Abstract
Pancreatic ductal adenocarcinoma (PDCA) is characterized by well-defined tubular units in the vast majority of the cases; however, variations in this theme do occur. It is important to recognize the morphologic spectrum of PDCA to avoid misdiagnosis especially in small specimens and also in metastatic foci. Here, we document a morphologic variant of PDCA that is characterized by a distinctive pattern of infiltrating cribriform nests in a distinctive “microcystic” or “secretory” pattern. Twenty-four cases of PDCA have been identified in a review of 505 cases diagnosed with PDCA. Histologically, this pattern was characterized by infiltrating nests of tumor cells with large vacuoles and “signet-ring” like appearance imparting a cribriform growth pattern. The vacuoles were one to five cells in size, often merging to form multilocular spaces separated by a thin rim of cell membrane. Many of these spaces contained CA19.9 positive granular secretory material. The nuclei were often pushed to the periphery and compressed in a pattern resembling adipocytes, although the nuclei were often densely hyperchromatic and displayed significant atypia. Especially in biopsies from the peripancreatic fat and peritoneum, these neoplastic cells had been misdiagnosed as degenerating adipocytes, and in the lymph nodes, they had been misinterpreted as lipogranulomas. Clinical findings of the patients were similar to that of conventional PDCA, except higher incidence of history of smoking (83% vs. 60%; p=0.034). In conclusion, vacuolated cell adenocarcinoma is a distinct morphologic variant of PDCA, and the presence of this peculiar pattern in a metastatic site, although not specific, should raise the suspicion of a PDCA.
Keywords: Pancreas, Pancreatobiliary, Ductal, Adenocarcinoma, Vacuolated, Lipoid, Signet ring, Cribriform
Introduction
Histopathologic diagnosis of pancreatic ductal adenocarcinoma (PDCA) is often challenging. This is partly due to the inaccessibility of the organ, which necessitates the diagnosis to be rendered in limited specimens, small biopsies, or frozen sections [1]. In addition, benign conditions of the pancreas, especially chronic pancreatitis, can closely mimic PDCA. Conversely, PDCA typically has an abundant stromal component (hence the synonym scirrhous carcinoma) which, on one hand, hampers the tumor tissue yield in small specimens and, on the other, complicates the microscopic evaluation by morphologically mimicking chronic pancreatitis [2]. This challenge may partly be overcome by recognizing the various morphologic patterns that PDCA exhibits.
It is also important to recognize the morphologic spectrum of PDCA for its correct diagnosis in the metastatic sites. A significant proportion of the PDCA patients presents with metastatic tumors and an unsuspected primary in the pancreas. In fact, studies have shown that PDCA is one of the most common, if not the most common, sources of “carcinoma of unknown origin” [3,4].
PDCA is characterized (and defined) by infiltrating tubular units lined by cuboidal cells, embedded in desmoplastic stroma. However, there are various cytologic and architectural variations that occur on top of this basic theme, which are included under the umbrella of pancreatobiliary carcinoma [4]. In this study, a distinctive morphologic variant characterized by infiltrating cribriform units that are formed by vacuolated tumor cells (resembling adipocytes or signet-ring cells) that contain CA19-9 positive secretory material is documented. The histologic, immunophenotypic, molecular, and clinical characteristics of this variant are analyzed and compared to those of the ordinary PDCA.
Materials and methods
Patients
Five hundred and five consecutive cases of PDCA seen in the files were reviewed. Twenty-four cases (18 pancreatectomies and six biopsies) that displayed this distinctive vacuolated/cribriform pattern in more than 25% of the tumors were identified. From the resection specimens, an average of 15 slides was available (range, one to 29) for examination on these cases.
Demographic features of the group were analyzed, and the clinical history of the patients for the presumed risk factors of PDCA such as diabetes mellitus, smoking, and alcohol were recorded, where available.
The findings were contrasted with those of conventional PDCA.
Histochemical analysis
Histochemical stains, namely Mayer s mucicarmine, high iron diamine–alcian blue (to differentiate sialated mucins from sulfated mucins), periodic acid–Schiff, and alcian blue–periodic acid–Schiff (AB-PAS, for acidic mucins), were performed.
Immunohistochemistry
All tumors for which material was available were studied for the mucin-related glycoproteins and oncoproteins using immunohistochemical stains with carcinoembryonic antigen (CEA; BioGenex, San Ramon, CA; 1:200), CA19-9 (Novo-castra, New Castle upon Tyne; 1:200), B72.3 (Signet, Dedham, MA; 1:40), MUC1 (Novocastra, New Castle upon Tyne; 1:160), and MUC2 (Novocastra, New Castle upon Tyne; 1:100), which have known expression patterns (and presumed diagnostic utility) in PDCA. High molecular weight cytokeratin, also known as HMWCK or CK34βE12, a marker that is positive in squamous change in pancreas tumors, was also tested to determine whether this pattern represents a form of abortive squamous differentiation.
Avidin–biotin–peroxidase method was used. Negative and positive controls were included with each batch of slides tested.
