Abstract
Purpose
Drugs are usually approved for a specific indication on the basis of randomized trials. However, once approved, these treatments are often used differently than as tested in trials. We performed an analysis to determine the patterns of use of erythropoiesis-stimulating agents (ESAs).
Methods
We used the Surveillance, Epidemiology, and End Results–Medicare database to identify patients age 65 years or older with breast, lung, or colon cancer diagnosed between 1995 and 2005 who had one ESA and chemotherapy claim. Associations of patient, tumor, and physician-related factors with receipt of ESAs were analyzed.
Results
Of 21,091 patients analyzed, 5,099 (24.2%) received ESAs for 1 week or less (misuse), and 1,601 (7.6%) received ESAs for more than 14 weeks (prolonged use). Receipt of ESAs while not actively receiving chemotherapy (off label) occurred in 2,876 patients (13.6%). In a multivariable analysis, ESA misuse was associated with MD degree, female sex of physician, and earlier year of medical school graduation. Private practice physicians (odds ratio [OR], 0.78; 95% CI, 0.72 to 0.84) and high-volume physicians (OR, 0.78; 95% CI, 0.72 to 0.85) were less likely to use 1 week or less of ESA treatment. Treatment by high-volume oncologists (OR, 1.33; 95% CI, 1.14 to 1.55) and by oncologists who graduated from US medical schools (OR, 1.26; 95% CI, 1.12 to 1.42) predicted prolonged-duration ESA use, whereas female oncologists (OR, 0.79; 95% CI, 0.68 to 0.93) were less likely to prescribe prolonged ESA treatment. Private practice physicians (OR, 1.18; 95% CI, 1.02 to 1.38) and high-volume providers (OR, 1.58; 95% CI, 1.33 to 1.87) were more likely to prescribe more than 24 weeks of ESA treatment.
Conclusion
Our study demonstrated widespread variability in the use of ESAs. Physician characteristics exerted substantial influence on ESA use. Policies to discourage inappropriate use of cancer therapies are needed.
INTRODUCTION
In 1993, the US Food and Drug Administration (FDA) approved the erythropoiesis-stimulating agent (ESA) epoetin alfa for patients with cancer.1 Approval of the long-acting erythropoietin preparation darbepoetin followed in 2002.2 FDA approval for both agents was granted based on reductions in transfusion requirements in placebo-controlled trials comparing 12 weeks of the respective ESA with placebo.1,2 Both agents were approved for use in patients with cancer while they are actively receiving cytotoxic chemotherapy. Often, drugs are approved for a specific indication based on data from randomized clinical trials, in which the agents are tested for specific indications and for specific durations. However, once approved, these treatments are used in a manner different from that studied in the clinical trials. Off-label and inappropriate use—which can take the form of under-, over-, or prolonged use—of drugs places patients at risk for toxicity without any proven benefit and represents a major source of excess health care expenditures.3–5
Despite an increasing number of studies questioning the safety of ESAs, their use in the United States increased by 340% between 2001and 2006.6–8 It is estimated that annual Medicare expenditures for ESAs exceed $1 billion.9 In 2007, prompted by emerging safety concerns, the FDA issued a black-box warning for ESAs. During the ESA review process, the FDA expressed significant concern regarding off-label and inappropriate use of ESAs. Although off-label use of oncologic drugs is common, the FDA review process for ESAs resulted in heightened scrutiny and changes in reimbursement.8,10
To facilitate appropriate ESA use, a number of professional societies have proposed guidelines for ESA administration.11–14 The American Society of Clinical Oncology (ASCO) first published ESA guidelines in 2002, and the European Organisation for Research and Treatment of Cancer endorsed recommendations in 2004.11,14 These guidelines have been regularly updated as emerging safety data have become available.12,13 However, despite ongoing debate regarding appropriate use of ESAs, relatively little is known about the patterns of ESA use among oncologists. We performed a population-based analysis to determine the patterns of use of ESAs in the United States. We examined patient and physician characteristics associated with off-label and unconventional use of ESAs.
METHODS
Data Source
We analyzed data from the Surveillance, Epidemiology, and End Results (SEER) –Medicare database.15 SEER provides information on tumor histology, location, stage of disease, treatment, and survival, along with SEER site at diagnosis and demographic and selected census tract-level information. The Medicare database includes Medicare A (inpatient) and B (outpatient) eligibility status, billed claims, and diagnoses. These two files are linked and provide the ability to determine who has been treated with ESAs and the dates of service. Exemption from the institutional review board of Columbia University was obtained.
Cohort Selection
We identified all individuals who were age 65 years or older, who had a pathologically confirmed primary diagnosis of breast, non–small-cell lung, or colon cancer16 from January 1, 1995, to December 31, 2005, and who were treated with chemotherapy. These cancers were thought to represent common cancers for which ESAs are frequently used. We excluded patients who were enrolled in non-Medicare health maintenance organizations.17 Patients who were enrolled in Medicare because of end-stage renal disease or dialysis as well as patients with other primary cancers were also excluded (Appendix Table A1, online only). Age at diagnosis was categorized into 5-year intervals. We recoded the SEER marital status variable as married, not married, or unknown.
Socioeconomic Status Score
We generated an aggregate socioeconomic status (SES) score from education, poverty level, and income data from the 2000 census tract data, as described previously by Du et al.18 Patient scores were ranked on a scale of 1 to 5 by use of a formula incorporating education, poverty, and income weighted equally, with 1 being the lowest value.
Assessment of Comorbid Disease
To assess the prevalence of comorbid disease in our cohort, we used the Klabunde adaptation of the Charlson comorbidity index.19,20 Medicare inpatient and outpatient claims were searched for diagnostic codes of the International Classification of Disease, Ninth Revision, Clinical Modification.16 Each condition was weighted, and patients were assigned a score based on the Klabunde–Charlson index method.20
Physician Characteristics
We matched treating physician to ESA claim by use of the unique physician identification number (UPIN) on the ESA claim; this was required to have a match in the American Medical Association file and indicate a primary or secondary specialty in oncology. Primary and secondary specialty codes for oncologists were defined as oncologist, hematologist, hematologist/oncologist, radiation oncologist, and surgical oncologist. Ninety-five percent of ESA claims were linked to valid UPINs, and for each tumor type, 90% to 92% of physicians associated with UPINs had a primary or secondary specialty of oncology. Oncologists characteristics analyzed based on variables in the American Medical Association master file included sex, year of graduation, primary employment setting (private v government or academic), location of training (United States v other), and type of degree (medical degree v doctor of osteopathic medicine). Physician ESA volumes were analyzed. Those physicians with approximately the highest quartile of patients receiving ESAs were considered high volume, and the cohort was dichotomized as one to nine or 10 or more patients accordingly.
