Abstract
Background
The prime-boost HIV vaccine regimen used in the recent RV144 trial resulted in modest efficacy of 31% over 3.5 years, but was substantially higher in the first year post-vaccination. We sought to explore the potential impact of a vaccine with rapidly waning efficacy in a South African population.
Methods
We explored two strategies using a dynamic compartmental epidemic model for heterosexual transmission of HIV: (1) vaccination of a single cohort (30%, 60% or 90% of the initial population), with potential booster vaccinations at 5-year or 2-year intervals over time, and (2) vaccination of the overall population at coverage levels (30%, 60% or 90%) that are constant over time, such that individuals are vaccinated or revaccinated to maintain effective coverage levels, given the rapidly waning protection of the vaccine. We also examined potential changes in post-vaccination condom use.
Results
The single cohort vaccination strategies did not have a substantial impact on HIV prevalence, although without boosters they still prevented 2–6% of the expected infections at 20 years, depending on the population coverage. The 5-year and 2-year booster strategies prevented 8–24% and 17–45% of the expected infections, respectively. The continuous vaccination strategies resulted in more substantial reductions in population HIV prevalence and greater numbers of infections prevented: HIV prevalence at 20 years was reduced from 23% to 8–14% and the number of expected infections was decreased by 34–59%, depending on the population coverage level. Moderate changes in post-vaccination condom use did not substantially affect these outcomes.
Conclusions
An HIV vaccine with partial efficacy and declining protection similar to the RV144 vaccine could prevent a substantial proportion of HIV infections if booster vaccinations were effective and available. Our estimates of the population impact of vaccination would be improved by further understanding of the duration of protection, the effectiveness of booster vaccination, and whether the vaccine efficacy varies between subpopulations at higher and lower risk of exposure.
Keywords: HIV vaccine, mathematical model, HIV prevention, South Africa, combination prevention
INTRODUCTION
The devastating effect of the HIV epidemic is nowhere more apparent than in South Africa, which has over 5.5 million people living with HIV (1). Dramatic gains in access to antiretroviral treatment (ART) have enabled approximately one million people to receive ART in South Africa, but HIV incidence remains high. With almost 500,000 new infections per year in South Africa (1), the need for effective preventive interventions is urgent.
Recent studies have provided encouragement about the prospects for development of an HIV vaccine (2). A large phase III trial of an ALVAC recombinant canarypox vector prime and AIDSVAX recombinant glycoprotein 120 subunit boost vaccine (RV144) found a 31% average reduction in new HIV infections among trial participants over the 3.5 year trial period (3). Reports of the successful development of broadly neutralizing antibodies have also provided cause for optimism (4, 5).
To understand the potential population benefits of an HIV vaccine with partial efficacy similar to RV144, the Centers for Disease Control and Prevention (CDC) and UNAIDS initiated a collaboration among investigators with an interest in model-based analyses of HIV vaccines. The purpose of the collaboration was to estimate the population outcomes of a partially efficacious vaccine in different populations, using a common set of assumptions about the efficacy and duration. Of particular importance is that the vaccine efficacy in the RV144 trial declined rapidly over time, with an overall efficacy of 31% for the 3.5 year trial period, but an efficacy of approximately 70% early after vaccination.
The enormous burden of HIV in South Africa makes it of special interest for HIV prevention interventions. We report here our analyses of the use of a partially protective HIV vaccine with efficacy similar to RV144 in South Africa, performed as part of the CDC-UNAIDS collaboration. We evaluated one-time vaccination programs, booster programs, and programs that maintained a constant level of vaccine coverage through continuous vaccination.
METHODS
Model description
We used a dynamic compartmental epidemic model to examine the impact of potential vaccination scenarios on heterosexual transmission of HIV. We have described the model specifications, equations, and inputs elsewhere (6) and provide a brief description here: The model contains twelve different compartments defined by sex (male, female), vaccination status (unvaccinated, vaccinated), and disease status (HIV negative, asymptomatic HIV positive, symptomatic HIV positive, AIDS). We did not explore additional vaccine effects beyond protection from infection, such as a decrease in infectivity or a delay in disease progression for those vaccinated individuals who are infected with HIV. Therefore, the compartments for symptomatic HIV positive and individuals with AIDS included both unvaccinated and vaccinated individuals. Movement between the various compartments, or states, is defined by differential equations; however, we implemented the model in Excel and therefore used difference equations to regulate the transitions between compartments. Individuals enter the model upon reaching sexual maturity and leave the model following death due to AIDS or non-AIDS related causes.
