9 | 9.6 | 11.0 | 10.1 | 11.6 | |||
| Marital status, % | < .001 | < .001 | < .001 | ||||||
| Married | 50.1 | 64.9 | 44.1 | 59.7 | 39.2 | 59.4 | |||
| Widowed | 35.3 | 21.7 | 40.8 | 25.7 | 45.1 | 25.9 | |||
| Single, separated, or divorced | 11.4 | 10.9 | 12.0 | 11.5 | 12.5 | 11.9 | |||
| Unknown | 3.2 | 2.6 | 3.1 | 3.1 | 3.1 | 2.8 | |||
| Residence location, % | .06 | .005 | .28 | ||||||
| Major metropolitan | 53.9 | 57.8 | 54.7 | 57.3 | 56.6 | 55.8 | |||
| Metropolitan or urban | 35.5 | 32.2 | 34.6 | 31.9 | 32.7 | 33.8 | |||
| Less urban or rural | 10.6 | 10.0 | 10.6 | 10.8 | 10.7 | 10.4 | |||
| Median household income (census tract), $ in thousands* | .07 | .01 | < .001 | ||||||
| Mean | 46.7 | 48.1 | 44.0 | 45.1 | 43.4 | 46.1 | |||
| SD | 23.3 | 23.9 | 21.7 | 22.1 | 21.0 | 22.5 | |||
| Less than 12-year education (census tract), %† | .97 | .61 | < .001 | ||||||
| Mean | 18.1 | 18.2 | 19.3 | 19.4 | 19.8 | 18.8 | |||
| SD | 12.1 | 12.3 | 12.5 | 12.9 | 12.9 | 12.5 | |||
| HCC comorbidity score | < .001 | < .001 | < .001 | ||||||
| Mean | 1.8 | 1.5 | 2.1 | 1.9 | 2.9 | 2.3 | |||
| SD | 1.2 | 1.1 | 1.4 | 1.2 | 1.6 | 1.3 | |||
| Tumor characteristics | |||||||||
| Year of diagnosis, % | < .001 | < .001 | < .001 | ||||||
| 1992-1995 | 18.5 | 16.9 | 23.2 | 21.3 | 22.6 | 18.2 | |||
| 1996-1999 | 18.6 | 19.7 | 21.5 | 22.5 | 19.8 | 20.1 | |||
| 2000-2002† | 28.2 | 35.3 | 28.9 | 32.0 | 27.4 | 31.2 | |||
| 2003-2005† | 34.7 | 28.0 | 26.4 | 24.2 | 30.1 | 30.5 | |||
| Tumor location, % | .002 | < .001 | < .001 | ||||||
| Right colon | 64.2 | 60.0 | 54.3 | 50.2 | 58.2 | 56.6 | |||
| Transverse colon | 10.1 | 8.6 | 11.8 | 10.8 | 10.9 | 9.1 | |||
| Left colon | 8.1 | 9.8 | 10.1 | 11.0 | 9.2 | 9.2 | |||
| Sigmoid colon | 16.0 | 20.0 | 22.4 | 26.4 | 20.0 | 23.5 | |||
| Unknown | 1.6 | 1.7 | 1.5 | 1.6 | 1.7 | 1.6 | |||
| Comorbidity and treatment measures, % | |||||||||
| Any hospitalization in the previous year | 28.1 | 19.0 | < .001 | 29.1 | 21.0 | < .001 | 32.7 | 22.0 | < .001 |
| Rehospitalization within 30 days of discharge | 11.0 | 8.4 | .009 | 12.8 | 9.2 | < 0.001 | 16.1 | 8.8 | < .001 |
| Any in-hospital surgical complication | 2.9 | 2.1 | .14 | 4.1 | 4.1 | .91 | 4.8 | 2.9 | < .001 |
| Oncologist visit within 30 days of surgery | 21.9 | 54.7 | < .001 | 23.3 | 54.6 | < .001 | 28.1 | 58.3 | < .001 |
| Poor prognostic features, % | |||||||||
| Emergent admission | 0.0 | 0.0 | 26.4 | 22.3 | < .001 | 26.6 | 16.9 | < .001 | |
| Intestinal obstruction on admission | 0.0 | 0.0 | 6.1 | 6.9 | .08 | 6.0 | 4.6 | < .001 | |
| Intestinal perforation on admission | 0.0 | 0.0 | 2.4 | 4.4 | < .001 | 2.7 | 1.6 | < .001 | |
| T stage | < .001 | < .001 | |||||||
| Tis, T0, T1, or T2 | 0.0 | 0.0 | 0.0 | 0.0 | 9.4 | 11.7 | |||
| T3 | 100.0 | 100.0 | 82.8 | 73.9 | 70.4 | 70.2 | |||
| T4 | 0.0 | 0.0 | 17.2 | 26.1 | 20.2 | 18.1 | |||
| No. of nodes resected, % | < .001 | < .001 | |||||||
| 0-11 | 0.0 | 0.0 | 72.5 | 69.5 | 50.7 | 47.8 | |||
| ≥ 12 | 100.0 | 100.0 | 27.5 | 30.5 | 49.3 | 52.2 | |||
| Tumor grade, % | .66 | .001 | .02 | ||||||
| Well differentiated | 8.5 | 8.1 | 7.4 | 6.9 | 4.8 | 5.0 | |||
| Moderately differentiated | 91.5 | 91.9 | 66.4 | 64.1 | 62.5 | 64.3 | |||
| Poorly or undifferentiated | 0.0 | 0.0 | 26.1 | 29.0 | 32.7 | 30.7 | |||
| No. of poor prognostic features, % | < .001 | < .001 | |||||||
| 0 | 100.0 | 100.0 | 0.0 | 0.0 | 19.4 | 24.0 | |||
| 1 | 0.0 | 0.0 | 60.5 | 56.5 | 39.0 | 43.2 | |||
| 2 | 0.0 | 0.0 | 30.1 | 31.0 | 28.1 | 23.7 | |||
| 3 | 0.0 | 0.0 | 7.9 | 10.7 | 10.9 | 7.6 | |||
| 4, 5, or 6 | 0.0 | 0.0 | 1.6 | 1.9 | 2.7 | 1.6 | |||
NOTE. Percentages may not sum to 100% due to rounding.
