Abstract
Non-pharmacological interventions for Myalgic Encephalomyelitis/chronic fatigue syndrome (ME/CFS) often emphasize gradual increases in activity to promote improvement in physical functioning and fatigue. The energy envelope theory may provide a framework for understanding the relationship between changes in activity level and outcomes for patients with ME/CFS. This study examined the relationship between energy envelope and changes in activity after non-pharmacological interventions in a sample of 44 adults with ME/CFS. Results showed that those who were within their energy envelope before treatment showed more improvement in physical functioning and fatigue compared to those outside of their energy envelope. These findings suggest that an assessment of perceived available and expended energy could help guide the development of individualized non-pharmacological interventions for people with ME/CFS.
Keywords: chronic fatigue syndrome, Myalgic Encephalomyelitis, actigraphy, non-pharmacological interventions
Fatigue is a common symptom in the general population and is reported across various medical and psychiatric illnesses. Exercise or increased activity has been found to significantly reduce fatigue among individuals with cancer (Mock et al., 2005; Schwartz, Mori, Gao, Nail, & King, 2001), depression (Lane & Lovejoy, 2001), and multiple sclerosis (Oken et al., 2004). Given the overall benefit of increased activity on fatigue, many non-pharmacological treatment interventions for Myalgic Encephalomyelitis/chronic fatigue syndrome (ME/CFS), an illness characterized by fatigue or energy depletion, have an integrated activity component (Whiting et al., 2001).
One example of a non-pharmacological intervention for ME/CFS with a considerable activity component is graded exercise therapy (GET; Edmonds, McGuire, & Price, 2004). GET involves gradual increases in aerobic activity over the course of the treatment period. While graded exercise interventions have led to improvements in fatigue and overall functioning in some patients with ME/CFS, it is also related with high treatment dropout rates and disapproval by patients (Edmonds et al., 2004; Wearden et al., 1998). Another non-pharmacological treatment for ME/CFS involving an activity component is cognitive behaviour therapy (CBT) with graded activity, which has demonstrated moderately superior outcomes to control interventions (Malouff, Thorsteinsson, Rooke, Bhullar, & Schutte, 2008). Although interventions promoting increased activity may be promising for some patients, it is evident that not all patients show a clinically significant response to these treatment approaches.
A recent analysis of three CBT trials for ME/CFS, Wiborg, Knoop, Stulemeijer, Prins, and Bleijenberg (2010) found that changes in physical activity were unrelated to participating in CBT. This indicates that while CBT protocols have a graded activity component, patients do not uniformly increase activity after treatment. The authors also found that although there were overall improvements in fatigue severity after CBT, these improvements were not associated with changes in physical activity. Consequently, the authors posit that improvements in fatigue may be related to cognitive changes that occur over the course of treatment. However, Wiborg et al. did not take into account individual factors that may influence the relationship between increased activity and treatment outcomes. For example, Friedberg and Sohl (2009) found that among patients with ME/CFS who reported improvement after CBT, some patients had increased activity while others had decreased or did not change their activity level. Based on findings from Friedberg and Sohl, it can be suggested that patients are diverse in the type of activity changes needed for optimal treatment outcomes. It is necessary to identify methods for determining which patients may benefit from increased activity and those who may benefit from decreased activity over the course of non-pharmacological interventions for ME/CFS.
The energy envelope theory (Jason et al., 1999; Pesek, Jason, & Taylor, 2000) may provide context for examining the relationship between increased activity and changes in fatigue among patients with ME/CFS. The energy envelope theory states that each individual has an amount of perceived available energy as well as an amount of energy that is perceived to be expended. If an individual perceives they expend only as much energy as they perceive they have available, then they will remain within their energy envelope. In doing so, the individual may prevent overexertion that can lead to a worsening of symptoms. Individuals who are within their energy envelope have been found to have better physical and psychological functioning (Jason, Muldowney, & Torres-Harding, 2008). In terms of non-pharmacological interventions for ME/CFS, Jason, Benton, Torres-Harding, and Muldowney (2009) found that individuals who were within their energy envelope 12 months after treatment had more improvement in physical functioning and fatigue severity. Because individuals who are inside of their energy envelope may experience positive outcomes while those who are outside of their energy envelope may have deleterious outcomes, an evaluation of energy envelope is an important variable for consideration.
