Abstract
We tested the hypothesis that differences in (+)-methamphetamine (METH) disposition during late rat pregnancy could lead to increased vulnerability to acute METH effects. The disposition of a single 1 mg/kg i.v. METH dose was studied during early (gestation day 7, GD7) and late (GD21) gestation. Results showed gestation time-dependent pharmacokinetics, characterized by a significantly higher area under the METH serum concentration versus time curve and a lower clearance on GD21 (p < 0.05; total, renal, and nonrenal clearance). The terminal elimination half-life (t1/2λz) of METH and (+)-amphetamine (AMP; a pharmacologically active metabolite of METH) were not different on GD7, but by GD21, AMP t1/2λz was 37% longer than METH t1/2λz (p < 0.05). To identify the mechanism for AMP metabolite changes, intravenous AMP pharmacokinetics on GD21 were compared with AMP metabolite pharmacokinetics after intravenous METH. The intravenous AMP t1/2λz was significantly shorter than metabolite AMP t1/2λz (p < 0.05), which suggested AMP metabolite formation (not elimination) was the rate-limiting process. To understand the medical consequence of METH use during late-stage pregnancy, timed-pregnant rats received an intravenous dose of saline or METH (1, 3, or 5.6 mg/kg) on GD21, 0 to 2 days antepartum. Although one rat died and another had stillbirths at term after the 5.6-mg/kg dose, the pharmacokinetic values for all of the other animals were not significantly different. In conclusion, late-gestational clearance reductions lengthen METH exposure time, possibly increasing susceptibility to adverse effects, including death.
Introduction
(+)-Methamphetamine (METH) abuse has evolved over the last few decades to include an expanding population of users (Anglin et al., 2000). A particularly troubling trend is the rise in female users, who now account for ∼45% of METH abusers (Cohen et al., 2007). Because the majority of these women are of child-bearing age and METH use impairs decision making (Kalechstein et al., 2003), it should not be surprising that there is also an increased use during pregnancy (Arria et al., 2006). In a study of 72 self-reported female METH users in the Infant, Development, Environment, and Lifestyle study, Smith et al. (2008) reported that 83% of the women use the drug during the first trimester and 42% use the drug during the third trimester. The mothers' choice to use METH places the fetus at risk for serious adverse effects including developmental problems, accidents, and addiction.
The powerful force of METH addiction can lead to a quick progression from infrequent use of low doses to “binge” use, which is characterized by shorter time intervals between escalating METH doses (Cho and Melega, 2002). During these periods of binge use, a substantial METH body burden can accumulate since the time interval between each new dose is short relative to the terminal elimination half-life (t1/2λz) of METH [12 h in humans (Cook et al., 1993)]. To add to the problem, METH metabolism leads to the formation of a pharmacologically active metabolite, (+)-amphetamine (AMP), which could also accumulate. When METH is used during pregnancy, the fetus is also vulnerable to METH-induced effects (Szeto, 1993). In an animal model of METH use, Burchfield et al. (1991) reported that METH crosses the placenta in ≤30 s after maternal METH administration in near-term pregnant sheep. Assuming similar rapid METH penetration into the fetus occurs in humans, METH exposure to mother and fetus could result in an increased risk of toxicity and death.
Pregnancy likely alters the degree of maternal and fetal exposure to METH through pregnancy-induced physiological changes (Loebstein et al., 1997). Some of these pregnancy-related maternal physiological changes include increased plasma volume, decreased plasma protein binding, delays in gastric emptying time (Mattison et al., 1991; Loebstein et al., 1997), and alterations in metabolic enzyme activity (Loebstein et al., 1997). These physiological changes evolve throughout pregnancy and can be organ specific. For instance, renal blood flow in rats increases during mid gestation but returns to nonpregnant levels by late gestation [rat gestation is 21–23 days (Reckelhoff et al., 1992)].
Studies from our laboratory in pregnant rats demonstrate that METH systemic clearance (corrected for bioavailability; Cl/F) significantly decreases near the end of pregnancy on gestational day 20 (GD20) and GD21 (just before delivery). In these previous studies, pregnant Sprague-Dawley rats received steady-state infusions of METH at 5.6, 10, or 13.2 mg/kg per day (White et al., 2009). The infusion of METH to steady-state levels provided a means to accurately determine METH (and AMP) partitioning and equilibration across the maternal and fetal blood-brain barrier and placental-blood barrier. However, most human METH users prefer more rapid routes of administration such as smoking, snorting, and injecting (Rawson and Condon, 2007) to significantly enhance the “rush” of pharmacological effects.
Because studies of the high and dangerous METH doses used by addicted humans (e.g., 1 g/day) (Cho, 1990; EROWID, http://www.erowid.org/) are not possible in pregnant or nonpregnant humans, animal models such as the rat must be used to help predict human adverse effects, including the potential for toxicity. Although the pregnant rat is not a perfect model for human pregnancy, the rat and human exhibit many comparable pregnancy-induced physiological changes (Conrad, 1987; Mattison et al., 1991; Dowell and Kauer, 1997) and have many placental similarities (DeSesso, 1997).
We tested the hypothesis that changes in METH (and AMP) disposition after intravenous METH administration occur during late-stage rat pregnancy. To assess the possibility of gestation time-dependent changes, pregnant Sprague-Dawley rats were treated with 1 mg/kg METH on GD7 (during early pregnancy) and 1 mg/kg METH or AMP on GD21 (late in pregnancy). To determine whether there were acute METH dose-dependent toxicological changes, we studied intravenous bolus doses of METH (1, 3, or 5.6 mg/kg) administered to gravid rats on GD21. Even though rats at the 5.6-mg/kg dose died, the pharmacokinetic values for the surviving animal groups were not significantly different. Results also showed there were late gestational-stage reductions in METH clearances that lengthened METH exposure time and thus increased the susceptibility to adverse effects, including the potential for lethality.
Materials and Methods
Drugs and Reagents.
(+)-Methamphetamine HCl, (+)-amphetamine sulfate, (±)-4-hydroxy-methamphetamine hydrochloride, and (±)-4-hydroxy-amphetamine hydrobromide were obtained from the National Institute on Drug Abuse (Rockville, MD). All drug concentrations were calculated as the free base form and prepared in sterile saline. (±)-Methamphetamine-d5 and (±)-amphetamine-d11 were purchased from Sigma-Aldrich (St. Louis, MO). All other reagents used in these studies were purchased from Thermo Fisher Scientific (Waltham, MA), unless specified otherwise.
Animals.
Timed-pregnant, female Sprague-Dawley rats with dual indwelling polyurethane jugular venous catheters [0.025 (i.d.) × 0.04 (o.d.) inches] arrived on GD3 from Charles River Laboratories, Inc. (Raleigh, NC). The catheters were imbedded in the subcutaneous space between the scapulae. One week after arrival, the animals were anesthetized with isoflurane, and their catheters were externalized. Catheters were flushed three times per week with 200 μl of saline followed by 50 μl of heparin/glycerol (500 U) to maintain patency. Rats were housed individually in an animal care facility with a 12-h light/dark cycle (6:00 AM to 6:00 PM) and an average 22°C ambient temperature. They received food (8640 Rodent Diet with 22% crude protein/5% crude fat; Harlan Teklad, Madison, WI) and water ad libitum. Animal protocols were in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the National Institutes of Health. They were approved by the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences (Little Rock, AR) before beginning the experiments.
Effect of Dose on GD21 Pharmacokinetics.
