Abstract
Introduction
Retrospective studies suggest that p53 alteration is prognostic for recurrence in patients with urothelial bladder cancer and predictive for benefit from combination methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) adjuvant chemotherapy.
Patients and Methods
Patients with pT1/T2N0M0 disease whose tumors demonstrated ≥ 10% nuclear reactivity on centrally performed immunohistochemistry for p53 were offered random assignment to three cycles of adjuvant MVAC versus observation; p53-negative patients were observed. By using a log-rank test with one-sided α = .05 and β = .10, 190 p53-positive patients were planned to be randomly assigned to detect an absolute improvement in probability of recurring by 3 years from 0.50 to 0.30.
Results
A total of 521 patients were registered, 499 underwent p53 assessment, 272 (55%) were positive, and 114 (42%) were randomly assigned. Accrual was halted on the basis of the data and safety monitoring board review of a futility analysis. Overall 5-year probability of recurring was 0.20 (95% CI, 0.16 to 0.24) with no difference on the basis of p53 status. Only 67% of patients randomly assigned to MVAC received all three cycles with 12 patients receiving no treatment. There was no difference in recurrence in the randomly assigned patients (hazard ratio, 0.78; 95% CI, 0.29 to 2.08; P = .62).
Conclusion
Neither the prognostic value of p53 nor the benefit of MVAC chemotherapy in patients with p53-positive tumors was confirmed, but the high patient refusal rate, lower than expected event rate, and failures to receive assigned therapy severely compromised study power.
INTRODUCTION
Significant advances in surgical care of patients with locally advanced urothelial cancer of the bladder have led to marked decreases in perioperative morbidity and mortality, greater options for urinary diversion, and high degrees of patient satisfaction.1 Nevertheless, long-term outcome continues to be compromised by the high risk of systemic recurrence with 40% to 50% of patients with pathologic stage III disease suffering recurrent incurable cancer. Several trials have addressed perioperative systemic treatment for patients with locally advanced disease, and a benefit from neoadjuvant cisplatin-based chemotherapy has been demonstrated.2,3 However, it has been difficult to demonstrate a significant benefit from adjuvant therapy, in large part because the available prospective trials have all been severely underpowered.4
Outcome for patients with urothelial bladder cancer is determined not only by clinical stage, surgical quality, and use of perioperative chemotherapy but also by molecular markers associated with bladder carcinogenesis. Several biomarkers have been investigated for their prognostic value, including the tumor suppressor proteins p53 and retinoblastoma (pRb), and their downstream effectors. Several studies have suggested that dysregulated p53 is prognostic, with the greatest effect seen in early-stage (pT1/T2N0) disease, which has a historical recurrence rate of about 30%.5–7 Many studies, however, have been hampered by the various technologies for assessing p53 dysregulation. The most common methodology is based on the finding that mutant p53 is stabilized and detectable by standard immunohistochemical (IHC) methods, but that normal p53 tends to be rapidly degraded and thus is not detectable by these methods.8 This assay depends on details of which IHC assay was used, is not sensitive to p53 mutations that lead to lack of protein expression, and correlates imperfectly with mutational status.
Molecular analysis of p53 alterations could provide a theoretical advantage, but until recently, technologies for mutational analysis of the large p53 gene were not applicable to large clinical cohorts. Subsequent studies have demonstrated that both mutations and IHC-detected expression are independent prognostic variables, suggesting that nonmutational p53 stabilization may have functional consequences as well.9 Downstream signaling partners of the p53 DNA damage response pathway, such as p21 expression, have thus also been investigated as clinical biomarkers. High expression of p21, reflecting an intact p53 pathway, has been associated with good prognosis in bladder cancer even in the context of apparent dysregulated p53 expression.10
In addition to p53 being prognostic, several studies have suggested that p53 inactivation may be predictive of benefit from DNA-damaging therapy. The literature regarding these effects is controversial and complex not only because of the aforementioned challenges with assessing p53 status but also because the effects may be dependent on the type of DNA damage that is induced. Nevertheless, several studies11 have demonstrated that normal p53 is necessary for successful response to DNA damage that leads to G2-M arrest and that lack of a normal p53 pathway leads to increased death when cancer cells are exposed to such agents. There have been few clinical studies assessing the potential for p53 to be a predictive biomarker for benefit from DNA-damaging therapy. However, in a retrospective analysis of patients with bladder cancer who were treated in a prospective adjuvant trial, the benefit from a cisplatin-based regimen was confined to patients with p53 inactivation.12 To further assess the hypothesis that in the setting of p53 inactivation there is clinical benefit from cisplatin-based therapy, a phase III trial of adjuvant combination methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) was conducted in patients with p53-altered T1/T2N0 urothelial cancer of the bladder. This trial also sought to prospectively compare the recurrence and overall survival of patients with and without tumors demonstrating p53 alterations.