K-ras mutation analysis
Frozen tumor tissue was available in eight cases and was used to search for the mutation in codon 12 of K-ras oncogene, which is very common in cases of conventional PDCA.
DNA extraction and PCR amplification
DNA extraction and PCR amplification for the analysis of mutations in codon 12 of the K-ras gene were accomplished following methods previously published [5]. The extract containing genomic DNA (10 μl) was subjected to PCR in a total volume of 50 μl of reaction mixture using specific primers. The primer set consisted of 5′-ATGACTGAATA TAAACTTGT-3′(forward) and 5′-CTATTGTTGGATCA TATT-3′ (reverse), and the PCR was carried out in a DNA thermal cylinder (Cetus-Perkin Elmer, Norwalk, CT) for 35 cycles. Each cycle of amplification consisted of 1 min of denaturation at 94°C, 1 min of annealing at 57°C, and 2 min of polymerization at 72°C. After the last cycle, polymerization was continued for an additional 7 min at 72°C.
Slot blotting and southern hybridization
Slot-blot southern analysis of PCR-amplified samples was carried out using 32P-labeled oligonucleotide probe panel following standard procedure [5]. Amplified DNA was denatured by adding 7 μl of the PCR reaction mixture to 100 μl of 0.4 M NaOH and 25 mM EDTA. The mixture was heated to 95°C for 2 min and placed on ice. One hundred microliters of 2 M Tris–HCl (pH 7.4) was added and slot blotted on a nylon membrane using the Schleicher & Schuell (Keane, NH) manifold-II apparatus. The membranes were prehybridized in the hybridization mixture (5× SSPE, 5× Denhardts, 0.5% sodium dodecyl sulfate (SDS), 100 mM sodium pyrophosphate, pH 7.5) for 1 h at 37°C on a shaker. 5′-end-32P-labeled oligonucleotide probe (wild-type probe and six specific probes to each base mutation) was added to the hybridization mix to a final concentration of 2×106 cpm/ml and hybridized overnight at 37°C in a hybridization incubator. The membranes were washed with 6× SCC and 3 M tetramethylammonium chloride (TMAC) wash solution (3 m TMAC, 50 mM Tris–HCl, 2 mM EDTA, 0.1% SDS) twice at 61°C for 30 min with constant shaking and were autoradiographed. Control samples for all the studies were taken from areas without tumor from the same histologic sections.
Statistical analysis and comparison with ordinary PDCA
Available demographic data (age, location of the tumor, as well as history of smoking, diabetes, and alcohol) of the patients with vacuolated cell pattern were compared with those of ordinary PDCA identified in the same database. For the comparison of age and tumor size, t test was applied, and for the remaining, chi-square test was utilized. Kaplan–Meier survival curves were made to compare the clinical outcome.
Results
Clinical findings
The mean age of the patients was 61 (range, 43 to 72 years) vs. 63 in PDCA. There was slight female predominance, with nine of the patients males and 15 females (F/M=1.7 vs. 1 in PDCA; Table 1). Fifteen were Caucasians, seven were African Americans, and two were unknown (ratio= 2.1 vs. 1.5 in PDCA). The tumors ranged in size from 2.0 to 6.0 cm (mean tumor size, 3.8 vs. 3.5 cm in PDCA). Six patients had biopsy diagnosis only, and their tumor sizes were based on reports from the CT examination. Among resection specimens, 12 tumors were located in the head, five in the tail, and one in the body of the pancreas.
Table 1.
Clinical findings
| Clinical findings | PDCA with vacuolated pattern (n=24) | PDCA with tubular pattern (n=481) |
|---|---|---|
| Demographic data | ||
| Mean age (years) | 61 | 63 |
| Female/male | 1.7 | 1 |
| Race | 68% Caucasian 32% African American |
60% Caucasian 40% African American |
| Tumor size (mean; cm) | 3.8 | 3.5 |
| Location | Head=67% Body/tail=33% |
Head=81% Tail=19% |
| Clinical history | ||
| Smoker (%) | 82 | 59 (p=0.034) |
| DM (%) | 30 | 27 |
| Alcohol abuse (%) | 21 | 22 |
| K-ras mutation (%) | 87.5 | 72 |
DM diabetes mellitus
In the available data on the past history of the patients, 14/16 were smokers (83% vs. 59% in PDCA), four of 13 had diabetes (30% vs. 27% in PDCA), and three of 14 (21% vs. 22% in PDCA) had alcohol abuse (Fig. 1). Only the difference in history of smoking was statistically significant (p=0.034).
Fig. 1.