On GD19, a predrug blood sample (80 μl) was collected to determine baseline hematocrit levels. On GD21, the gravid animals were treated with an intravenous bolus dose of saline or METH (1, 3, or 5.6 mg/kg; n = 5 per dose) over 15 s via the right jugular venous catheter. The catheter was then flushed with 200 μl of saline. Blood was drawn at predetermined times of 1, 5, 15, 30, 60, 120, 210, 300, 390, and 510 min after METH administration. This sampling period was based on previous experiments from our laboratory in which the METH t1/2λz was determined to be ∼70 min in male and nonpregnant female rats dosed with 1 or 3 mg/kg i.v. METH (Milesi-Hallé et al., 2005), thus >99% of METH would be cleared by 510 min. Blood sample volumes consisted of 50 μl for earlier time points (1–210 min) and 100 μl for later time points; however, an additional 30 μl was collected at 30 and 510 min for determination of hematocrits. Sterile saline was administered to the animals in volumes equal to the volume of blood collected after each time point to prevent hypovolemia. The total volume of blood collected from each animal during the pharmacokinetic study was no more than 5% of the rat's total blood volume, based on the animal's weight on GD21 (320–446 g). Serum was collected after centrifugation and stored at −80°C until analyzed.
In addition to the experimental saline-treated control group, two additional control groups were used for monitoring procedural and health stress. A noncatheterized group was used to control for procedural stresses (i.e., catheter implantation and externalization; anesthesia). No drugs were administered, and no blood was collected for this group. A third control group served to control for potential changes in the health of the experimental animals because of procedures (e.g., effects of blood loss from sampling) and handling. These rats were exposed to the same procedures as the saline group, with the exception of blood sampling for pharmacokinetic analysis. Minimal volumes of blood were drawn (30 μl) to monitor hematocrit levels at times that coincided with blood samples drawn from the treatment groups. As mentioned previously, these time points included GD19 and GD21 (30 and 510 min), as well as postnatal day 1 (PND1), PND4, and PND7.
Urinary Excretion and Pharmacokinetic Studies on GD7 and GD21.
Pregnant catheterized rats received a 15-s intravenous bolus injection of 1 mg/kg METH on GD7 or GD21, or a dose of AMP (1.1 mg/kg) equimolar to 1 mg/kg METH on GD21 (n = 5 per group). After drug administration and the first blood collection at 5 min, the animals were placed in metabolism cages (Nalgene; Nalge Nunc International, Rochester, NY) where they had ad libitum access to food and water. Animals were removed briefly from their cages for blood sampling. Sampling times, volumes, and processing were the same as in the previous experiment.
Animals remained in the metabolism cages until the final urine samples were collected (24 h after drug administration). For the first hour of the pharmacokinetic experiment, each urine void was quickly collected. After the first hour, urine samples were collected when volumes were >500 μl. Urine pH, appearance, volume, and time of collection were noted at each collection. After centrifugation, urine was stored at −80°C until analysis.
Myoglobin Detection in Urine and Serum on GD7 and GD21.
The urine collected during the pharmacokinetic experiments on GD7 and GD21 was darker than normal or red-colored on some occasions for all of the animals, which suggested the possibility of hemoglobin in the urine. The presence of hemoglobin was determined by analysis with urine test strips (Multistix 10 SG Reagent Strips for Urinalysis; Bayer Corp., Pittsburgh, PA); however, these test strips do not differentiate between the presence of myoglobin or free hemoglobin. To determine which was present, the solubility of the proteins was assessed by ammonium sulfate precipitation. Hemoglobin precipitates out of the urine in the presence of an 80% saturation with ammonium sulfate, whereas myoglobin precipitates only after 100% saturation (Hamilton et al., 1989). Ammonium sulfate precipitation (80% saturation) was performed by adding 0.56 g of ammonium sulfate to a 1-ml specimen of fresh uncentrifuged urine. All crystals were dissolved by mixing the solution for 5 min, followed by centrifugation at 2300 rpm for 3 min. The supernatant from the solution of urine was again tested with a Multistix SG10 test strip, and if still positive, the test was reported as positive for myoglobin in the urine. If negative, it was reported as positive for hemoglobin in the urine. Myoglobin from equine skeletal muscle was used as a positive control (BioChemika; Sigma-Aldrich).
Quantitation of METH and AMP Concentrations.
Determination of METH and AMP concentration in rat serum was based on the liquid-liquid phase extraction (LLE) method developed by Hendrickson et al. (2006). In brief, 25 or 50 μl of each serum sample was diluted to 100 μl using drug-free normal rat serum (Pel-Freez Biologicals, Rogers, AR). Calibration (0.3–1000 ng/ml) and quality control (3–800 ng/ml) drug standards were also prepared using drug-free normal rat serum. Internal standards (10 μl), consisting of a mixture of 100 μl of (±)-methamphetamine-d5 and (±)-amphetamine-d11, were added to all samples and standards. Proteins were precipitated with the addition of ice-cold trichloroacetic acid (100 μl of 20% w/v). The samples were vortex mixed, rotated, and centrifuged. The supernatant was then collected and filtered.
Analysis of METH and AMP concentrations in urine was accomplished using solid-phase extraction (Hendrickson et al., 2004). To summarize, urine samples were centrifuged at 10,000 rpm for 5 min. Because the METH and AMP concentrations were expected to be very high, the supernatant was diluted 100-fold in drug-free normal rat serum. Internal standard (10 μl) was added to diluted samples (100 μl), along with calibration and quality control standards. A 100-μl aliquot of 8 M guanidine-HCl and 200 μl of 10% w/v ZnSO4-7H2O were added to each sample to denature and precipitate the proteins, followed by vortexing and centrifugation. The supernatants were loaded and washed using conditioned Oasis HLB (1 ml × 30 mg) solid-phase extraction columns (Waters, Milford, MA). Analytes were eluted with 1 ml of acetonitrile containing 0.1% (v/v) acetic acid. The eluent was evaporated using N2 vaporizer at 38°C for ∼20 min. The residue was resuspended in 100 μl of 5 mM ammonium acetate (pH 3.7) containing 5% (v/v) acetonitrile.
The drug-containing solutions from LLE were injected (20 μl) onto a 3-μm BDS Hypersil C8 column (Thermo Fisher Scientific) and analytically separated using a binary linear gradient (Hendrickson et al., 2006). The flow rate was 0.3 ml/min. A Waters Alliance 2695 liquid chromatography (LC) coupled to a Waters/Micromass Quattro LC triple quadrupole mass spectrometer (Waters) with an electrospray interface and a Mark II source in the positive ion mode was used for analyzing part of the serum concentrations after 1, 3, or 5.6 mg/kg METH. Retention times were 8.22 ± 0.04 and 7.95 ± 0.04 min for METH and AMP, respectively.
A SIL-HTa autosampler (Shimadzu, Columbia, MD) coupled to a Waters/Micromass Quattro Premiere triple quadrupole mass spectrometer with an electrospray interface in the positive-ion mode was used for the remaining serum and urine samples. Samples (20 μl) were injected onto a 3-μm BDS Hypersil C8 column, and the column was maintained at 40°C using a CTO-10A column oven (Shimadzu). The analytes were separated using a binary gradient with a flow rate of 0.3 ml/min through two LC 10-ADVP pumps (Shimadzu). Solvent A was 5 mM ammonium acetate buffer (pH 3.7) with 5% (v/v) acetonitrile. Solvent B was 5 mM ammonium acetate buffer (pH 3.7) with 95% (v/v) acetonitrile. The initial conditions consisted of 100% solvent A for the first 2.5 min after the sample was injected. Subsequent increases of solvent B to 65% were achieved over the next 5 min and held for 2.5 min. Solvent B was reduced back to 0% over the next 2 min. The first 4 min of the chromatographic run were directed to waste, followed by a run time of 11 min. The retention times for the LLE method were 7.86 ± 0.06 min for AMP and 8.28 ± 0.2 min for METH. The retention times were longer for the solid-phase extraction method, 8.09 ± 0.06 and 8.37 ± 0.06 min for AMP and METH, respectively. Both systems were controlled using the computer software Masslynx 4.1 (Waters). No attempt was made to determine the concentrations of Phase II metabolites.
