Abstract
Purpose
Multiple myeloma (MM) is an incurable plasma-cell neoplasm for which most treatments involve a therapeutic agent combined with dexamethasone. The preclinical combination of lenalidomide with the mTOR inhibitor CCI-779 has displayed synergy in vitro and represents a novel combination in MM.
Patients and Methods
A phase I clinical trial was initiated for patients with relapsed myeloma with administration of oral lenalidomide on days 1 to 21 and CCI-779 intravenously once per week during a 28-day cycle. Pharmacokinetic data for both agents were obtained, and in vitro transport and uptake studies were conducted to evaluate potential drug-drug interactions.
Results
Twenty-one patients were treated with 15 to 25 mg lenalidomide and 15 to 20 mg CCI-779. The maximum-tolerated dose (MTD) was determined to be 25 mg lenalidomide with 15 mg CCI-779. Pharmacokinetic analysis indicated increased doses of CCI-779 resulted in statistically significant changes in clearance, maximum concentrations, and areas under the concentration-time curves, with constant doses of lenalidomide. Similar and significant changes for CCI-779 pharmacokinetics were also observed with increased lenalidomide doses. Detailed mechanistic interrogation of this pharmacokinetic interaction demonstrated that lenalidomide was an ABCB1 (P-glycoprotein [P-gp]) substrate.
Conclusion
The MTD of this combination regimen was 25 mg lenalidomide with 15 mg CCI-779, with toxicities of fatigue, neutropenia, and electrolyte wasting. Pharmacokinetic and clinical interactions between lenalidomide and CCI-779 seemed to occur, with in vitro data indicating lenalidomide was an ABCB1 (P-gp) substrate. To our knowledge, this is the first report of a clinically significant P-gp–based drug-drug interaction with lenalidomide.
INTRODUCTION
Treatment for resistant multiple myeloma (MM) is still palliative, justifying pursuit of novel therapeutic strategies. Cell-line and xenograft models have demonstrated that the phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (mTOR) pathway is important in MM1–4; therefore, clinical evaluation of mTOR inhibitors and their combination with standard treatment is warranted.
Akt and mTOR activation lead to accumulation of cyclin D1 and cell proliferation through phosphorylation of the ribosomal subunit p70S6k and eukaryotic initiation factor 4 binding protein 1 (4E-BP1).5,6 CCI-779 (temsirolimus), an ester prodrug of the macrocyclic immunosuppressive agent rapamycin (sirolimus), targets mTOR. CCI-779 has shown in vitro activity against MM cell lines, specifically those with PTEN mutations,7,8 and in vivo activity in xenograft models.9 A recent phase II study in 16 patients with MM treated with single-agent CCI-779 25 mg resulted in one partial and five minor responses using Bladé et al10 criteria and associated reduction in phosphorylated p70S6K and eukaryotic initiation factor 4 binding protein 1.
Lenalidomide is an immunomodulatory agent with tumoricidal and immunomodulatory activity and is a standard treatment in initial and relapsed MM.11–13 In vitro studies have demonstrated synergy between lenalidomide and rapamycin in inhibiting proliferation and inducing apoptosis in drug-resistant MM cell lines and primary patient cells.14 This provided the rationale for combining these agents in the treatment of MM.
In this study, we report safety, tolerability, clinical response, and pharmacokinetic data for the combination of lenalidomide and CCI-779 in patients with relapsed MM. We also present in vitro data that provide evidence for a lenalidomide–CCI-779 interaction involving P-glycoprotein (P-gp), relevant for lenalidomide combinations with other P-gp substrates or inducers.
PATIENTS AND METHODS
Clinical Study
A phase I clinical trial was initiated with lenalidomide and CCI-779 supplied by the National Cancer Institute Cancer Therapy Evaluation Program. The study protocol was approved by the Cancer Therapy Evaluation Program and institutional review board, and informed consent was obtained from all enrolled patients. Patients were required to have relapsed myeloma after at least one prior therapy (which could have contained CCI-779 or lenalidomide but not both), Eastern Cooperative Oncology Group performance status of 2 or greater, neutrophil count greater than 1.5 × 109/L, platelet count greater than 100 × 1012/L, creatinine less than 2 mg/dL, bilirubin less than 1.5× the upper limit of normal, AST/ALT less than 3× the upper limit of normal, fasting serum cholesterol less than 350 mg/dL, fasting serum triglycerides less than 400 mg/dL, and all of the following: immunofixation positive serum and/or urine or an abnormal serum free light chain ratio, clonal plasma cells on bone marrow aspirate or core biopsy, and any sign of related organ or tissue impairment attributable to myeloma. Patients with active infections and a history of recurrent venous thromboembolism (VTE) or VTE occurring while receiving therapeutic levels of anticoagulation were excluded. Because of the known association between hypertriglyceridemia and CCI-779, patients with fasting serum triglycerides greater than 200 mg/dL or nonfasting serum triglycerides greater than 350 mg/dL were treated with gemfibrozil 600 mg twice daily.
Trial Design and Treatment
This was a standard three-plus-three phase I study designed to establish the maximum tolerable dose (MTD) and evaluate toxicity for this treatment in patients with previously treated MM. The dose combination determined to be the MTD was expanded to 10 patients to better define efficacy and pharmacokinetics. Dose escalation of oral lenalidomide started at 20 mg on days 1 to 21 of a 28-day cycle. CCI-779 was administered intravenously over 30 minutes starting at 15 mg on days 1, 8, 15, and 22. Patients were evaluated for dose-limiting toxicities (DLTs) during cycle one, and the dose level at which fewer than two of six patients experienced DLTs was defined as the MTD. Appropriate VTE prophylaxis was required in all patients. Treatment proceeded until occurrence of disease progression after at least two cycles of lenalidomide and CCI-779 or occurrence of unacceptable adverse events (AEs) or DLTs.