The probability of becoming infected with HIV depends on a number of factors, including the number of partners per year, the HIV status and disease stage (if infected) of the partner, the probability that HIV is transmitted in a partnership, the level of condom use including the likelihood of condom failure, the change in condom use post-vaccination, whether the person has been vaccinated, and the efficacy of the vaccine used. We explored the impact of a preventive vaccine such that all vaccinated HIV-negative individuals are protected from HIV infection in their partnerships with HIV-positive individuals according to the efficacy level of the vaccine. Therefore, all vaccinated individuals are protected against a proportion of their exposures to HIV, which is determined by the protective efficacy of the vaccine. We examined post-vaccination risk behavior change by exploring changes in condom use behaviors. Because of the gender and power dynamics related to condom negotiation in Soweto, we assumed that negotiation of condom use in a sexual partnership was determined by the male partner (regardless of whether a male or female condom was used). Therefore, post-vaccination changes in condom use in sexual partnerships were determined by the vaccinated male partner in the model.
RV144 trial parameters
We simulated the impact of an HIV vaccine with properties that approximated the vaccine candidate tested in the RV144 trial. Although the trial findings indicated an average vaccine efficacy of approximately 31% over 42 months based on a modified intention-to-treat analysis, the trial data show a rapidly waning effect with higher efficacy over the first 12 months and substantially lower impact thereafter (3). Additional analysis has shown average efficacy over the first year to be approximately 60%, with events truncated at 12 months (personal communication, Donald Stablein). The trial data were approximated by an exponential decay function for vaccine efficacy, VE=0.78exp[−0.06t] where t is time in months since vaccination (see accompanying editorial in this issue for further details).
Reference case
Because several research groups are examining the potential impact of the RV144 vaccine candidate as part of the CDC/UNAIDS vaccine modeling consensus group, common inputs and outcome measures were chosen for a reference case to allow relative comparisons between different models—although they will still differ greatly in terms of structure, assumptions, and other inputs. For the reference case, we simulated a scenario in which 30% or 60% of the adult population was vaccinated at time zero and no further vaccinations were conducted after that year. Rapidly waning vaccine efficacy was assumed according to the equation described above for those individuals vaccinated in the single-year campaign, and no booster vaccinations were considered. For the reference case we explored only general population vaccination strategies and assumed that no changes in risk behavior occurred post-vaccination. The common outcome measure was the percentage of expected infections averted by the vaccination program over a 10-year period.
Vaccination scenarios
We explored two general types of vaccination strategies: (1) vaccination of a single cohort of individuals, with potential booster vaccinations over time for those individuals who were vaccinated initially, and (2) vaccination of the overall population at coverage levels that are constant over time, such that individuals are revaccinated or new individuals are vaccinated to maintain a certain effectively vaccinated population coverage level. In the latter strategy, we allow for constant revaccination because otherwise the vaccinated percentage of the population will decrease over time due to population increases or mortality and, more importantly, due to the very short duration of protection of the vaccine candidate we are modeling in these scenarios. When vaccine protection wanes, individuals who were previously vaccinated receive no further protection and therefore are no longer ‘effectively’ vaccinated; for modeling purposes, these individuals return to the ‘unvaccinated’ populations. As such, individual members of the population must be continuously vaccinated (or revaccinated) to maintain this effectively vaccinated population coverage level. We examined all outcomes in terms of infections prevented over time at 5, 10, 15, and 20 years, as well as the impact on population-level HIV prevalence over a 50-year period.
For the single cohort vaccination strategy, we assumed 30%, 60%, or 90% of the adult population was vaccinated in a single year and that vaccine efficacy decayed over time at the individual level as specified by the above equation. All individuals who were vaccinated received partial protection from the vaccine. We also explored the consequences of booster vaccinations for those individuals who were originally vaccinated and simulated boosting at 5-year or more frequent 2-year intervals. Individuals who were not vaccinated in the first round were thus not eligible for subsequent booster vaccinations. We assumed that revaccination resulted in the same efficacy (according to the exponential decay curve above) at preventing infection over time as the original vaccination. We did not explore changes in risk behavior for this vaccination strategy.
For the continuous vaccination strategy, we assumed that 30%, 60%, or 90% of the adult population was vaccinated initially, and that these population coverage levels were maintained over time as described above. As such, individuals who were previously vaccinated but whose protection had waned could be revaccinated, and individuals who were vaccine-naïve could be vaccinated, such that overall effectively vaccinated population coverage levels remained constant. For this strategy, we assumed that vaccine efficacy decayed over time at the population level. We also explored the impact of post-vaccination changes in risk behavior by simulating scenarios in which condom use increased or decreased by 50%.