This study examined the relationship between energy envelope and changes in activity after non-pharmacological interventions. It was hypothesized that individuals who started treatment outside of their energy envelope would not demonstrate improvements in fatigue severity and physical functioning if they increased activity. In contrast, participants within their energy envelope at baseline were expected to show improvements with increased activity, particularly if they remained within their energy envelope at the follow-up assessment.
Method
Participants
The present investigation utilized data derived from a larger longitudinal study of nonpharmacological treatment interventions for ME/CFS (Jason et al., 2007). Participants were recruited from physician referrals, media advertisements, and ME/CFS support groups. Participants were required to be at least 18 years of age, not pregnant, able to read and speak English, and considered to be physically capable of attending scheduled appointments. Participants were included if they met the Fukuda et al. (2004) criteria for CFS as diagnosed by the study physician (see Jason et al., 2007 for details on diagnostic procedures). A total of 114 participants were recruited and enrolled in the original study. All procedures were approved by the DePaul University Institutional Review Board. Informed consent was given by all participants.
In the present study, 70 participants did not have complete data at baseline and the 12-month follow-up period, leaving a total of 44 participants for analysis. The large amount of attrition occurred due to the burden of collecting actigraphy data. Participants were required to wear the actigraph for a one-week period at both baseline and the 12-month follow-up, and many participants only participated in the actigraphy data collection at one timepoint. Actigraphy data collection required participants to remember to wear the actigraph daily as well as ensure the technology was working properly, and this task was difficult for some participants to complete. The 44 participants included in the present study did not significantly differ from the 70 who had missing data on any demographic variables.
Materials
CFS Questionnaire
The CFS Questionnaire was used to collect demographic, health status, medication usage, and symptom data. This screening scale has demonstrated adequate validity and inter-rater and test-retest reliability (Hawk, Jason, & Torres-Harding, 2007; Jason et al., 1997).
Energy Envelope
Energy envelope data were collected at baseline and 12-month assessments and was computed by comparing perceived available versus perceived expended energy. Participants were asked to rate available energy and expended energy over the past week on a 100-point scale, with 0 = no energy and 100 = abundant energy similar to when the person was completely well. Test-retest reliabilities for weekly perceived available and expended energy have been reported at .81 and .64, respectively (Hawk et al., 2007). Perceived available energy referred to the participants’ estimation of their available energy resources. Perceived expended energy was defined as the participants’ estimation of the total amount of energy exerted. Expended energy can be greater than available energy, particularly when participants push themselves over their energy limits. The participants’ expended energy was divided by their available energy, and this number was then multiplied by 100. This represents the energy quotient for weekly ratings. A score of equal to or below 150 at baseline was considered to be within one's energy envelope. While an energy quotient of 100 or below is the most precise indicator of an individual being within their energy envelope, only 11 participants in the current sample fell into this range. The cutoff of 150 has been utilized in a previous study (Jason et al., 2009), and increasing the cutoff to 150 yielded more equivalent groups for analysis. In addition, given that the average energy quotient for this sample was 239.04 (SD = 233.95), an energy quotient of 150 is substantially below that of the average participant. Participants were grouped based on their baseline energy envelope ratings as either inside or outside of their energy envelope.
Actigraph
Participants wore an actigraph for a one week period at baseline and at the 12-month follow-up. Participants wore the actigraph on their waist at all times except when bathing or sleeping. An actigraph is a small, lightweight, cost-efficient activity monitor worn on the waist. It continuously collects data over 24-hour periods for 22 days before its memory reaches capacity (Tryon & Williams, 1996). The actigraph records the intensity of movements using an accelerometer. An 8-bit analog-to-digital (A/D) converter quantifies these measurements into 128 levels of positive acceleration and 128 levels of negative acceleration 10 times each second. Integration over the resulting sampling time of 0.1 s in combination with other details provided by Tryon and Williams (1996) would result in measurement units of 1.664 milli-g/activity activity count. A/D counts are retained as activity units. The average of 600 absolute A/D values is stored in memory at the end of every minute. Changes in actigraphy units were calculated by subtracting baseline actigraphy units from 12-month follow-up actigraphy units. Negative change scores indicated a decrease in activity and positive change scores indicated an increase in activity. Based on baseline to 12-month follow-up comparisons of actigraphy units, participants were divided into two groups: increased activity or decreased activity.