Abbreviations: Chemo, chemotherapy; SEER, Surveillance, Epidemiology, and End Results; SD, standard deviation; HCC, Hierarchical Condition Categories.
A total of 2,243 individuals with missing data are not included in these means.
Three SEER registries (Kentucky, Louisiana, New Jersey) were added in 2000.
In the stage II with any poor prognostic features group, higher proportions of patients with perforation, T4 stage, and poor histology were seen in the chemotherapy group compared with the no chemotherapy group. In contrast, equal or smaller proportions of patients with emergent admission, obstruction, or fewer than 12 nodes received chemotherapy.
Given the clear group differences by chemotherapy receipt, propensity scores describing the likelihood of chemotherapy receipt contingent on covariates were used to reweight the patient population for each stage group. Many distributional differences, such as age, were eliminated, yielding treated and untreated groups that were more similar, as would be anticipated in a randomized trial (Appendix Table A2, online only).
Survival Benefit of Chemotherapy
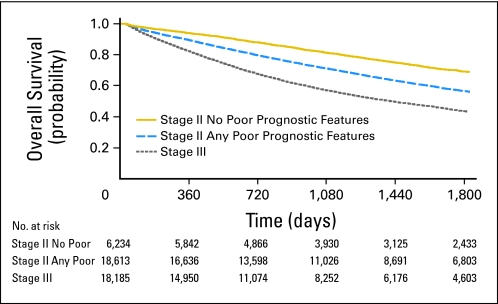
Kaplan-Meier unadjusted survival analysis was conducted to compare 5-year overall survival between the three stage groups (Fig 1). The presence of poor prognostic features identified a subgroup of stage II patients with significantly reduced survival at 5 years (stage II with no poor prognostic features, 69% [95% CI, 67.9% to 70.5%]; stage II with any poor prognostic features, 57% [95% CI, 55.8% to 57.4%]; stage III, 44% [95% CI, 42.8% to 44.4%]).
Fig 1.
Overall survival by stage group (Kaplan-Meier). Cox regression–based test for equality of survival curves confirms significant differences between stage groups (likelihood-ratio χ2 = 1540.59; P < .001).
To examine the stage-specific survival benefit attributable to chemotherapy, we used Cox proportional hazards regression methods. We first examined the baseline model predicting 5-year overall mortality, including all covariates and a stage group–chemotherapy interaction term. This interaction was significant (likelihood-ratio χ2 = 34.5, P < .0001), suggesting that we should proceed with separate regression models by stage group (Table 3). The unadjusted models, containing chemotherapy as the explanatory variable, showed a significant survival benefit for all stage groups from adjuvant chemotherapy, as demonstrated by HRs less than 1.0. However, when important patient and disease characteristics were included as covariates, only the stage III group retained significant mortality reductions (HR, 0.64; 95% CI, 0.61 to 0.68). When propensity scores were included in the covariate-adjusted regression models as weights, the stage-specific 5-year survival benefit from chemotherapy remained unchanged, with a significant HR seen only for stage III patients (HR, 0.64; 95% CI, 0.60 to 0.67).
Table 3.
Overall 5-Year Mortality by Stage Group (Cox regression model)
| Model | Stage II With No Poor Prognostic Features |
Stage II With Any Poor Prognostic Features |
Stage III |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Unadjusted | 0.67 | 0.59 to 0.77 | < .001 | 0.67 | 0.63 to 0.71 | < .001 | 0.48 | 0.46 to 0.5 | < .001 |
| Adjusted for covariates* | 1.05 | 0.9 to 1.22 | .53 | 0.96 | 0.89 to 1.03 | .24 | 0.64 | 0.61 to 0.68 | < .001 |
| Adjusted for covariates* with propensity score weighting | 1.02 | 0.84 to 1.25 | .80 | 1.03 | 0.94 to 1.13 | .47 | 0.64 | 0.6 to 0.67 | < .001 |
Abbreviation: HR, hazard ratio.