In the analysis for the single cohort vaccination strategy, we adapted our model structure to allow the specification of the vaccine efficacy at each time step according to the exponential decay equation used for this efficacy. As such, the exact time-dependent efficacy was modeled for this group. In the analysis for the continuous vaccination strategy, new cohorts of individuals were being vaccinated or revaccinated every year and we were not able to specify the vaccine efficacy for each cohort at each time step due to limitations with the structure of the model. Therefore, we instead used a fixed efficacy and average duration of protection calculated from the exponential decay function to approximate the impact of waning efficacy at the population level. Although this does not produce an identical result as modeling waning efficacy over time, this method provides a good approximation at the population level (data not shown).
Simulations
We initialized the model with parameters from published sources as well as assumptions to simulate the late-stage, generalized heterosexual HIV epidemic in the township of Soweto, South Africa (6): We assumed the vaccine stimulated an immune response resulting in partial protection from infection for all vaccinated individuals. We examined varying infectivity values (0.06 to 0.27) for the annual per-partner (not per sex act) probability of HIV transmission, depending on the disease stage and direction (male-to-female or female-to-male) of HIV transmission (7–10). We assumed 0–3 sexual partners per year, depending on the disease stage. We assumed baseline condom use levels of 50% and an overall condom failure rate of 14% per partnership (11). We calculated the time from HIV infection to death due to AIDS as 10.2 years, divided into asymptomatic, symptomatic and AIDS stages (12). We simulated the impact of vaccination programs on a population of 823,000 sexually active adults aged 17 years or older (13) with a mean age of 25.1 years (14), a life expectancy of 60.8 years in the absence of AIDS-related mortality (14), and a non-AIDS annual mortality rate of 0.028 (14). Individuals left the model population as a result of AIDS- or non-AIDS-related mortality. Although the prevalence of HIV is unknown in Soweto, we used national and provincial data to estimate initial HIV prevalence rates of 11.6% for men and 20.0% for women in an urban township setting (15).
We first calculated the number of infections that were expected over time and the population HIV prevalence in the absence of vaccination. We assumed the vaccine was available immediately and then simulated the various vaccination scenarios to calculate the percentage of expected infections prevented and the changes in population HIV prevalence over time. We implemented the model using difference equations and a one-year time step in Excel® 2003 spreadsheet software (Microsoft Corporation, Redmond WA)
RESULTS
Baseline scenario
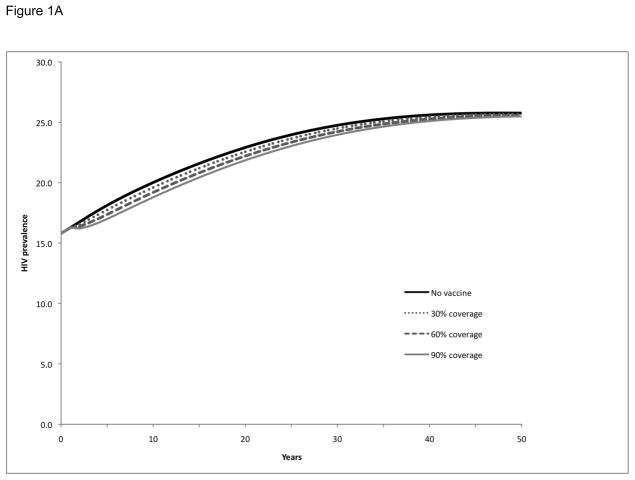
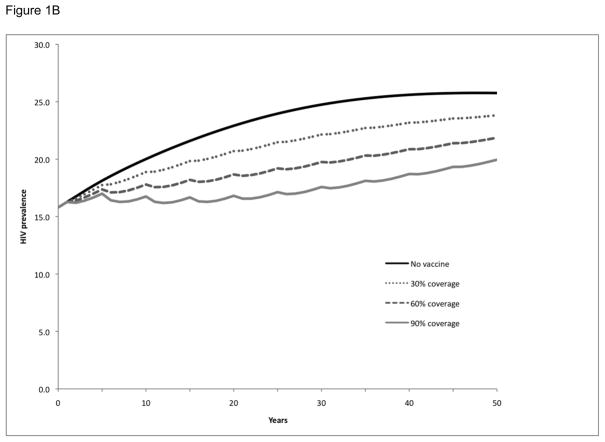
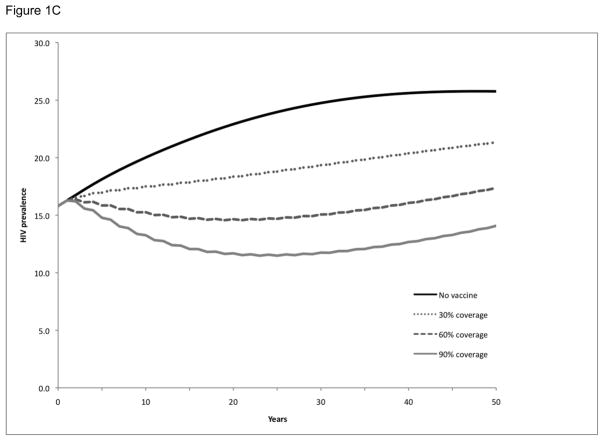
Baseline projections were prepared to simulate the number of new HIV infections and prevalence of HIV in the absence of a vaccination program or other interventions to prevent HIV infection. Over a 20-year period, 318,000 new adult HIV infections are expected to occur and the prevalence of HIV in Soweto will increase from 16% to 23%. HIV prevalence will continue to increase and eventually stabilize over a 50-year period at 26% (see ‘no vaccine’ simulation in Figure 1).