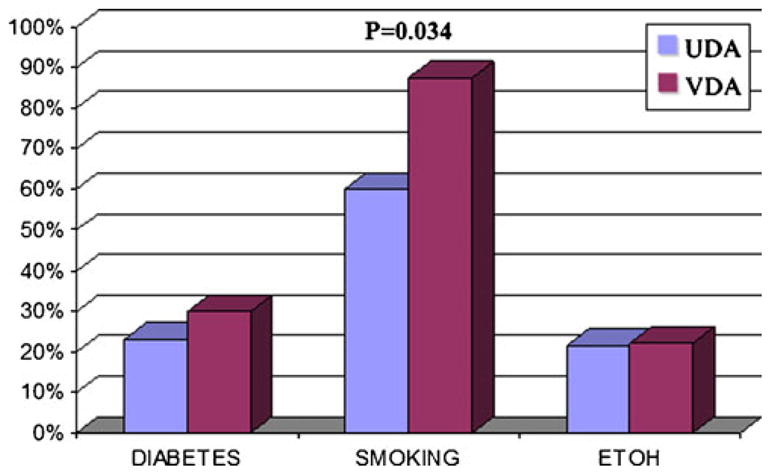
When comparing the incidence of diabetes mellitus, smoking, and alcohol in vacuolated cell variant (VDA) and ordinary ductal adenocarcinoma (UDA), only the difference in history of smoking was statistically significant (p=0.034)
Histopathologic findings
Microscopically, this variant was characterized by infiltrating clusters of tumor cells that contained large vacuoles imparting them a cribriform architecture (Fig. 2). The vacuoles were one to five cells in size, often merging to form multilocular spaces separated by a thin rim of cell membrane (Figs. 2 and 3). These microcystic spaces pushed the nuclei to the periphery of the cells, compressing them and indenting the nuclear membrane. This created a pattern reminiscent of adipocytes (lipoid pattern) or signet-ring cells (Fig. 4). Many of these spaces contained material composed of cellular debris (necrotic cells, Fig. 2) and mucin (Fig. 5; positive with histochemical and immunohistochemical stains; see below). The nuclei, upon close inspection, were often densely hyperchromatic and displayed significant atypia, enlargement, and pleomorphism (Figs. 4 and 6). In two cases, in the biopsies from the peripancreatic fat and peritoneum, this pattern has been misdiagnosed as degenerating adipocytes. In two other cases, the metastatic foci in the lymph nodes were missed (Fig. 7), possibly because they were misinterpreted as lipogranulomas or sinusoidal lipid, which they closely resembled.
Fig. 2.
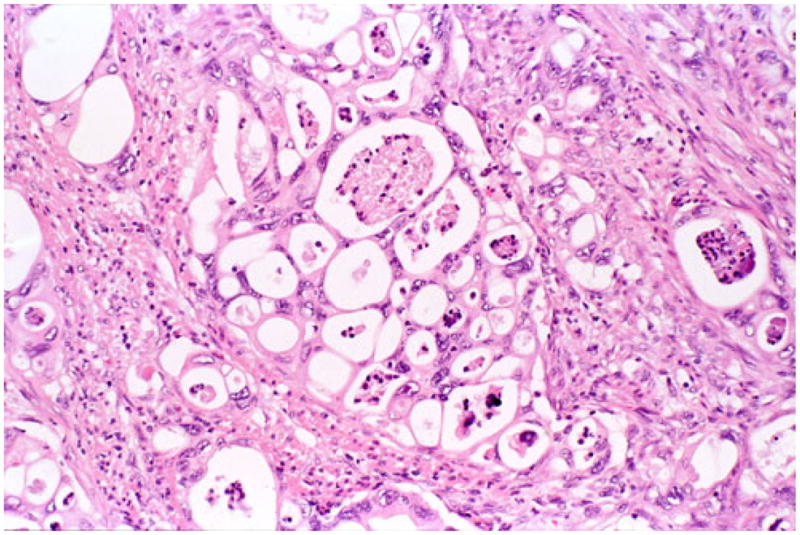
In vacuolated variant of ductal adenocarcinoma, infiltrating glandular elements often form a cribriform pattern created by multiple-cell-size vacuoles that are separated by thin strands of cytoplasm. The vacuoles often contain necrotic debris admixed with neutrophils
Fig. 3.
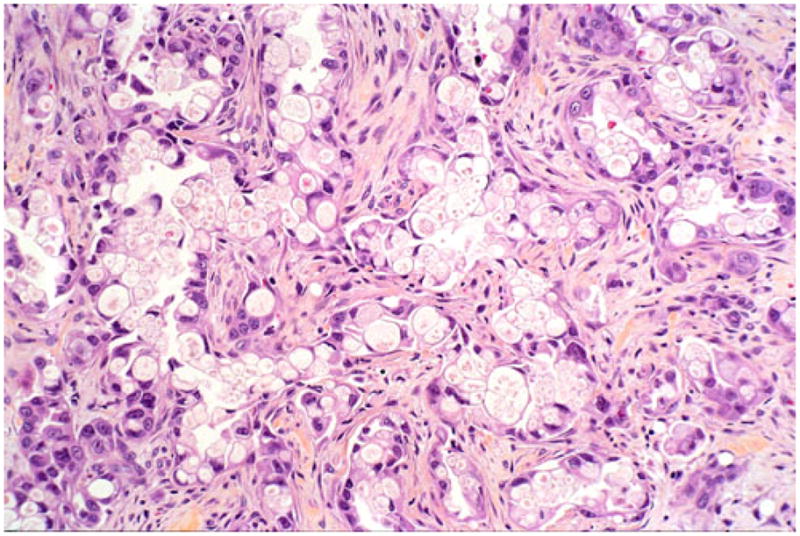
In some examples, the vacuoles impart a signet-ring-like appearance to the cells and may even have targetoid mucin in some areas
Fig. 4.