Treatment Characteristics
We extracted information on chemotherapy from date of diagnosis from the Medicare files by searching the Level II Healthcare Common Procedure Coding System; Current Procedural Terminology codes; International Classification of Disease, Ninth Revision, Clinical Modification, diagnostic codes; and procedure, diagnostic-related group, and center codes from physician claims files, hospital outpatient claims files, or Medicare provider review files. We searched for Level II Healthcare Common Procedure Coding System codes corresponding to ESAs erythropoietin and darbopoietin (Q0136-7, J0880-2, and J0885-6). All patients had at least one claim for ESAs, and we excluded patients who received their first ESA before they received chemotherapy. Use of ESAs was categorized by number of consecutive weeks of therapy, total number of weeks, and total number of claims. Continuous use of ESAs was defined as the length of time receiving ESAs with no more than 4 weeks between claims, starting with the first ESA. Continuous use was divided into three groups: 1 week or less (misuse), 2 to 14 weeks (standard use based on clinical trials), and more than 14 weeks (prolonged use). An ESA was defined as received with concurrent chemotherapy if a claim was filed within 8 weeks of the chemotherapy claim, as per the current guidelines, or as off label if claims continued beyond 8 weeks after completion of chemotherapy. Misuse, prolonged use, and off-label use were considered inappropriate use.
We classified patients into the following three groups: nonmetastatic (those who received chemotherapy only), metastatic (those who received chemotherapy only with metastatic or recurrent cancer), and both (those who received chemotherapy in both settings). Patients were classified as nonmetastatic if they had stage 1 to 3 breast, non–small-cell lung, or colon cancer when they were treated. They were classified as metastatic if they had stage 4 breast, non–small-cell lung, or colon cancer. If chemotherapy was administered after the first 12 months, the patient was categorized as having a recurrence.
Statistical Analysis
Treatment duration of ESAs was compared using χ2 tests and univariate regression, with respect to clinical and demographic variables. We used Generalized Estimating Equations (GEE) methodology to account for the correlations of outcome measures among patients who had the same physician. Unit of analysis was the patient. For each patient, the UPIN was used as the clustering variable. GEE was used to analyze the association of underuse with standard use of ESAs with clinical variables, and then overuse with standard use of ESAs. A similar approach was taken to evaluate concurrent and off-label use of ESAs. We performed similar analyses to determine predictors of more than 12 weeks and more than 24 weeks of continuous ESA use. In the multivariate GEE analysis, we included physician characteristics, clinical characteristics, and demographic variables that we thought might be clinically significant in the model. All analyses were conducted with SAS, version 9.13 (SAS Institute, Cary, NC). All statistical tests were two sided.
RESULTS
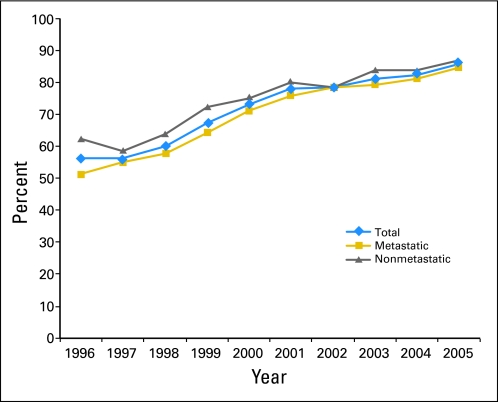
A total of 21,091 patients were included in the duration-of-use analysis. During the years encompassed by the study, ESA use increased; 70.5% of patients who received chemotherapy in 1995 were treated with ESAs compared with 85.6% in 2005. Figure 1 displays yearly ESA use for all patients and also for patients within each disease stage. Overall, 5,099 patients (24.2%) received ESAs for 1 week or less. Table 1 lists demographic, clinical, and physician characteristics associated with duration of use. Clinical factors associated with misuse (≤ 1 week) included nonwhite race, treatment in the later years of the study, nonmetropolitan residence, lower SES, high-grade tumor, metastatic disease, and colon cancer (P < .05). Physician factors associated with ESA use of 1 week or less were MD degree, female sex, academic practice, and lower volume of ESA use (P < .05). In our multivariable model, the only clinical factors that remained associated with short duration were black race, year of diagnosis, colon cancer, nonmetropolitan area, lower SES, and presence of metastatic disease (Table 2). Physician characteristics associated with ESA misuse included MD degree (odds ratio [OR], 1.43; 95% CI, 1.18 to 1.73), female sex (OR, 1.10; 95% CI, 1.01 to 1.20), and earlier year of medical school graduation. In contrast, US medical school graduates (OR, 0.92; 95% CI, 0.85 to 0.99), private practice physicians (OR, 0.78; 95% CI, 0.72 to 0.84), and physicians who used high volumes of ESAs (OR, 0.78; 95% CI, 0.72 to 0.85) were less likely to administer ESAs for 1 week or less.
Fig 1.
Percentages of patients with cancer treated with chemotherapy who also received erythropoiesis-stimulating agents, stratified by year of treatment (N = 24,112).