Covariates: age, sex, ethnicity, marital status, Surveillance, Epidemiology, and End Results registry, residence location, tumor location, diagnosis year, census-tract median household income and education, Hierarchical Condition Categories score, prior-year hospitalizations, 30-day rehospitalizations, in-hospital complications, 30-day oncologist visits.
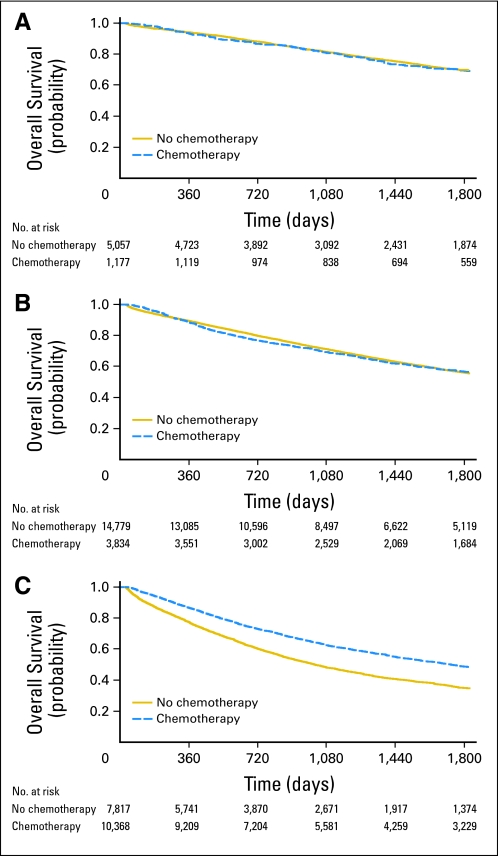
Kaplan-Meier survival analysis with propensity-score weighting was used to compare 5-year overall survival between treated and untreated patients in each stage group (Figs 2A through 2C). Given the sample size available for this analysis, we would have been able to detect an absolute difference in 5-year overall survival among the stage II with any poor prognostic features group of 2%. However, no difference in 5-year survival was observed between treated and untreated patients for this group (56.7% v 56.1%) or for stage II with no poor prognostic features (70.0% v 69.5%). In contrast, stage III patients derived significant survival benefit from chemotherapy (48.9% v 35.2%).
Fig 2.
Overall survival by chemotherapy administration status and stage group (Kaplan-Meier) with propensity score weighting. Cox regression–based test for equality of survival curves: (A) stage II with no poor prognostic features: Wald χ2 = 0.01, P = .94; (B) stage II with any poor prognostic features: Wald χ2 = 0.20, P = .65; (C) stage III: Wald χ2 = 232.80, P < .001.
DISCUSSION
Our primary aim was to determine the degree of 5-year overall survival benefit conferred by adjuvant chemotherapy on patients with stage II colon cancer having poor prognostic features. Although these characteristics do distinguish a subgroup of stage II patients with worse survival than the rest of the stage II cohort, they do not predict measurable mortality benefit from chemotherapy in this study population. Our sample size in this analysis of Medicare beneficiaries allows us to detect, if present, a 2% absolute difference in 5-year survival attributable to chemotherapy; our lack of statistical significance implies that any benefit that exists is quite small. Interestingly, chemotherapy use does not differ between stage II patients with or without poor prognostic features, suggesting that these are not central to the treatment decision.