Pharmacokinetic Analysis.
To determine the pharmacokinetic parameters of METH and AMP, log concentration versus time curves for each animal were analyzed by model-dependent and model-independent methods using WinNonlin (Pharsight, Mountain View, CA). For model-dependent analyses of METH, bi- and tri-exponential curves were fit successively to the individual data sets using no weighting, 1/y and 1/y2 weighting functions, where y was the predicted concentration. The best-fit line was chosen by statistical analysis, visual inspection, analysis of the residuals, and examination of the coefficients of variation for each parameter.
For the model-independent analyses, pharmacokinetic parameters were derived from the log concentration versus time graph of METH and AMP concentrations in the terminal elimination phase with a first-order input function and no weighting, 1/y and 1/y2 weighting functions. The best-fit line was determined visually and statistically based on the best-fit line to the concentration time data points. Pharmacokinetic parameters from the chosen model-dependent analysis of each animal were compared with the parameters determined from the model-independent analysis to assure validity of both methods.
METH pharmacokinetic parameters were determined using the following equations: Dose = ClT × AUC, Vd = ClT × λz, t1/2λz = 0.693/λz, ClT = intravenous dose/AUC0∞, MRT = 1/λz, ClR = fu × ClT, Vdss = MRT × ClT, and ClNR = ClT − ClR, where λz is the terminal elimination rate constant, MRT is mean residence time, Vdss is volume of distribution at steady state, Vd is the apparent volume of distribution, AUC0∞ is area under the serum concentration versus time curve from time 0 to infinity, ClR is renal clearance, fu is the fraction of unchanged METH in the urine, and ClNR is the nonrenal clearance.
AMP pharmacokinetic parameters consisted of AUC0∞, t1/2λz, maximum AMP serum concentrations (Cmax), time to reach maximum serum AMP concentrations (Tmax), and fraction of parent drug converted to a metabolite (fm). The calculation used to determine fm is given by fm = [(AUCm)p/Dp] × [Dm/(AUCm)m], where (AUCm)p refers to the total AUC of metabolite (AMP) after a single intravenous dose of the parent drug (METH), (AUCm)m is the total AUC of AMP after a single intravenous dose of the metabolite (AMP), Dp is the dose of the parent drug, and Dm is an equimolar dose of the metabolite.
General Experimental Observations.
Once the animals completed the pharmacokinetic experiment (GD21–GD22), they were returned to their home cages and monitored four to five times per day to determine time of parturition. This time point was then designated as PND0. Beginning on the morning of PND1 at approximately 9:00 AM, postnatal observations (5-min duration) were performed at the animal's home cage with minimal disruptions to behavior and were not blinded. These observations consisted of monitoring nonmaternal activities (i.e., eating, drinking, grooming, and sleeping) and maternal activities (i.e., grooming pups, carrying pups, and nursing). Activity ratings were scored based on the absence or presence of a particular behavior. Subsequently, daily measurements of litter size and weight were determined. Postnatal maternal observations were conducted, as described previously, at approximately 3:00 PM. Dam and litter measurements continued daily from PND1 through PND7. As mentioned previously, hematocrits were monitored on PND1, PND4, and PND7 in all catheterized dams (after litter size and weight measurements). Once the last postnatal observation was complete on the afternoon of PND7, all dams and litters were humanely sacrificed by decapitation under isoflurane anesthesia.
Statistical Analysis.
All values are represented as the means ± S.D.; however, harmonic means and “pseudo” S.D. values were calculated for the t1/2λz values (Lam et al., 1985). A Grubbs' test was used to detect any outliers among the METH concentrations at 510 min after 1, 3, or 5.6 mg/kg i.v. METH. A one-way analysis of variance (ANOVA) with dose as the main factor was used to test for any significant differences for each pharmacokinetic parameter of the dose-range-finding pharmacokinetics studies conducted on GD21. For litter weight and size data, as well as hematocrit measurements in pregnant rats, a two-way repeated-measures ANOVA was performed with one within factor (GD or PND) and one between factor (dose). To determine whether statistically significant differences exist in the average maternal activity ratings, a two-way ANOVA was used with one within factor (activity rate) and one between factor (dose). This statistical test was also conducted to detect any significant differences among METH and its metabolite AMP t1/2λz on GD7 and GD21. If any of the parameters were found to be significant, a Student-Newman-Keuls post hoc test was conducted. In addition, a t test was performed on each pharmacokinetic parameter of METH and AMP after an intravenous bolus dose of 1 mg/kg METH on GD7 or GD21. Finally, statistical comparisons were made using a t test for METH and AMP pharmacokinetic parameters after an intravenous bolus dose of 1 mg/kg METH or an equimolar dose of AMP on GD21. ANOVA and t tests were conducted with SigmaStat version 2.03 software (SPSS Inc., Chicago, IL). For all analyses, a value of p < 0.05 was considered to be statistically significant.
Results
Effect of Gestation Time on METH and AMP Pharmacokinetics.
To determine whether gestation time affects METH pharmacokinetic properties, we first studied the pharmacokinetics of a 1-mg/kg i.v. METH dose on GD7 and GD21, as representative of time points for early and late pregnancy. Comparisons of METH pharmacokinetic values derived from model-independent and model-dependent analyses of these data were very similar, with less than a 9% difference between pharmacokinetic parameters (data not shown). Model-dependent pharmacokinetic models can be complex and sometimes difficult to interpret, especially in pregnancy (Krauer and Krauer, 1977); therefore, we chose to use the pharmacokinetic values from the noncompartmental analysis. In most of the pharmacokinetic concentration versus time figures, we present data from the single animal that best represents the average values of all animals in their treatment groups. Average data and the associated variance for all the animals in each study are shown in the tables.
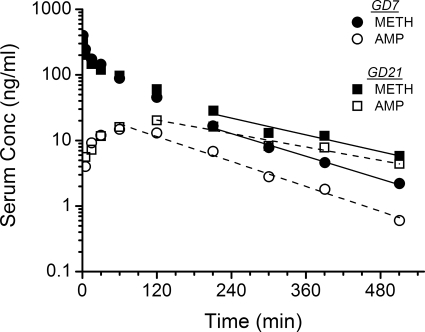
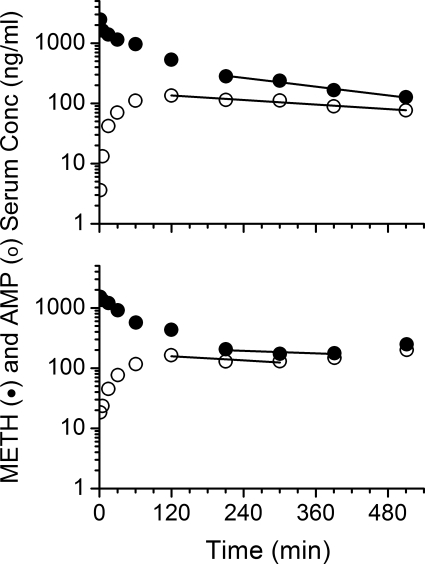
Representative average METH and AMP serum concentration versus time curves from GD7 and GD21 after a 1-mg/kg dose of METH are shown in Fig. 1. A bi-exponential decline in concentrations, similar to GD21, was seen for METH on GD7; however, the terminal elimination phase for GD7 declined more rapidly. Consequently, the AUC was significantly smaller (p < 0.05) on GD7 than GD21. These values, along with the other average METH and AMP pharmacokinetic parameters, are summarized in Table 1.