Table 1.
Univariate Analysis of Factors Associated With Short- (≤ 1 week) and Prolonged-Duration (> 14 weeks) ESA Use
| Factor | ≤ 1 Week |
2 to 14 Weeks |
P* | > 14 Weeks |
P† | |||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||
| Total | 5,099 | 24.2 | 14,391 | 68.2 | 1,601 | 7.6 | ||
| Age at diagnosis, years | .28 | .42 | ||||||
| 65-69 | 1,416 | 27.8 | 3,968 | 27.6 | 437 | 27.3 | ||
| 70-74 | 1,647 | 32.3 | 4,829 | 33.6 | 564 | 35.2 | ||
| 75-79 | 1,297 | 25.4 | 3,629 | 25.2 | 401 | 25.1 | ||
| > 80 | 739 | 14.5 | 1,965 | 13.7 | 199 | 12.4 | ||
| Race | .001 | .15 | ||||||
| White | 4,333 | 85.0 | 12,546 | 87.2 | 1,403 | 87.6 | ||
| Black | 433 | 8.5 | 1,036 | 7.2 | 127 | 7.9 | ||
| Hispanic | 59 | 1.2 | 157 | 1.1 | 11 | 0.7 | ||
| Missing or other | 274 | 5.4 | 652 | 4.5 | 60 | 3.8 | ||
| Year of diagnosis | .015 | < .001 | ||||||
| 2005 | 880 | 17.3 | 2,183 | 15.2 | 178 | 11.1 | ||
| 2004 | 883 | 17.3 | 2,320 | 16.1 | 193 | 12.1 | ||
| 2003 | 799 | 15.7 | 2,397 | 16.7 | 226 | 14.1 | ||
| 2002 | 700 | 13.7 | 2,042 | 14.2 | 250 | 15.6 | ||
| 2001 | 619 | 12.1 | 1,904 | 13.2 | 256 | 16.0 | ||
| 2000 | 559 | 11.0 | 1,641 | 11.4 | 220 | 13.7 | ||
| 1999 | 203 | 4.0 | 591 | 4.1 | 77 | 4.8 | ||
| 1998 | 155 | 3.0 | 436 | 3.0 | 60 | 3.8 | ||
| 1997 | 121 | 2.4 | 372 | 2.6 | 58 | 3.6 | ||
| 1996 | 90 | 1.8 | 268 | 1.9 | 52 | 3.3 | ||
| 1995 | 90 | 1.8 | 237 | 1.7 | 31 | 1.9 | ||
| Residence | < .001 | .13 | ||||||
| Metropolitan | 4,658 | 91.4 | 13,362 | 92.9 | 1,503 | 93.9 | ||
| Nonmetropolitan | 441 | 8.7 | 1,029 | 7.2 | 98 | 6.1 | ||
| Marital status | .25 | .68 | ||||||
| Married | 2,915 | 57.2 | 8,353 | 58.0 | 941 | 58.8 | ||
| Unmarried | 2,039 | 40.0 | 5,619 | 39.0 | 619 | 38.7 | ||
| Unknown | 145 | 2.8 | 419 | 2.9 | 41 | 2.6 | ||
| Socioeconomic status | < .001 | .76 | ||||||
| First (lowest) quartile | 623 | 12.2 | 1,421 | 9.9 | 174 | 10.9 | ||
| Second quintile | 902 | 17.7 | 2,573 | 17.9 | 283 | 17.7 | ||
| Third quintile | 1,079 | 21.2 | 3,145 | 21.9 | 351 | 21.9 | ||
| Fourth quintile | 1,145 | 22.5 | 3,324 | 23.1 | 370 | 23.1 | ||
| Fifth (highest) quartile | 1,350 | 26.5 | 3,928 | 27.3 | 423 | 26.4 | ||
| Comorbidity score | .45 | .02 | ||||||
| 0 | 4,294 | 84.2 | 12,170 | 84.6 | 1,361 | 85.0 | ||
| 1 | 608 | 11.9 | 1,633 | 11.4 | 181 | 11.3 | ||
| > 1 | 197 | 3.9 | 588 | 4.1 | 59 | 3.7 | ||
| Tumor site | < .001 | .017 | ||||||
| Breast | 1,489 | 29.2 | 4,478 | 31.1 | 445 | 27.8 | ||
| Colon | 1,276 | 25.0 | 3,036 | 21.1 | 340 | 21.2 | ||
| Lung | 2,334 | 45.8 | 6,877 | 47.8 | 816 | 51.0 | ||
| Tumor grade | .13 | .06 | ||||||
| High | 1,957 | 38.4 | 5,369 | 37.3 | 603 | 37.7 | ||
| Low | 1,829 | 35.9 | 5,311 | 37.0 | 531 | 33.2 | ||
| Unknown | 1,313 | 25.8 | 3,711 | 25.8 | 467 | 29.2 | ||
| Treatment | .02 | < .001 | ||||||
| Recurrent/metastatic | 2,708 | 53.1 | 7,374 | 51.2 | 992 | 62.0 | ||
| Early | 2,391 | 46.9 | 7,017 | 48.8 | 609 | 38.0 | ||
| Oncologist training | .78 | < .001 | ||||||
| Non–United States | 1,573 | 30.9 | 4,470 | 31.1 | 588 | 36.7 | ||
| United States | 3,526 | 69.2 | 9,921 | 68.9 | 1,013 | 63.3 | ||
| Oncologist degree | < .001 | .45 | ||||||
| DO | 150 | 2.9 | 581 | 4.0 | 71 | 4.4 | ||
| MD | 4,949 | 97.1 | 13,810 | 96.0 | 1,530 | 95.6 | ||
| Oncologist sex | .15 | .002 | ||||||
| Male | 4,264 | 83.6 | 12,156 | 84.5 | 1,400 | 87.5 | ||
| Female | 835 | 16.4 | 2,235 | 15.5 | 201 | 12.6 | ||
| Oncologist year of graduation | .07 | .012 | ||||||
| 1990s | 741 | 14.5 | 2,182 | 15.2 | 209 | 13.1 | ||
| 1980s | 1,788 | 35.1 | 5,267 | 36.6 | 554 | 34.6 | ||
| 1970s | 1,954 | 38.3 | 5,285 | 36.7 | 640 | 40.0 | ||
| 1960s | 616 | 12.1 | 1,657 | 11.5 | 198 | 12.4 | ||
| Oncologist practice setting | < .001 | .87 | ||||||
| Academic | 1,224 | 24.0 | 2,875 | 20.0 | 317 | 19.8 | ||
| Private | 3,875 | 76.0 | 11,516 | 80.0 | 1,284 | 80.2 | ||
| Patient volume | < .001 | < .001 | ||||||
| 1-9 | 1,130 | 22.2 | 2,511 | 17.5 | 213 | 13.3 | ||
| > 10 | 3,969 | 77.8 | 11,880 | 82.6 | 1,388 | 86.7 | ||
Abbreviations: DO, doctor of osteopathic medicine; ESA, erythropoiesis-stimulating agent; MD, doctor of medicine.
Comparison of ≤ 1 week v 2 to 14 weeks of use.
Comparision of > 14 weeks v 2 to 14 weeks of use.
Table 2.
Multivariable Analysis of Factors Associated With Short- (≤ 1 week) and Prolonged-Duration (> 14 weeks) ESA Use
| Factor | ≤ 1 Week |
> 14 Weeks |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age at diagnosis, years | ||||||
| 65-69 | Referent | Referent | ||||
| 70-74 | 0.96 | 0.89 to 1.05 | .39 | 1.03 | 0.90 to 1.18 | .63 |
| 75-79 | 1.00 | 0.92 to 1.10 | .98 | 0.98 | 0.85 to 1.13 | .77 |
| > 80 | 1.02 | 0.92 to 1.14 | .66 | 0.89 | 0.74 to 1.07 | .21 |
| Race | ||||||
| White | Referent | Referent | ||||
| Black | 1.17 | 1.02 to 1.33 | .02 | 1.04 | 0.84 to 1.28 | .74 |
| Hispanic | 0.99 | 0.73 to 1.34 | .93 | 0.62 | 0.33 to 1.16 | .14 |
| Missing or other | 1.12 | 0.95 to 1.31 | .18 | 0.89 | 0.66 to 1.20 | .44 |