PATIENTS AND METHODS
All patients provided written informed consent, and the protocol was approved by the local institutional review board or ethics review panel at all participating institutions.
Patients
Patients with stage pT1/T2N0M0 urothelial cancer who had undergone a radical cystectomy and bilateral pelvic lymphadenectomy within the prior 9 weeks were eligible. Patients were required to have ≥ 15 lymph nodes removed or a normal computed tomography if fewer nodes were identified. Patients with Pa, P0, or Pis disease were included if T1, T2a, or T2b disease was present on the precystectomy transurethral resection specimen. Pathology and operative reports were centrally reviewed. Patients were also required to have a performance status of 0 or 1, a chest x-ray free from metastatic disease within 6 weeks of cystectomy, and normal organ function, including a WBC count ≥ 4,000/μL; platelet count ≥ 150,000/μL; creatinine ≤ 1.8 mg/dL; AST, ALT, and alkaline phosphatase ≤ 2× the upper limit of normal; and normal total bilirubin. Patients with serious arrhythmias or congestive heart failure or who had received prior systemic chemotherapy or local pelvic radiotherapy were not eligible. Patients with previous or concomitant malignancies were also excluded with the exception of pT2 (Gleason score ≤ 7) prostate cancer, basal or squamous cell carcinoma of the skin, in situ cervical cancer, or other malignancies definitively treated more than 5 years before registration without any evidence of recurrence.
p53 Analysis and Randomization
Pathology specimens were centrally assessed for p53 and p21 expression by a single pathologist (R.J.C.). The formalin-fixed, paraffin-embedded tissue was sectioned at 5-μm intervals, deparaffinized, rehydrated through graded alcohols, and subject to antigen retrieval in heated citrate buffer. Following blocking with horse serum, the primary monoclonal antibody was applied (1:200 for oncogene PAb1801 p53 antibody and 1:40 for oncogene p21 antibody). Following incubation and wash, a biotinylated secondary antibody was applied and visualized with an avidin-biotin complex immunoperoxidase system (Vector Laboratories, Burlingame, CA) that used 0.03% diaminobenzidine (DAB) as the chromagen and hematoxylin as the counterstain. Both external and internal controls were used to assess the quality of the IHC reaction. Patients with ≥ 10% nuclear immunoreactivity were considered altered for p53 (p53 positive) and patients with less than 10% nuclear immunoreactivity were considered altered for p21.
Patients with altered p53 were asked to reconsent to random assignment. Allocation was based on a minimization/randomization method stratified by age (< 65 v ≥ 65 years), stage (pT1 v pT2), grade (1 and 2 v 3 and 4), and p21 status.13 Patients assigned to receive MVAC were to begin chemotherapy within 2 weeks of random assignment and within 12 weeks of cystectomy.
Treatment and Monitoring
MVAC was administered for three cycles.14 Growth factor support was not routinely administered, and toxicity was reported by using Common Terminology Criteria for Adverse Events (CTCAE) v2.0 criteria. All patients were followed every 3 months for the first year, every 6 months for the following 4 years, and annually thereafter. Clinical follow-up required a physical examination, routine laboratory tests, and a chest x-ray; computed tomography scans were at the discretion of the treating physician.