Response Assessment
Response to treatment was assessed by serum and urine M-protein quantification every 4 weeks. Two consecutive assessments of response using International Myeloma Working Group criteria15 were required for confirmation.
AEs
The National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 was used to characterize toxicities. DLTs were defined as AEs related to lenalidomide or CCI-779 occurring within cycle one and fulfilling one of the following criteria: grade 4 hematologic toxicity (neutropenia or thrombocytopenia) lasting longer than 1 week; grade 3 or 4 nonhematologic toxicity (except alopecia, fatigue, and controllable nausea/vomiting or lipid abnormalities); intolerable grade 2 nonhematologic or grade 3 hematologic toxicities requiring dose reduction during the first 28 days of treatment; and AEs resulting in treatment delay longer than 1 week during the first 28 days of treatment.
Pharmacokinetics
Separate blood samples (6 mL) were collected into EDTA tubes for lenalidomide and CCI-779 at predose and various times up to 24 hours after concurrent lenalidomide ingestion and start of CCI-779 infusion on cycle one, day 1. Plasma samples were immediately separated and stored at −70°C until analysis using validated liquid chromatography/tandem mass spectrometry assays for lenalidomide16 and CCI-779 (Data Supplement). Noncompartmental and compartmental pharmacokinetic analyses and modeling were performed using WINNonlin (Scientific Consultant, Apex, NC) Professional v.5.2 (Pharsight, Mountain View, CA). Creatinine clearance (CLCr) was calculated as previously described.17
In Vitro Lenalidomide Transport and Uptake
Lenalidomide for in vitro studies was obtained by extraction from donated patient capsules, as previously reported.18 Control and human ABCB1-transfected MDCKII cells (MDCKII v MDCKII/P-gp) were kindly provided by Alfred Schinkel, PhD (the Netherlands Cancer Institute, Amsterdam, the Netherlands). HL-60 and P-gp–overexpressing HL-60/VCR cells were kindly provided by Kapil Bhalia, MD, to J.C.B. MDCKII and MDCKII/P-gp cells were grown on polycarbonate Transwell membranes (Corning Co, Corning, NY) and incubated for 1 hour with 10 μmol/L lenalidomide added to the donor compartment (either apical or basolateral), followed by quantification of lenalidomide in the receiver compartment using liquid chromatography–tandem mass spectrometry methods modified for the cell-culture matrix. For intracellular accumulation, HL-60 and HL-60/VCR cells were incubated with 3 μmol/L lenalidomide for 2 hours, followed by quantification of intracellular lenalidomide. To specifically inhibit P-gp expression, HL-60/VCR cells were transfected with siRNA for ABCB1 (siRNA 4123, 225 nmol/L; Applied Biosystems, Carlsbad, CA) and silencer negative control (siRNA 1, 225 nmol/L) using the Amaxa Cell Line Nucleofector Kit R (Lonza Group, Basel, Switzerland) according to manufacturer protocols. ABCB1 mRNA and P-gp protein levels were measured, and uptake of lenalidomide (3 μmol/L) was evaluated 48 hours after transfection. Details of the materials and methods used in these assays are provided in the Data Supplement.
Statistical Analysis
Descriptive summaries were provided for the primary end points of MTD and toxicity. Secondary end points of estimated pharmacokinetic parameters were analyzed as continuous measures, and associations between outcomes and groups were evaluated. When either group was small or variances were not homogeneous in the comparison groups, nonparametric Wilcoxon rank-sum (Mann-Whitney) or Kruskal-Wallis tests were used. Otherwise, ANOVA or two-sample t test were employed for comparisons of groups. Linear regression was used to explore the relationship between CLCr and observed apparent oral clearance (CL/F). Data are expressed as mean (with standard deviation [SD]).
RESULTS
Demographics and Determination of MTD
A total of 21 relapsed patients were treated per protocol, with baseline characteristics listed in the Data Supplement. The median number of prior therapies was three (range, one to six therapies). Prior therapies received at any time point included dexamethasone (100%), thalidomide (43%), bortezomib (48%), lenalidomide (19%), melphalan (66%), and autologous stem-cell transplantation (43%). Of the four patients who had previously been administered lenalidomide, only one had progressed while receiving it. Although a 4-week dexamethasone washout period was encouraged before therapy, three patients reported dexamethasone use within 1 month of treatment initiation.
Four dose levels were administered, ranging from 15 mg each of lenalidomide and CCI-779 to 25 mg lenalidomide with 20 mg CCI-779. At the maximum administered dose level, two of four patients suffered DLTs (pneumonia and thrombocytopenia with clinically significant urinary hemorrhage; Table 1). A total of 10 patients were treated at the MTD of 25 mg lenalidomide with 15 mg CCI-779 (Table 2).
Table 1.
