An endemic form of PF (pemphigus foliaceus) exists and is virtually exclusive to certain inland states of Brazil, where it is known as Fogo Selvagem (FS) (Diaz et al., 1989b). FS shows similar clinical, histological and immunological features to those observed in non-endemic PF (Diaz et al., 1989a; Stanley et al., 1986). The etiology of FS is unknown, but blister formation is mediated predominantly by IgG4 (Rock et al., 1989). Moreover, a progression from preclinical to clinical disease is associated with a sharp rise in IgG4 anti-Dsg1 (Warren et al., 2003), and these autoantibodies are predictors of FS (Qaqish et al., 2009). Emergence of self-reactive IgG4 anti-Dsg1 in FS is a mystery; however, there are some promising circumstantial clues that point toward the etiology of the disease. For example, the antigen-selected nature of anti-Dsg1 (Qian et al., 2009) and the presence of IgM anti-Dsg1 in healthy individuals in endemic areas of FS (Diaz et al., 2008) strongly suggest a recent or an ongoing exposure of settlers in these endemic regions to an environmental antigen(s) (Aoki et al., 2004; Diaz et al., 2004; Diaz et al., 2008)
IgG4 and IgE antibodies develop in individuals chronically exposed to environmental allergens or during immunotherapy of patients with allergic diseases (Lichtenstein et al., 1968; Muller, 2005). Little is known about the IgE anti-Dsg1 response in FS, but recently Nagel et al (Nagel et al., 2009) have reported that IgE anti-Dsg3 correlated with disease activity in Pemphigus Vulgaris patients. In this investigation, we have evaluated the IgE, IgM and IgG4 anti-Dsg1 responses in a large cohort of FS patients and control individuals. A total of 558 sera were tested for IgE anti-Dsg1. Patient cohorts included 143 FS patients (Brazil), 39 non-endemic PF patients (USA and Japan), and 59 PV patients (USA and Japan). Healthy control (HC) cohorts included 161 healthy individuals living in endemic areas of FS (Brazil), 57 healthy individuals from non-endemic areas (Brazil), and 99 healthy individuals from USA (n= 76) and Japan (n= 23). Dsg1-specific ELISA were carried out as described (Qian et al., 2007) with a minor modification, i.e. the use of biotin labelled mouse anti-human IgE and HRP conjugated streptavidin (SouthernBiotech, Birmingham, AL). The levels of IgE anti-Dsg1 were expressed as index value units as reported by Amagai et al (Amagai et al., 1999) and Diaz et al (Diaz et al., 2008; Qaqish et al., 2009).
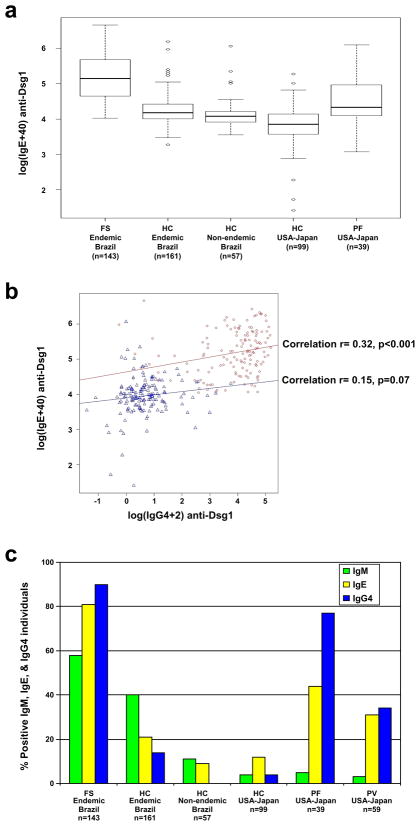
The distribution of IgE index values in sera from FS, PF, and healthy individuals from Brazilian, Japanese and US donors is shown as box plots (Figure 1a). The statistical comparisons of these groups are shown in Table 1. The IgE anti-Dsg1 index values in the FS group were higher than in the other sets, while HC from US and Japan showed the lowest values. The differences in means between the FS group and all other groups tested were statistically significant at the 0.05 level. The difference between HC from endemic regions of Brazil and non-endemic regions of Brazil was not significant. However, the difference between HC from Brazil and USA/Japan was statistically significant. The significantly different IgE anti-Dsg1 levels between the FS group and the PF USA/Japan indicate that the IgE anti-Dsg1 response in FS is a distinctive serological feature of this disease. The significant difference in IgE anti-Dsg1 between HC Brazil (endemic and non-endemic) and HC USA/Japan needs to be explored in the future. The lack of difference between HC endemic Brazil and HC non-endemic is likely explained by the fact that these groups share certain living conditions, and perhaps environmental antigens. These antigens however may not be present in USA and Japan.
Figure 1. IgE and IgG4 anti-Dsg1 in Fogo Selvagem patients and Control groups.