Statistical Plan
The primary objective was to compare recurrence in patients with p53-positive tumors randomly assigned to MVAC (arm 1) versus observation (arm 2); the secondary objective was to compare recurrence in patients with p53-positive (arms 1 and 2, and group 4) versus p53-negative tumors (group 3). For the latter groups, survival was calculated as the time from registration to time of death due to any cause, and time to recurrence (TTR) was calculated as the time from registration to the first observation of disease recurrence, censuring patients who died of unrelated causes. When comparing patients randomly assigned to MVAC versus those randomly assigned to observation, the times were calculated from the date of random assignment. The statistical design specified a 0.20 absolute reduction in probability of recurrence at 3 years (from an estimated 0.50 to 0.30), corresponding to a hazard ratio of 0.52. Under the assumption of an exponential TTR distribution, 3 years of accrual, a 2.0-year minimum follow-up, a one-sided α error of .05, and using a log-rank statistic, 190 patients were planned to be randomly assigned to provide a power of 0.90. All primary analyses were specified to be on an intent-to-treat basis. The probabilities of survival plots, estimates, and 95% CIs were based on the Kaplan-Meier product-limit method; the probabilities of recurring plots, estimates, and 95% CIs were based on cumulative incidence curves, with death from non–bladder cancer as a competing cause of treatment failure.15 An interim analysis was planned after 100 patients had been randomly assigned and followed for 1 year. At the time of the interim analysis, the independent Data and Safety Monitoring Board reviewed the results of the first 110 randomly assigned patients and recommended study closure. Four patients who were randomly assigned after the time of the data lock for the interim analysis but before the Board recommendation are included in the final analysis.
RESULTS
Accrual and Follow-Up
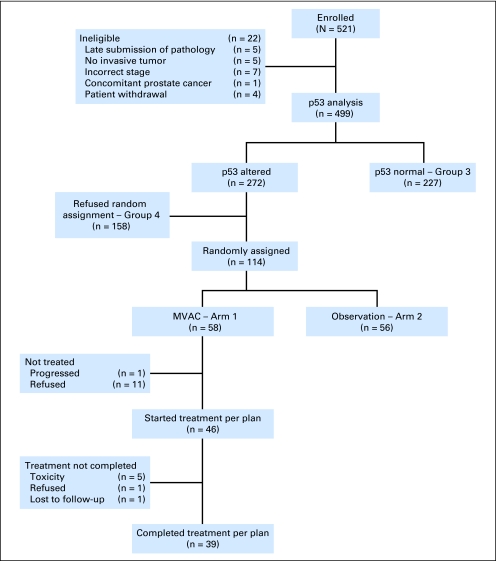
Study conduct is summarized in Figure 1. Between August 1997 and January 2006, 521 patients from 39 institutions were enrolled and 499 were eligible. Reasons for exclusion included late submission of tumor block (n = 5), no invasive tumor in submitted specimen (n = 5), incorrect staging or histology on central review (n = 7), presence of clinically significant prostate cancer (n = 1), and patient decision to withdraw (n = 4). Forty-five percent of eligible patients were p53 negative and were observed. Of 272 patients with p53-positive tumors, 114 (42%) agreed to random assignment. Twelve of 58 patients randomly assigned to chemotherapy never received treatment because of rapid disease recurrence in one patient and refusal in the other 11. None of the patients with p53-negative tumors and none of the patients randomly assigned to observation received adjuvant therapy. Median follow-up at the time of final analysis for the entire cohort was 5.4 years (95% CI, 5.1 to 5.9 years). Of 365 patients who have neither recurred nor died, 198 (54%) have more than 5 years and 305 (84%) have more than 3 years of follow-up.
Fig 1.
CONSORT diagram describing cohort, p53 analysis, randomization, and treatment. MVAC, methotrexate, vinblastine, doxorubicin, and cisplatin.