Adverse Events for All Patients Who Received Any Protocol Treatment
| Toxicity | Grade* |
Patients (%) | |||
|---|---|---|---|---|---|
| 4 | 3 | 2 | 1 | ||
| Some grade 3 to 4 severity | |||||
| Fatigue | 0 | 1 | 16 | 2 | 90 |
| Neutropenia | 2 | 5 | 8 | 2 | 81 |
| Anemia | 0 | 2 | 9 | 6 | 81 |
| Hypophosphatemia | 3 | 9 | 3 | 1 | 76 |
| Anorexia | 0 | 2 | 11 | 2 | 71 |
| Lymphopenia | 1 | 4 | 9 | 1 | 71 |
| Thrombocytopenia | 0 | 2 | 6 | 6 | 67 |
| Hypokalemia | 0 | 6 | 0 | 7 | 62 |
| Hypertriglyceridemia | 2 | 0 | 0 | 10 | 57 |
| Infection | 0 | 4 | 6 | 0 | 48 |
| Hypercholesterolemia | 0 | 2 | 1 | 3 | 29 |
| Diarrhea | 0 | 1 | 4 | 0 | 24 |
| Hyperglycemia | 0 | 1 | 1 | 1 | 14 |
| Febrile neutropenia | 0 | 2 | 0 | 0 | 10 |
| Grade 1 to 2 | |||||
| Rash | 12 | 4 | 76 | ||
| Nausea | 8 | 5 | 62 | ||
| Taste alterations | 7 | 5 | 57 | ||
| Constipation | 7 | 2 | 43 | ||
| ALT/AST | 3 | 6 | 43 | ||
| Depression | 2 | 0 | 10 | ||
| Insomnia | 1 | 1 | 10 | ||
| Dizziness | 0 | 1 | 5 | ||
NOTE. Adverse events with attribution of possible, likely, or definite are listed. Maximum grade for each toxicity per patient (n = 21) was recorded; each toxicity was recorded only once per patient, regardless of No. of recurrences. Infection events were two pneumonias, four upper respiratory infections, one sinusitis, one cellulitus, one urinary tract infection, and one oral herpes outbreak.
According to National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
Table 2.
Mean Pharmacokinetic Parameters of Lenalidomide and CCI-779 by Dose Level
| Dose Level (mg) | No. of Patients | AUClast(hr × μM)* |
CL/F (L/hr)* |
K01 (1/hr)† |
K10(1/hr)† |
K10_HL (hr)† |
Cmax (μM)† |
Tmax (hr)† |
V/F (L)† |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | Range | Mean | SD | Mean | SD | Mean | SD | ||
| Lenalidomide | |||||||||||||||||
| 15/15 | 1 | 5.08 | 10.8 | 1.76 | 0.23 | 0.39 | 0.75 | 1.33 | 56.8 | ||||||||
| 20/15 | 6 | 4.44 | 2.58 | 22.3 | 12.3 | 0.76 | 0.96 | 0.26 | 0.09 | 3.01 | 1.90-5.62 | 0.52 | 0.20 | 3.07 | 1.37 | 80.5 | 33.2 |
| 25/15 | 9 | 5.34 | 1.29 | 18.4 | 4.69 | 0.95 | 1.00 | 0.23 | 0.05 | 3.11 | 2.28-4.24 | 0.66 | 0.23 | 3.04 | 1.75 | 80.4 | 30.1 |
| 25/20 | 4 | 7.35 | 1.78 | 13.5 | 3.99 | 0.21 | 0.06 | 0.20 | 0.06 | 3.61 | 2.47-4.89 | 0.83 | 0.36 | 5.15 | 1.38 | 49.7 | 22.4 |
| No. of Patients | AUClast(hr × nM)* |
CL (L/hr)* |
K10_HL (hr)‡ |
Alpha(1/hr)‡ |
Beta (1/hr)‡ |
Alpha_HL (hr)‡ |
Beta_HL (hr)‡ |
A (nM)‡ |
B (nM)‡ |
Cmax (nM)‡ |
Vss (L)‡ |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | Range | Mean | SD | Mean | SD | Mean | Range | Mean | Range | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| CCI-779 | |||||||||||||||||||||||
| 15/15 | 1 | 81.5 | 176 | 0.26 | 3.32 | 0.09 | 0.21 | 7.70 | 156 | 1.17 | 157 | 632 | |||||||||||
| 20/15 | 6 | 103 | 59.7 | 198 | 131 | 0.49 | 0.36-0.75 | 2.29 | 0.29 | 0.06 | 0.03 | 0.31 | 0.26-0.36 | 18.1 | 7.68-49.3 | 126 | 94.4 | 1.31 | 0.83 | 127 | 95.2 | 2,560 | 2,867 |
| 25/15 | 10 | 217 | 105.5 | 83.9 | 34.1 | 0.37 | 0.20-0.47 | 2.58 | 0.64 | 0.11 | 0.06 | 0.28 | 0.18-0.38 | 7.25 | 2.78-10.1 | 321 | 149 | 4.08 | 3.33 | 325 | 151 | 322 | 230 |
| 25/20 | 4 | 165 | 81.4 | 116 | 43.1 | 0.35 | 0.24-0.52 | 2.47 | 0.52 | 0.16 | 0.06 | 0.29 | 0.23-0.38 | 4.84 | 2.85-7.12 | 280 | 147 | 2.71 | 1.61 | 283 | 148 | 257 | 281 |
Abbreviations: AUC, area under the concentration-time curve; Cmax, maximum concentration; CL, clearance (for lenalidomide, CL is clearance divided by bioavailability or CL/F); HL, half-life; SD, standard deviation; Tmax, the time of maximal plasma concentration; V/F, apparent volume of distribution; Vss, the steady-state volume of distribution.
Noncompartmental analysis.
One-compartmental analysis.
Two-compartmental analysis.
AEs
AEs attributed to protocol therapy (possible, probable, definite) are listed in Table 1. The most frequent AE was fatigue, primarily grade 2. Grade 3 or 4 neutropenia and anemia occurred in 33% and 10% of patients, respectively. Hypophosphatemia and hypokalemia were frequent, occurring in 76% and 62% of the study population, respectively (grade 3 or 4 in 57% and 29% of patients, respectively), as was rash in 76% of patients. Thirteen severe AEs were reported. Five were related to infection, and two were associated with grade 3 or 4 neutropenia (one febrile). Three severe AEs were associated with grade 3 hypokalemia and grade 4 hypophosphatemia. Reasons for discontinuation of protocol therapy included disease progression (six patients), alternate therapy such as autologous peripheral blood stem-cell transplantation (two patients), prolonged hematologic toxicities (four patients, all at or above MTD), intolerable symptoms (six patients, three at or above the MTD), and orthopedic surgery (one patient). Among those citing intolerable toxicities, GI AEs of anorexia (71%; grade 3 or 4 in 10%), nausea (62%), and taste alterations (57%) were common.