Fig. 1.
Serum concentration (Conc) versus time profile of METH and AMP after intravenous bolus dose of METH at 1 mg/kg in pregnant female Sprague-Dawley rats on GD7 or GD21. Semilogarithmic graphs are representative of one animal from each group (n = 5 per dose group) that portrays the mean pharmacokinetic values. The best-fit lines for the serum concentration versus time profile of METH (solid line) and AMP (dashed line) were determined by a noncompartmental model in the terminal elimination phase.
TABLE 1.
METH and AMP metabolite pharmacokinetics in timed-pregnant Sprague-Dawley rats after an intravenous bolus dose of 1 mg/kg METH on GD7 or GD21
Pharmacokinetic parameters were determined from model-independent analyses of the METH serum concentration versus time profiles.
| METH | GD7 | GD21 |
|---|---|---|
| AUC (ng-min/ml) | 17,997 ± 1489 | 24,445 ± 4477c |
| t1/2λz (min) | 91 ± 16a | 115 ± 6a,c |
| MRT (min) | 95 ± 3 | 157 ± 19c |
| Vd (l/kg) | 7 ± 1 | 7 ± 1 |
| Vdss (l/kg) | 5 ± 0.3 | 6 ± 1 |
| ClT (ml · min−1 · kg−1) | 55 ± 5 | 39 ± 6c |
| ClR (ml · min−1 · kg−1) | 6 ± 1 | 3 ± 1c |
| ClNR (ml · min−1 · kg−1) | 50 ± 5 | 36 ± 5c |
| METH dose in urine (%) | 10 ± 3 | 8 ± 2 |
| AMP metabolite | ||
| AUC (ng-min/ml) | 3459 ± 926 | 7025 ± 1625c |
| t1/2λz (min) | 95 ± 12a | 189 ± 11a,c,d |
| Tmax (min) | 72 ± 27 | 156 ± 49c |
| Cmax (ng/ml) | 16 ± 4 | 22 ± 4c |
| AMP dose in urine (%) | 2 ± 0.4 | 3 ± 0.6 |
| Molar ratio of AMP/METH AUCb | 0.21 ± 0.05 | 0.31 ± 0.03c |
Harmonic mean and pseudo S.D. (Lam et al., 1985).
The molar ratio of AMP/METH AUC was achieved by dividing the nmol/ml per hour AMP AUC value by the nmol/ml per hour METH AUC value.
p < 0.05 when compared to GD7 values.
p < 0.05 when compared to METH values on GD21.
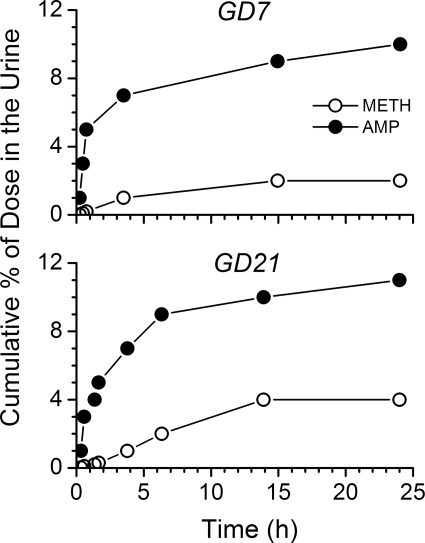
The GD21 values for ClT, ClNR, and ClR were significantly decreased (p < 0.05) compared with values on GD7 (Table 1). The cumulative percentage of METH and AMP eliminated unchanged in the urine was not affected by the gestation stage (Table 1; Fig. 2). METH t1/2λz was significantly prolonged (by 26%) on GD21, which resulted from a significantly decreased ClT without a change in the Vd. AMP metabolite t1/2λz was also significantly longer on GD21 compared with GD7 values (99% longer; Table 1 and Fig. 1).
Fig. 2.
Representative plot of the cumulative percentage of METH (●) and AMP (○) excreted unchanged in the urine of a pregnant rat on GD7 (top) and GD21 (bottom) after an intravenous METH dose of 1 mg/kg on GD7 and GD21. Urine was collected up to 24 h after drug treatment.
Rate-Limiting Step of AMP Elimination on GD21.
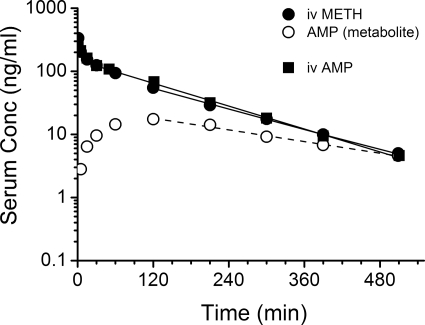
To help determine whether AMP metabolite disposition was formation or elimination rate limited, an equimolar dose of AMP was administered to a separate group of animals on GD21. As shown in Fig. 3, the serum concentration versus time profiles of intravenous AMP and intravenous METH were virtually superimposable, and pharmacokinetic parameters calculated for intravenous AMP (Table 2) and intravenous METH (Table 1) were not significantly different. Furthermore, after administering AMP to pregnant rats on GD21, fm was calculated as 0.32 ± 0.06. This was not different from the AMP/METH AUC molar ratio determined on GD21 (0.31 ± 0.03; Table 1) after METH administration.
Fig. 3.
Serum concentration (Conc) versus time curve of AMP from one animal representative of the average pharmacokinetics after an intravenous bolus dose of 1 mg/kg AMP to pregnant rats on GD21 and the serum concentration versus time curves of METH and its metabolite AMP (from Fig. 4) after a 1-mg/kg METH dose on GD21. The best-fit lines for the serum concentration versus time profile of intravenous METH and intravenous AMP (solid line) and AMP metabolite (dashed line) were determined by a noncompartmental model in the terminal elimination phase.
TABLE 2.
Intravenous AMP pharmacokinetics in timed-pregnant Sprague-Dawley rats after an intravenous-bolus dose of 1 mg/kg AMP on GD21
Pharmacokinetic parameters were determined from model-independent analyses of the AMP serum concentration versus time profiles.
| Pharmacokinetic Parameter (Intravenous AMP) | GD21 |
|---|---|
| AMP AUC (ng-min/ml) | 23,952 ± 2909 |
| t1/2λz (min) | 108 ± 8a |
| MRT (min) | 141 ± 17 |
| Vd (l/kg) | 6 ± 1 |
| Vdss (l/kg) | 6 ± 0.4 |
| ClT (ml · min−1 · kg−1) | 41 ± 6 |
| ClR (ml · min−1 · kg−1) | 3 ± 1 |
| ClNR (ml · min−1 · kg−1) | 38 ± 6 |
| AMP dose in urine (%) | 7 ± 2 |
Harmonic mean and pseudo S.D. (Lam et al., 1985).