| Year of diagnosis | ||||||
| 2005 | Referent | Referent | ||||
| 2004 | 0.94 | 0.84 to 1.05 | .30 | 1.00 | 0.81 to 1.24 | .99 |
| 2003 | 0.82 | 0.74 to 0.94 | < .001 | 1.09 | 0.89 to 1.34 | .41 |
| 2002 | 0.84 | 0.74 to 0.94 | .003 | 1.45 | 1.18 to 1.77 | < .001 |
| 2001 | 0.80 | 0.71 to 0.90 | < .001 | 1.55 | 1.27 to 1.90 | < .001 |
| 2000 | 0.84 | 0.74 to 0.95 | .006 | 1.52 | 1.23 to 1.88 | < .001 |
| 1999 | 0.82 | 0.68 to 0.99 | .03 | 1.56 | 1.16 to 2.09 | .003 |
| 1998 | 0.83 | 0.68 to 1.02 | .08 | 1.57 | 1.14 to 2.16 | .006 |
| 1997 | 0.78 | 0.63 to 0.98 | .03 | 1.77 | 1.28 to 2.45 | < .001 |
| 1996 | 0.79 | 0.61 to 1.02 | .07 | 2.25 | 1.59 to 3.18 | < .001 |
| 1995 | 0.88 | 0.68 to 1.15 | .37 | 1.52 | 1.01 to 2.30 | .05 |
| Residence | ||||||
| Metropolitan | Referent | Referent | ||||
| Nonmetropolitan | 1.32 | 1.14 to 1.51 | < .001 | 0.81 | 0.63 to 1.04 | .10 |
| Marital status | ||||||
| Married | Referent | Referent | ||||
| Unmarried | 1.03 | 0.96 to 1.11 | .37 | 0.98 | 0.88 to 1.10 | .77 |
| Unknown | 0.97 | 0.80 to 1.19 | .79 | 0.84 | 0.60 to 1.18 | .32 |
| Socioeconomic status | ||||||
| First (lowest) quartile | Referent | Referent | ||||
| Second quintile | 0.81 | 0.70 to 0.94 | .005 | 0.95 | 0.74 to 1.20 | .65 |
| Third quintile | 0.78 | 0.67 to 0.90 | < .001 | 0.88 | 0.69 to 1.13 | .32 |
| Fourth quintile | 0.79 | 0.68 to 0.92 | .002 | 0.87 | 0.67 to 1.11 | .26 |
| Fifth (highest) quartile | 0.79 | 0.67 to 0.91 | .002 | 0.86 | 0.67 to 1.10 | .23 |
| Comorbidity score | ||||||
| 0 | Referent | Referent | ||||
| 1 | 0.97 | 0.87 to 1.08 | .57 | 1.04 | 0.87 to 1.24 | .66 |
| > 1 | 0.84 | 0.71 to 1.00 | .05 | 0.92 | 0.69 to 1.23 | .59 |
| Tumor site | ||||||
| Breast | Referent | Referent | ||||
| Colon | 1.24 | 1.12 to 1.36 | < .001 | 1.01 | 0.86 to 1.19 | .90 |
| Lung | 0.96 | 0.88 to 1.05 | .40 | 1.08 | 0.94 to 1.24 | .31 |
| Tumor grade | ||||||
| High | Referent | Referent | ||||
| Low | 1.00 | 0.93 to 1.08 | .98 | 0.89 | 0.79 to 1.02 | .09 |
| Unknown | 1.04 | 0.94 to 1.14 | .47 | 1.06 | 0.92 to 1.23 | .43 |
| Treatment | ||||||
| Recurrent/metastatic | Referent | Referent | ||||
| Early | 0.93 | 0.87 to 1.00 | .04 | 0.71 | 0.64 to 0.80 | < .001 |
| Oncologist training | ||||||
| Non–United States | Referent | Referent | ||||
| United States | 0.92 | 0.85 to 0.99 | .02 | 1.26 | 1.12 to 1.42 | < .001 |
| Oncologist degree | ||||||
| DO | Referent | Referent | ||||
| MD | 1.43 | 1.18 to 1.73 | < .001 | 0.94 | 0.72 to 1.22 | .63 |
| Oncologist sex | ||||||
| Male | Referent | Referent | ||||
| Female | 1.10 | 1.01 to 1.20 | .04 | 0.79 | 0.68 to 0.93 | .005 |
| Oncologist year of graduation | ||||||
| 1990s | Referent | Referent | ||||
| 1980s | 1.06 | 0.96 to 1.18 | .24 | 0.97 | 0.82 to 1.16 | .75 |
| 1970s | 1.21 | 1.09 to 1.34 | < .001 | 1.06 | 0.89 to 1.27 | .49 |
| 1960s | 1.24 | 1.09 to 1.42 | .002 | 0.98 | 0.79 to 1.22 | .85 |
| Oncologist practice setting | ||||||
| Academic | Referent | Referent | ||||
| Private | 0.78 | 0.72 to 0.84 | < .001 | 0.94 | 0.82 to 1.08 | .40 |
| Patient volume | ||||||
| 1-9 | Referent | Referent | ||||
| > 10 | 0.78 | 0.72 to 0.85 | < .001 | 1.33 | 1.14 to 1.55 | < .001 |
NOTE. Models also adjusted for Surveillance, Epidemiology, and End Results site.
Abbreviations: DO, doctor of osteopathic medicine; ESA, erythropoiesis-stimulating agent; MD, doctor of medicine; OR, odds ratio.
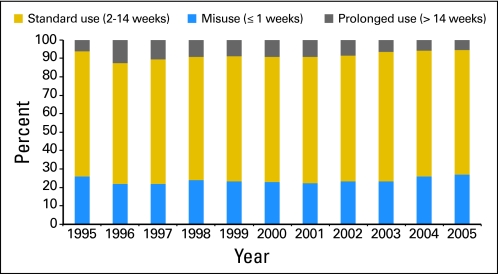
ESAs were administered for more than 14 weeks (prolonged use) in 1,601 patients (7.6%). Characteristics associated with prolonged use are listed in Table 1. In the adjusted model, year of diagnosis, treatment by a high-volume oncologist (OR, 1.33; 95% CI, 1.14 to 1.55), and treatment by a graduate of a US medical school (OR, 1.26; 95% CI, 1.12 to 1.42) significantly predicted prolonged-duration ESA use (Table 2). Female oncologists (OR, 0.79; 95% CI, 0.68 to 0.93) were less likely to prescribe prolonged ESAs, and patients with early-stage disease (OR, 0.71; 95% CI, 0.64 to 0.80) were less likely to receive prolonged ESAs. Figure 2 displays the temporal trends of misuse, standard use, and prolonged use of ESAs.
Fig 2.
Use, misuse, and prolonged use of erythropoiesis-stimulating agents stratified by year of diagnosis.
We then examined off-label use of ESAs (ESA use > 8 weeks after completion of chemotherapy; Table 3). Of the 21,091 patients included, 2,876 (13.6%) received off-label ESAs. Patient characteristics were strong predictors of off-label ESA use. Off-label use of ESAs was noted in 11.8% of patients age 65 to 69 years versus 15.6% of those age 80 years or older (adjusted OR, 1.40; 95% CI, 1.23 to 1.59). Likewise, black patients (OR, 1.42; 95% CI, 1.22 to 1.66) and patients with greater comorbidity (OR, 1.28; 95% CI, 1.05 to 1.55) were more likely to receive ESAs while off treatment. Physicians with high ESA claims volume (OR, 1.19; 95% CI, 1.07 to 1.33) were more likely to prescribe off-label ESAs.
Table 3.