Our analysis has several important advantages over prior investigations. In contrast to prior trials that predominantly recruited patients with stage III disease, our study population has sufficient power to detect clinically significant differences in survival for patients with stage II disease, addressing concerns about inadequate sample size. In addition, this is the only study to date that specifically examines the subgroup of stage II patients with poor prognostic features, thereby addressing the population heterogeneity that was thought to confound prior conclusions of minimal survival benefit.1–10,12,15 The median age of colon cancer diagnosis is 70 years, yet many older adults were explicitly excluded from randomized chemotherapy trials or were otherwise deemed ineligible as a result of age-related comorbidity, provider bias, or patient preference.1–10 Our study population importantly presents a more real-world age distribution (68% over age 75 years at diagnosis) and includes patients with greater levels of comorbidity and risk than typically allowed in randomized trials, facilitating better generalizability to the population of senior adults.32 These factors, particularly our inclusion of older individuals and those with shorter postoperative survival compared with the Schrag studies, may explain the relatively low utilization of chemotherapy identified in our analysis, even among stage III patients.13
This investigation uses existing data to address a research question that would require substantial resources and time to answer through a randomized trial. Limitations to our study include our inability to investigate the role of two known poor prognostic features, preoperative CEA and lymphovascular invasion, as these characteristics are not available within the SEER-Medicare database. These features may represent more locally advanced disease, but it is unclear how they influence the likelihood of chemotherapy receipt and any subsequent survival benefit. Although our models included several important patient- and disease-related variables, administrative data lack direct measurement of factors that may influence treatment assignment, such as patient preferences or provider care patterns. There is some suggestion that chemotherapy administration is related to provider practice: Hershman et al33 found that among elderly women with breast cancer, chemotherapy was more commonly received by those treated by younger private-practice oncologists. Although these factors may influence treatment receipt, their relationship to survival is unknown. Finally, our study examines only Medicare beneficiaries age 66 years and older at the time of diagnosis, limiting its applicability to younger patients with colon cancer, such as those with familial syndromes. However, because more than 60% of colorectal cancer diagnoses are made in the 65-years-and-older population, our findings are likely appropriate for the majority of patients with colon cancer.32
This study retrospectively examines the use of chemotherapy as identified through Medicare claims data using a “one claim” algorithm.22,23 This creates a heterogeneous population in which some patients received a substandard duration of therapy for unrecorded reasons, possibly including patient/provider preference or adverse effects, whereas other patients receive a complete course. We speculate that those patients who stop chemotherapy early may have other features related to poor survival (older age, comorbid conditions, and so on), and that the inclusion of both subsets of patients within a single analysis group likely leads to a reduction in the survival benefit observed with chemotherapy. However, the “none versus any” approach used to assign treatment status provides a window into the effectiveness of chemotherapy in real world practice, in which an individual's likelihood of completing the treatment course is not known at the outset of the study. We also note that chemotherapeutic recommendations have changed since the time period of this analysis, and many patients in our study population likely did not receive oxaliplatin, thus limiting the applicability of our results to today's standard regimens. Further investigation into patient characteristics predicting therapy completion, the influence of chemotherapy duration on survival, and the benefit of oxaliplatin in practice may be warranted.
The poor prognostic features considered in this analysis and described in published guidelines represent an imprecise clinical mechanism for identifying patients with high-risk stage II colon cancer. Recently, high-frequency DNA microsatellite instability from mismatch repair defects has emerged as an important predictor of better prognosis as well as resistance to fluorouracil therapy.12,34–37 Older patients may also demonstrate increased prevalence of a methylator phenotype (CIMP), which may be correlated with higher disease stages and sporadic cases of microsatellite instability.38,39 These and other histologic and genetic factors are the subject of investigation in ongoing basic science and prospective clinical trials that may identify any subset of stage II patients for whom chemotherapy offers significant survival benefit.40
In conclusion, we find that patients with stage II colon cancer, even those with any of six identified poor prognostic features, do not have a significant survival benefit from chemotherapy. Given the frequent use of chemotherapy in this generalizable population of older adults, this suggests that, in practice, many patients may be receiving chemotherapy with a disadvantageous risk-benefit ratio. Given the statistical power of this study, clinicians may wish to counsel high-risk older adults with colon cancer that any possible survival benefit is likely less than 2% at 5 years.
Acknowledgment
We thank Carol Weidel, SAS programmer, Health Innovation Program, for her assistance in preparing the data for analysis, and Colleen Brown, research specialist, Health Innovation Program, for her assistance in formatting and proofing the manuscript (the University of Wisconsin Health Innovation Program assisted with manuscript preparation and data management). This study used the linked Surveillance, Epidemiology, and End Results (SEER) –Medicare database. We thank the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
The work presented here was carried out while Dr O'Connor was a Primary Care Research Fellow supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin (UW) Department of Family Medicine (Dr Bruce Barrett, principal investigator), with additional salary support provided by the University of Wisconsin Department of Surgery. Additional support was provided by the University of Wisconsin Carbone Cancer Center (UWCCC) Support Grant from the National Cancer Institute –National Institutes of Health, (grant number P30CA014520-34), and the Health Innovation Program and the Community -Academic Partnerships core of the University of Wisconsin Institute for Clinical and Translational Research (UW ICTR), grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health. Additionally, funding for this project was provided by the UW School of Medicine and Public Health from the Wisconsin Partnership Program. The UW Health Innovation Program assisted with manuscript preparation and data management. No other funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Appendix
Table A1.