Because the Tmax values for the AMP metabolite was significantly later on GD21 than GD7 (p < 0.05; Table 1), the AMP metabolite formation rate was slower on GD21 (Fig. 1). In addition, AMP concentrations on GD21 declined significantly more slowly than on GD7, which resulted in a 64% longer AMP metabolite t1/2λz compared with METH. The combined effect of a slower formation and longer AMP t1/2λz resulted in significant accumulation of AMP metabolite on GD21. This is best revealed by the 48% increase in the molar ratio of AMP/METH AUC on GD21 versus GD7.
Considered together, this series of experiments showed the rate-limiting step for AMP systemic elimination was AMP formation, and not the AMP elimination rate constant.
Effect of METH Dose on METH and AMP Pharmacokinetics on GD21.
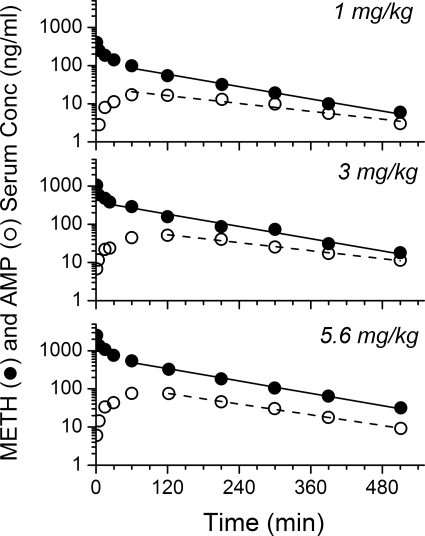
Representative rat METH and AMP serum concentration versus time curves after an intravenous bolus dose of 1, 3, or 5.6 mg/kg METH are shown in Fig. 4 (n = 5 per group). A total of seven rats was used for the 5.6 mg/kg METH dose because two of the seven developed METH-induced toxicities, which resulted in lethality. The METH and AMP serum concentrations in these two rats are shown in Fig. 5. One rat (along with the pups) died ∼30 min after completion of blood sampling; the other rat survived, but the pups delivered as stillbirths on GD25. The data from these two animals are not included in the detailed report of these studies in Table 3.
Fig. 4.
Serum concentration (Conc) versus time profile of METH and AMP after intravenous bolus dose of METH at 1, 3, or 5.6 mg/kg in pregnant female Sprague-Dawley rats on GD21. Semilogarithmic graphs were representative of one animal from each group (n = 5 per dose group) that portrays the mean pharmacokinetic values. The best-fit lines for the serum concentration versus time profile of METH (solid line) and AMP (dashed line) were determined by a noncompartmental model in the terminal elimination phase.
Fig. 5.
Serum concentration (Conc) versus time graph of METH and AMP after intravenous bolus METH (5.6 mg/kg) administration on GD21 to pregnant rats that developed lethal toxicity after METH administration. Top, METH and AMP concentrations for a pregnant rat that died approximately 9 h after METH administration. Bottom, METH and AMP concentrations for a pregnant rat that delivered stillbirths on GD25. The best-fit lines for the serum concentration versus time profile of METH (solid line) and AMP (dashed line) were determined by a noncompartmental model in the terminal elimination phase. These METH and AMP concentrations were compared to the concentrations for the pregnant rat representative of the mean pharmacokinetic values (n = 5) of this dose group shown previously in Fig. 4 (bottom). However, data from these two rats were not included with the surviving, apparently normal rats (n = 5) in the pharmacokinetic data analysis in Table 3.
TABLE 3.
METH and AMP metabolite pharmacokinetics in timed-pregnant Sprague-Dawley rats after an intravenous bolus dose of 1, 3, or 5.6 mg/kg METH on GD21
Pharmacokinetic parameters were determined from model-independent analyses of the METH and AMP serum concentration versus time profiles.
| 1 mg/kg | 3 mg/kg | 5.6 mg/kg | |
|---|---|---|---|
| METH | |||
| AUC (normalized for dose) [(ng-min/ml)/(mg/kg)] | 22565 ± 5278 | 21832 ± 1163 | 24756 ± 3212 |
| t1/2λz (min) | 110 ± 17a | 117 ± 5a | 109 ± 19a |
| MRT (min) | 131 ± 14 | 142 ± 8 | 127 ± 15 |
| Vd (l/kg) | 7 ± 2 | 7 ± 1 | 6 ± 2 |
| Vdss (l/kg) | 6 ± 1 | 6 ± 1 | 5 ± 1 |
| ClT (ml · min−1 · kg−1) | 44 ± 10 | 44 ± 2 | 38 ± 6 |
| AMP | |||
| AUC (normalized for dose) [(ng-min/ml)/(mg/kg)] | 5369 ± 1532 | 5370 ± 881 | 5768 ± 1282 |
| t1/2λz (min) | 170 ± 31a,c | 180 ± 10a,c | 151 ± 17a,c |
| Tmax (min) | 108 ± 27 | 120 ± 1 | 108 ± 27 |
| Cmax (ng/ml) | 17 ± 3 | 51 ± 8 | 129 ± 32 |
| Molar ratio of AMP/METH AUCb | 0.26 ± 0.02 | 0.27 ± 0.03 | 0.26 ± 0.08 |
Harmonic mean and pseudo S.D. (Lam et al., 1985).
The molar ratio of AMP/METH AUC was achieved by dividing the nmol/ml per hour AMP AUC value by the nmol/ml per hour METH AUC value.
p < 0.05 versus METH t1/2λz.
When the first death occurred unexpectedly during the study of the third rat in this dose group, we considered the possibility that this animal's outcome was an experimental outlier. However, when we continued to study additional rats to obtain a total of five animals in this group, we discovered another animal with toxicity in the form of stillborn pups. Because these experiments were not designed to determine a METH LD50 value in pregnant rats, we did not continue to study more rats after we successfully completed a series of five animals without deaths. However, later determination of the METH and AMP concentrations versus time curves in these rats (Fig. 5), especially after approximately 120 min, revealed the t1/2λz for the animal that delivered stillbirths was significantly prolonged at 249 min for METH and 484 min for AMP. Because the METH and AMP concentrations were higher at 510 min than 390 min for the pregnant rat that died, meaningful pharmacokinetic analysis could not be accomplished. Nonetheless, to show the magnitude of METH and AMP concentrations of the animals, the average METH and AMP AUC0510min of the five animals were 1376 and 507 ng-h/ml, respectively. In comparison, the METH and AMP AUC0510min of the animal that delivered stillbirths were 1978 and 794 ng-h/ml and of the animal that died were 2380 and 1111 ng-h/ml, respectively.
The remaining five animals administered 5.6 mg/kg METH completed the studies without serious complications; however, they did exhibit temporary chromodacryorrhea and stereotypic behavior such as excessive sniffing and licking. The average serum pharmacokinetic parameters for METH and AMP after the 1, 3, or 5.6 mg/kg METH doses (n = 5 per group) are presented in Table 3. There were no significant differences in the pharmacokinetic values determined for METH and AMP in the total of 15 rats studied at these three doses (after excluding the two animals from the 5.6-mg/kg dose). This suggested dose-independent pharmacokinetics in 15 of 17 rats.
The results from these intravenous bolus doses of METH in the current studies are consistent with the values for Cl/F in pregnant rats receiving continuous subcutaneous infusions of METH (5.6, 10, or 13.2 mg/kg per day) from GD7 to GD21 (White et al., 2009). Cl/F values corrected for fraction of the dose absorbed after subcutaneous infusions were similar from GD8 to GD17 (ranged from 41 ± 12 to 59 ± 16 ml · min−1 · kg−1; p ≥ 0.05). However, these values on GD20 and GD21 (ranged from 25 ± 4 to 36 ± 9 ml · min−1 · kg−1) were significantly decreased versus the values on GD8, GD10, and GD13. Thus, regardless of the route of METH administration (intravenous or subcutaneous infusion), there are late-gestational reductions in METH clearance.