Multivariable Analysis of Associations Between Clinical, Demographic, and Physician Characteristics and Off-Label ESA Use
| Characteristic | Off-Chemotherapy ESA Use |
P | |||
|---|---|---|---|---|---|
| No. of Patients | % | Multivariable OR | 95% CI | ||
| Total | 2,876 | 13.6 | |||
| Age at diagnosis, years | |||||
| 65-69 | 686 | 11.8 | Referent | ||
| 70-74 | 960 | 13.6 | 1.20 | 1.08 to 1.33 | < .001 |
| 75-79 | 777 | 14.6 | 1.30 | 1.16 to 1.45 | < .001 |
| > 80 | 453 | 15.6 | 1.40 | 1.23 to 1.59 | < .001 |
| Race | |||||
| White | 2,430 | 13.3 | Referent | ||
| Black | 269 | 16.9 | 1.42 | 1.22 to 1.66 | < .001 |
| Hispanic | 33 | 14.5 | 1.00 | 0.69 to 1.47 | .98 |
| Missing or other | 144 | 14.6 | 1.07 | 0.88 to 1.30 | .51 |
| Year of diagnosis | |||||
| 2005 | 369 | 11.4 | Referent | ||
| 2004 | 458 | 13.5 | 1.23 | 1.06 to 1.42 | .007 |
| 2003 | 557 | 16.3 | 1.49 | 1.29 to 1.72 | < .001 |
| 2002 | 44 | 13.7 | 1.23 | 1.05 to 1.43 | .009 |
| 2001 | 403 | 14.5 | 1.30 | 1.11 to 1.52 | < .001 |
| 2000 | 314 | 13.0 | 1.12 | 0.95 to 1.32 | .17 |
| 1999 | 107 | 12.3 | 1.16 | 0.92 to 1.47 | .22 |
| 1998 | 91 | 14.0 | 1.29 | 1.00 to 1.67 | .05 |
| 1997 | 74 | 13.4 | 1.22 | 0.92 to 1.60 | .16 |
| 1996 | 47 | 11.5 | 1.03 | 0.74 to 1.43 | .87 |
| 1995 | 45 | 12.6 | 1.16 | 0.83 to 1.62 | .40 |
| Residence | |||||
| Metropolitan | 2,682 | 13.7 | Referent | ||
| Nonmetropolitan | 194 | 12.4 | 1.17 | 0.97 to 1.41 | .10 |
| Marital status | |||||
| Married | 1,643 | 13.5 | Referent | ||
| Unmarried | 1,147 | 13.9 | 0.97 | 0.89 to 1.06 | .49 |
| Unknown | 1.20 | 0.94 to 1.52 | .15 | ||
| Socioeconomic status | |||||
| First (lowest) quartile | 325 | 14.7 | Referent | ||
| Second quintile | 484 | 12.9 | 1.17 | 0.97 to 1.42 | .10 |
| Third quintile | 618 | 13.5 | 1.25 | 1.02 to 1.52 | .03 |
| Fourth quintile | 652 | 13.5 | 1.25 | 1.02 to 1.52 | .03 |
| Fifth (highest) quartile | 797 | 14.0 | 1.26 | 1.04 to 1.54 | .02 |
| Comorbidity score | |||||
| 0 | 2,367 | 13.3 | Referent | ||
| 1 | 368 | 15.2 | 1.12 | 0.99 to 1.27 | .08 |
| > 1 | 141 | 16.7 | 1.28 | 1.05 to 1.55 | .01 |
| Tumor site | |||||
| Breast | 946 | 14.8 | Referent | ||
| Colon | 661 | 14.2 | 0.84 | 0.75 to 0.95 | .005 |
| Lung | 1,269 | 12.7 | 0.83 | 0.75 to 0.93 | < .001 |
| Tumor grade | |||||
| High | 1,173 | 14.8 | Referent | ||
| Low | 969 | 12.6 | 0.84 | 0.76 to 0.93 | < .001 |
| Unknown | 0.93 | 0.83 to 1.05 | .24 | ||
| Treatment | |||||
| Recurrent/metastatic | 1,569 | 14.2 | Referent | ||
| Early | 1,307 | 13.1 | 0.87 | 0.80 to 0.95 | .001 |
| Oncologist training | |||||
| Non–United States | 903 | 13.6 | Referent | ||
| United States | 1,973 | 13.6 | 0.96 | 0.88 to 1.06 | .42 |
| Oncologist degree | |||||
| DO | 119 | 14.8 | Referent | ||
| MD | 2,757 | 13.6 | 0.85 | 0.69 to 1.05 | .13 |
| Oncologist sex | |||||
| Male | 2,389 | 13.4 | Referent | ||
| Female | 487 | 14.9 | 1.11 | 0.99 to 1.24 | .06 |
| Oncologist year of graduation | |||||
| 1990s | 449 | 14.9 | Referent | ||
| 1980s | 1,014 | 13.3 | 0.92 | 0.81 to 1.04 | .17 |
| 1970s | 1,066 | 13.5 | 0.95 | 0.84 to 1.08 | .40 |
| 1960s | 347 | 14.0 | 0.99 | 0.84 to 1.16 | .91 |
| Oncologist practice setting | |||||
| Academic | 624 | 14.1 | Referent | ||
| Private | 2,252 | 13.5 | 0.94 | 0.85 to 1.04 | .25 |
| Patient volume | |||||
| 1-9 | 482 | 12.5 | Referent | ||
| > 10 | 2,394 | 13.9 | 1.19 | 1.07 to 1.33 | .002 |
NOTE. Model also adjusted for Surveillance, Epidemiology, and End Results site.
Abbreviations: DO, doctor of osteopathic medicine; ESA, erythropoiesis-stimulating agent; MD, doctor of medicine; OR, odds ratio.
Total (lifetime) ESA use for the cohort was then examined. A total of 4,432 patients (21.0%) received more than 12 weeks of ESA treatment, whereas 1,389 (7.1%) received ESAs for longer than 24 weeks. Year of diagnosis was a strong predictor of use for longer than 12 and longer than 24 weeks (Table 4). Patients in nonmetropolitan areas (OR, 0.69; 95% CI, 0.51 to 0.92), those with lung cancer (OR, 0.71; 95% CI, 0.61 to 0.83), and those with early-stage disease (OR, 0.34; 95% CI, 0.30 to 0.39) were less likely to receive ESAs for either more than 12 or more than 24 weeks. Patients treated by private practice physicians (OR, 1.18; 95% CI, 1.02 to 1.38) as well as those treated by high-volume providers (OR, 1.58; 95% CI, 1.33 to 1.87) were more likely to have had more than 24 weeks of treatment. Sensitivity analysis with removal of nonsignificant variables from the models did not result in any significant changes in associations between variables of interest and patterns of ESA use.
Table 4.