Prognostic Value of p53 Positivity
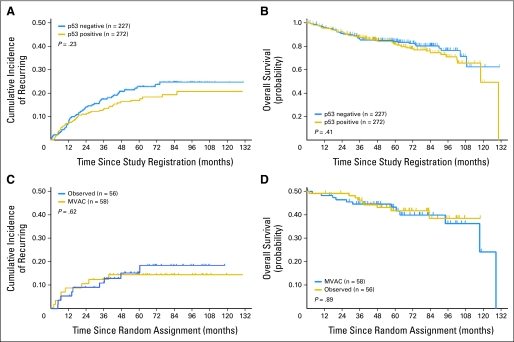
Table 1 provides baseline characteristics for p53-positive versus p53-negative patients. There were no major differences in age, sex, race/ethnicity, or stage, but patients with p53-positive tumors had a minimally higher rate of grade 3 to 4 tumors. There was also no difference in clinical prognostic factors, including the number of lymph nodes identified, lymphovascular invasion, or associated carcinoma in situ. As expected, patients with p53-altered tumors had a lower rate of p21 expression. A total of 95 patients progressed and 98 patients died with 63% of the deaths due to bladder cancer. Overall 5-year probability of recurring for all 499 eligible patients was 0.20 (95% CI, 0.16 to 0.24). Figure 2 shows that there was no difference in TTR or overall survival between patients with p53-positive versus those with p53-negative tumors.
Table 1.
Comparison of Patients With p53-Positive and p53-Negative Tumors According to Baseline Characteristics
| Characteristic | Total |
p53 Negative |
p53 Positive |
P* | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Total patients | 499 | 227 | 100 | 272 | 55 | ||
| Age, years | .59 | ||||||
| < 65 | 286 | 57 | 127 | 56 | 159 | 58 | |
| ≥ 65 | 213 | 43 | 100 | 44 | 113 | 42 | |
| Sex | .43 | ||||||
| Female | 99 | 20 | 49 | 22 | 50 | 18 | |
| Male | 400 | 80 | 178 | 78 | 222 | 82 | |
| Race/ethnicity | .24 | ||||||
| White | 454 | 91 | 203 | 89 | 251 | 92 | |
| Black | 22 | 4 | 13 | 6 | 9 | 3 | |
| Asian | 9 | 2 | 6 | 3 | 3 | 1 | |
| Hispanic | 11 | 2 | 3 | 1 | 8 | 3 | |
| Other | 3 | 1 | 2 | 1 | 1 | 1 | |
| Stage | .64 | ||||||
| pT1 | 185 | 37 | 87 | 38 | 98 | 36 | |
| pT2 and pT2a | 314 | 63 | 140 | 62 | 174 | 64 | |
| Grade | .037 | ||||||
| 1 or 2 | 24 | 5 | 16 | 7 | 8 | 3 | |
| 3 or 4 | 473 | 95 | 210 | 93 | 263 | 97 | |
| Missing | 2 | 1 | 1 | ||||
| No. of nodes identified | .11 | ||||||
| < 15 | 168 | 34 | 68 | 30 | 100 | 37 | |
| ≥ 15 | 331 | 66 | 159 | 70 | 172 | 63 | |
| p21 status | < .001 | ||||||
| Absent | 145 | 29 | 35 | 16 | 110 | 41 | |
| Present | 350 | 71 | 190 | 84 | 160 | 59 | |
| Missing | 4 | 2 | 2 | ||||
| Lymphovascular invasion | .97 | ||||||
| No | 259 | 52 | 117 | 52 | 142 | 52 | |
| Yes | 102 | 20 | 46 | 20 | 56 | 21 | |
| Unknown | 138 | 28 | 64 | 28 | 74 | 27 | |
| Bladder carcinoma in situ | .66 | ||||||
| No | 124 | 25 | 60 | 26 | 64 | 24 | |
| Yes | 302 | 61 | 137 | 60 | 165 | 61 | |
| Unknown | 73 | 14 | 30 | 13 | 43 | 16 | |
P value based on Pearson χ2 test.
Fig 2.
Effect of p53 immunohistochemistry on recurrence and survival. (A) Estimated cumulative incidence curves for time to recurrence and (B) Kaplan-Meier curves for overall survival, based on p53 nuclear immunoreactivity (negative is wild type and positive is altered). (C) Estimated cumulative incidence curves for time to recurrence and (D) Kaplan-Meier curves for overall survival among patients with p53-altered tumors in patients randomly assigned to observation versus chemotherapy with methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC; intent-to-treat analysis). Two-sided P values are based on the log-rank test for (A) and (B) and on the stratified log-rank test (stratified by age (< 65 v ≥ 65 years), stage (pT1 v pT2), grade (1 to 2 v 3 to 4), and p21 status for (C) and (D).