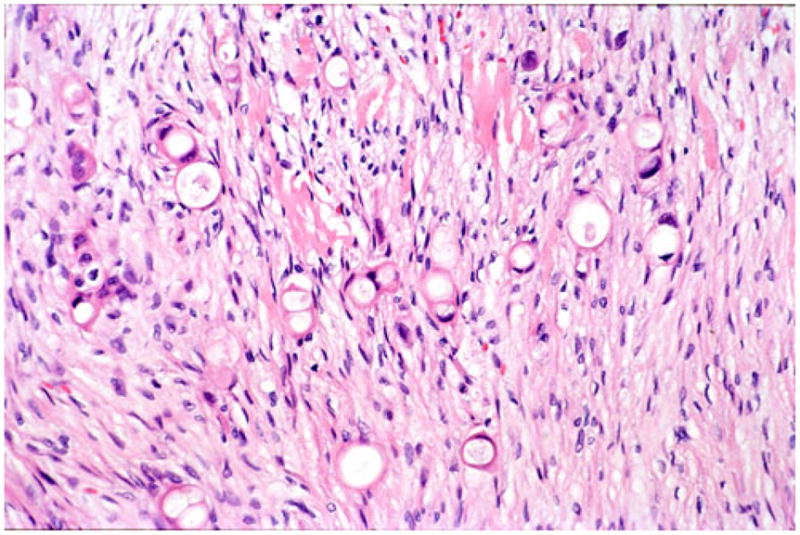
In areas where the infiltrative units are smaller, tangential cut of the vacuolated glands may give the impression of individual cell infiltration mimicking diffuse infiltrative-type (“poorly cohesive cell” or “signet-ring” cell) carcinoma; however, the presence of larger cohesive glandular elements establish the diagnosis that this is merely the vacuolated variant of ductal adenocarcinoma
Fig. 5.
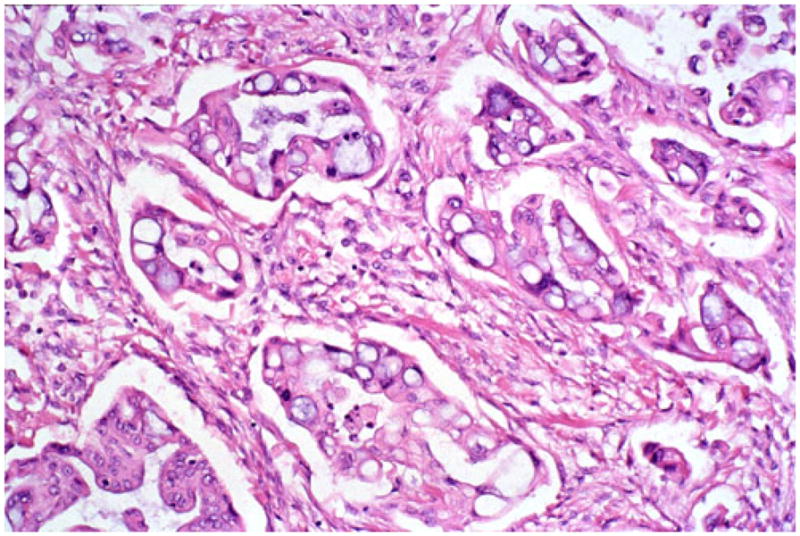
In rare areas, transition from more mucin-filled vacuoles to the more characteristic empty vacuoles can be observed. In this particular case, artifactual clefting around the infiltrative glands created a pseudo-micropapillary carcinoma appearance
Fig. 6.
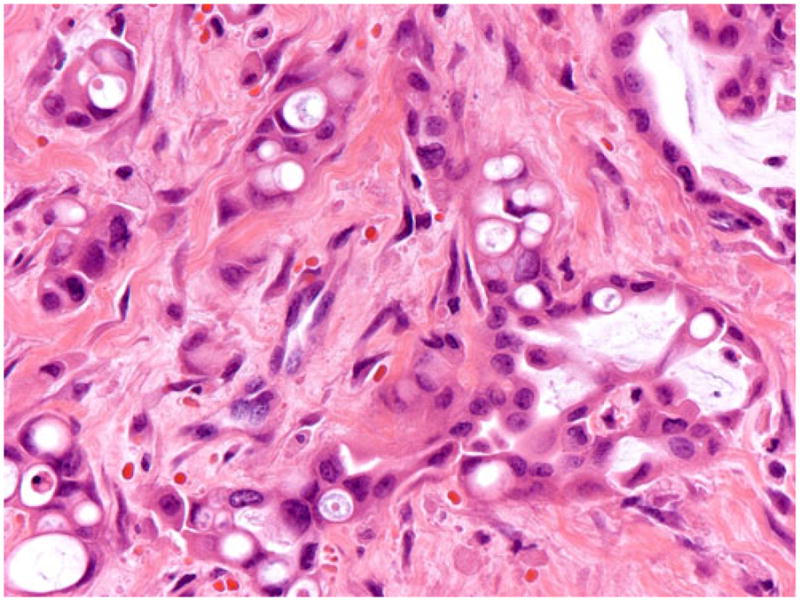
In some areas, the vacuoles were smaller and overtly intracytoplasmic, some containing targetoid mucin. The nuclei were often displayed significant atypia, enlargement, and pleomorphism
Fig. 7.