Multivariable Analysis of Factors Associated With Total ESA Use of > 12 Weeks and > 24 Weeks
| Factor | > 12 Weeks |
> 24 Weeks |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Total | ||||||
| No. | 4,432 | 1,389 | ||||
| % | 21.0 | 7.1 | ||||
| Age at diagnosis, years | ||||||
| 65-69 | Referent | Referent | ||||
| 70-74 | 1.07 | 0.98 to 1.17 | .14 | 1.05 | 0.91 to 1.21 | .53 |
| 75-79 | 1.01 | 0.92 to 1.11 | .89 | 1.10 | 0.94 to 1.28 | .24 |
| > 80 | 0.99 | 0.88 to 1.11 | .87 | 1.00 | 0.83 to 1.21 | .99 |
| Race | ||||||
| White | Referent | Referent | ||||
| Black | 1.23 | 1.08 to 1.41 | .002 | 1.22 | 0.98 to 1.51 | .08 |
| Hispanic | 1.31 | 0.96 to 1.79 | .09 | 1.67 | 1.07 to 2.62 | .03 |
| Missing or other | 1.09 | 0.91 to 1.30 | .35 | 0.75 | 0.54 to 1.04 | .09 |
| Year of diagnosis | ||||||
| 2005 | Referent | Referent | ||||
| 2004 | 1.08 | 0.94 to 1.23 | .29 | 1.38 | 1.06 to 1.79 | .02 |
| 2003 | 1.47 | 1.29 to 1.67 | < .001 | 2.08 | 1.63 to 2.65 | < .001 |
| 2002 | 1.50 | 1.31 to 1.71 | < .001 | 2.26 | 1.77 to 2.89 | < .001 |
| 2001 | 1.71 | 1.50 to 1.95 | < .001 | 2.49 | 1.95 to 3.18 | < .001 |
| 2000 | 1.68 | 1.47 to 1.93 | < .001 | 2.31 | 1.80 to 2.97 | < .001 |
| 1999 | 1.62 | 1.34 to 1.96 | < .001 | 2.20 | 1.58 to 3.05 | < .001 |
| 1998 | 1.54 | 1.24 to 1.90 | < .001 | 2.28 | 1.61 to 3.22 | < .001 |
| 1997 | 1.72 | 1.38 to 2.14 | < .001 | 2.69 | 1.90 to 3.81 | < .001 |
| 1996 | 1.76 | 1.38 to 2.25 | < .001 | 2.31 | 1.55 to 3.43 | < .001 |
| 1995 | 1.46 | 1.11 to 1.91 | .006 | 2.52 | 1.67 to 3.80 | < .001 |
| Residence | ||||||
| Metropolitan | Referent | Referent | ||||
| Nonmetropolitan | 0.84 | 0.72 to 0.99 | .04 | 0.69 | 0.51 to 0.92 | .01 |
| Marital status | ||||||
| Married | Referent | Referent | ||||
| Unmarried | 0.95 | 0.88 to 1.02 | .16 | 0.92 | 0.82 to 1.04 | .17 |
| Unknown | 0.96 | 0.78 to 1.18 | .70 | 0.87 | 0.61 to 1.24 | .44 |
| Socioeconomic status | ||||||
| First (lowest) quartile | Referent | Referent | ||||
| Second quintile | 1.02 | 0.87 to 1.19 | .85 | 1.23 | 0.94 to 1.62 | .13 |
| Third quintile | 0.99 | 0.84 to 1.17 | .92 | 1.17 | 0.89 to 1.55 | .27 |
| Fourth quintile | 1.00 | 0.85 to 1.18 | .97 | 1.25 | 0.94 to 1.66 | .12 |
| Fifth (highest) quartile | 0.99 | 0.84 to 1.16 | .89 | 1.17 | 0.88 to 1.56 | .28 |
| Comorbidity score | ||||||
| 0 | Referent | Referent | ||||
| 1 | 1.09 | 0.98 to 1.22 | .13 | 1.11 | 0.93 to 1.32 | .27 |
| > 1 | 0.94 | 0.79 to 1.13 | .51 | 1.03 | 0.77 to 1.36 | .87 |
| Tumor site | ||||||
| Breast | Referent | Referent | ||||
| Colon | 1.04 | 0.94 to 1.15 | .43 | 0.91 | 0.77 to 1.07 | .26 |
| Lung | 0.88 | 0.80 to 0.96 | .006 | 0.71 | 0.61 to 0.83 | < .001 |
| Tumor grade | ||||||
| High | Referent | Referent | ||||
| Low | 0.97 | 0.90 to 1.06 | .53 | 0.92 | 0.80 to 1.05 | .22 |
| Unknown | 1.13 | 1.03 to 1.25 | .01 | 1.04 | 0.89 to 1.22 | .60 |
| Treatment | ||||||
| Recurrent/metastatic | Referent | Referent | ||||
| Early | 0.54 | 0.50 to 0.58 | < .001 | 0.34 | 0.30 to 0.39 | < .001 |
| Oncologist training | ||||||
| Non–United States | Referent | Referent | ||||
| United States | 0.81 | 0.75 to 0.88 | < .001 | 0.74* | 0.65 to 0.83 | < .001 |
| Oncologist degree | ||||||
| DO | Referent | Referent | ||||
| MD | 0.95 | 0.79 to 1.13 | .54 | 0.84 | 0.63 to 1.13 | .25 |
| Oncologist sex | ||||||
| Male | Referent | Referent | ||||
| Female | 0.84 | 0.76 to 0.93 | < .001 | 0.80 | 0.68 to 0.95 | .01 |
| Oncologist year of graduation | ||||||
| 1990s | Referent | Referent | ||||
| 1980s | 0.99 | 0.89 to 1.11 | .92 | 0.92 | 0.76 to 1.12 | .42 |
| 1970s | 1.00 | 0.90 to 1.12 | .99 | 1.07 | 0.89 to 1.30 | .47 |
| 1960s | 1.05 | 0.92 to 1.21 | .47 | 1.07 | 0.85 to 1.34 | .59 |
| Oncologist practice setting | ||||||
| Academic | Referent | Referent | ||||
| Private | 1.11 | 1.01 to 1.21 | .03 | 1.18 | 1.02 to 1.38 | .03 |
| Patient volume | ||||||
| 1-9 | Referent | Referent | ||||
| > 10 | 1.48 | 1.35 to 1.63 | < .001 | 1.58 | 1.33 to 1.87 | < .001 |
NOTE. Models also adjusted for Surveillance, Epidemiology, and End Results site.
Abbreviations: DO, doctor of osteopathic medicine; ESA, erythropoiesis-stimulating agent; MD, doctor of medicine; OR, odds ratio.
P value < .05.
DISCUSSION
Our findings suggest that variability in use of ESAs is widespread. Nearly 25% of the patients in our cohort received 1 week or less of treatment, a dose that would provide negligible if any clinical effect. Likewise, we noted that 8% of patients received prolonged continuous ESA treatment for more than 14 weeks, and nearly 14% of the cohort continued to receive ESA treatment for more than 2 months after completion of chemotherapy. In addition to subjecting patients to toxicity, these patterns of use impose a significant financial burden to the health care system. Our findings raise concern in that actual use of ESAs deviates significantly from clinical trials and FDA labeling.
The off-label use of drugs, particularly in oncology, is common.3–5 One investigation noted that 38% of patients treated for bladder cancer in 2002 received an off-label agent, and 58% of men with hormone-refractory prostate cancer received an off-label drug.4 Off-label use is particularly prevalent for new drugs entering the market. In the early 2000s, 22% of Australian women with metastatic breast cancer received off-label trastuzumab, and 75% of patients treated in the late 1990s with rituximab received the drug off label.21,22 Inappropriate drug use also seems to be a problem for supportive care measures.23,24 In a survey of the ASCO membership, Bennett et al23 noted that many oncologists were using colony-stimulating factors in scenarios and dosing schedules that evidence and guidelines did not support.
Prior data examining the patterns of ESA use have predominantly focused on compliance with recommendations for target hemoglobin levels.8,25–30 A majority of these studies have found fair to moderate compliance with recommended hemoglobin targets.8,26–30 An evaluation of patients in the United States treated with ESAs between 2002 and 2006 noted that 24% of patients who received ESAs had hemoglobin levels greater than 12 gm/dL.29 We focused our analysis on documenting use patterns that were clearly inappropriate and of questionable clinical utility. We noted that relatively large proportions of patients received either ultra-short courses of ESAs or prolonged-duration ESA therapy. An additional 14% of our cohort continued to receive ESA treatment well after completion of chemotherapy, treatment clearly at odds with current ESA labeling.
Although patient and tumor factors influence treatment decisions, it is becoming increasingly clear that physician characteristics are also important determinants of care.31,32 In our analysis, physician characteristics including medical school training and physician sex, volume, and practice setting all affected use of ESAs. A prior study of patterns of erythropoietin use noted that practice setting was the most important predictor of ESA use; those physicians in fee-for-service settings were more than twice as likely to use ESAs frequently.25 We noted that oncologists in private practice settings were less likely to prescribe ESAs for 1 week or less and 20% more likely to administer ESAs for more than 6 months continuously.