Detection of Hemoglobin and Protein in Urine on GD7 and GD21.
Each animal treated with 1 mg/kg METH on both GD7 and GD21 had noticeably dark urine at times (three were found to have light red-colored urine), suggesting METH-induced hematuria. Dipstick testing was conducted, which confirmed the presence of heme in the urine, as well as protein. Results from ammonium sulfate precipitation tests showed that urine was negative for myoglobin, suggesting rhabdomyolysis was not the cause. Saline-treated control pregnant rats (n = 2) experienced the same experimental conditions as METH-treated rats, and heme and protein were also detected in the urine of these animals. In addition, no differences were found in urine pH (range, 5.5–9) or in average total urine volume (METH administration on GD7, 18.4 ± 4.8 ml; METH administration on GD21, 12.1 ± 3.7 ml; AMP administration on GD21, 16.7 ± 3.7 ml), respectively.
General Observations during Parturition and Postnatal.
All of the animals, with the exception of the two animals treated with 5.6 mg/kg, delivered within the normal time frame (GD22–GD23; data not shown). Average litter size and pup weight on PND1 are shown in Table 4. No significant differences were found between groups.
TABLE 4.
Average litter size and pup weight on PND1 for pups exposed prenatally to an intravenous bolus dose of saline or METH (1, 3, or 5.6 mg/kg) on GD21 or no treatment (control rats noncatheterized and without METH dosing)
| Average Litter Size | Average Pup Weight (g) | |
|---|---|---|
| Noncatheterized | 13 ± 3 | 6.9 ± 0.8 |
| Saline-hematocrit only | 12 ± 2 | 6.4 ± 0.3 |
| Saline | 12 ± 2 | 6.5 ± 0.6 |
| 1 mg/kg METH | 12 ± 1 | 7.1 ± 0.5 |
| 3 mg/kg METH | 13 ± 1 | 6.4 ± 0.6 |
| 5.6 mg/kg METH | 10 ± 2 | 6.4 ± 0.5 |
Maternal activity ratings (nursing, caring for self, and caring for pups) were determined for animals in the three METH dose groups, as well as the experimental control group (saline treated), the health control group (saline treated; hematocrits taken only), and the procedural control group (noncatheterized; no drug treatment) (data not shown). Activity ratings were scored in the animal's home cage and based on the presence or absence of an activity for 5-min observations at 9:00 AM and 3:00 PM from PND1 to PND7. There were no significant differences found for the average activity ratings of the combined data collected from PND1 to PND7.
Finally, hematocrit values were not significantly different between study animals with blood sampling and study animals in which blood was only collected for determination of hematocrits (data not shown). This comparison was made from GD19 to the end of the study on PND7. Average hematocrits, excluding the two rats with lethal events, were 38 ± 2% on GD19 (before drug administration), 35 ± 2% on GD21 (30 min), 35 ± 4% on GD21 (510 min), 32 ± 2% on GD22, 35 ± 2% on PND1, 40 ± 3% on PND4, and 41 ± 3% on PND7. By PND7, the hematocrits of the animals had returned to levels of nonpregnant female Sprague-Dawley rats [41% (Giknis, 2006)].
Discussion
We wanted to understand the possible mechanism(s) for increased vulnerability to METH use for pregnant dams and pups. To accomplish this goal, we studied effects of METH doses during early (GD7) and late (GD21) pregnancy. We found METH ClT was significantly decreased and t1/2λz was increased in late-stage pregnancy (Table 1; Fig. 1), without any change in Vd. This occurred despite the fact that the pharmacokinetic properties were found to be METH dose independent on GD21 (Table 3).
The three METH doses were chosen for study because low and middle doses produce only moderate (1 mg/kg) to high (3 mg/kg) pharmacological effects (e.g., locomotor activity) without any overt toxicity in male and nonpregnant female Sprague-Dawley rats (Milesi-Hallé et al., 2005, 2007). A higher METH intravenous dose of 5.6 mg/kg in drug naive male rats produces intense focused stereotypy and severe chromodacryorrhea, which are overt signs of METH toxicity (Byrnes-Blake et al., 2003). Intravenous doses of 10 mg/kg are sometimes lethal. Thus, studying METH doses at the threshold of toxicity (e.g., ≤3 mg/kg) gave us the best chance to discover changes at the interface between pharmacological and toxicological events. This also represented a model of human use in which binge use of METH by addicts is often at the boundary between a desired pharmacological high and serious toxicity.
In the current studies, similar effects were observed in pregnant rats administered 5.6 mg/kg METH as in male rats treated with 10 mg/kg METH (Byrnes-Blake et al., 2003). Indeed, deaths occurred in two of seven animals administered 5.6 mg/kg METH (one dam died on GD21 shortly after the last blood sample; the other delivered stillbirths on GD25; Fig. 5). This finding appears to suggest that late-stage pregnant rats were more susceptible to harmful METH effects than male rats. Although the exact cause of death on GD21 was not determined, METH intoxication in humans causes shock, hyperthermia, and organ failure (Lan et al., 1998). It is likely that one or more of these METH-induced complications contributed to the rise in METH and AMP concentrations found at 510 min in the pregnant rat that died (Fig. 5). No attempt was made to increase the number of study animals or METH doses to determine a METH LD50.
It is interesting to note that the other five animals in the 5.6-mg/kg dose group not only recovered quickly from the METH-induced chromodacryorrhea and stereotypy, they displayed pharmacokinetic profiles that were not significantly different from animals treated with lower doses (Table 3). In related studies, White et al. (2009) found pregnant rats chronically infused with 17.8 mg/kg per day s.c. METH from GD7 to GD21 died by GD11, yet animals infused with 13.2 mg/kg per day became tolerant to the METH-induced effects by mid gestation. These and the current studies suggest that chronic and acute METH administration at high doses results in a steep dose-response lethality curve.
Because METH pharmacokinetics were dose independent at 1, 3, and 5.6 mg/kg on GD21, we used a 1-mg/kg METH dose to examine the effect of gestational time on METH and AMP pharmacokinetics. GD7 was chosen as the early time point for comparison with the GD21 late time point because GD7 is after implantation (GD5–GD6) but before early differentiation (GD10) (DeSesso, 1997). In addition, the placenta-blood barrier is not mature until GD12 (de Rijk et al., 2002). These developmental events were considered important in our choice of an early time point for the studies, because placental permeability, along with the rate of maternal drug elimination, are important determinants of drug disposition in pregnancy (Szeto, 1993).
The pharmacokinetic properties were dependent on gestation time, with both METH and AMP elimination occurring more slowly as pregnancy advanced (Fig. 1; Table 1). The main mechanism for decreased elimination was a 28% reduction in ClNR. Although ClR decreased by 50% on GD21 (6 versus 3 ml · min−1 · kg−1), this route of elimination contributed only 8 to 11% of the ClT on GD7 and GD21. These gestation time-dependent clearance reductions could arise from pregnancy-induced physiological changes in plasma protein binding and hemodynamics. However, METH serum protein binding is unaltered throughout pregnancy [∼10% (White et al., 2009)]. Because glomerular filtration rate and renal blood flow increase during early and mid gestation and decline before delivery (Reckelhoff et al., 1992), renal hemodynamic changes likely contribute to the reduction in ClR seen on GD21.