Codes Used for Variable Definitions
| Category | Source and Type | Codes |
|---|---|---|
| Colon cancer histology | SEER, PEDSF Histology ICD-03 | 8140-7, 8210-1, 8220-1, 8260-3, 8480-1, 8490* |
| Colon cancer primary tumor sites | SEER, PEDSF Site | C18.0-C18.9 |
| Surgical resection | Medicare, ICD-9-CM Procedure | 45.7x (partial excision large intestine), 45.8x(total intra-abdominal colectomy) |
| Radiation therapy | Medicare, ICD-9-CM Diagnosis | Medical evaluation: V58.0, V66.1, V67.1 |
| Medicare, ICD-9-CM Procedure | Administration: 92.21-92.29 | |
| Medicare, Revenue center codes | Administration: 0330, 0333 | |
| Medicare, HCPCS/CPT | Administration: 77,401-77499, 77,750-77799 | |
| Chemotherapy | Medicare, ICD-9-CM Diagnosis | Medical evaluation: V58.1, V66.2, V67.2, E9331 |
| Medicare, ICD-9-CM Procedure | Administration: 99.25 | |
| Medicare, Revenue center codes | Administration: 0331, 0332, 0335 | |
| Medicare, CPT | Administration: 96,400-96599 | |
| Medicare, HCPCS | Administration: C8953-5, S9329-31, G0355-363, Q0083-5, E0781 Agents: J8530-8999, J9000-9999; J0640, J8510, J8520-1, J9190, J9200, J9206, J9263, Q0177 | |
| Distant recurrence of cancer | Medicare, ICD-9-CM Diagnosis | Diagnosis of secondary malignancy: 197.0-.3, 197.8, 198.3-.5, 198.41, 198.45, 198.48, 198.51, 197.04, 197.08 |
| Medicare, ICD-9-CM Procedure | Treatment of liver metastases: 50.20-50.22, 50.29, 50.3, 50.4; 197.7 | |
| Medicare, CPT | Treatment of liver metastases: 36,246, 36,247, 36,260, 47,100, 47,120, 47,122, 47,125, 47,130, 47,370, 47,371, 47380-2, 76,362, 76,394, 76,490, 77,013, 77,022, 76,940 | |
| Poor prognostic features | ||
| Obstruction | Medicare, ICD-9-CM Diagnosis | 560.89, 560.90 |
| Perforation | Medicare, ICD-9-CM Diagnosis | 569.83 |
| Emergent admission | SEER, MedPAR variable 118 | |
| T4 stage | SEER, PEDSF EOD Tumor Extent (1992–2003);PEDSF CS Extension (2004+) | 50, 55, 57, 60, 65, 66, 70, 75, 80 |
| Poor/undifferentiated histology | SEER, PEDSF Grade | |
| Lymph nodes < 12 | SEER, PEDSF EOD-10 Number Nodes Examined |
Abbreviations: SEER, Surveillance, Epidemiology, and End Results; PEDSF, Patient Entitlement and Diagnosis Summary File; ICD-9-CM, International Classification of Disease, 9th edition, Clinical Modification; HCPCS, Healthcare Common Procedure Coding System; CPT, Current Procedural Terminology; MedPAR, Medicare Provider Analysis and Review file; EOD, extent of disease; CS, collaborative staging.
Patients with mucinous cystadenocarcinoma (histology code 8470) were excluded because the natural history of this disease, which occurs in the appendix and is associated with pseudomyxomaperitoneii, is different from other histologic subtypes of colon adenocarcinoma.
Table A2.
Characteristics of Patients Undergoing Resection for Stage II/III Colon Cancer After Propensity Score Weighting
| Characteristic | Stage II With No Poor Prognostic Features (n = 6,234) |
Stage II With Any Poor Prognostic Features (n = 18,613) |
Stage III (n = 18,185) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| No Chemo (81%) | Chemo (19%) | P | No Chemo (79%) | Chemo (21%) | P | No Chemo (43%) | Chemo (57%) | P | |
| Patient characteristics | |||||||||
| Age, years, % | .93 | .98 | .99 | ||||||
| 65-69 | 13.0 | 13.1 | 10.6 | 10.7 | 13.4 | 13.3 | |||
| 70-74 | 20.8 | 20.5 | 19.1 | 19.1 | 21.8 | 21.8 | |||
| 75-79 | 25.5 | 25.1 | 24.1 | 24.4 | 25.1 | 25.2 | |||
| 80-84 | 23.1 | 22.3 | 23.2 | 22.9 | 21.6 | 21.7 | |||
| 85+ | 17.7 | 19.0 | 23.1 | 22.9 | 18.1 | 18.1 | |||
| Male sex, % | 43.2 | 47.0 | .10 | 39.6 | 48.2 | < .001 | 40.8 | 43.0 | .02 |
| Race/ethnicity, % | .08 | .07 | < .001 | ||||||
| White | 86.6 | 87.7 | 86.6 | 87.1 | 81.3 | 85.2 | |||
| Black | 5.8 | 3.5 | 6.4 | 5.0 | 9.3 | 5.7 | |||
| Asian or Pacific Islander | 4.1 | 5.0 | 3.4 | 3.4 | 5.1 | 4.9 | |||
| Hispanic or other | 3.5 | 3.7 | 3.7 | 4.5 | 4.3 | 4.2 | |||
| SEER registry, % | .10 | .14 | < .001 | ||||||
| California | 26.5 | 31.7 | 28.7 | 27.3 | 27.0 | 28.1 | |||
| Connecticut | 8.8 | 9.9 | 9.8 | 10.3 | 9.1 | 11.2 | |||
| Detroit | 2.5 | 2.6 | 10.7 | 11.9 | 11.8 | 10.9 | |||