MVAC Compliance
Only 46 of 58 patients assigned to MVAC therapy received any treatment. Of these, eight began chemotherapy later than the specified 12 weeks post cystectomy (seven at 13 weeks and one at 14 weeks); 41patients began cycle 2 and 39 began cycle 3. Reasons for not beginning a subsequent cycle of chemotherapy included toxicity (n = 5), patient refusal (n = 1), and loss to follow-up (n = 1). Of the 39 patients who started all three cycles of therapy, the median percent of total planned drug doses administered for methotrexate, vinblastine, doxorubicin, and cisplatin were 71%, 71%, 86%, and 100%, respectively. No unexpected or fatal toxicities were observed.
Outcome in p53-Positive Randomly Assigned Arms
Table 2 provides baseline characteristics for randomly assigned p53-positive patients. There was no major difference in baseline age, sex, stage, or grade, the number of lymph nodes removed, presence of lymphovascular invasion, associated carcinoma in situ, or p21 expression. Overall probability of recurring by 5 years for all randomly assigned patients was 0.15 (95% CI, 0.09 to 0.23), and the overall probability of surviving 5 years was 0.85 (95% CI, 0.78 to 0.92). No difference in TTR or survival could be detected (Figs 2C and 2D).
Table 2.
Comparison of Patients Randomly Assigned to MVAC (arm 1) and Control/Observation (arm 2) According to Baseline Characteristics
| Characteristic | MVAC(arm 1) |
Observation(arm 2) |
Total | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Total patients | 58 | 51 | 56 | 49 | 114 |
| Sex | |||||
| Female | 7 | 12 | 9 | 16 | 16 |
| Male | 51 | 88 | 47 | 84 | 98 |
| Age, years | |||||
| < 65 | 37 | 64 | 41 | 73 | 78 |
| ≥ 65 | 21 | 36 | 15 | 27 | 36 |
| Stage | |||||
| pT1 | 21 | 36 | 16 | 29 | 37 |
| pT2 | 37 | 64 | 40 | 71 | 77 |
| Grade | |||||
| 1 or 2 | 2 | 3 | 1 | 2 | 3 |
| 3 or 4 | 56 | 97 | 55 | 98 | 111 |
| No. of nodes identified | |||||
| < 15 | 22 | 38 | 14 | 25 | 36 |
| ≥ 15 | 36 | 62 | 42 | 75 | 78 |
| p21 status | |||||
| Absent | 24 | 41 | 22 | 39 | 46 |
| Present | 34 | 59 | 34 | 61 | 68 |
| Lymphovascular invasion | |||||
| No | 33 | 57 | 25 | 45 | 58 |
| Yes | 13 | 22 | 14 | 25 | 27 |
| Unknown | 12 | 21 | 17 | 30 | 29 |
| Bladder carcinoma in situ | |||||
| No | 16 | 27 | 12 | 21 | 28 |
| Yes | 34 | 59 | 32 | 57 | 66 |
| Unknown | 8 | 14 | 12 | 21 | 20 |
Abbreviation: MVAC, methotrexate, vinblastine, doxorubicin, and cisplatin.
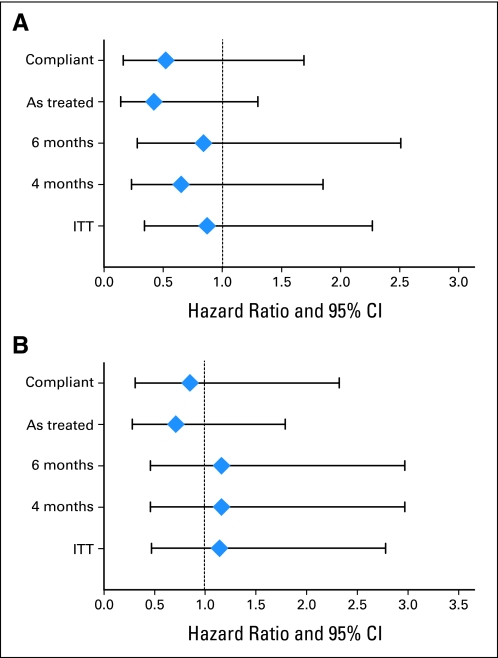
Because the 21% noncompliance with assignment to MVAC therapy had the potential for diluting the power of the prespecified intent-to-treat analysis, four additional exploratory/sensitivity analyses were performed. We analyzed TTR, excluding all noncompliant patients, by the treatment received instead of as assigned, and by using a landmark analysis that analyzed only those patients in each arm as assigned who survived without recurrence to a 4- or 6-month landmark (Fig 3). There was a numerical improvement in time to recurrence using the first two post hoc analyses, but this did not reach the usual criteria for statistical significance and was not apparent with the landmark analysis.