Physical Functioning
The Physical Functioning subscale of the Medical Outcomes Study Short Form-36 Health Survey (SF-36; Ware & Sherbourne, 1992) was used to measure overall physical disability. Scores on the Physical Functioning subscale range from 0 to 100, with higher scores indicating better functioning. Reliability and validity studies for the SF-36 have shown adequate internal consistency, discriminant validity among subscales, and substantial differences between patient and nonpatient populations in the pattern of scores (McHorney, Ware, Lu, & Sherbourne, 1994; McHorney, Ware, & Raczek, 1993).
Fatigue
The Fatigue Severity Scale (FSS; Krupp, LaRocca, Muir-Nash, & Steinberg, 1989) was used to assess fatigue severity. This inventory consists of 9 scale items rated on seven-point scales, with severity scores ranging from 1 to 7, with 1 indicating no fatigue and 7 indicating very severe fatigue. The final severity score is determined by averaging the 9 self-reported scores. The scale items primarily concern the behavioral effects of fatigue, and measure different facets of fatigue including the extent of physical, social, and emotional impairment. The FSS was shown to have adequate internal consistency (α = .88) and test-retest reliability (Krupp et al., 1989).
Procedure
Participants were administered the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 1995) to diagnose any Axis I disorders and rule out exclusionary psychological disorders according to the Fukuda et al. (1994) criteria. A physician screening and laboratory tests were also conducted to determine the presence of exclusionary medical illnesses (Fukuda et al., 1994). Once study eligibility criteria were met, participants were randomly assigned to four treatment conditions. Participants completed a battery of self-report measures and wore actigraphs at baseline and 12 months after treatment was completed. For additional details regarding study procedures and treatment protocols, see Jason et al. (2007).
Treatment Protocols
Two nurse-clinicians trained in each of the protocols, administered 13, forty five minute sessions of cognitive behavior therapy with graded exercise, anaerobic activity, cognitive coping skills, or relaxation once every two weeks. Participants attended an average of 10 out of a possible 13 sessions, with a range from one to 13. The average dropout rate of 25% was not significantly different across the treatment conditions. Given the comparability of outcomes across the four treatment conditions (Jason et al., 2007), the treatment conditions were collapsed in the present study.
Analyses
Data analysis was conducted using the 44 participants with complete data at baseline and 12-month follow-up. Two independent variables were used: baseline energy envelope (inside vs. outside) and actigraphy change (increased vs. decreased). Two 2 x 2 repeated measures analyses of variance (RM-ANOVA) were carried out with two outcome variables measured at baseline and 12-month follow-up: physical functioning and fatigue severity. The following were examined for each outcome: the main effect of time, time by energy envelope interaction, time by actigraphy change interaction, and the three-way time by energy envelope by actigraphy change interaction. If a significant three-way interaction was observed, simple effects of time and post hoc pairwise comparisons were conducted for that outcome. For simple effects and pairwise comparisons, four groups of participants were compared: inside-increased, inside-decreased, outside-increased, and outside-decreased. Due to the large number of pairwise comparisons, a Bonferroni correction was used and the significance level was set at p < .008.
Since the four treatment conditions were collapsed in the analyses, an evaluation of equal distribution of the four envelope/actigraphy change groups across treatment conditions was conducted using SAS Proc Freq Fisher's Exact Test. No significant differences were found across the four groups in terms of treatment assignment (p = .54).
Results
Sample Characteristics
Among the 44 participants in the sample, 81.8% were female. The average age was 45.6 years. Regarding ethnicity, 90.9% were White, 4.5 % were Asian American, 2.3% were African American, and 2.3% were Latino. As for marital status, 50% were married or living with someone, 27.2% were either divorced or separated, and 22.7% were single. In terms of work status, 68.2% were not working or were part-time students, and 31.8% were working or full-time students. In terms of education, 52.3% had earned a standard college degree, 22.7% had a graduate or professional degree, 20.5% had partial college, and 4.5% had a high school/GED degree or less.
The average energy quotient at baseline was 244.66 (SD = 245.90). A total of 17 participants were inside their energy envelope at baseline, and 27 were outside of their energy envelope at baseline. In terms of changes in actigraphy counts, participants reduced their activity from baseline to 12-month follow-up by an average of 717.80, although there was large variability across the sample (SD = 3058.51). Twenty-three participants increased activity, and 21 participants decreased their activity.