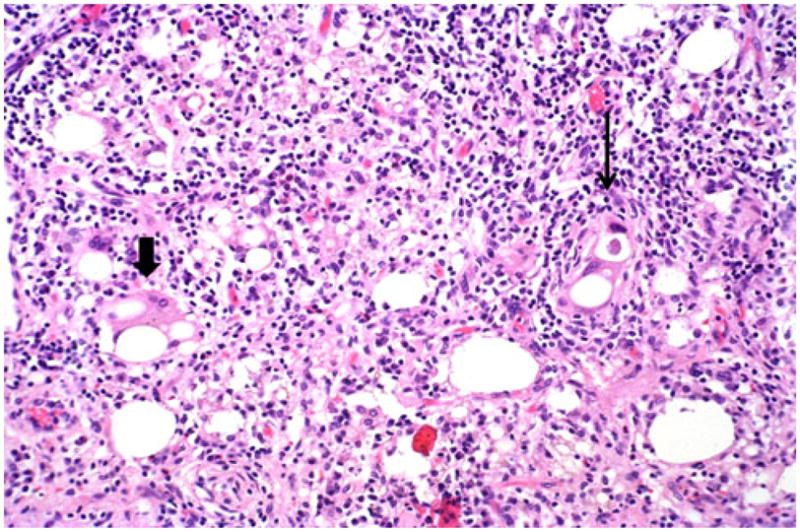
Vacuolated cell adenocarcinomas (right, thin arrow) can be mistaken as lipogranulomas (left, thick arrow) because of their mimicry of fat. Close inspection, however, typically elucidates the severe degree of cytologic atypia including marked pleomorphism and hyperchromatism, which is characteristically present
Histochemical findings
Mucicarmine, high iron diamine (Fig. 8), and AB-PAS stains [mostly blue (sialated acidic mucin) with focal black (sulfated acidic mucin)] were all positive in the vacuolated cells of 11 cases examined (11/11).
Fig. 8.
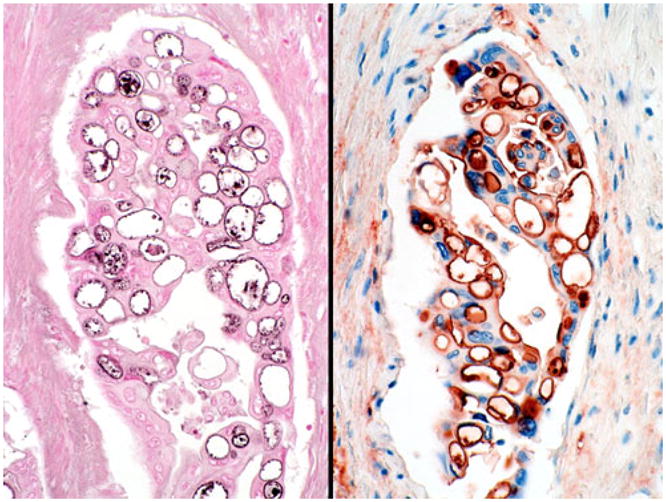
Histochemical and immunohistochemical stains confirm that these vacuoles are in fact intraglandular lumen formations that are lined by secretory apparatus containing mucin (left, high iron diamine) and mucin-related glycoproteins (right, CA19-9)
Immunohistochemical findings
Immunohistochemical phenotype of the vacuolated pattern was similar to that of PDCA. The tumor cells were positive uniformly for CA19-9 (11/11, Fig. 8) and MUC1 (11/11) and, in some cases, also for CEA (8 of 11, two of which was focal) and B72.3 (5 of 11). CK34βE12, which is usually negative in PDCA, however, was detected in 6 of 11 cases. Only one case revealed focal MUC2 positivity, which was limited to goblet cell metaplasia area (1 of 11).
Molecular analysis
Mutation in codon 12 of K-ras oncogene, was detected in seven of the eight (87.5%) cases studied (vs. 56 of 78 (72%) in PDCA; p=0.676).
Survival analysis
Median survival after diagnosis was 13.7 months (vs. 13.8 months in PDCA; Fig. 9).
Fig. 9.