Fig 3.
Post hoc analysis and forest plots of time to recurrence and overall survival hazard ratios with 95% CIs to assess the impact of noncompliance with assigned therapy in the arm treated with methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC). Compliant excludes patients who refused assigned treatment; as treated includes all patients and analyzes them according to treatment received; 4-month and 6-month landmark analyses assessed only those patients in each arm as assigned who survived without recurrence to a 4- or 6-month landmark, respectively; and ITT represents protocol-specified intent-to-treat analysis.
DISCUSSION
To the best of our knowledge, this was the first study in bladder cancer to base a treatment decision on a molecular alteration and was, at the time, the largest randomized study of adjuvant chemotherapy. The focus was on patients with T1/T2N0 disease, a group not usually treated with adjuvant therapy but for whom recurrence rates are historically high. This study demonstrated a 5-year probability of recurring of approximately 0.20, which is better than that observed in older surgical series but nevertheless emphasizes the highly malignant nature of the disease and the need to improve outcomes for these patients.
There are basically three ways to accomplish this goal. The first is to develop more effective systemic agents and regimens. Despite their inadequacies and multiple efforts to develop alternatives, the MVAC and gemcitabine-cisplatin regimens remain the best available for this disease. The second is to develop better prognostic biomarkers. Under the assumption that the relative benefit of treatment is equivalent across prognostic groups, a greater absolute benefit will be accomplished in patients with the worst prognosis. The last is to develop better predictive biomarkers for benefit from the available regimens.
This trial attempted to confirm p53 IHC as a prognostic biomarker and to determine whether patients with pathologic pT1/T2N0 urothelial bladder cancer who also had p53-altered tumors would benefit from MVAC. The trial was based on extensive preliminary but retrospective data suggesting the value of p53 for these indications and was performed in a prospective manner with a standardized centrally performed biomarker assay. The trial was, however, unable to demonstrate either a prognostic or a predictive value for p53 overexpression. Notably, the better overall patient outcome than predicted limited the power of a single biomarker. In addition, and in retrospect, the data for the predictive value of p53 were somewhat limited by current clinical trial standards.12 It is also important to note that the lack of random assignment to treatment in the p53-negative group means that a pure predictive value of p53 IHC could not be assessed even if a major difference in the p53-positive randomized arms were demonstrated.
Another possible factor for the observed results was the higher rate of p53 alterations observed compared with earlier studies, possibly because of changes in technique such as better antigen retrieval methods. Whether mutation analysis and/or more comprehensive p53 pathway analysis may be more useful is the subject of current research with the collected samples. Nevertheless, this is, to the best of our knowledge, the only prospective study of p53 as a prognostic or predictive biomarker that uses a centrally assessed standardized assay methodology.
Perhaps the most important factor in the failure of the trial to support the original hypothesis is that the number of patients randomly assigned and, more importantly, who received treatment as assigned, was much lower than anticipated. Under the original plan, patients were to agree to biomarker analysis, and the therapeutic aspects of the trial immediately postoperatively for the biomarker analysis was to be completed in a timely manner. To then address the ethical challenges of consenting to a potentially toxic therapy in the postoperative state, a reconsent process was put into place. It was anticipated that the majority of patients would reconsent, but in reality only 40% did, leading to a much larger trial than anticipated. Equally concerning is the fact that only 39 of 58 patients assigned to MVAC therapy actually received three cycles as planned. Legitimate toxicity and disease considerations contributed to this observation, but the vast majority of patients not receiving therapy simply declined. Although the challenges of randomizing between a toxic therapy and observation with deferred therapy at time of progression are well known and anticipated, it is unclear whether the nature of the patient population or biases of treating physicians contributed to these observations.