Association between physician characteristics and ESA use is likely caused by a multitude of factors. Patients seen by private practice physicians are more likely to have commercial insurance, higher SES, and fewer medical comorbidities. Any or all of these factors may have influenced the prescribing patterns we noted. We also found a strong association between high practice volume and prolonged and off-chemotherapy ESA use. Finally, it also seems likely that economic considerations play a role in the allocation of ESAs. In the survey of oncologists reported by Adams et al,25 37% of US physicians reported that financial considerations affected their decision to use ESAs. In addition, physicians in fee-for-service settings were more likely to withhold ESAs because of reimbursement considerations. The current system in which private practice physicians purchase ESAs and generate profit from their administration has raised concerns regarding conflicts of interest.
Safety matters aside, misuse of ESAs is of concern, because ESAs represent a major source of drug-associated health care expenditures. It is estimated that Medicare expenditures for ESAs are more than $1 billion annually.9 A recent study that modeled conservative use of ESAs reported that they were not cost effective, noting that the incremental cost per quality-adjusted life year gained with ESA treatment was $267,000.33,34 Given the widespread misuse of ESAs that we found, the financial consequences surrounding ESA use are even greater in real-world practice.
Our findings of widespread variability in use of ESAs are somewhat surprising. Previous work examining guideline compliance by physicians has yielded mixed results.24,35,36 In an effort to facilitate guideline compliance, ESA reimbursement has been limited in the United States, and the FDA has established a risk evaluation and mitigation strategy program to improve evidence-based use.8,10 More work will be needed to monitor the efficacy of these efforts. Although ESAs are the only drugs currently being regulated in this way, our findings raise the question of whether other drugs should be more tightly regulated from the onset.
We acknowledge several important limitations of our study and of the SEER-Medicare database in general.37 It is possible that not all patients who received ESAs were captured with Medicare claims. However, because of the substantial expense associated with ESAs, we believe that this would have occurred relatively infrequently. Patterns of ESA use may differ among younger patients and those with commercial insurance. The SEER-Medicare database lacks data on hemoglobin levels. As such, we could not calculate the number of patients receiving ESAs who had high hemoglobin levels. We used the overall number of patients treated with ESAs as a surrogate for physician prescribing volume. We recognize that this may not be representative of a physician's entire practice. As with any analysis of administrative data, it is impossible to determine individual patient and physician preferences that may have influenced patterns of use. Finally, given the widespread recognition of the safety concerns of ESAs, patterns of use have likely shifted in the last 5 years. More studies are clearly warranted to examine the influence of new regulations on ESA use.
Our study demonstrates widespread variability in use of ESAs in the United States. Short treatment duration providing little clinical efficacy and prolonged use were common. We noted that even after completing chemotherapy, a substantial number of patients continued to receive ESAs. Although patient-related factors affected patterns of ESA use, physician characteristics exerted substantial influence on the way ESAs were administered. Recent regulatory changes as well as limitations on reimbursement may drive more rational ESA use; however, further interventions to encourage guideline-based use of ESAs and other cancer-related drugs are needed.
Acknowledgment
We thank the Applied Research Branch, Division of Cancer Prevention and Population Science, National Cancer Institute; the Office of Information Services and Office of Strategic Planning, Health Care Financing Administration; Information Management Services; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries for their efforts in the creation of the SEER-Medicare database.
Appendix
Table A1.
Cohort Selection by Type of Cancer
| Characteristic | Breast | Colon | Lung |
|---|---|---|---|
| Histology codes | 800, 801, 802, 805, 814, 821, 823, 826, 848-854, 856, 857 | 800.0, 800.1, 802, 814, 822, 823, 848, 857 | 800-805, 807, 812, 814, 825, 826, 831, 832, 843, 848, 849, 851, 856, 857, 898 |
| Total cohort | |||
| Patients with first primary cancer diagnosed between 1991 and 2005 | 492,795 | 418,286 | 418,065 |
| Clinical selection | 187,457 | 137,700 | 201,286 |
| Diagnosis not based on autopsy | |||
| Age ≥ 65 years | |||
| Medicare enrollment because of age | |||
| Date of death between SEER registry and Medicare enrollment ≤ 3 months | |||
| Demographic selection | 121,308 | 90,049 | 119,157 |
| No HMO enrollment in year before diagnosis to 1 year after diagnosis | |||
| Enrolled in Medicare parts A and B before and after diagnosis | |||
| Selected histologies | 119,209 | 71,705 | 100,995 |
| Stages I to IV | 95,329 | 65,154 | 86,369 |
| Patients undergoing dialysis deleted | 92,353 | 62,508 | 83,268 |
| Early-stage disease | |||
| Stages I to III with ESA claim | 7,340 | 3,904 | 6,583 |
| Removal of patients with ESA claim before cancer diagnosis | 7,248 | 3,871 | 6,486 |
| Patients with ESA claim and physician listed in AMA file | 7,137 | 3,706 | 6,393 |
| Physician with oncology specialty | 6,271 | 3,182 | 5,732 |
| Total with removal of patients who received ESAs before 1994 and those with missing SES | 14,763 | ||
| Advanced and recurrent disease | |||
| Stage I to III recurrent and stage IV with ESA claim | 3,493 | 3,477 | 7,357 |
| Patients with ESA claim and physician listed in AMA file | 3,443 | 3,435 | 7,243 |
| Physician with oncology specialty | 3,088 | 3,058 | 6,615 |
| Total with removal of patients who received ESAs before 1994 and those with missing SES | 12,003 | ||
| All patients (early and late) | 26,766 | ||
| Removal of duplicate patients in early-stage group who developed metastatic disease and were moved to late group | 24,112 | ||
| Selection of patients who received some or all ESAs with chemotherapy | 21,091 | ||
| Final cohort | 21,091 |
Abbreviations: AMA, American Medical Association; ESA, erythropoiesis-stimulating agent; HMO, health maintenance organization; SEER, Surveillance, Epidemiology, and End Results; SES, socioeconomic status.
Footnotes
Supported in part by Grant No. R01CA134964 from the National Cancer Institute (D.L.H.).