Figure 1. HIV prevalence following vaccination and booster vaccination of a single cohort in Soweto.
HIV prevalence over time following vaccination of a single cohort (30%, 60%, or 90% of the adult population at time zero) in Soweto (Panel A). Booster vaccinations were considered at 5-year (Panel B) and 2-year (Panel C) intervals for the initial cohort (30%, 60%, or 90% of the adult population at time zero) receiving the vaccinations. All simulations considered a vaccine with efficacy and duration of protection approximated to RV144 trial conditions.
Reference case
For the reference case, we explored the impact of a single-year vaccination campaign that reaches either 30% or 60% of the population (other coverage levels are explored outside of the reference case, below), with no booster vaccinations or changes in behavior post-vaccination. Vaccinating 30% of the population in a single year would prevent 3% of the cumulative expected infections over a 10-year period, while vaccinating 60% of the population in a single year would prevent 6% of the cumulative expected infections over a 10-year period (see ‘single cohort vaccination’, 10 year outcomes, 30% and 60% population coverage in Table 1).
Table 1.
Percentage of cumulative infections prevented over 20 years following vaccination and booster vaccination of a single cohort in Soweto: Sensitivity analysis on initial population coverage levels achieved and vaccination boosting strategya
| Vaccination strategy and time period for measurement of program outcomes b | Population coverage for single cohort vaccination c |
||
|---|---|---|---|
| 30% | 60% | 90% | |
| Single cohort vaccination | |||
| 5 years | 4% | 8% | 12% |
| 10 years | 3% | 6% | 8% |
| 15 years | 2% | 5% | 7% |
| 20 years | 2% | 4% | 6% |
| Single cohort vaccination with booster vaccination every 5 years | |||
| 5 years | 4% | 8% | 12% |
| 10 years | 7% | 13% | 19% |
| 15 years | 8% | 15% | 22% |
| 20 years | 8% | 16% | 24% |
| Single cohort vaccination with booster vaccination every 2 years | |||
| 5 years | 11% | 22% | 33% |
| 10 years | 14% | 27% | 39% |
| 15 years | 16% | 31% | 44% |
| 20 years | 17% | 32% | 45% |
Percentages reflect the proportion of cumulative expected adult infections prevented by each program scenario compared with a no vaccine baseline. All simulations assume a vaccine with waning efficacy calculated from RV144 trial data.
Simulations assume initial vaccination of cohort at time zero and outcomes calculated at 5, 10, 15 and 20 year intervals.
Population coverage refers to the proportion of the adult population at time zero which is vaccinated. For booster vaccination scenarios, only individuals who were initially vaccinated are revaccinated at 2- and 5-year intervals.
Vaccination scenarios: single cohort strategy
We examined the impact of single cohort vaccination campaigns on HIV prevalence for three different population coverage levels—30%, 60%, and 90%—without boosters (Figure 1A), with booster vaccination ever 5 years (Figure 1B), and with more frequent booster vaccination every 2 years (Figure 1C). Single cohort vaccination without boosters did not have a substantial effect on HIV prevalence over time, regardless of the level of population coverage: prevalence at 20 years was 23% and the vaccination program resulted in a prevalence of 22–23%, although the program did provide somewhat better interim benefits in the first few years post-vaccination. However, the single cohort strategy with booster vaccinations for those individuals who had originally received the vaccine had a more substantial impact on HIV prevalence: revaccinating every 5 years resulted in a decrease in prevalence to 17–21%, depending on the level of population coverage, and revaccinating every 2 years resulted in the prevalence decreasing to 12–18%. Although these prevalence reductions lessened over time as the single cohort originally vaccinated aged, they were still significant 50 years later.