Outcomes
Means and standard deviations for the two RM-ANOVAs are reported in Table 1. For physical functioning, a significant time by baseline energy envelope interaction was revealed, with those who started treatment within their energy envelope demonstrating significantly more improvement in physical functioning than those who were outside of their energy envelope, F(1, 40) = 8.08, p = .007, ηp2 = .17. In terms of the clinical significance of these findings, participants who were inside of their energy envelope had a 37.6% improvement in physical functioning, compared to a 6.0% improvement for those who started treatment outside of their energy envelope. There was no significant time by actigraphy change interaction, nor was there a significant three-way time by energy envelope by actigraphy change interaction.
Table 1.
Means (and Standard Deviations) of Outcomes for Baseline Energy Envelope × Actigraphy Change × Time (N = 44)
| Outcomes | Inside Energy Envelope at Baseline |
Outside Energy Envelope at Baseline |
||
|---|---|---|---|---|
| Decreased Actigraphy (n = 10) | Increased Actigraphy (n = 7) | Decreased Actigraphy (n = 11) | Increased Actigraphy (n = 16) | |
| Baseline | ||||
| Physical Functioning1 | 38.78 (17.90) | 57.14 (25.47) | 35.01 (18.00) | 46.88 (24.69) |
| Fatigue Severity2 | 6.02 (0.62) | 6.02 (0.61) | 6.39 (0.74) | 6.10 (0.66) |
| 12-Month Follow-up | ||||
| Physical Functioning1 | 55.50 (28.33) | 76.43 (26.73) | 38.64 (25.01) | 48.13 (26.64) |
| Fatigue Severity2 | 5.63 (1.44) | 4.49 (1.30)a | 6.16 (1.03) | 6.00 (0.76)a |
Higher numbers are better
Lower numbers are better
Significant pairwise post hoc difference (p = .004)
For fatigue severity, Figure 1 shows a significant three-way time by energy envelope by actigraphy interaction, F(1, 40) = 4.46, p = .04, ηp2 = .10. Pairwise post hoc analyses did not reveal any significant differences at baseline. A significant difference was found at the 12-month follow-up between the inside-increased group and the outside-increased group, such that those who were inside and increased had lower fatigue severity (mean difference = 1.51; p = .004). None of the other pairwise comparisons were significant at the p < .008 level. In terms of simple effects of time, only those who were in the inside-increased group significantly reduced fatigue severity from baseline to 12-month follow-up, F(1, 40) = 17.89, p < .001, ηp2 = .31.
Figure 1.
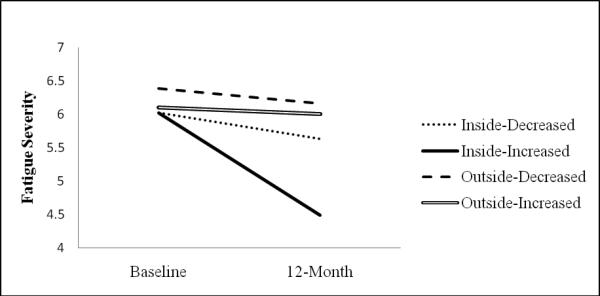
Fatigue Severity Scale Means for Pairwise Groups
For Fatigue Severity, there was also a significant time by baseline energy envelope interaction, with those who started treatment within their energy envelope demonstrating significantly more improvement in fatigue severity than those who were outside their energy envelope, F(1, 40) = 6.93, p = .01, ηp2 = .15. In terms of the clinical significance, participants who were inside of their energy envelope had a 16.0% improvement in fatigue severity, compared to a 2.7% improvement for those who started treatment outside of their energy envelope. No significant time by actigraphy change interaction was revealed.
Discussion
The aim of this study was to determine whether increasing physical activity after nonpharmacological interventions has a positive effect on physical functioning and fatigue severity among patients with ME/CFS. An objective measure of activity level, the actigraph, was used to determine increases and decreases in activity from baseline to 12-month follow-up. Results from this study found no significant relationship between increasing or decreasing activity and changes in physical functioning and fatigue severity. These findings do not provide support for treatment models of ME/CFS which suggest that increases in activity are necessary for patients with ME/CS to show improvement (e.g., Fulcher & White, 1997; Wearden et al., 1998).