Response
Two patients met criteria for partial response (both treated with 25 mg lenalidomide), and 15 patients had stable disease. Six patients (29%) had marginal response by Bladé et al10 criteria. Details of monoclonal protein response are shown in the Data Supplement. Three patients did not complete a full cycle and were not evaluable for response. One additional patient did not meet criteria for measurable disease by International Myeloma Working Group criteria.
Pharmacokinetics
Totals of 180 and 168 plasma concentration observations from 20 and 21 patients were available for lenalidomide and CCI-779 pharmacokinetics, respectively. Plasma concentration-time profiles are presented in the Data Supplement. A summary of estimated pharmacokinetic parameters is presented in Table 2. The overall ranges of concentrations and pharmacokinetic parameter values for both lenalidomide and CCI-779 were comparable to previously reported data.19–24
Mean increases in lenalidomide area under the concentration-time curve (34%) and maximum concentration (Cmax; 37%) were observed when the dose was increased from 20 to 25 mg, and a visual trend was observed between lenalidomide CL/F and CLCr. No trends were observed for CCI-779 pharmacokinetic parameters with respect to dose. Summaries of these results are presented in the Data Supplement. To assess the relationship between pharmacokinetics and outcome, scatter plots were generated, and visual trends were further evaluated with analysis of variance. No significant associations were identified in this analysis.
Pharmacokinetic Interaction Between Lenalidomide and CCI-779
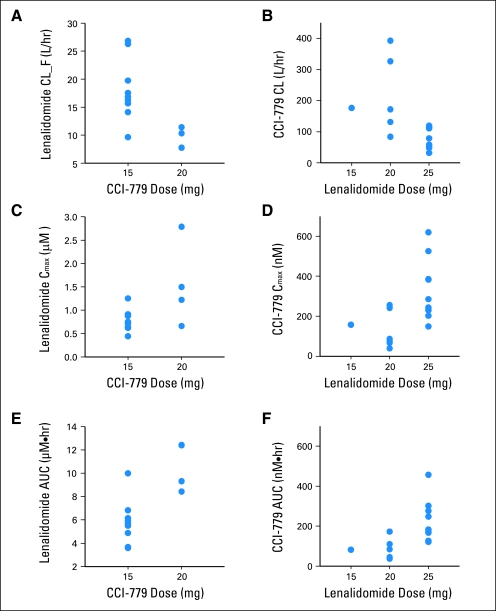
Pharmacokinetic interaction between lenalidomide and CCI-779 was evaluated by comparison of estimated pharmacokinetic parameters for fixed doses of each drug with respect to multiple doses of the other. Mean pharmacokinetic parameters (CL, CL/F, Cmax, and area under the concentration-time curve) for each drug were significantly different among the dose groups of the other drug. Between the 15-mg (nine patients) and 20-mg (four patients) dose groups of CCI-779, there were nearly two-fold differences in compartmental pharmacokinetic parameter estimates for lenalidomide (fixed dose of 25 mg). Similarly, significant differences were also observed for CCI-779 parameters at a fixed dose of 15 mg between the 20-mg (six patients) and 25-mg (10 patients) doses of lenalidomide. These comparisons are presented in Figure 1 and Table 3.
Fig 1.
Pharmacokinetic interaction of lenalidomide with CCI-779. Relationship between CCI-779 dose and lenalidomide (A) apparent oral clearance (CL/F), (C) maximum concentration (Cmax), and (E) area under the concentration-time curve (AUC) as well as lenalidomide dose and CCI-779 (B) CL, (D) Cmax, and (F) AUC.
Table 3.
Summary of Pharmacokinetic Interactions of Lenalidomide With CCI-779
| Dose (mg) | No. of Patients | Pharmacokinetic Parameters of Lenalidomide (25 mg) |
|||||
|---|---|---|---|---|---|---|---|
| CL/F (L/hr) |
Cmax (μM) |
AUC(μM × hr) |
|||||
| Mean | SD | Mean | SD | Mean | SD | ||
| CCI-779 | |||||||
| 15 | 9 | 18.1 | 5.53 | 0.79 | 0.23 | 5.79 | 1.91 |
| 20 | 4 | 9.30 | 1.85 | 1.54 | 0.90 | 10.6 | 2.1 |
| P | .01 | .03 | .002 | ||||
| No. of Patients | Pharmacokinetic Parameters of CCI-779 (15 mg) |
||||||
| CL (L/hr) |
Cmax (nM) |
AUC(nM × hr) |
|||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Lenalidomide | |||||||
| 15 | 1 | 176 | 157 | 82 | |||
| 20 | 6 | 198 | 131 | 127 | 95 | 103 | 60 |
| 25 | 10 | 84 | 34 | 325 | 151 | 217 | 106 |
| P | .03 | .02 | .02 | ||||
NOTE. P values determined by analysis of variance or Student's t test.
Abbreviations: AUC, area under the concentration-time curve; Cmax, maximum concentration; CL, clearance (for lenalidomide, CL is clearance divided by bioavailability or CL/F); SD, standard deviation.