We next examined the impact of the above 9 vaccination scenarios (3 boosting strategies and 3 population coverage levels) on the number of expected HIV infections at 5-, 10-, 15-, and 20-year intervals (Table 1). Although the single cohort vaccination strategy without boosters did not have a substantial impact on HIV prevalence, it still prevented 4–12% of the expected infections at 5 years, depending on the population coverage, and 2–6% of the expected infections at 20 years. The 5-year booster strategy prevented 8–24% of the expected infections at 20 years, while the 2-year booster strategy prevented 17–45% of the expected infections at 20 years.
Vaccination scenarios: continuous strategy
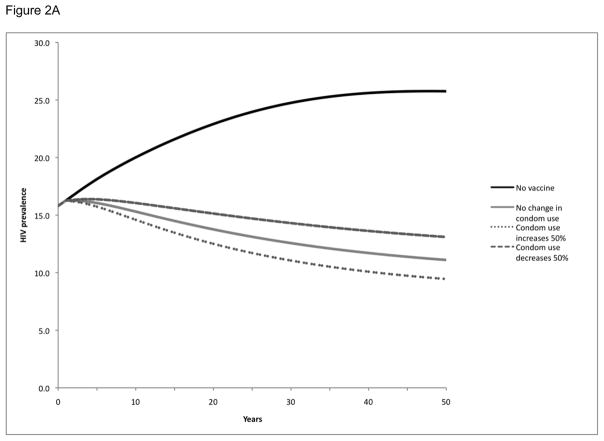
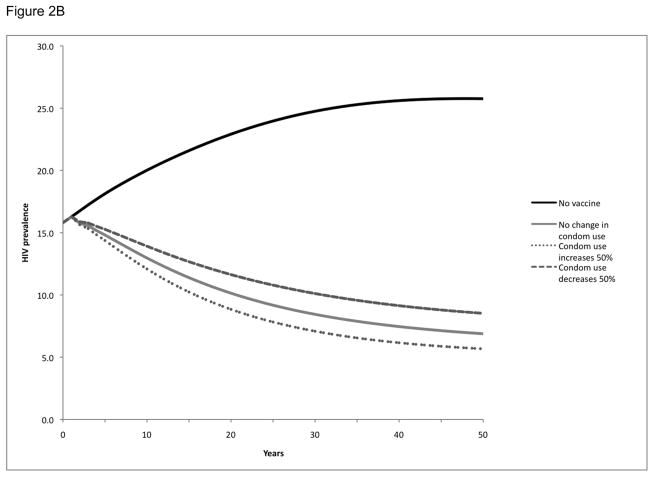
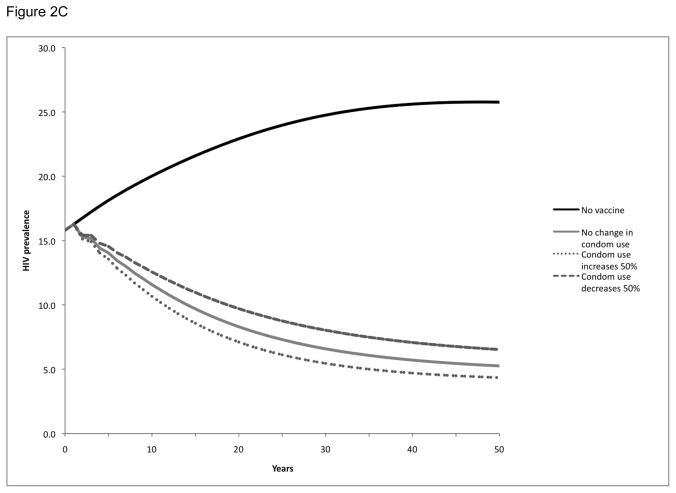
We also examined the impact of maintaining population coverage levels at 30% (Figure 2A), 60% (Figure 2B), and 90% (Figure 2C) over time through vaccination of new individuals as well as revaccination of individuals who had been previously vaccinated but in whom protection had waned. As might be expected, this strategy resulted in more substantial reductions in population HIV prevalence than the single cohort vaccination strategy: HIV prevalence at 20 years was reduced from 23% to 8–14%, depending on the population coverage level. This strategy also had a greater impact in terms of infections prevented: over a 20-year period, these vaccination programs would avert 34–59% of the expected HIV infections in the population, depending on the population level of vaccination coverage maintained (Table 2).
Figure 2. HIV prevalence following vaccination with population coverage levels maintained over time by continuous revaccination in Soweto.