Panel a: Box plots of IgE anti-Dsg1 in FS and PF patients and healthy controls (HC). The horizontal lines within the box plots indicate the median values; the lower and upper ends of the boxes represent the 25th and 75th percentiles. Panel b. The correlation between anti-Dsg1 IgE and IgG4 in FS patients and healthy controls. Blue triangles and blue line, healthy controls; red circles and red line, FS patients. Panel c. IgM, IgE and IgG4 anti-Dsg1 in FS, PF, PV, BP and healthy controls (HC). Percentages of positive anti-Dsg1 sera for each group are shown in green bars (IgM), yellow bars (IgE) and blue bars (IgG4).
Table 1.
1 Comparison of the IgE anti-Dsg1 Autoantibodies in FS and Control Groups
| Comparison | Difference Between Means of Index Value | Simultaneous 95% Confidence Limits | p Values | |
|---|---|---|---|---|
| FS vs PF USA/Japan2 | 0.69 | 0.42 | 0.96 | 0.05 |
| FS vs HC Endemic area-Brazil2 | 0.90 | 0.73 | 1.07 | 0.05 |
| FS vs HC Non-Endemic area-Brazil | 1.01 | 0.78 | 1.25 | 0.05 |
| FS vs HC USA/Japan | 1.32 | 1.13 | 1.51 | 0.05 |
| PF USA/Japan vs HC USA/Japan | 0.63 | 0.35 | 0.91 | 0.05 |
| HC Endemic area-Brazil vs HC Non-endemic area-Brazil | 0.12 | −0.11 | 0.34 | ns2 |
| HC USA/Japan vs HC Endemic area-Brazil | −0.42 | −0.61 | −0.23 | 0.05 |
| HC USA/Japan vs HC Non-endemic area -Brazil | −0.31 | −0.55 | −0.06 | 0.05 |
Analysis of differences between groups used one-way analysis of variance with adjustment for multiple comparisons by Tukey’s studentized range test. Analysis was done on log(IgE+40) and log(IgG4+2) as these transformed variables were approximately normally distributed. Analysis was done in the R 2.9 software (Team, 2009).
FS = Fogo Selvagem, PF = Pemphigus foliaceus, HC = Healthy controls, ns = Non significant comparison
We then analyzed the correlation between the IgE and IgG4 anti-Dsg1 responses in 143 FS and 317 HC. Figure 1b shows a plot of IgE against IgG4 anti-Dsg1, with superimposed regression lines, in the FS (red) and HC groups (blue). We found a correlation between IgE and IgG4 in the FS group (Spearman correlation r= 0.32, p<0.001), and less correlation in the HC group (r= 0.15, p= 0.07) suggesting that generation of these autoantibodies in FS may be linked and arising due to sensitizing to a common environmental allergen. These patterns of IgG4 and IgE responses are similar to those observed in allergic patients. Ongoing studies may demonstrate a stronger correlation between IgE and IgG4 anti-Dsg1 systems when testing sera from patients at different clinical and immunological stages of evolution.
To evaluate the humoral IgM, IgG4 and IgE anti-Dsg1 responses in FS and HC, we extracted data on IgM and IgG4 anti-Dsg1 for the same subjects utilized in previous publications (Diaz et al., 2008; Qaqish et al., 2009). An ROC curve for IgM, IgE and IgG4 anti-Dsg1 index values was used to determine a cutoff point for sensitivity and specificity as described (Diaz et al., 2008; Qaqish et al., 2009). The percentages of positive sera for IgM, IgE and IgG4 anti-Dsg1 in the different groups were plotted and shown in Figure 1c. It is apparent that the triple response of IgM, IgG4 and IgE anti-Dsg1 is much higher in FS patients than in HC from the same endemic regions and HC from non-endemic regions of Brazil and USA/Japan (χ2, p<0.05, for IgM, IgE, and IgG4 anti-Dsg1). The percentages of positive individuals for IgM and IgE anti-Dsg1 were higher in the FS group (58% and 81% respectively) than in all other groups (χ2, p<0.05). As expected and also shown in Figure 1c, IgG4 anti-Dsg1 was detected in a large number of FS, PF and PV (90%, 77% and 33% respectively), which is a known feature of these diseases. These findings suggest a persistent antigenic stimulation of the immune system of individuals living in the endemic areas of FS.
In summary, our findings further support the notion that the anti-Dsg1 response in FS patients may be initiated by sensitization to an environmental allergen(s). The cross-reactive IgE, IgM and pathogenic IgG4 anti-Dsg1 response may be the serological markers of these immunological responses.
Acknowledgments
Funding support is provided by NIH grants R01-AR30281, RO1-AR32599, T32 AR07369 (LAD), and K01-AR056378 (YQ). Dr. Qian’s research is also supported by a Dermatology Foundation Research Award and an American Skin Association Alice P. Melly Research Grant.