This study used the linked Surveillance, Epidemiology, and End Results–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Jennifer Malin, Amgen (C) Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Jason D. Wright, Alfred I. Neugut, Dawn L. Hershman
Financial support: Dawn L. Hershman
Administrative support: Dawn L. Hershman
Provision of study materials or patients: Dawn L. Hershman
Collection and assembly of data: Dawn L. Hershman
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Henry DH, Brooks BJ, Jr, Case DC, Jr, et al. Recombinant human erythropoietin therapy for anemic cancer patients receiving cisplatin chemotherapy. Cancer J Sci Am. 1995;1:252–260. [PubMed] [Google Scholar]
- 2.Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94:1211–1220. doi: 10.1093/jnci/94.16.1211. [DOI] [PubMed] [Google Scholar]
- 3.Leveque D. Off-label use of anticancer drugs. Lancet Oncol. 2008;9:1102–1107. doi: 10.1016/S1470-2045(08)70280-8. [DOI] [PubMed] [Google Scholar]
- 4.Levêque D, Michallat AC, Schaller C, et al. Off label drug use in adult patients treated by anticancer chemotherapy [in French] Bull Cancer. 2005;92:498–500. [PubMed] [Google Scholar]
- 5.Mullins CD, Montgomery R, Tunis S. Uncertainty in assessing value of oncology treatments. Oncologist. 2010;15(suppl 1):58–64. doi: 10.1634/theoncologist.2010-S1-58. [DOI] [PubMed] [Google Scholar]
- 6.Bohlius J, Schmidlin K, Brillant C, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: A meta-analysis of randomised trials. Lancet. 2009;373:1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- 7.Bohlius J, Schmidlin K, Brillant C, et al. Erythropoietin or darbepoetin for patients with cancer: Meta-analysis based on individual patient data. Cochrane Database Syst Rev. 2009;3:CD007303. doi: 10.1002/14651858.CD007303.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett CL, McKoy JM, Henke M, et al. Reassessments of ESAs for cancer treatment in the US and Europe. Oncology (Williston Park) 2010;24:260–268. [PubMed] [Google Scholar]
- 9.Cotter DJ. Reevaluating erythropoiesis-stimulating agents. N Engl J Med. 2010;362:1743. author reply, 1743–1744. [PubMed] [Google Scholar]
- 10.Mitka M. New oversight put in place for physicians giving anemia drugs to patients with cancer. JAMA. 2010;303:1355–1356. doi: 10.1001/jama.2010.359. [DOI] [PubMed] [Google Scholar]
- 11.Bokemeyer C, Aapro MS, Courdi A, et al. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer. Eur J Cancer. 2004;40:2201–2216. doi: 10.1016/j.ejca.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Bokemeyer C, Aapro MS, Courdi A, et al. EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer. 2007;43:258–270. doi: 10.1016/j.ejca.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Rizzo JD, Brouwers M, Hurley P, et al. American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol. 2010;28:4996–5010. doi: 10.1200/JCO.2010.29.2201. [DOI] [PubMed] [Google Scholar]
- 14.Rizzo JD, Lichtin AE, Woolf SH, et al. Use of epoetin in patients with cancer: Evidence-based clinical practice guidelines of the American Society of Clinical Oncology and the American Society of Hematology. J Clin Oncol. 2002;20:4083–4107. doi: 10.1200/JCO.2002.07.177. [DOI] [PubMed] [Google Scholar]
- 15.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) http://www.cdc.gov/nchs/icd/icd9cm.htm.
- 17.Hershman DL, Buono DL, Malin J, et al. Patterns of use and risks associated with erythropoiesis-stimulating agents among Medicare patients with cancer. J Natl Cancer Inst. 2009;101:1633–1641. doi: 10.1093/jnci/djp387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110:660–669. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Sax FL, MacKenzie CR, et al. Assessing illness severity: Does clinical judgment work? J Chronic Dis. 1986;39:439–452. doi: 10.1016/0021-9681(86)90111-6. [DOI] [PubMed] [Google Scholar]
- 20.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 21.Kocs D, Fendrick AM. Effect of off-label use of oncology drugs on pharmaceutical costs: The rituximab experience. Am J Manag Care. 2003;9:393–400. quiz, 401-402. [PubMed] [Google Scholar]
- 22.Pearson SA, Ringland CL, Ward RL. Trastuzumab and metastatic breast cancer: Trastuzumab use in Australia—Monitoring the effect of an expensive medicine access program. J Clin Oncol. 2007;25:3688–3693. doi: 10.1200/JCO.2007.11.2516. [DOI] [PubMed] [Google Scholar]
- 23.Bennett CL, Weeks JA, Somerfield MR, et al. Use of hematopoietic colony-stimulating factors: Comparison of the 1994 and 1997 American Society of Clinical Oncology surveys regarding ASCO clinical practice guidelines—Health Services Research Committee of the American Society of Clinical Oncology. J Clin Oncol. 1999;17:3676–3681. doi: 10.1200/JCO.1999.17.11.3676. [DOI] [PubMed] [Google Scholar]
- 24.Ramsey SD, McCune JS, Blough DK, et al. Colony-stimulating factor prescribing patterns in patients receiving chemotherapy for cancer. Am J Manag Care. 2010;16:678–686. [PubMed] [Google Scholar]
- 25.Adams JR, Elting LS, Lyman GH, et al. Use of erythropoietin in cancer patients: Assessment of oncologists' practice patterns in the United States and other countries. Am J Med. 2004;116:28–34. doi: 10.1016/j.amjmed.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Hess G, Nordyke RJ, Hill J, et al. Effect of reimbursement changes on erythropoiesis-stimulating agent utilization and transfusions. Am J Hematol. 2010;85:838–843. doi: 10.1002/ajh.21837. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig H, Aapro M, Bokemeyer C, et al. Treatment patterns and outcomes in the management of anaemia in cancer patients in Europe: Findings from the Anaemia Cancer Treatment (ACT) study. Eur J Cancer. 2009;45:1603–1615. doi: 10.1016/j.ejca.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Luo W, Nordstrom BL, Fraeman K, et al. Adherence to guidelines for use of erythropoiesis-stimulating agents in patients with chemotherapy-induced anemia: Results of a retrospective study of an electronic medical-records database in the United States, 2002-2006. Clin Ther. 2008;30:2423–2435. doi: 10.1016/j.clinthera.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 29.Nordstrom BL, Luo W, Fraeman K, et al. Use of erythropoiesis-stimulating agents among chemotherapy patients with hemoglobin exceeding 12 grams per deciliter. J Manag Care Pharm. 2008;14:858–869. doi: 10.18553/jmcp.2008.14.9.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinmetz T, Totzke U, Söling U, et al. Hemoglobin levels that trigger erythropoiesis-stimulating agent treatment decisions for cancer-associated anemia: Examination of practice in Germany. Curr Med Res Opin. 2008;24:2751–2756. doi: 10.1185/03007990802377057. [DOI] [PubMed] [Google Scholar]
- 31.Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 32.Hershman DL, Buono D, McBride RB, et al. Influence of private practice setting and physician characteristics on the use of breast cancer adjuvant chemotherapy for elderly women. Cancer. 2009;115:3848–3857. doi: 10.1002/cncr.24448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klarenbach S, Manns B, Reiman T, et al. Economic evaluation of erythropoiesis-stimulating agents for anemia related to cancer. Cancer. 2010;116:3224–3232. doi: 10.1002/cncr.25052. [DOI] [PubMed] [Google Scholar]
- 34.Sheffield R, Sullivan SD, Saltiel E, et al. Cost comparison of recombinant human erythropoietin and blood transfusion in cancer chemotherapy-induced anemia. Ann Pharmacother. 1997;31:15–22. doi: 10.1177/106002809703100101. [DOI] [PubMed] [Google Scholar]
- 35.Graham ID, Evans WK, Logan D, et al. Canadian oncologists and clinical practice guidelines: A national survey of attitudes and reported use—Provincial Lung Disease Site Group of Cancer Care Ontario. Oncology. 2000;59:283–290. doi: 10.1159/000012184. [DOI] [PubMed] [Google Scholar]
- 36.Malin JL, Schneider EC, Epstein AM, et al. Results of the National Initiative for Cancer Care Quality: How can we improve the quality of cancer care in the United States? J Clin Oncol. 2006;24:626–634. doi: 10.1200/JCO.2005.03.3365. [DOI] [PubMed] [Google Scholar]
- 37.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(suppl 8):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]