| Hawaii | 12.6 | 10.8 | 1.2 | 0.8 | 2.2 | 1.8 | |||
| Iowa | 2.7 | 2.3 | 14.6 | 13.5 | 12.0 | 12.4 | |||
| New Mexico | 7.5 | 6.4 | 2.3 | 2.3 | 3.1 | 2.6 | |||
| Seattle | 3.4 | 3.2 | 7.4 | 6.5 | 7.0 | 6.5 | |||
| Utah | 4.1 | 3.7 | 2.7 | 2.4 | 3.4 | 3.1 | |||
| Atlanta and rural Georgia | 5.5 | 5.0 | 4.0 | 3.6 | 4.3 | 3.4 | |||
| Kentucky | 4.3 | 5.2 | 4.4 | 5.2 | 5.5 | 4.5 | |||
| Louisiana | 10.5 | 11.5 | 4.3 | 5.2 | 4.6 | 4.2 | |||
| New Jersey | 26.5 | 31.7 | 9.9 | 11.0 | 10.1 | 11.4 | |||
| Marital status, % | .20 | .33 | .80 | ||||||
| Married | 53.1 | 56.9 | 47.4 | 49.5 | 50.6 | 51.6 | |||
| Widowed | 32.6 | 30.7 | 37.5 | 35.2 | 34.0 | 33.4 | |||
| Single, separated, or divorced | 11.2 | 8.7 | 11.9 | 12.2 | 12.5 | 12.1 | |||
| Unknown | 3.1 | 3.7 | 3.1 | 3.2 | 2.9 | 2.9 | |||
| Residence location, % | .02 | .73 | .38 | ||||||
| Major metropolitan | 53.8 | 60.1 | 55.3 | 55.6 | 57.1 | 55.7 | |||
| Metropolitan or urban | 35.5 | 30.6 | 34.0 | 33.2 | 32.8 | 33.6 | |||
| Less urban or rural | 10.8 | 9.3 | 10.7 | 11.2 | 10.2 | 10.7 | |||
| Median household income (census tract), $ in thousands | .07 | .006 | < .001 | ||||||
| Mean* | 46.8 | 48.8 | 44.2 | 44.1 | 43.8 | 45.6 | |||
| 95% CI | 46.1, 47.5 | 46.4, 51.2 | 43.8, 44.6 | 43.0, 45.1 | 43.1, 44.5 | 45.0, 46.2 | |||
| Less than 12 yr education (census tract), % | .97 | .61 | < .001 | ||||||
| Mean* | 18.1 | 18.2 | 19.4 | 19.6 | 20.0 | 19.2 | |||
| 95% CI | 17.8, 18.5 | 17.1, 19.4 | 19.2, 19.6 | 19.0, 20.2 | 19.6, 20.4 | 18.8, 19.5 | |||
| HCC comorbidity score | < .001 | < .001 | < .001 | ||||||
| Mean | 1.7 | 1.8 | 2.1 | 2.1 | 2.6 | 2.6 | |||
| 95% CI | 1.7, 1.8 | 1.7, 1.9 | 2.1, 2.1 | 2.0, 2.1 | 2.5, 2.6 | 2.5, 2.6 | |||
| Tumor characteristics | |||||||||
| Year of diagnosis, % | .12 | .78 | .95 | ||||||
| 1992-1995 | 18.0 | 15.4 | 22.7 | 22.1 | 19.6 | 19.7 | |||
| 1996-1999 | 18.8 | 17.3 | 21.7 | 21.0 | 19.7 | 20.1 | |||
| 2000-2002† | 29.6 | 29.1 | 29.6 | 30.2 | 29.6 | 29.5 | |||
| 2003-2005† | 33.6 | 38.2 | 26.0 | 26.7 | 31.2 | 30.7 | |||
| Tumor location, % | .47 | .10 | .08 | ||||||
| Right colon | 63.9 | 63.4 | 53.7 | 52.9 | 56.3 | 57.8 | |||
| Transverse colon | 10.1 | 8.1 | 11.7 | 9.9 | 10.8 | 9.3 | |||
| Left colon | 8.1 | 9.1 | 10.2 | 10.9 | 9.3 | 8.8 | |||
| Sigmoid colon | 16.3 | 17.9 | 22.9 | 24.5 | 21.9 | 22.2 | |||
| Unknown | 1.6 | 1.5 | 1.5 | 1.8 | 1.7 | 1.9 | |||
| Comorbidity and treatment measures | |||||||||
| Any hospitalization in the previous year, % | 26.4 | 28.0 | .48 | 27.4 | 26.6 | .49 | 26.6 | 26.4 | .79 |
| Rehospitalization within 30 days of discharge, % | 10.8 | 8.2 | .04 | 12.1 | 12.1 | .94 | 11.8 | 12.2 | .47 |
| Any in-hospital surgical complication, % | 2.8 | 2.9 | .92 | 4.1 | 4.4 | .58 | 3.7 | 3.7 | .94 |
| Oncologist visit within 30 days of surgery, % | 28.1 | 27.6 | .80 | 29.7 | 29.9 | .89 | 45.2 | 45.5 | .82 |
| Poor prognostic features | |||||||||
| Emergent admission, % | NA | NA | 25.7 | 23.6 | .07 | 24.1 | 18.8 | < .001 | |
| Intestinal obstruction on admission, % | NA | NA | 6.0 | 6.5 | .31 | 5.5 | 4.7 | .07 | |
| Intestinal perforation on admission, % | NA | NA | 2.4 | 4.1 | < .001 | 2.3 | 1.7 | .03 | |
| T stage, % | < .001 | .16 | |||||||
| Tis, T0, T1, or T2 | NA | NA | 0.0 | 0.0 | 10.2 | 11.2 | |||
| T3 | 0.5 | 0.5 | 82.8 | 74.0 | 70.4 | 70.4 | |||
| T4 | NA | NA | 17.2 | 26.0 | 19.4 | 18.4 | |||
| No. of nodes resected, % | .01 | .32 | |||||||
| 0-11 | NA | NA | 72.5 | 69.7 | 49.8 | 48.8 | |||
| ≥ 12 | 0.5 | 0.5 | 27.5 | 30.3 | 50.3 | 51.2 | |||
| Tumor grade, % | .86 | .05 | .68 | ||||||
| Well differentiated | 0.0 | 0.0 | 7.5 | 7.1 | 5.4 | 5.1 | |||
| Moderately differentiated | 0.5 | 0.5 | 66.7 | 64.2 | 63.9 | 63.6 | |||
| Poorly or undifferentiated | NA | NA | 25.9 | 28.8 | 30.8 | 31.3 | |||
| No. of poor prognostic features, % | < .001 | < .001 | |||||||
| 0 | 0.5 | 0.5 | NA | NA | 21.5 | 22.8 | |||
| 1 | NA | NA | 61.2 | 56.5 | 39.9 | 42.3 | |||
| 2 | NA | NA | 29.7 | 30.6 | 26.7 | 25.3 | |||
| 3 | NA | NA | 7.6 | 10.9 | 9.6 | 7.7 | |||
| 4, 5, or 6 | NA | NA | 1.5 | 2.0 | 2.3 | 1.9 | |||
NOTE. Percentages may not sum to 100% because of rounding.