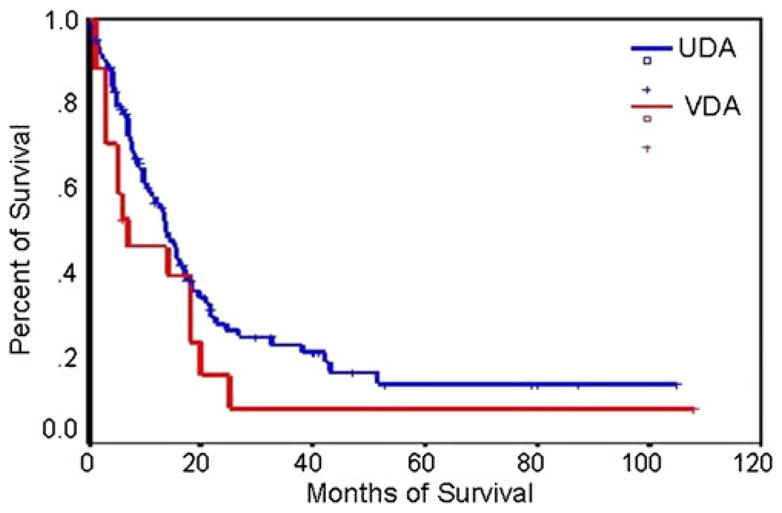
Survival rates of vacuolated cell variant (VDA) and ordinary ductal adenocarcinoma (UDA)
Discussion
Morphologic repertoire of pancreatobiliary-type adenocarcinoma originating in the pancreas (invasive ductal adeno-carcinoma, PDCA) includes a hitherto unrecognized distinctive pattern characterized by infiltrating cribriform clusters of cells with microcystic formations. In this variant, the microcysts appear to result from the fusion of intracellular lumenae, many of which contain CA19-9 positive secretory material admixed with nuclear debris and mucin. These spaces compress the cytoplasmic organelles to the periphery of the cell, forming a thin bridging membrane. The nuclei are often compressed and indented, creating a picture reminiscent of signet-ring cells or adipocytes, to an extent that these tumor cells have been mistaken for lipogranulomas or sinusoidal fat, both of which are common findings in peripancreatic lymph nodes. They may also be mistaken for fat necrosis in biopsies from peripancreatic tissue or peritoneal surfaces to which PDCA often disseminates.
It is important to recognize this pattern because it may be diagnostically important in limited specimens or in biopsies from metastatic foci, especially since many PDCA patients present with distant tumor and an unsuspected primary in the pancreas. The authors have seen several examples in the liver and other sites including bone marrow, in which this pattern led to the correct diagnosis of otherwise unsuspected PDCA. Differential diagnosis include signet-ring cell carcinoma, tumors with lipoblast-like or signet-ring cells from other primary sites, specifically breast and stomach, and liposar- coma (alone or as part of sarcomatoid carcinoma). All these distinctions have significant therapeutic implications.
Although uncommon, this pattern may also be encountered in tumors from other sites, especially in surface epithelial organs. The presence of distinctive large vacuoles that compress the nucleus forming a cribriform architecture is a typical finding in adenomatoid tumors (the so-called localized mesothelioma of gynecologic tract) [6,7], but may also be rarely seen in mesotheliomas [8]. This pattern is also well recognized as a variant of meningioma in the meninges [9], another site with a highly specialized “lining” properties, similar in that sense to mesothelium. The authors have also encountered this pattern in laryngeal and GE-junction carcinomas, both of which are sites with transition from a glandular to squamous epithelium, raising the question of this pattern being a result of lumen forming cells that are also showing immature squamous properties. The positivity of CK34βE12, a marker of squamous differentiation in the pancreas, in 6 of 11 cases reported here may lend further support to this possibility. On the other hand, in none of these cases was frank evidence of squamous differentiation such as keratin formation, squamous pearls, or histologic evidence of desmosomal junctions identified. Therefore, it appears that the process is likely to be recapitulating attenuated “lining” cells akin to those seen in adenomatoid tumors or mesotheliomas, rather than true squamous differentiation.
A pattern similar to the one described here may also be rarely seen in solid glandular organs including the salivary glands [10,11], and we have noted it occasionally even in the colon. In the ovary, it has been documented as a rare variant that was reported as “mixed-epithelial carcinoma with microcystic pattern and signet ring cells” [12], a term that has been coined for this pattern in the meninges [9]. Since ovarian involvement in disseminated PDCA is not uncommon [13–15] (and vice versa is also true [16]), it is important to be aware that this pattern may be exhibited by the tumors from both organs. A similar tumor type has been also noted in the urinary bladder under the same heading (microcystic transitional cell carcinomas) [5,17,18] along with the lipid cell variant of urothelial carcinoma with histologic appearance similar to lipoblasts [19–22].
The term microcystic, in the pancreas, however, is used specifically for serous cystadenomas (and its look-alikes) that are characterized by macroscopically visible small cysts (only a few millimeters in diameter) that form a distinctive sieve-like arrangement. Therefore, although “microcystic” is probably the best descriptive term for this pattern, it ought to be avoided for this variant of PDCA since it is already fully identified with a benign and distinctive tumor in this organ. Also, although the cells in this pattern have “signet-ring” appearance; we, as many others, are of the opinion that the term “signet ring” ought to be avoided outside the setting of diffuse infiltrative-type (E-cadherin related) carcinoma that is characterized by individual cell infiltration. In the void of the liberty to use these terms, the best descriptive names to designate this pattern are felt to be vacuolated or cribriform adenocarcinoma. The latter has been associated with a distinctive and well-differentiated tumor in the breast and may be misleading as well.