In Vitro Lenalidomide Transcellular Transport and Intracellular Accumulation
Although not expected based on the published pharmacology of both agents, the pharmacokinetic data in this study suggested that CCI-779 and lenalidomide may interact. Given that lenalidomide is not metabolized,25 a direct interaction would likely involve drug transport. CCI-779 is a clinically relevant P-gp substrate,26–28and we therefore aimed to determine if the drugs interacted at this transporter site. Our in vitro results in MDCKII monolayers indicated an apical-directed transport of lenalidomide, which was significantly increased across P-gp–overexpressing MDCKII/P-gp monolayers. Figure 2A shows an approximately two-fold higher basolateral (BP) -to-apical (AP) flux, compared with AP-to-BL flux for lenalidomide in MDCKII monolayers (mean, 8.25 × 10−7 [SD, 1.99 × 10−8] v mean, 4.31 × 10−7 [SD, 1.53 × 10−7] cm/s, respectively; P < .05). This resulted in a net efflux ratio (ratio of AP/BL permeability) of 1.91, demonstrating an apically directed active transport of lenalidomide. In MDCKII/P-gp monolayers, the difference between BL to AP and AP to BL was increased (mean, 1.87 × 10−6 [SD, 1.99 × 10−7] v mean, 5.11 × 10−7 [SD, 5.36 × 10−8] cm/s, respectively; P < .001; efflux ratio, 3.66).
Fig 2.
Lenalidomide is a substrate of P-glycoprotein (P-gp). (A) Apparent permeability (Papp) of lenalidomide (10 μmol/L) across MDCKII and MDCKII/P-gp cell monolayers after 1-hour incubation; (B) intracellular accumulation of lenalidomide (3 μmol/L) in HL-60 and HL-60/VCR cells after 2-hour incubation. Error bars indicate standard deviation (n = 3). P values were calculated using two-sample t test. AP, apical; BL, basolateral.
Further evaluation of lenalidomide uptake in HL-60/VCR cells overexpressing P-gp was performed. After a 2-hour incubation with 3 μmol/L lenalidomide, intracellular drug accumulation was approximately two-fold higher in HL-60 compared with HL-60/VCR cells (mean, 38.4 [SD, 13.2] v mean, 19.2 [SD, 9.6] pmol/mg protein, respectively; P = .005). Coincubation of lenalidomide with verapamil 100 μmol/L increased lenalidomide accumulation slightly, whereas coincubation with CCI-779 10 μmol/L resulted in lenalidomide accumulation (mean, 37.2 [SD, 6.0] pmol/mg protein; P = .002) equivalent to that in HL-60 cells. Figure 2B summarizes these uptake results. Validation of human ABCB1 and canine ABCB1 mRNA expression, P-gp protein expression, and functional P-gp–mediated dye exclusion for these cell lines is presented in the Data Supplement.
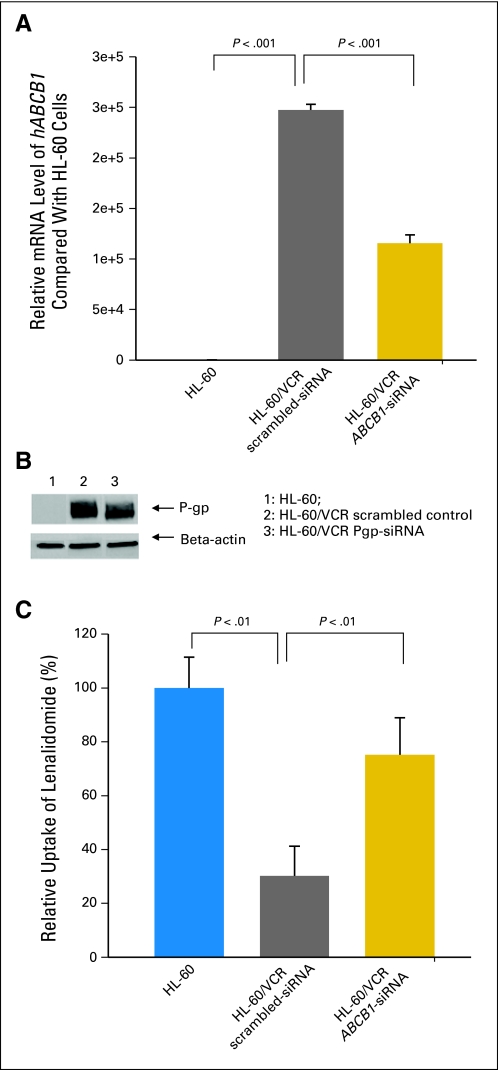
siRNA knockdown of ABCB1 in HL-60/VCR cells resulted in approximately a 50% reduction in ABCB1 mRNA levels and 30% reduction in protein levels 48 hours after transfection, compared with levels in HL-60/VCR cells transfected with scrambled control. Similarly, uptake of lenalidomide was significantly increased more than two-fold in HL-60/VCR cells transfected with ABCB1-targeted siRNA, compared with no increase in those transfected with scrambled control (Fig 3).
Fig 3.
siRNA knockdown of ABCB1 in HL-60/VCR cells. (A) mRNA level of hABCB1 in HL-60, scrambled, and ABCB1-silenced HL-60/VCR cells. (B) Western blot of P-glycoprotein (P-gp) protein in HL-60, scrambled, and ABCB1-silenced HL-60/VCR cells. A 30% decrease in relative band intensity was observed after siRNA-mediated knockdown of ABCB1 compared with scrambled HL-60/VCR cells. (C) Uptake of lenalidomide (3 μmol/L) in HL-60, scrambled, and ABCB1-silenced HL-60/VCR cells. Data are expressed as means; error bars in (A), (C) indicate standard deviation (n = 3).