These challenges limited the ability for the trial to evaluate the potential of p53 overexpression to define a group that might be sensitive to cisplatin-based chemotherapy. The low randomization rate and poor compliance with assigned treatment made the trial's power to detect any biologically plausible predictive value negligible. Post hoc analyses of time to recurrence on the basis of treatment as assigned or by elimination of patients who refused therapy give some hint that p53 expression may have modest predictive value. However, this was not apparent with landmark analyses, which are less susceptible to the well-known biases associated with these approaches. It must thus be concluded that the study was unable to determine whether p53 overexpression alone can define a subgroup of patients who benefit from adjuvant MVAC therapy.
Nevertheless, the extensive sample collection along with the well-annotated clinical and outcome data all obtained in a prospective manner will form the basis of further exploratory biomarker studies. The carefully controlled nature of this trial will make it much more likely that biomarkers identified in this effort will be clinically qualified in future prospective confirmatory studies.
Supplementary Material
Acknowledgment
We thank the University of Southern California study coordinators, Rebecca Gutierrez, Sola Odusote, Charlotte Lee, Ellenie Tuazon, and Carmela Villajin-Busque. Debra Hawes, MD; Clive Taylor, MD, PhD; Anirban Mitra; and Lillian Young assisted in the immunohistochemical evaluation of the specimens. We also thank the members of the Data Safety Monitoring Committee (Pat Loehrer, Jean deKernion, Fred Waldman, and Mark Krailo) and patient advocate Brian Meyer.
Appendix
The following are principal investigators who participated in this study along with their institutions: Timothy G. Wilson, City of Hope, Los Angeles, CA; Peter Venner, Cross Cancer Institute, Edmonton, Alberta, Canada; Howard Gross, Dayton Community Clinical Oncology Program (CCOP), Dayton, OH; Wayne Harris, Emory University, Atlanta, GA; Marc-Oliver Grim, Heinrich Heine Universität, Duesseldorf, Germany; Kenneth Steven, Herlev Hospital, Herlev, Denmark; Arnulf Stenzl, University Innsbruck, Innsbruck, Austria; Mark Schoenberg, Johns Hopkins University, Baltimore, MD; Hendrik Van Poppel, University Leuven, Leuven, Belgium; Robert Ruckle, Loma Linda University, Loma Linda, CA; Joseph Chin, London Health Sciences Center, London, Ontario, Canada; Dennis Venable, Louisiana State University-Shreveport, Shreveport, LA; Steven Campbell, Loyola University, Maywood, IL; Colin Dinney, MD Anderson Cancer Center, Houston, TX; Benjamin Marchello, Montana Cancer Consortium CCOP, Billings, MT; Julio M. Pow-Sang, Moffit Cancer Center, Tampa, FL; Cora Sternberg, San Camillo Forlaninin Hospitals, Rome, Italy; Thomas Keane, Medical University of South Carolina, Charleston, SC; Andreas Hinkel, Ruhr University, Bochum, Germany; Laurence Klotz, Sunnybrook and Women's College Health Sciences Centre, Toronto, Ontario, Canada; Markus Kuczyk, Universität Tübingen, Tübingen, Germany; Vincenzo Pagliarulo, University Bari, Bari, Italy; Christopher Kane, University of California-San Francisco, San Francisco, CA; David Crawford, University of Colorado, Denver, CO; Markus Hohenfellner, Universität Heidelberg, Heidelberg, Germany; Michael O'Donnell, University of Iowa, Iowa City, IA; Brantley Thrasher, University of Kansas, Kansas City, KS; David Wood, University of Michigan, Ann Arbor, MI; Raj Pruthi, University of North Carolina-Chapel Hill, Chapel Hill, NC; Daniel Culkin, University of Oklahoma, Oklahoma City, OK; Bruce Malkowicz, University of Pennsylvania, Philadelphia, PA; Arthur Sagalowsky, University of Texas Southwestern, Dallas, TX; Michael Jewett, University of Toronto, Princess Margaret Hospital, Toronto, Ontario, Canada; Thomas R. Pritchett, Virginia Mason University, Seattle, WA; Michael Cher, Wayne State University, Detroit, MI.