Fortunately, the name one chooses to describe this variant may not be that important because the findings in this study suggest that this is a morphologic variant of pancreatobiliary-type adenocarcinoma, not a separate tumor type. Its clinical characteristics are not too different from PDCA, with the exception of higher incidence of smoking history. The association with smoking may be of interest because studies in other organs are now revealing that smoking triggers different pathways of carcinogenesis [23,24] and may have closer association with certain glandular subtypes [25]. Along the same lines, although it did not reach statistical significance, the incidence of K-ras mutation appeared to be higher in vacuolated carcinomas in our population. Whether this has a role in the development of this distinctive secretory/ vacuolated pattern needs further scrutiny because it is becoming increasingly clear that K-ras is an oncogene commonly involved in glandular neoplasia of various organs [26], and different types of K-ras mutation may be responsible for different patterns and degrees of secretory properties.
The clinical course of vacuolated variant appears to be slightly more aggressive than the average PDCA, putting it in the more poorly differentiated category. Breast carcinomas with similar pattern also reported to have worse prognosis, particularly in elderly patients [27]. While this may be surprising based on the degree of glandular secretory differentiation, it is not at all if the nuclear grade is taken into consideration because this variant is often associated with high nuclear grade; despite their compressed (attenuated) appearance, the nuclei are often very atypical, hyperchromatic, and irregular, an important clue for the diagnosis of this pattern.
In conclusion, the morphologic spectrum of PDCA includes a vacuolated (cribriform) pattern that ought to be recognized because it may be important in the often challenging differential diagnosis of this tumor type both in limited specimens from the pancreas and metastatic sites.
Acknowledgments
This study is supported in part by the National Cancer Institute Specialized Program in Research Excellence (SPORE) CA101936 in Pancreas Cancer (PAR-02-068) and in part by the Georgia Cancer Coalition Distinguished Cancer Clinicians and Scientists Program.
Footnotes
Conflict of interest The authors have no conflict of interest.
This study was presented in part at the annual meeting of the United States and Canadian Academy of Pathology in Atlanta, GA in 2000.
Contributor Information
Nevra Dursun, Department of Pathology, Istanbul Education and Research Hospital, Istanbul, Turkey.
Jining Feng, Department of Pathology, Karmanos Cancer Institute and Wayne State University, Detroit, MI, USA.
Olca Basturk, Department of Pathology, Memorial Sloan–Kettering Cancer Center, New York, NY, USA.
Sudeshna Bandyopadhyay, Department of Pathology, Karmanos Cancer Institute and Wayne State University, Detroit, MI, USA.
Jeanette D. Cheng, Department of Pathology, Piedmont Hospital, Atlanta, GA, USA
Volkan N. Adsay, Email: volkan.adsay@emory.edu, nadsay@emory.edu, Department of Pathology and Laboratory Medicine, Emory University Hospital, 1364 Clifton Road NE, Room H-180B, Atlanta, GA 30322, USA
References
- 1.Adsay N, Bandyopadhyay S, Basturk O, Kilinc N, Andea A, Cheng J. Chronic pancreatitis or pancreatic ductal adeno-carcinoma? Histopathologic criteria for the differential diagnosis. Semin Diagn Pathol. 2005;21(4):268–276. [Google Scholar]
- 2.Adsay N, Basturk O, Klimstra D, Klöppel G. Pancreatic pseudotumors: non-neoplastic solid lesions of the pancreas that clinically mimic pancreas cancer. Semin Diagn Pathol. 2005;21(4):260–267. doi: 10.1053/j.semdp.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Cubilla A, Fitzgerald PJ. Pancreas cancer. I. Duct adenocarcinoma. A clinical-pathologic study of 380 patients. Pathol Annu. 1978;13:241–289. [PubMed] [Google Scholar]
- 4.Hruban RH, Pitman MB, Klimstra DS. Tumors of the pancreas. ARP; Washington: 2007. [Google Scholar]
- 5.Sari A, Uyaroglu MA, Ermete M, Oder M, Girgin C, Dincer C. Microcystic urothelial carcinoma of the urinary bladder metastatic to the penis. Pathol Oncol Res. 2007;13(2):170–173. doi: 10.1007/BF02893496. [DOI] [PubMed] [Google Scholar]
- 6.Delahunt B, Eble JN, Nacey JN, Thornton A. Immunohistochemical evidence for mesothelial origin of paratesticular adenomatoid tumour. Histopathology. 2001;38(5):479. doi: 10.1046/j.1365-2559.2001.1163a.x. [DOI] [PubMed] [Google Scholar]
- 7.Young RH. Testicular tumors—some new and a few perennial problems. Arch Pathol Lab Med. 2008;132(4):548–564. doi: 10.5858/2008-132-548-TTNAAF. [DOI] [PubMed] [Google Scholar]