Abbreviations: Chemo, chemotherapy; SEER, Surveillance, Epidemiology, and End Results; SD, standard deviation; HCC, Hierarchical Condition Categories; NA, not applicable.
A total of 2,243 individuals with missing data were not included in these means.
Three SEER registries (Kentucky, Louisiana, New Jersey) were added in 2000.
Footnotes
See accompanying editorial on page 3346; listen to the podcast by Dr Saltz at www.jco.org/podcasts
The ideas and opinions expressed herein are those of the author(s), and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred.
Support sources for this work include University of Wisconsin (UW) Department of Family Medicine Primary Care Research Fellowship, Health Resources and Services Administration National Research Service Award (T32HP10010); UW Carbone Cancer Center Support Grant, National Cancer Institute-National Institutes of Health (NIH) (P30CA014520-34); UW Institute for Clinical and Translational Research, Clinical and Translational Science Award, National Center for Research Resources-NIH (1UL1RR025011); Wisconsin Partnership Program; and UW Health Innovation Program.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Noelle K. LoConte, sanofi-aventis, Bayer Pharmaceuticals, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Erin S. O'Connor, David Yu Greenblatt, Noelle K. LoConte, Maureen A. Smith
Administrative support: Maureen A. Smith
Provision of study materials or patients: Maureen A. Smith
Collection and assembly of data: Erin S. O'Connor, Maureen A. Smith
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer: International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol. 1999;17:1356–1363. [PubMed] [Google Scholar]
- 2.Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: Who benefits and by how much? J Clin Oncol. 2004;22:1797–1806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 3.Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: Observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872–877. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mamounas E, Wieand S, Wolmark N, et al. Comparative efficacy of adjuvant chemotherapy in patients with Dukes' B versus Dukes' C colon cancer: Results from four National Surgical Adjuvant Breast and Bowel Project adjuvant studies (C-01, C-02, C-03, and C-04) J Clin Oncol. 1999;17:1349–1355. doi: 10.1200/JCO.1999.17.5.1349. [DOI] [PubMed] [Google Scholar]
- 5.Sharif S, O'Connell MJ, Yothers G, et al. FOLFOX and FLOX regimens for the adjuvant treatment of resected stage II and III colon cancer. Cancer Invest. 2008;26:956–963. doi: 10.1080/07357900802132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: Results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol. 1993;11:1879–1887. doi: 10.1200/JCO.1993.11.10.1879. [DOI] [PubMed] [Google Scholar]
- 7.Wolmark N, Rockette H, Mamounas E, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes' B and C carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol. 1999;17:3553–3559. doi: 10.1200/JCO.1999.17.11.3553. [DOI] [PubMed] [Google Scholar]
- 8.Quasar Collaborative Group. Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 9.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 10.Efficacy of adjuvant fluorouracil and folinic acid in colon cancer: International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet. 1995;345:939–944. [PubMed] [Google Scholar]
- 11.Wilkinson NW, Yothers G, Lopa S, et al. Long-term survival results of surgery alone versus surgery plus 5-fluorouracil and leucovorin for stage II and stage III colon cancer: Pooled analysis of NSABP C-01 through C-05—A baseline from which to compare modern adjuvant trials. Ann Surg Oncol. 2010;17:959–966. doi: 10.1245/s10434-009-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benson AB, 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 13.Schrag D, Rifas-Shiman S, Saltz L, et al. Adjuvant chemotherapy use for Medicare beneficiaries with stage II colon cancer. J Clin Oncol. 2002;20:3999–4005. doi: 10.1200/JCO.2002.11.084. [DOI] [PubMed] [Google Scholar]
- 14.Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: A secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912–2919. doi: 10.1200/JCO.2003.05.062. [DOI] [PubMed] [Google Scholar]