DISCUSSION
Herein, we report, to our knowledge, the first phase I study of combined lenalidomide and CCI-779 in patients with relapsed MM. The MTD was identified as 25 mg lenalidomide administered daily for 21 days with CCI-779 15 mg administered weekly on a 28-day cycle. Administration of this combination demonstrated a higher frequency of electrolyte abnormalities, rash, and other constitutional symptoms than would be expected for either agent alone in this population.27,28
Pharmacokinetic profiles of CCI-779 and lenalidomide were evaluated at two and three dose levels, respectively. Renal excretion is the major route of lenalidomide elimination.21 Of note, we observed that lenalidomide clearance exceeded patients' glomerular filtration rate, suggesting active tubular secretion might be involved in the renal elimination of lenalidomide. In addition, pharmacokinetic observations suggested a potential interaction between lenalidomide and CCI-779. The disposition of CCI-779 is mediated by CYP3A4/5 and P-gp.28,29 Although reports have indicated little or no metabolism of lenalidomide in vitro or in vivo, especially with respect to CYP enzyme activity,25,30 published studies evaluating interactions with P-gp are lacking. Furthermore, clinical evidence for such an interaction has been limited to a single trial evaluating the possible interaction of lenalidomide and digoxin, a known P-gp substrate.13 In this study, a slight increase in digoxin Cmax was reported (14%).13,31 Details of this trial have not been published.
We conducted in vitro studies to evaluate the presence of active transport and determine specifically if lenalidomide is transported by P-gp. Our collective studies in P-gp–overexpressing cell models demonstrated active lenalidomide transport, which was inhibited in vitro by CCI-779 and to a lesser extent by verapamil. Furthermore, siRNA knockdown of ABCB1 in overexpressing HL-60/VCR cells significantly increased lenalidomide uptake. Combined with our clinical observations, these data provide evidence that lenalidomide is a P-gp substrate.
The identification of lenalidomide as a P-gp substrate does not rule out other potential transporter-mediated interactions with these agents, but it does provide some rationale for the observed pharmacokinetic data. No direct evidence of active lenalidomide tubular secretion has been presented previously, but the higher lenalidomide clearance compared with CLCr observed in clinical pharmacokinetic studies suggests this may occur.21 The role of P-gp in renal lenalidomide excretion requires further study. The apparent increased systemic concentrations and exposure of lenalidomide with the 20-mg versus 15-mg dose of CCI-779 may be the results of direct inhibition of lenalidomide efflux during absorption or excretion processes. The observed increased drug levels for CCI-779 at the higher lenalidomide dose may provide additional evidence for this competitive process, although some patients did report use of comedications that were CYP3A4/5/7 inhibitors or inducers and P-gp inhibitors, which may have also contributed to the variability in CCI-779 pharmacokinetics. Given the small number of patients and doses evaluated in this study, additional clinical pharmacokinetic data will be required to characterize the true impact of P-gp–mediated interactions on lenalidomide disposition. Nonetheless, identification of lenalidomide as a P-gp substrate has numerous implications for lenalidomide use in MM and other diseases. Our evaluation of seven MM cell lines suggested variable ABCB1 expression (Data Supplement). Overexpression of P-gp is believed to contribute to drug resistance in MM and may serve as an indicator for poor outcome with therapy,32–36 although few trials evaluating P-gp inhibitors have shown benefit.37 A recent report has suggested other multidrug resistance transporters may be associated with a resistance phenotype in MM.38 Comedications that are P-gp substrates or inducers may affect outcome with lenalidomide therapy by elevating or prolonging systemic exposure and increasing lenalidomide-associated toxicities. Direct evaluation of P-gp– lenalidomide interactions in primary tumor cells will be required to understand the relevance of P-gp–mediated lenalidomide transport in tumors.
The effects of P-gp interactions in the prevalent combination of lenalidomide and dexamethasone in MM warrant special consideration. Dexamethasone is a weak substrate but potentially strong inducer of P-gp.39–42 Our group did not observe direct interactions between dexamethasone and lenalidomide in our transport and uptake assays (Data Supplement), although we have observed evidence of significant dexamethasone-induced P-gp upregulation in kidney cell models (Data Supplement). These data are consistent with other reports of dexamethasone-induced P-gp expression.43–45 Thorough preclinical and clinical characterizations of this and other potential interactions with various P-gp substrates will be important for further lenalidomide development in various diseases and across broad dose ranges.
In summary, we report a phase I study with lenalidomide and CCI-779 in patients with MM, in which modest activity and increased toxicity was observed. Pharmacokinetic data suggested a potential drug-drug interaction. Subsequent in vitro evaluation provided evidence that lenalidomide is a substrate for the multidrug resistance transporter P-gp, and its associated efflux from cells is directly inhibited by CCI-779. Because of the pharmacokinetic interactions and increased toxicities observed in this study, future study designs combining lenalidomide and CCI-779 should consider sequential therapy. Clinical characterization of potential interactions with dexamethasone and other agents as well as the impact of ABCB1 pharmacogenetics on lenalidomide outcomes with therapy will be critical for the continued optimal development of lenalidomide as an antitumor and immunomodulatory agent.
Supplementary Material
Footnotes
Supported by a Leukemia and Lymphoma Society Specialized Center of Research grant and the D. Warren Brown Foundation (J.C.B.); National Institutes of Health/National Cancer Institute (NCI) Grants No. 5 U01 CA76576 and 2 P01 CA81534 (M.R.G.), 1 P50 CA140158 (J.C.B.), and 5KL2RR025754 (M.P.); and NCI Grant No. K12CA133250 (C.C.H., A.J.J.).