It is unlikely that urine volume or pH contributed to METH ClR changes, because these physiological factors were not significantly different between GD7 and GD21. The late-gestational reduction in ClR was also not a result of proteinuria or hematuria, because proteinuria and hematuria were detected on both GD7 and GD21. The hematuria could also be caused by mechanical stress, along with administration of low doses of heparin used to maintain long-term catheter patency (Schuster and Lewis, 1987).
Hormonal fluctuations during pregnancy can affect transporters and enzymes. The organic cation transporter 3 is involved in the transport of METH across the brush-border membrane in rat kidneys and is inhibited by estradiol (Wu et al., 1998). Before delivery, estradiol concentrations increase compared to nulliparous and early-stage pregnant rats (Garland et al., 1987). Thus, competitive inhibition by estradiol in late-stage pregnancy could lead to less binding of METH to the transporters and reduced METH ClR.
A possible mechanism for reduced ClNR is decreased metabolic capacity. Neale and Parke (1973) reported a 25% reduction in CYP450 in late-gestational rats (GD19–GD20) relative to virgins. Ward and Pollack (1996) found in vitro metabolism of methanol in pregnant Sprague-Dawley rat (GD20) liver homogenates is significantly lower than nonpregnant rats, with no change in Km values.
When a metabolite such as AMP is eliminated more slowly than the parent drug, the rate-limiting step is the metabolite's elimination. Because AMP t1/2λz was significantly longer than METH t1/2λz on GD21 (Table 1), it seemed that the decline in AMP serum concentrations was elimination rate limited. Yet, the significantly longer AMP Tmax on GD21 compared to GD7 suggested AMP metabolite was formation rate limited. To determine the correct mechanism for the change on GD21, an equimolar dose of intravenous AMP was administered to pregnant rats to allow comparison of intravenous AMP and AMP metabolite kinetics. The results showed intravenous AMP declined at the same rate as intravenous METH, and not at the same rate as AMP metabolite (Fig. 3; Table 2). Therefore, AMP metabolite disposition is formation rate limited on GD7 and GD21.
Gestation stage-dependent pharmacokinetic patterns in the present study were consistent with previous studies using pregnant rats chronically infused with METH (White et al., 2009). Yet, METH pharmacokinetics in pregnant rats are different from nonpregnant rats (Milesi-Hallé et al., 2005), because dose-dependent pharmacokinetics of METH and AMP in nonpregnant females are found at intravenous doses of 1 or 3 mg/kg METH.
The potential for enhanced exposure during late-stage pregnancy prompted us to examine the health and pharmacological effects of METH on dams and pups at parturition and postnatally. Contrary to reports citing that prenatal METH use can increase the risk of premature delivery (Eriksson et al., 1978), a significant difference in parturition time compared with control dams was not found. Indeed, one rat treated with 5.6 mg/kg METH delivered stillbirths 2 to 4 days after normal parturition time. Slamberova et al. (2005) also observed the length of gestation was longest (GD23–GD24) in rats treated with 5 mg/kg METH s.c. throughout gestation.
In addition to increased risk of delivering prematurely, female METH users neglect and abuse their children (Connell-Carrick, 2007). Animal research aimed at understanding maternal behavior show rats treated with METH, cocaine, or opiates throughout gestation display attenuations in maternal behaviors, such as nursing, grooming pups, and nest building (Johns et al., 1994; Stafisso-Sandoz et al., 1998; Slamberova et al., 2005). We observed no significant differences in maternal behavior. Possible weaknesses of our experimental design include the limited range of maternal behavior monitored and the use of only a single treatment of METH; however, a single dose of cocaine administered on PND0 disrupts the onset of maternal behavior on PND1 (Johns et al., 1994).
These studies show changes in METH clearance during late-stage pregnancy are a possible cause of increased vulnerability to METH effects, if they exist, especially in human METH addicts that frequently binge (Cho and Melega, 2002). This can lead to substantial accumulation if the pregnant user does not increase the length of time between doses, as during nonpregnancy. The gestational time-dependent differences consisted of significant decreases in METH clearance and prolongation of t1/2λz on GD21 compared with GD7. The mechanism of organ clearance changes were attributed to significant reductions of ClNR and ClR as pregnancy advanced. Whereas the ClR was a minor elimination process in rats (8–11%), in humans it is a major mechanism of METH clearance with 37–45% of the METH dose excreted unchanged in urine (Cook et al., 1993). These METH clearance reductions in late-stage pregnant rats lengthen METH exposure time and thus increase the susceptibility to adverse effects, including the potential for maternal and fetal lethality.
Acknowledgments
We thank Melinda Gunnell, Sherri Wood, Jonathan Hubbard, Eric Peterson, Mike West, Michael Hambuchen, Amber Hampton, and Krishna Chimalakonda for assistance with these studies.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA07610]; the National Institutes of Health National Institute of Environmental Health Sciences [Grant T32-EA07310]; the National Institutes of Health National Center for Research Resources [Grant UL1-RR029884]; and a graduate fellowship from GlaxoSmithKline.
S.M.O. and W.B.G. have financial interests in and serve as Chief Scientific Officer and Chief Medical Officer, respectively, of InterveXion Therapeutics LLC (Little Rock, AR), a pharmaceutical biotechnology company focused on treating human drug addiction with antibody-based therapy.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.111.039446.
- METH
- (+)-methamphetamine
- ANOVA
- analysis of variance
- AMP
- (+)-amphetamine
- AUC0∞
- area under the serum concentration-versus-time curve from time zero to infinity
- ClNR
- nonrenal clearance
- ClR
- renal clearance
- ClT
- total body clearance
- Cl/F
- systemic clearance corrected for bioavailability
- GD
- gestation day
- fm
- fraction of parent drug converted to a metabolite
- fu
- fraction of unchanged parent drug in urine
- GD
- gestational day
- λz
- terminal elimination rate constant
- LC
- liquid chromatography
- LLE
- liquid-liquid phase extraction
- MRT
- mean residence time
- PND
- postnatal day
- t1/2λz
- terminal elimination half-life
- Vd
- volume of distribution
- Vdss
- volume of distribution at steady state.
Authorship Contributions
Participated in research design: White, Laurenzana, Hendrickson, Gentry, and Owens.
Conducted experiments: White, Hendrickson, and Laurenzana.
Contributed new reagents or analytic tools: Hendrickson.
Performed data analysis: White and Owens.
Wrote or contributed to the writing of the manuscript: White, Laurenzana, Hendrickson, Gentry, and Owens.