- 8.Umezu H, Kuwata K, Ebe Y, Yamamoto T, Naito M, Yamato Y, et al. Microcystic variant of localized malignant mesothelioma accompanying an adenomatoid tumor-like lesion. Pathol Int. 2002;52(5–6):416–422. doi: 10.1046/j.1440-1827.2002.01357.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuchna I, Matyja E, Wierzba-Bobrowicz T, Mazurowski W. Microcystic meningioma—a rarely occurring morphological variant of meningioma. Folia Neuropathol. 1994;32(4):259–263. [PubMed] [Google Scholar]
- 10.Skalova A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34 (5):599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 11.Ghannoum JE, Freedman PD. Signet-ring cell (mucin-producing) adenocarcinomas of minor salivary glands. Am J Surg Pathol. 2004;28(1):89–93. doi: 10.1097/00000478-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Che M, Tornos C, Deavers MT, Malpica A, Gershenson DM, Silva EG. Ovarian mixed-epithelial carcinomas with a microcystic pattern and signet-ring cells. Int J Gynecol Pathol. 2001;20 (4):323–328. doi: 10.1097/00004347-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Young RH, Hart WR. Metastases from carcinomas of the pancreas simulating primary mucinous tumors of the ovary. A report of seven cases. Am J Surg Pathol. 1989;13(9):748–756. doi: 10.1097/00000478-198909000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Prat J. Ovarian carcinomas, including secondary tumors: diagnostically challenging areas. Mod Pathol. 2005;18(Suppl 2):S99–S111. doi: 10.1038/modpathol.3800312. [DOI] [PubMed] [Google Scholar]
- 15.Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis. Am J Surg Pathol. 2003;27(7):985–993. doi: 10.1097/00000478-200307000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Adsay NV, Andea A, Basturk O, Kilinc N, Nassar H, Cheng JD. Secondary tumors of the pancreas: an analysis of a surgical and autopsy database and review of the literature. Virchows Arch. 2004;444(6):527–535. doi: 10.1007/s00428-004-0987-3. [DOI] [PubMed] [Google Scholar]
- 17.Young RH, Zukerberg LR. Microcystic transitional cell carcinomas of the urinary bladder. A report of four cases. Am J Clin Pathol. 1991;96(5):635–639. doi: 10.1093/ajcp/96.5.635. [DOI] [PubMed] [Google Scholar]
- 18.Leroy X, Leteurtre E, De La Taille A, Augusto D, Biserte J, Gosselin B. Microcystic transitional cell carcinoma: a report of 2 cases arising in the renal pelvis. Arch Pathol Lab Med. 2002;126(7):859–861. doi: 10.5858/2002-126-0859-MTCC. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Beltran A, Sauter G, Gasser T. Infiltrating urothelial carcinoma. IARC; Lyon: 2004. [Google Scholar]
- 20.Soylu A, Aydin NE, Yilmaz U, Kutlu R, Gunes A. Urothelial carcinoma featuring lipid cell and plasmacytoid morphology with poor prognostic outcome. Urology. 2005;65(4):797. doi: 10.1016/j.urology.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Leroy X, Gonzalez S, Zini L, Aubert S. Lipoid-cell variant of urothelial carcinoma: a clinicopathologic and immunohisto-chemical study of five cases. Am J Surg Pathol. 2007;31(5):770–773. doi: 10.1097/01.pas.0000213410.48805.16. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Beltran A, Amin MB, Oliveira PS, Montironi R, Algaba F, McKenney JK, et al. Urothelial carcinoma of the bladder, lipid cell variant: clinicopathologic findings and LOH analysis. Am J Surg Pathol. 2010;34(3):371–376. doi: 10.1097/PAS.0b013e3181cd385b. [DOI] [PubMed] [Google Scholar]
- 23.Paris C, Clement-Duchene C, Vignaud JM, Gislard A, Stoufflet A, Bertrand O, et al. Relationships between lung adenocarcinoma and gender, age, smoking and occupational risk factors: a case–case study. Lung Cancer. 2010;68(2):146–153. doi: 10.1016/j.lungcan.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Mountzios G, Fouret P, Soria JC. Mechanisms of disease: signal transduction in lung carcinogenesis—a comparison of smokers and never-smokers. Nat Clin Pract Oncol. 2008;5(10):610–618. doi: 10.1038/ncponc1181. [DOI] [PubMed] [Google Scholar]
- 25.Borras J, Borras JM, Galceran J, Sanchez V, Moreno V, Gonzalez JR. Trends in smoking-related cancer incidence in Tarragona, Spain, 1980–96. Cancer Causes Control. 2001;12(10):903–908. doi: 10.1023/a:1013764220293. [DOI] [PubMed] [Google Scholar]
- 26.Porta M, Crous-Bou M, Wark PA, Vineis P, Real FX, Malats N, et al. Cigarette smoking and K-ras mutations in pancreas, lung and colorectal adenocarcinomas: etiopathogenic similarities, differences and paradoxes. Mutat Res. 2009;682(2–3):83–93. doi: 10.1016/j.mrrev.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Tavassoli FA, Norris HJ. Secretory carcinoma of the breast. Cancer. 1980;45(9):2404–2413. doi: 10.1002/1097-0142(19800501)45:9<2404::aid-cncr2820450928>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]