Content is solely the responsibility of the authors and does not represent the official views of the National Cancer Institute or National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00398515.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Sherif S. Farag, Celgene Research Funding: Sherif S. Farag, Celgene Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Amy J. Johnson, Don M. Benson Jr, Eric H. Kraut, Kenneth K. Chan, Sherif S. Farag, Michael R. Grever, John C. Byrd
Financial support: Kenneth K. Chan, Michael R. Grever, John C. Byrd, Mitch A. Phelps
Provision of study materials or patients: Craig C. Hofmeister, Celia L. Garr, Don M. Benson Jr, Eric H. Kraut, William J. Hicks, Ching-Shih Chen, Michael R. Grever, John C. Byrd
Collection and assembly of data: Craig C. Hofmeister, Xiaoxia Yang, Flavia Pichiorri, Ping Chen, Darlene M. Rozewski, Seungsoo Lee, Zhongfa Liu, Celia L. Garr, John C. Byrd, Mitch A. Phelps
Data analysis and interpretation: Craig C. Hofmeister, Xiaoxia Yang, Flavia Pichiorri, Ping Chen, Darlene M. Rozewski, Amy J. Johnson, Seungsoo Lee, Erinn M. Hade, Jia Ji, Larry J. Schaaf, Don M. Benson Jr, Eric H. Kraut, William J. Hicks, Kenneth K. Chan, Ching-Shih Chen, Michael R. Grever, John C. Byrd, Mitch A. Phelps
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Peterson TR, Laplante M, Thoreen CC, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumann P, Mandl-Weber S, Oduncu F, et al. The novel orally bioavailable inhibitor of phosphoinositol-3-kinase and mammalian target of rapamycin, NVP-BEZ235, inhibits growth and proliferation in multiple myeloma. Exp Cell Res. 2009;315:485–497. doi: 10.1016/j.yexcr.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Frost P, Shi Y, Hoang B, et al. AKT activity regulates the ability of mTOR inhibitors to prevent angiogenesis and VEGF expression in multiple myeloma cells. Oncogene. 2007;26:2255–2262. doi: 10.1038/sj.onc.1210019. [DOI] [PubMed] [Google Scholar]
- 4.Stromberg T, Dimberg A, Hammarberg A, et al. Rapamycin sensitizes multiple myeloma cells to apoptosis induced by dexamethasone. Blood. 2004;103:3138–3147. doi: 10.1182/blood-2003-05-1543. [DOI] [PubMed] [Google Scholar]
- 5.Dancey JE. Molecular targeting: PI3 kinase pathway. Ann Oncol. 2004;15(suppl 4):iv233–iv239. doi: 10.1093/annonc/mdh932. [DOI] [PubMed] [Google Scholar]
- 6.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 7.Gera JF, Mellinghoff IK, Shi Y, et al. AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem. 2004;279:2737–2746. doi: 10.1074/jbc.M309999200. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Gera J, Hu L, et al. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 2002;62:5027–5034. [PubMed] [Google Scholar]
- 9.Frost P, Moatamed F, Hoang B, et al. In vivo antitumor effects of the mTOR inhibitor CCI-779 against human multiple myeloma cells in a xenograft model. Blood. 2004;104:4181–4187. doi: 10.1182/blood-2004-03-1153. [DOI] [PubMed] [Google Scholar]
- 10.Bladé J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation: Myeloma Subcommittee of the EBMT—European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 11.Bonkowski J, Vermeulen LC, Kolesar JM. The clinical utility of lenalidomide in multiple myeloma and myelodysplastic syndromes. J Oncol Pharm Pract. 2010;16:223–232. doi: 10.1177/1078155209351967. [DOI] [PubMed] [Google Scholar]
- 12.Galustian C, Dalgleish A. Lenalidomide: A novel anticancer drug with multiple modalities. Expert Opin Pharmacother. 2009;10:125–133. doi: 10.1517/14656560802627903. [DOI] [PubMed] [Google Scholar]
- 13.Hazarika M, Rock E, Williams G, et al. Lenalidomide in combination with dexamethasone for the treatment of multiple myeloma after one prior therapy. Oncologist. 2008;13:1120–1127. doi: 10.1634/theoncologist.2008-0077. [DOI] [PubMed] [Google Scholar]
- 14.Raje N, Kumar S, Hideshima T, et al. Combination of the mTOR inhibitor rapamycin and CC-5013 has synergistic activity in multiple myeloma. Blood. 2004;104:4188–4193. doi: 10.1182/blood-2004-06-2281. [DOI] [PubMed] [Google Scholar]
- 15.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 16.Liu Q, Farley KL, Johnson AJ, et al. Development and validation of a highly sensitive liquid chromatography/mass spectrometry method for simultaneous quantification of lenalidomide and flavopiridol in human plasma. Ther Drug Monit. 2008;30:620–627. doi: 10.1097/FTD.0b013e318185813d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cockcroft D, Gault M. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 18.Lapalombella R, Yu B, Triantafillou G, et al. Lenalidomide down-regulates the CD20 antigen and antagonizes direct and antibody-dependent cellular cytotoxicity of rituximab on primary chronic lymphocytic leukemia cells. Blood. 2008;112:5180–5189. doi: 10.1182/blood-2008-01-133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farag SS, Zhang S, Jansak BS, et al. Phase II trial of temsirolimus in patients with relapsed or refractory multiple myeloma. Leuk Res. 2009;33:1475–1480. doi: 10.1016/j.leukres.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahut WL, Aragon-Ching JB, Woo S, et al. Phase I study of oral lenalidomide in patients with refractory metastatic cancer. J Clin Pharmacol. 2009;49:650–660. doi: 10.1177/0091270009335001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen N, Lau H, Kong L, et al. Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J Clin Pharmacol. 2007;47:1466–1475. doi: 10.1177/0091270007309563. [DOI] [PubMed] [Google Scholar]