In order to provide an alternative perspective on activity level and improvements, this study explored the role of energy envelope in physical functioning and fatigue severity changes. Results showed that there was a significant increase in physical functioning and decrease in fatigue severity for those individuals who were within their energy envelope at baseline compared to those who were outside of their energy envelope. Individuals who started treatment outside of their energy envelope showed minimal improvement in physical functioning (6.0%) and fatigue severity (2.7%). It is possible that individuals who began the intervention already within their energy envelope were likely to remain inside of their energy envelope after the intervention and, therefore, were more likely to show improvements in physical functioning and fatigue. As support for this hypothesis, the energy quotients for participants who were within their energy envelope at baseline were, on average, still within their energy envelope at the 12-month follow-up (Inside-Decreased M = 121.97; Inside-Increased M = 91.36), while those who were outside of their energy envelope at baseline were also outside at the follow-up (Outside-Decreased M = 244.09; Outside-Increased M = 284.38).
This study did not support the concept of increasing activity universally for all patients with ME/CFS in order to improve functioning, nor did these findings support Wiborg et al.'s (2010) conclusion that increased activity during CBT is unrelated to treatment outcomes. However, findings did suggest that increasing activity is indicated for some patients and not indicated for others, depending on one's energy envelope. Results from this study showed that individuals who started treatment within their energy envelope and increased their activity level demonstrated significantly greater improvements in fatigue severity at the 12-month follow-up than those who started outside of their energy envelope and increased activity. The inside-increased group was the only group demonstrating a significant improvement in fatigue over time. Moreover, those who were inside and increased activity remained, on average, inside their energy envelope at the follow-up; while those who were outside and increased remained outside of their energy envelope at the follow-up despite increases or decreases in activity.
According to the energy envelope theory (Jason et al., 1999), results from this study are congruent with the notion that those individuals who only expend as much energy as they have available will show the most significant improvements, while those who are overextended and increase activity will not improve. Further, Friedberg and Sohl (2009) found that the group of patients reporting improvement after a CBT with graded activity had both shown increases and decreases in actigraphy counts. The authors suggest that patients with ME/CFS are diverse and careful assessment is necessary to understand the relationship among treatment elements and various outcomes. Based on the present study, it is recommended that assessment of energy envelope be incorporated in non-pharmacological intervention trials.
A major limitation to this study was that the sample sizes in some of the groups may have been too small to detect significant differences between groups. Moreover, the effect sizes were small, suggesting that while some findings were statistically significant, the importance of the differences should be interpreted with caution. A further concern was that differential effects across the four treatment conditions were not evaluated. Yet, collapsing the treatment conditions provided a broader examination of activity changes after non-pharmacological interventions. Future research with larger sample sizes should explore potential improvements related to decreasing activity among those who are outside of their energy envelope within various treatment interventions.
This study provides clarification of the role of increased activity during non-pharmacological interventions for patients with ME/CFS. It was found that increasing activity was associated with more improvements for those who started treatment within their energy envelope compared to those outside of their energy envelope. Consequently, treatment models emphasizing increases in activity may only be beneficial for a select group of patients, particularly if they remain within their energy envelope. An assessment of energy envelope should be incorporated into research on non-pharmacological interventions, as well as used in clinical settings that provide treatment to patients with ME/CFS.
Acknowledgments
The authors appreciate the financial assistance provided by the National Institute of Allergy and Infectious Diseases (grant numbers AI36295 and AI49720).