- 15.Buyse M, Piedbois P. Should Dukes' B patients receive adjuvant therapy? A statistical perspective. Semin Oncol. 2001;28(suppl 1):20–24. doi: 10.1016/s0093-7754(01)90247-7. [DOI] [PubMed] [Google Scholar]
- 16.NIH consensus conference: Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–1450. [PubMed] [Google Scholar]
- 17.Quah HM, Chou JF, Gonen M, et al. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum. 2008;51:503–507. doi: 10.1007/s10350-008-9246-z. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute. SEER Program Overview. http://www.seer.cancer.gov/about/
- 19.Warren J, Klabunde C, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(suppl 8):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 20.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 21.Greenblatt DY, Weber SM, O'Connor ES, et al. Readmission after colectomy for cancer predicts one-year mortality. Ann Surg. 2010;251:659–669. doi: 10.1097/SLA.0b013e3181d3d27c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradley CJ, Given CW, Dahman B, et al. Adjuvant chemotherapy after resection in elderly Medicare and Medicaid patients with colon cancer. Arch Intern Med. 2008;168:521–529. doi: 10.1001/archinternmed.2007.82. [DOI] [PubMed] [Google Scholar]
- 23.Dobie SA, Baldwin LM, Dominitz JA, et al. Completion of therapy by Medicare patients with stage III colon cancer. J Natl Cancer Inst. 2006;98:610–619. doi: 10.1093/jnci/djj159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ash AS, Ellis RP, Pope GC, et al. Using diagnoses to describe populations and predict costs. Health Care Financ Rev. 2000;21:7–28. [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 26.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 27.D'Agostino RJ. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 28.Rubin D. The design versus the analysis of observational studies for causal effects: Parallels with the design of randomized trials. Stat Med. 2007;26:20–36. doi: 10.1002/sim.2739. [DOI] [PubMed] [Google Scholar]
- 29.Zuckerman IH, Rapp T, Onukwugha E, et al. Effect of age on survival benefit of adjuvant chemotherapy in elderly patients with Stage III colon cancer. J Am Geriatr Soc. 2009;57:1403–1410. doi: 10.1111/j.1532-5415.2009.02355.x. [DOI] [PubMed] [Google Scholar]
- 30.Cucino C, Buchner AM, Sonnenberg A. Continued rightward shift of colorectal cancer. Dis Colon Rectum. 2002;45:1035–1040. doi: 10.1007/s10350-004-6356-0. [DOI] [PubMed] [Google Scholar]
- 31.Saltzstein SL, Behling CA. Age and time as factors in the left-to-right shift of the subsite of colorectal adenocarcinoma: A study of 213,383 cases from the California Cancer Registry. J Clin Gastroenterol. 2007;41:173–177. doi: 10.1097/01.mcg.0000225550.26751.6a. [DOI] [PubMed] [Google Scholar]
- 32.National Cancer Institute. SEER Stat Fact Sheets: Cancer of the Colon and Rectum. http://www.seer.cancer.gov/statfacts/html/colorect.html.
- 33.Hershman DL, Buono D, McBride RB, et al. Influence of private practice setting and physician characteristics on the use of breast cancer adjuvant chemotherapy for elderly women. Cancer. 2009;115:3848–3857. doi: 10.1002/cncr.24448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim GP, Colangelo LH, Wieand HS, et al. Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: A National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol. 2007;25:767–772. doi: 10.1200/JCO.2006.05.8172. [DOI] [PubMed] [Google Scholar]
- 35.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 36.Sinicrope FA, Sargent DJ. Clinical implications of microsatellite instability in sporadic colon cancers. Curr Opin Oncol. 2009;21:369–373. doi: 10.1097/CCO.0b013e32832c94bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samowitz WS, Albertsen H, Herrick J, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–845. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 40.Oxaliplatin, leucovorin, and fluorouracil with or without bevacizumab in treating patients who have undergone surgery for stage II colon cancer. http://clinicaltrials.gov/ct2/results?term=NCT00217737.