- 22.Hidalgo M, Buckner JC, Erlichman C, et al. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin Cancer Res. 2006;12:5755–5763. doi: 10.1158/1078-0432.CCR-06-0118. [DOI] [PubMed] [Google Scholar]
- 23.Boni JP, Leister C, Bender G, et al. Population pharmacokinetics of CCI-779: Correlations to safety and pharmacogenomic responses in patients with advanced renal cancer. Clin Pharmacol Ther. 2005;77:76–89. doi: 10.1016/j.clpt.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Chang SM, Kuhn J, Wen P, et al. Phase I/pharmacokinetic study of CCI-779 in patients with recurrent malignant glioma on enzyme-inducing antiepileptic drugs. Invest New Drugs. 2004;22:427–435. doi: 10.1023/B:DRUG.0000036685.72140.03. [DOI] [PubMed] [Google Scholar]
- 25.Kumar G, Lau H, Laskin O. Lenalidomide: In vitro evaluation of the metabolism and assessment of cytochrome P450 inhibition and induction. Cancer Chemother Pharmacol. 2009;63:1171–1175. doi: 10.1007/s00280-008-0867-7. [DOI] [PubMed] [Google Scholar]
- 26.Arceci RJ, Stieglitz K, Bierer BE. Immunosuppressants FK506 and rapamycin function as reversal agents of the multidrug resistance phenotype. Blood. 1992;80:1528–1536. [PubMed] [Google Scholar]
- 27.Hoof T, Demmer A, Christians U, et al. Reversal of multidrug resistance in Chinese hamster ovary cells by the immunosuppressive agent rapamycin. Eur J Pharmacol. 1993;246:53–58. doi: 10.1016/0922-4106(93)90009-x. [DOI] [PubMed] [Google Scholar]
- 28.Lampen A, Zhang Y, Hackbarth I, et al. Metabolism and transport of the macrolide immunosuppressant sirolimus in the small intestine. J Pharmacol Exp Ther. 1998;285:1104–1112. [PubMed] [Google Scholar]
- 29.Sattler M, Guengerich FP, Yun CH, et al. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab Dispos. 1992;20:753–761. [PubMed] [Google Scholar]
- 30.Fine HA, Kim L, Albert PS, et al. A phase I trial of lenalidomide in patients with recurrent primary central nervous system tumors. Clin Cancer Res. 2007;13:7101–7106. doi: 10.1158/1078-0432.CCR-07-1546. [DOI] [PubMed] [Google Scholar]
- 31.US Food and Drug Administration Drug Approvals and Databases; 2005. Dec, Drug information for Revlimid (lenalidomide): Application No. (NDA) 021880. [Google Scholar]
- 32.Tucci M, Quatraro C, Dammacco F, et al. Role of active drug transporters in refractory multiple myeloma. Curr Top Med Chem. 2009;9:218–224. doi: 10.2174/156802609787521625. [DOI] [PubMed] [Google Scholar]
- 33.Yang HH, Ma MH, Vescio RA, et al. Overcoming drug resistance in multiple myeloma: The emergence of therapeutic approaches to induce apoptosis. J Clin Oncol. 2003;21:4239–4247. doi: 10.1200/JCO.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Huang YW, Hamilton A, Arnuk OJ, et al. Current drug therapy for multiple myeloma. Drugs. 1999;57:485–506. doi: 10.2165/00003495-199957040-00004. [DOI] [PubMed] [Google Scholar]
- 35.Sonneveld P. Drug resistance in multiple myeloma. Pathol Biol (Paris) 1999;47:182–187. [PubMed] [Google Scholar]
- 36.Pilarski LM, Mant MJ, Belch AR. Drug resistance in multiple myeloma: Novel therapeutic targets within the malignant clone. Leuk Lymphoma. 1999;32:199–210. doi: 10.3109/10428199909167381. [DOI] [PubMed] [Google Scholar]
- 37.Sonneveld P, Durie BG, Lokhorst HM, et al. Modulation of multidrug-resistant multiple myeloma by cyclosporine: The Leukaemia Group of the EORTC and the HOVON. Lancet. 1992;340:255–259. doi: 10.1016/0140-6736(92)92353-h. [DOI] [PubMed] [Google Scholar]
- 38.Jakubikova J, Adamia S, Kost-Alimova M, et al. Lenalidomide targets clonogenic side population in multiple myeloma: Pathophysiologic and clinical implications. Blood. 2011;117:4409–4419. doi: 10.1182/blood-2010-02-267344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mark PJ, Waddell BJ. P-glycoprotein restricts access of cortisol and dexamethasone to the glucocorticoid receptor in placental BeWo cells. Endocrinology. 2006;147:5147–5152. doi: 10.1210/en.2006-0633. [DOI] [PubMed] [Google Scholar]
- 40.Ueda K, Okamura N, Hirai M, et al. Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J Biol Chem. 1992;267:24248–24252. [PubMed] [Google Scholar]
- 41.Perloff MD, von Moltke LL, Greenblatt DJ. Ritonavir and dexamethasone induce expression of CYP3A and P-glycoprotein in rats. Xenobiotica. 2004;34:133–150. doi: 10.1080/00498250310001630215. [DOI] [PubMed] [Google Scholar]
- 42.Lin JH, Chiba M, Chen IW, et al. Effect of dexamethasone on the intestinal first-pass metabolism of indinavir in rats: Evidence of cytochrome P-450 3A [correction of P-450 A] and p-glycoprotein induction. Drug Metab Dispos. 1999;27:1187–1193. [PubMed] [Google Scholar]
- 43.Chieli E, Santoni-Rugiu E, Cervelli F, et al. Differential modulation of P-glycoprotein expression by dexamethasone and 3-methylcholanthrene in rat hepatocyte primary cultures. Carcinogenesis. 1994;15:335–341. doi: 10.1093/carcin/15.2.335. [DOI] [PubMed] [Google Scholar]
- 44.Fardel O, Lecureur V, Guillouzo A. Regulation by dexamethasone of P-glycoprotein expression in cultured rat hepatocytes. FEBS Lett. 1993;327:189–193. doi: 10.1016/0014-5793(93)80167-s. [DOI] [PubMed] [Google Scholar]
- 45.Micuda S, Fuksa L, Mundlova L, et al. Morphological and functional changes in P-glycoprotein during dexamethasone-induced hepatomegaly. Clin Exp Pharmacol Physiol. 2007;34:296–303. doi: 10.1111/j.1440-1681.2007.04558.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.