References
- Edmonds M, McGuire H, Price J. The Cochrane Library. John Wiley & Sons, Ltd.; Chichester, UK: 2004. Exercise therapy for chronic fatigue syndrome (Cochrane Review). [DOI] [PubMed] [Google Scholar]
- Fulcher KY, White PD. Randomised controlled trial of graded exercise in patients with the chronic fatigue syndrome. British Medical Journal. 1997;314:1647–1665. doi: 10.1136/bmj.314.7095.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg F, Sohl S. Cognitive-Behavior Therapy in Chronic Fatigue Syndrome: Is Improvement Related to Increased Physical Activity? Journal of Clinical Psychology. 2009;65:423–442. doi: 10.1002/jclp.20551. doi: 10.1002/jclp.20551. [DOI] [PubMed] [Google Scholar]
- Hawk C, Jason LA, Torres-Harding S. Reliability of a chronic fatigue syndrome questionnaire. Journal of Chronic Fatigue Syndrome. 2007;13:41–66. doi: 10.1300/J092v13n04_05. [Google Scholar]
- Jason LA, Benton M, Torres-Harding S, Muldowney K. The impact of energy modulation on physical functioning and fatigue severity among patients with ME/CFS. Patient Education and Counseling. 2009;77:237–241. doi: 10.1016/j.pec.2009.02.015. doi:10.1016/j.pec.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jason LA, Melrose H, Lerman A, Burroughs V, Lewis K, King CP, et al. Managing chronic fatigue syndrome: Overview and case study. AAOHN Journal. 1999;47:17–21. [PubMed] [Google Scholar]
- Jason LA, Muldowney K, Torres-Harding S. The energy envelope theory and Myalgic Encephalomyelitis/chronic fatigue syndrome. AAOHN Journal. 2008;56:189–195. doi: 10.3928/08910162-20080501-06. [DOI] [PubMed] [Google Scholar]
- Jason LA, Torres-Harding S, Friedberg F, Corradi K, Njoku MG, Donalek J, et al. Non-pharmacologic interventions for CFS: A randomized trial. Journal of Clinical Psychology in Medical Settings. 2007;14:275–296. doi: 10.1007/s10880-007-9090-7. [Google Scholar]
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- Lane AM, Lovejoy DJ. The effects of exercise on mood changes: The moderating effect of depressed mood. Journal of Sports Medicine and Physical Fitness. 2001;41(4):539–45. [PubMed] [Google Scholar]
- Malouff JM, Thorsteinsson EB, Rooke SE, Bhullar N, Schutte NS. Efficacy of cognitive behavioral therapy for chronic fatigue syndrome: A meta-analysis. Clinical Psychology Review. 2008;28:736–745. doi: 10.1016/j.cpr.2007.10.004. doi: 10.1016/j.cpr.2007.10.004. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Lu AW, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Medical Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Mock V, Frangakis C, Davidson NE, Ropka ME, Pickett M, Poniatowski B. Exercise manages fatigue during breast cancer treatment: A randomized controlled trial. Psycho-Oncology. 2005;14:464–477. doi: 10.1002/pon.863. doi: 10.1002/pon.863. [DOI] [PubMed] [Google Scholar]
- Oken BS, Kishiyama S, Zajdel D, Bourdette D, Carlsen J, Haas M. Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology. 2004;62:2058–2064. doi: 10.1212/01.wnl.0000129534.88602.5c. [DOI] [PubMed] [Google Scholar]
- Pesek JR, Jason LA, Taylor RR. An empirical investigation of the envelope theory. Journal of Human Behavior in the Social Environment. 2000;3:59–77. doi: 10.1300/J137v03n01_04. [Google Scholar]
- Schwartz AL, Mori M, Gao R, Nail LM, King ME. Exercise reduces daily fatigue in women with breast cancer receiving chemotherapy. Medicine and Science in Sports and Exercise. 2001;33:718–723. doi: 10.1097/00005768-200105000-00006. [DOI] [PubMed] [Google Scholar]
- Wearden AJ, Morriss RK, Mullis R, Strickland PL, Pearson DJ, Appleby L. Randomised, double-blind, placebo-controlled treatment trial of fluoxetine and graded exercise for chronic fatigue syndrome. British Journal of Psychiatry. 1998;172:485–490. doi: 10.1192/bjp.172.6.485. doi: 10.1192/bjp.172.6.485. [DOI] [PubMed] [Google Scholar]
- Whiting P, Bagnall AM, Snowden AJ, Cornell JE, Mulrow CD, Ramirez G. Interventions for the treatment and management of chronic fatigue syndrome. A systematic review. JAMA. 2001;286:1360–1368. doi: 10.1001/jama.286.11.1360. doi: 10.1001/jama.286.11.1360. [DOI] [PubMed] [Google Scholar]
- Wiborg JF, Knoop H, Stulemeijer M, Prins JB, Bleijenberg G. How does cognitive behaviour therapy reduce fatigue in patients with chronic fatigue syndrome? The role of physical activity. Psychological Medicine. 2010 doi: 10.1017/S0033291709992212. doi:10.1017/S0033291709992212. [DOI] [PubMed] [Google Scholar]