HIV prevalence over time following vaccination with population coverage levels maintained over time at 30% (Panel A), 60% (Panel B), or 90% (Panel C). Vaccination or revaccination of individuals maintains population coverage at a constant level over time. All scenarios explored potential behavior change, specifically a 50% increase or 50% decrease in condom use following vaccination.
Table 2.
Percentage of cumulative infections prevented over 20 years with population vaccination coverage levels maintained over time in Soweto: Sensitivity analysis on population coverage levels achieved and post-vaccination changes in condom use behaviorsa
| Risk behavior change and time period for measurement of program outcomes b | Population coverage maintained over time c |
||
|---|---|---|---|
| 30% | 60% | 90% | |
| No change in condom use behavior | |||
| 5 years | 21% | 33% | 41% |
| 10 years | 27% | 41% | 50% |
| 15 years | 31% | 46% | 55% |
| 20 years | 34% | 50% | 59% |
| Condom use behavior increases by 50% post-vaccination | |||
| 5 years | 24% | 37% | 46% |
| 10 years | 31% | 47% | 55% |
| 15 years | 36% | 52% | 61% |
| 20 years | 39% | 56% | 65% |
| Condom use behavior decreases by 50% post-vaccination | |||
| 5 years | 17% | 29% | 36% |
| 10 years | 23% | 35% | 44% |
| 15 years | 26% | 40% | 49% |
| 20 years | 29% | 44% | 53% |
Percentages reflect the proportion of cumulative expected adult infections prevented by each program scenario compared with a no vaccine baseline. All simulations assume vaccine efficacy and average duration of protection calculated from RV144 trial data.
Simulations assume initial vaccination of population at time zero and outcomes calculated at 5, 10, 15 and 20 year intervals. Post-vaccination changes in condom use (increase or decrease by 50%) assume change in male-negotiated condom use in vaccinated individuals.
Population coverage refers to the proportion of the adult population which is effectively vaccinated. Individuals are vaccinated or revaccinated over time to maintain the specified overall population coverage level.
In these scenarios, we also considered the impact of post-vaccination changes in risk behavior by exploring a 50% increase or decrease in condom use (Figure 2, Table 2). While an increase in condom use augmented the benefits of the vaccination program and a decrease in condom use decreased the benefits of the vaccination program, neither effect was significant enough to negate the impact of the program. With a 50% increase in condom use post-vaccination, the HIV prevalence over 20 years would be reduced even further to 7–13% and an even greater number (39–65%) of infections would be prevented, depending on the population level of coverage maintained. Conversely, with a 50% decrease in condom use post-vaccination, the HIV prevalence would be reduced only to 10–15% and the number of infections prevented would be reduced (29–53%), depending on the population level of coverage maintained.
DISCUSSION
We analyzed how the use of an HIV vaccine with rapidly declining efficacy, similar to that observed in the RV144 trial, would affect HIV prevalence and infections averted in South Africa. Our assumption about vaccine efficacy is important in interpreting our results, as it bears directly on the importance of booster vaccination. Consistent with the RV144 trial, which showed an overall average efficacy of 31% for the 3.5 year trial period, we assumed that the vaccine protection would decline exponentially and would have an instantaneous efficacy of approximately 70% shortly after vaccination, declining to less than 10% at three years.
The number of infections prevented by such a vaccine depends on the proportion of the population vaccinated and on the frequency and efficacy of booster vaccinations. A one-time vaccination with 60% coverage and no booster vaccination would prevent 8% of new HIV infections over a 5-year time horizon, but only 4% of new infections over 20 years as the population impact of the single vaccination campaign is diluted over time. It is important to recognize that even this worst-case scenario nonetheless prevents a substantial number of infections during the first five years after vaccination. Booster vaccination markedly increases the number of infections averted, with vaccination at 2-year intervals preventing 22% and 32% of HIV infections at 5 years and 20 years, respectively. Maintaining population coverage leads to greater numbers of infections prevented over time. Although difficult and potentially expensive, vaccination of 90% of the population every 2 years would prevent 45% of HIV infections.
The effect of vaccination on HIV prevalence varies depending on whether vaccination is performed for a single cohort (that may or may not be revaccinated) or whether a constant level of population coverage is maintained by vaccinating successive cohorts. A one-time vaccination without boosting does not substantially reduce prevalence. As booster vaccination becomes more frequent or coverage increases, the effect on prevalence becomes more pronounced, but prevalence remains high even with frequent booster vaccination and substantial coverage in our single cohort analysis. In contrast, if vaccination of a fixed proportion of the population is maintained over time by vaccinating successive cohorts, the effect on prevalence can be substantial if frequent booster vaccination is performed. However, due to the rapidly declining protection associated with this vaccine, this strategy would involve substantial, although not insurmountable, public health outreach efforts depending on the population coverage level to be maintained. Overall, the main implication of our analysis is that a partially protective vaccine of quite modest efficacy would provide substantial health benefit if booster vaccination is feasible and is as efficacious as the initial vaccination.