Footnotes
Supported by Grants No. CA71921 (R.J.C.), CA70903 (R.J.C.), and CA14089 and in part by Public Health Service Cooperative Agreement Grants No. CA32102, CA38926, CA58882, CA46368, CA12644, CA27057, CA35192, CA14028, CA35090, CA46282, CA67575, and CA42777 from the National Cancer Institute, Department of Health and Human Services.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00005047.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Derek Raghavan, sanofi-aventis (C), Eli Lilly (U) Stock Ownership: None Honoraria: None Research Funding: Derek Raghavan, Eli Lilly Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Seth P. Lerner, Susan Groshen, John P. Stein, Derek Raghavan, David Esrig, Donald G. Skinner
Administrative support: Susan Groshen
Provision of study materials or patients: Walter M. Stadler, Seth P. Lerner, Derek Raghavan, Gary Steinberg, David Wood, Laurence Klotz, Craig Hall, Donald G. Skinner
Collection and assembly of data: Walter M. Stadler, Seth P. Lerner, Susan Groshen, John P. Stein, Gary Steinberg, David Wood, Laurence Klotz, Craig Hall, Donald G. Skinner
Data analysis and interpretation: Walter M. Stadler, Seth P. Lerner, Susan Groshen, John P. Stein, Shan-Rong Shi, Derek Raghavan, Richard J. Cote
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 2.Advanced Bladder Cancer Overview Collaboration. Neoadjuvant chemotherapy for invasive bladder cancer. Cochrane Database Syst Rev. 2005;2:CD005246. doi: 10.1002/14651858.CD005246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 4.Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Adjuvant chemotherapy for invasive bladder cancer (individual patient data) Cochrane Database Syst Rev. 2006;2:CD006018. doi: 10.1002/14651858.CD006018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esrig D, Elmajian D, Groshen S, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med. 1994;331:1259–1264. doi: 10.1056/NEJM199411103311903. [DOI] [PubMed] [Google Scholar]
- 6.Goebell PJ, Groshen SG, Schmitz-Dräger BJ, et al. p53 immunohistochemistry in bladder cancer: A new approach to an old question. Urol Oncol. 2010;28:377–388. doi: 10.1016/j.urolonc.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Malats N, Bustos A, Nascimento CM, et al. P53 as a prognostic marker for bladder cancer: A meta-analysis and review. Lancet Oncol. 2005;6:678–686. doi: 10.1016/S1470-2045(05)70315-6. [DOI] [PubMed] [Google Scholar]
- 8.Esrig D, Spruck CH, 3rd, Nichols PW, et al. p53 nuclear protein accumulation correlates with mutations in the p53 gene, tumor grade, and stage in bladder cancer. Am J Pathol. 1993;143:1389–1397. [PMC free article] [PubMed] [Google Scholar]
- 9.George B, Datar RH, Wu L, et al. p53 gene and protein status: The role of p53 alterations in predicting outcome in patients with bladder cancer. J Clin Oncol. 2007;25:5352–5358. doi: 10.1200/JCO.2006.10.4125. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee SJ, Datar R, Youssefzadeh D, et al. Combined effects of p53, p21, and pRb expression in the progression of bladder transitional cell carcinoma. J Clin Oncol. 2004;22:1007–1013. doi: 10.1200/JCO.2004.05.174. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira CG, Tolis C, Giaccone G. p53 and chemosensitivity. Ann Oncol. 1999;10:1011–1021. doi: 10.1023/a:1008361818480. [DOI] [PubMed] [Google Scholar]
- 12.Cote RJ, Esrig D, Groshen S, et al. p53 and treatment of bladder cancer. Nature. 1997;385:123–125. doi: 10.1038/385123b0. [DOI] [PubMed] [Google Scholar]
- 13.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 14.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 15.Gaynor JJ, Feuer EJ, Tan CC, et al. On the use of cause-specific failure and conditional failure probabilities: Examples from clinical oncology data. J Am Stat Assoc. 1993;88:400–409. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.