References
- Anglin MD, Burke C, Perrochet B, Stamper E, Dawud-Noursi S. (2000) History of the methamphetamine problem. J Psychoactive Drugs 32:137–141 [DOI] [PubMed] [Google Scholar]
- Arria AM, Derauf C, Lagasse LL, Grant P, Shah R, Smith L, Haning W, Huestis M, Strauss A, Della Grotta S, et al. (2006) Methamphetamine and other substance use during pregnancy: preliminary estimates from the Infant Development, Environment, and Lifestyle (IDEAL) study. Matern Child Health J 10:293–302 [DOI] [PubMed] [Google Scholar]
- Burchfield DJ, Lucas VW, Abrams RM, Miller RL, DeVane CL. (1991) Disposition and pharmacodynamics of methamphetamine in pregnant sheep. JAMA 265:1968–1973 [PubMed] [Google Scholar]
- Byrnes-Blake KA, Laurenzana EM, Carroll FI, Abraham P, Gentry WB, Landes RD, Owens SM. (2003) Pharmacodynamic mechanisms of monoclonal antibody-based antagonism of (+)-methamphetamine in rats. Eur J Pharmacol 461:119–128 [DOI] [PubMed] [Google Scholar]
- Cho AK. (1990) Ice: a new dosage form of an old drug. Science 249:631–634 [DOI] [PubMed] [Google Scholar]
- Cho AK, Melega WP. (2002) Patterns of methamphetamine abuse and their consequences. J Addict Dis 21:21–34 [DOI] [PubMed] [Google Scholar]
- Cohen JB, Greenberg R, Uri J, Halpin M, Zweben JE. (2007) Women with methamphetamine dependence: research on etiology and treatment. J Psychoactive Drugs November; Suppl 4:347–351 [DOI] [PubMed] [Google Scholar]
- Connell-Carrick K. (2007) Methamphetamine and the changing face of child welfare: practice principles for child welfare workers. Child Welfare 86:125–144 [PubMed] [Google Scholar]
- Conrad KP. (1987) Possible mechanisms for changes in renal hemodynamics during pregnancy: studies from animal models. Am J Kidney Dis 9:253–259 [DOI] [PubMed] [Google Scholar]
- Cook CE, Jeffcoat AR, Hill JM, Pugh DE, Patetta PK, Sadler BM, White WR, Perez-Reyes M. (1993) Pharmacokinetics of methamphetamine self-administered to human subjects by smoking S-(+)-methamphetamine hydrochloride. Drug Metab Dispos 21:717–723 [PubMed] [Google Scholar]
- de Rijk EP, van Esch E, Flik G. (2002) Pregnancy dating in the rat: placental morphology and maternal blood parameters. Toxicol Pathol 30:271–282 [DOI] [PubMed] [Google Scholar]
- DeSesso JM. (1997) Comparative embryology, in Handbook of Developmental Toxicology (Hood RD. ed) pp 111–174, CRC Press, Boca Raton [Google Scholar]
- Dowell RT, Kauer CD. (1997) Maternal hemodynamics and uteroplacental blood flow throughout gestation in conscious rats. Methods Find Exp Clin Pharmacol 19:613–625 [PubMed] [Google Scholar]
- Eriksson M, Larsson G, Winbladh B, Zetterström R. (1978) The influence of amphetamine addiction on pregnancy and the newborn infant. Acta Paediatr Scand 67:95–99 [DOI] [PubMed] [Google Scholar]
- Garland HO, Atherton JC, Baylis C, Morgan MR, Milne CM. (1987) Hormone profiles for progesterone, oestradiol, prolactin, plasma renin activity, aldosterone and corticosterone during pregnancy and pseudopregnancy in two strains of rat: correlation with renal studies. J Endocrinol 113:435–444 [DOI] [PubMed] [Google Scholar]
- Giknis M. (2006) Clinical Laboratory Parameters for CR1:CD(SD) Rats, Charles River Laboratories, Wilmington, MA [Google Scholar]
- Hamilton RW, Hopkins MB, 3rd, Shihabi ZK. (1989) Myoglobinuria, hemoglobinuria, and acute renal failure. Clin Chem 35:1713–1720 [PubMed] [Google Scholar]
- Hendrickson H, Laurenzana E, Owens SM. (2006) Quantitative determination of total methamphetamine and active metabolites in rat tissue by liquid chromatography with tandem mass spectrometric detection. AAPS J 8:E709–E717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson HP, Milesi-Hallé A, Laurenzana EM, Owens SM. (2004) Development of a liquid chromatography-tandem mass spectrometric method for the determination of methamphetamine and amphetamine using small volumes of rat serum. J Chromatogr B Analyt Technol Biomed Life Sci 806:81–87 [DOI] [PubMed] [Google Scholar]
- Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. (1994) Effects of chronic and acute cocaine treatment on the onset of maternal behavior and aggression in Sprague-Dawley rats. Behav Neurosci 108:107–112 [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Green M. (2003) Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. J Neuropsychiatry Clin Neurosci 15:215–220 [DOI] [PubMed] [Google Scholar]
- Krauer B, Krauer F. (1977) Drug kinetics in pregnancy. Clin Pharmacokinet 2:167–181 [DOI] [PubMed] [Google Scholar]
- Lan KC, Lin YF, Yu FC, Lin CS, Chu P. (1998) Clinical manifestations and prognostic features of acute methamphetamine intoxication. J Formos Med Assoc 97:528–533 [PubMed] [Google Scholar]
- Lam FC, Hung CT, Perrier DG. (1985) Estimation of variance for harmonic mean half-lives. J Pharm Sci 74:229–231 [DOI] [PubMed] [Google Scholar]
- Loebstein R, Lalkin A, Koren G. (1997) Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet 33:328–343 [DOI] [PubMed] [Google Scholar]
- Mattison DR, Blann E, Malek A. (1991) Physiological alterations during pregnancy: impact on toxicokinetics. Fundam Appl Toxicol 16:215–218 [DOI] [PubMed] [Google Scholar]
- Milesi-Hallé A, Hendrickson HP, Laurenzana EM, Gentry WB, Owens SM. (2005) Sex- and dose-dependency in the pharmacokinetics and pharmacodynamics of (+)-methamphetamine and its metabolite (+)-amphetamine in rats. Toxicol Appl Pharmacol 209:203–213 [DOI] [PubMed] [Google Scholar]
- Milesi-Hallé A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM. (2007) Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol Biochem Behav 86:140–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MG, Parke DV. (1973) Effects of pregnancy on the metabolism of drugs in the rat and rabbit. Biochem Pharmacol 22:1451–1461 [DOI] [PubMed] [Google Scholar]
- Rawson RA, Condon TP. (2007) Why do we need an Addiction supplement focused on methamphetamine. Addiction 102:1–4 [DOI] [PubMed] [Google Scholar]
- Reckelhoff JF, Yokota SD, Baylis C. (1992) Renal autoregulation in midterm and late-pregnant rats. Am J Obstet Gynecol 166:1546–1550 [DOI] [PubMed] [Google Scholar]
- Schuster GA, Lewis GA. (1987) Clinical significance of hematuria in patients on anticoagulant therapy. J Urol 137:923–925 [DOI] [PubMed] [Google Scholar]
- Slamberová R, Charousová P, Pometlová M. (2005) Methamphetamine administration during gestation impairs maternal behavior. Dev Psychobiol 46:57–65 [DOI] [PubMed] [Google Scholar]
- Smith LM, Lagasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Della Grotta S, et al. (2008) Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol 30:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafisso-Sandoz G, Polley D, Holt E, Lambert KG, Kinsley CH. (1998) Opiate disruption of maternal behavior: morphine reduces, and naloxone restores, c-fos activity in the medial preoptic area of lactating rats. Brain Res Bull 45:307–313 [DOI] [PubMed] [Google Scholar]
- Szeto HH. (1993) Kinetics of drug transfer to the fetus. Clin Obstet Gynecol 36:246–254 [DOI] [PubMed] [Google Scholar]
- Ward KW, Pollack GM. (1996) Comparative toxicokinetics of methanol in pregnant and nonpregnant rodents. Drug Metab Dispos 24:1062–1070 [PubMed] [Google Scholar]
- White SJ, Laurenzana EM, Gentry WB, Hendrickson HP, Williams DK, Ward KW, Owens SM. (2009) Vulnerability to (+)-methamphetamine effects and the relationship to drug disposition in pregnant rats during chronic infusion. Toxicol Sci 111:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Kekuda R, Huang W, Fei YJ, Leibach FH, Chen J, Conway SJ, Ganapathy V. (1998) Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J Biol Chem 273:32776–32786 [DOI] [PubMed] [Google Scholar]