The finding that HIV vaccines with only partial efficacy can provide substantial health benefits is consistent with analyses we have done for other settings and with differing assumptions about vaccine efficacy (6, 16–21). It is also consistent with other analyses performed for South Africa as well as Thailand, Australia, and the United States as part of the CDC-UNAIDS HIV vaccine modeling collaboration. We emphasize this finding because a vaccine efficacy of 30% for conditions other than HIV would often be considered a failure. Because of the high mortality from HIV in resource limited settings and the high cost of HIV-related care in high-income countries (21), a vaccine does not need to have high efficacy to provide important health or economic benefit.
One concern about a partially protective vaccine is that risk compensation might mitigate the benefit of vaccination: individuals who are vaccinated could feel protected and decrease their condom use or increase their number of sex partners or risky sexual acts. However, it is also possible that individuals who are vaccinated might decrease their risky behaviors as a result of the education and counseling associated with a mass vaccination campaign. Consistent with prior analyses (6), our study indicates that the effect of behavior change associated with a vaccination campaign can either amplify or diminish the benefits of a vaccine. For a vaccine of modest efficacy, as analyzed here, increases or reductions in risk behavior have a modest impact on infections averted, and even with a 50% decrease in condom use, a vaccine still provides substantial net benefit. For vaccines of higher efficacy, change in risk behavior is less important. Fortunately, there was not evidence of increased risk behavior in the RV144 trial and there is no evidence that risk compensation might occur in a larger vaccination campaign.
Our analysis has several limitations. Our assumptions about declining efficacy of the vaccine reflect the findings of the RV144 trial, but the trial did not assess subsequent booster vaccination. Thus, our analyses of booster vaccination should be considered illustrative only, as there are no empirical observations about the efficacy of booster vaccination. We assumed that booster vaccination would return immunity to the level achieved by initial vaccination; if booster vaccination is less effective than initial vaccination, our analyses overestimate the benefit of vaccination strategies. We also assumed that the efficacy results from the trial, which was conducted in a heterosexual population at relatively low risk for HIV infection, would be applicable to the generalized epidemic in Soweto where multiple partnerships are more common and individuals are at relatively high risk for heterosexual HIV infection. However, it is possible that differential vaccine efficacy could exist in differing subpopulations of varying risk (e.g. sex workers and their clients) and with different modes of transmission (e.g. men who have sex with men or people who inject drugs). Our modeling framework also uses a dynamic transmission model to capture the effect of vaccination on the HIV epidemic. This framework is well suited for capturing overall transmission, but does not capture the full complexity of sexual networks. Therefore, we cannot evaluate strategies that aim to target specific sexual networks.
In addition, we did not consider the cost effectiveness of vaccination, which is particularly relevant if frequent booster vaccinations would be needed to maintain immunity. The cost effectiveness would be strongly influenced by vaccine cost, which is unknown. Analyses for the United States suggest that revaccination would be potentially cost effective only if targeted to subpopulations at higher risk of exposure, given a vaccine cost of $500 (20); however, such a cost would be prohibitive in much of the world. Finally, our analysis evaluates the use of an HIV vaccine apart from other preventive and treatment interventions. To the extent that treatment and preventive interventions become widespread, the effect of a vaccine may be less than we estimated.
In summary we find that a partially protective HIV vaccine with declining efficacy similar to the RV144 vaccine could prevent a substantial proportion of HIV infections if booster vaccinations were effective and available. Our estimates of the population impact of a vaccination would be improved by further understanding of the duration of protection, the effectiveness of booster vaccination, and whether the efficacy of the vaccine varies between subpopulations at higher or lower risk of exposure.
Acknowledgments
The authors thank the anonymous reviewers who have provided valuable insights on the content of the paper, as well as Robert Chen, John Glasser, Catherine Hankins, Don Stablein, and the other members of the CDC/UNAIDS vaccine modeling consensus group for their valuable discussions on the implications of the RV144 trial.
This work was supported by the National Institute on Drug Abuse (RO1DA015612). Dr. Owens has been a consultant to Sanofi-Aventis on comparative effectiveness in diabetes. The other authors do not have any associations that may pose a potential conflict of interest.