Abbreviations
- Dsg1
desmoglein 1
- Dsg3
desmoglein 3
- FS
Fogo selvagem
- PF
pemphigus foliaceus
- PV
pemphigus vulgaris
- rDsg1
recombinant Dsg1
- ROC
receiver operating characteristic
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Amagai M, Komai A, Hashimoto T, Shirakata Y, Hashimoto K, Yamada T. Usefulness of enzyme-linked immunosorbent assay using recombinant desmogleins 1 and 3 for serodiagnosis of pemphigus. Br J Dermatol. 1999;140:351–7. doi: 10.1046/j.1365-2133.1999.02752.x. [DOI] [PubMed] [Google Scholar]
- Aoki V, Millikan RC, Rivitti EA, Hans-Filho G, Eaton DP, Warren SJ, et al. Environmental risk factors in endemic pemphigus foliaceus (fogo selvagem) J Investig Dermatol Symp Proc. 2004;9:34–40. doi: 10.1111/j.1087-0024.2004.00833.x. [DOI] [PubMed] [Google Scholar]
- Diaz L, Sampaio S, Rivitti E, Martins C, Cunha P, Lombardi C, et al. Endemic pemphigus foliaceus (fogo selvagem). I. Clinical features and immunopathology. J Am Acad Dermatol. 1989a;20:657–69. doi: 10.1016/s0190-9622(89)70079-7. [DOI] [PubMed] [Google Scholar]
- Diaz LA, Arteaga LA, Hilario-Vargas J, Valenzuela JG, Li N, Warren S, et al. Anti-desmoglein-1 antibodies in onchocerciasis, leishmaniasis and Chagas disease suggest a possible etiological link to Fogo selvagem. J Invest Dermatol. 2004;123:1045–51. doi: 10.1111/j.0022-202X.2004.23438.x. [DOI] [PubMed] [Google Scholar]
- Diaz LA, Prisayanh PS, Dasher DA, Li N, Evangelista F, Aoki V, et al. The IgM anti-desmoglein 1 response distinguishes Brazilian pemphigus foliaceus (fogo selvagem) from other forms of pemphigus. J Invest Dermatol. 2008;128:667–75. doi: 10.1038/sj.jid.5701121. [DOI] [PubMed] [Google Scholar]
- Diaz LA, Sampaio SA, Rivitti EA, Martins CR, Cunha PR, Lombardi C, et al. Endemic pemphigus foliaceus (Fogo Selvagem): II. Current and historic epidemiologic studies. J Invest Dermatol. 1989b;92:4–12. doi: 10.1111/1523-1747.ep13070394. [DOI] [PubMed] [Google Scholar]
- Lichtenstein L, Holtzman N, Burnett L. A Quantitative in Vitro Study of the Chromatographic Distribution and Immunoglobulin Characteristics of Human Blocking Antibody. J Immunol. 1968;101:317. [PubMed] [Google Scholar]
- Muller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005;5:343–7. doi: 10.1097/01.all.0000173783.42906.95. [DOI] [PubMed] [Google Scholar]
- Nagel A, Lang A, Engel D, Podstawa E, Hunzelmann N, de Pita O, et al. Clinical activity of pemphigus vulgaris relates to IgE autoantibodies against desmoglein 3. Clin Immunol. 2009;134:320–30. doi: 10.1016/j.clim.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Qaqish BF, Prisayanh P, Qian Y, Andraca E, Li N, Aoki V, et al. Development of an IgG4-based predictor of endemic pemphigus foliaceus (fogo selvagem) J Invest Dermatol. 2009;129:110–8. doi: 10.1038/jid.2008.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Clarke SH, Aoki V, Hans-Filhio G, Rivitti EA, Diaz LA. Antigen selection of anti-DSG1 autoantibodies during and before the onset of endemic pemphigus foliaceus. J Invest Dermatol. 2009;129:2823–34. doi: 10.1038/jid.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Diaz LA, Ye J, Clarke SH. Dissecting the anti-desmoglein autoreactive B cell repertoire in pemphigus vulgaris patients. J Immunol. 2007;178:5982–90. doi: 10.4049/jimmunol.178.9.5982. [DOI] [PubMed] [Google Scholar]
- Rock B, Martins CR, Theofilopoulos AN, Balderas RS, Anhalt GJ, Labib RS, et al. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem) N Engl J Med. 1989;320:1463–9. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]
- Stanley J, Klaus-Kovtun V, Sampaio S. Antigenic Specificity of Fogo Selvagem Autoantibodies Is Similar to North American Pemphigus Foliaceus and Distinct from Pemphigus Vulgaris Autoantibodies. J Invest Dermatol. 1986;87:197–201. doi: 10.1111/1523-1747.ep12695334. [DOI] [PubMed] [Google Scholar]
- Warren S, Arteaga L, Rivitti E, Aoki V, Hans-Filho G, Qaqish B, et al. The Role of Subclass Switching in the Pathogenesis of Endemic Pemphigus Foliaceus. J Invest Dermatol. 2003;120:104–08. doi: 10.1046/j.1523-1747.2003.12017.x. [DOI] [PubMed] [Google Scholar]