Footnotes
Preliminary data from this study presented at:
AIDS Vaccine 2010 Conference Satellite Symposium, “Preparing for the Availability of a Partially Effective HIV Vaccine”, Atlanta, Georgia, September 28 – October 1, 2010
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kyeen M. Andersson, Email: kyeen@aya.yale.edu.
A. David Paltiel, Email: david.paltiel@yale.edu.
Douglas K. Owens, Email: owens@standford.edu.
References
- 1. [accessed 2-8-11]; http://www.unaids.org/en/Regionscountries/Countries/SouthAfrica/
- 2.Wayne CK, Berkley SF. The renaissance in HIV vaccine development--future directions. N Engl J Med. 2010 Jul 29;363(5):e7. doi: 10.1056/NEJMp1007629. [DOI] [PubMed] [Google Scholar]
- 3.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009 Dec 3;361(23):2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 4.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010 Aug 13;329(5993):856–61. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010 Aug 13;329(5993):811–7. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson KM, Owens DK, Vardas E, Gray GE, McIntyre JA, Paltiel AD. Predicting the impact of a partially effective HIV vaccine and subsequent risk behavior change on the heterosexual HIV epidemic in low- and middle-income countries: a South African example. Journal of Acquired Immunodeficiency Syndrome. 2007;46:78–90. doi: 10.1097/QAI.0b013e31812506fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray RH, Li X, Wawer MJ, et al. Stochastic simulation of the impact of antiretroviral therapy and HIV vaccines on HIV transmission; Rakai, Uganda. AIDS. 2003;17:1941–1951. doi: 10.1097/00002030-200309050-00013. [DOI] [PubMed] [Google Scholar]
- 7.Owens DK, Edwards DE, Shachter RD. Population effects of preventive and therapeutic HIV vaccines in early- and late-stage epidemics. AIDS. 1998;12:1057–1066. [PubMed] [Google Scholar]
- 8.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1 discordant couples in Rakai, Uganda. Lance. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 9.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 10.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Eng J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 11.Workshop Summary: Scientific Evidence of Condom Effectiveness for Sexually Transmitted Disease (STD) Prevention. Herndon, VA: National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services; 2000. [Google Scholar]
- 12.Morgan D, Mahe C, Mayanja B, et al. HIV-1 infection in rural Africa: is there a difference in median time to AIDS and survival compared with that in industrialized countries? AIDS. 2002;16:597–603. doi: 10.1097/00002030-200203080-00011. [DOI] [PubMed] [Google Scholar]
- 13.Statistics South Africa. 2001 South African census. 2004 Available at http://www.statssa.gov.za.
- 14.Pretoria, South Africa: Statistics South Africa. 2004. Mid-Year Population Estimates, South Africa: 2004. [Google Scholar]
- 15.Shisan O, Rehle T, Simbayi LC, et al. South African National HIV Prevalence, HIV Incidence, Behaviour and Communication Survey, 2005. Cape Town, South Africa: HSRC Press; 2005. [Google Scholar]
- 16.Long EF, Brandeau ML, Owens DK. Potential population health outcomes and expenditures of HIV vaccination strategies in the United States. Vaccine. 2009;27:5402–5410. doi: 10.1016/j.vaccine.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owens DK, Edwards DM, Shachter RD. Costs and benefits of imperfect HIV vaccines: Implications for vaccine development and use. In: Kaplan EH, Brookmeyer R, editors. Quantitative Evaluation of HIV Prevention Programs. Yale Press; 2001. pp. 143–171. [Google Scholar]
- 18.Owens DK, Edwards DM, Cavallaro JF, Shachter RD. The cost effectiveness of partially effective HIV vaccines. In: Brandeau ML, Sainfort F, Pierskalla WP, editors. Operations Research and Health Care: A Handbook of Methods and Applications. Kluwer Academic Publishers; 2004. pp. 403–418. [Google Scholar]
- 19.Edwards DM, Shachter RD, Owens DK. A dynamic HIV-transmission model for evaluating the costs and benefits of vaccine programs. Interfaces. 1998;28:144–166. [Google Scholar]
- 20.Long EF, Owens DK. The cost effectiveness of a modestly effective HIV vaccine in the United States. Vaccine. 2011 doi: 10.1016/j.vaccine.2011.04.013. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnett PG, Chow A, Joyce VR, Bayoumi AM, Griffin SC, Nosyk B, Sun H, Holodniy M, Brown ST, Cameron DW, Sculpher M, Anis AH, Owens DK. Determinants of the cost of health services used by patients with HIV. Medical Care. 2011 doi: 10.1097/MLR.0b013e31821b34c0. (in press) [DOI] [PubMed] [Google Scholar]