Abstract
Current therapies for the autoimmune demyelinating disease multiple sclerosis (MS) target inflammation but do not directly address oligodendrocyte protection or myelin repair. The gp130 family cytokines CNTF, LIF and IL-11 have been identified as oligodendrocyte growth factors, and IL-11 is also strongly immunoregulatory, but their underlying mechanisms of action are incompletely characterized. Here, we demonstrate that these effects of IL-11 are mediated via differential regulation of apoptosis in oligodendrocytes versus antigen-presenting dendritic cells (DCs), and are dependent on lineage-specific activity of the transcription factors Stat1 versus Stat3. Focal demyelinating lesions induced in cerebral cortices of IL-11Rα−/− mice using stereotactic microinjection of lysolecithin were larger than in controls, and remyelination was delayed. In IL-11Rα−/− mice, lesions displayed extensive oligodendrocyte loss and axonal transection, and increased infiltration by inflammatory cells including CD11c+ DCs, CD3+ lymphocytes and CD11b+ phagocytes. In oligodendrocyte progenitor cell (OPC) cultures, IL-11 restricted caspase 9 activation and apoptosis, and increased myelination in OPC-neuron co-cultures. Importantly, siRNA inhibition of Stat1 enhanced the anti-apoptotic effects of IL-11 on OPCs, but IL-11 induced apoptosis in the presence of Stat3 silencing. In contrast, IL-11 augmented caspase activation and apoptosis in cultures of CD11c+ DCs, but not in CD11b+ or CD3+ cells. Inhibition of Stat3 exacerbated the pro-apoptotic effects of IL-11 on DCs, whereas they were ablated in Stat1−/− cultures. Collectively, these findings reveal novel mechanisms underlying the actions of a neuroprotective and immunoregulatory member of the gp130 cytokine family, and suggest avenues to enhance oligodendrocyte viability and restrict CNS inflammation in MS.
Keywords: oligodendrocyte, myelination, dendritic cell, differentiation, apoptosis
Introduction
Multiple sclerosis (MS) is a presumed autoimmune demyelinating disease of the CNS characterized by inflammation, loss of myelin and oligodendrocytes, axonal transection and reactive astrogliosis (1). Conduction block is believed to underlie early neurological dysfunction (2), whereas axonal transection is responsible for more permanent later deficits (3). Remyelination occurs in early lesions and is associated with functional recovery (4), but fails with disease progression (5). Notably, existing therapies focus on immunoregulation and reduce the frequency of clinical relapse, but do not directly address protection of oligodendrocytes and neurons, or myelin repair (6).
Members of the gp130 family of neurotrophic cytokines, including ciliary neurotrophic factor (CNTF), leukemia inhibitory factor (LIF) and interleukin-11 (IL-11), have been identified as survival factors for oligodendrocytes (7–9), and as potential therapeutic avenues for MS (10–12). The family also encompasses IL-6, IL-27, cardiotrophin-1 (CT-1) and oncostatin M (OSM). All members bind to receptors containing the common gp130 subunit and activate downstream Jak-Stat signaling and Stat-independent pathways (13). Each member shows a distinct spectrum of activity. CNTF−/− mice with experimental autoimmune encephalomyelitis (EAE), a model of MS, display more severe disease than controls, with increased oligodendrocyte apoptosis and axonal transection (10). LIFRβ+/−Δgp130+/− and LIF−/− mice exhibit a similar phenotype both in EAE and in cuprizone-induced demyelination (11), although LIF−/− mice also show attenuated later disease (14). In a third pattern, we recently found that IL-11 is expressed by reactive astrocytes in MS plaques (9), and that mice deficient for IL-11 receptor α (IL-11Rα−/−) display exacerbated EAE, associated with increased size of demyelinated lesions and also with potentiated CNS inflammation (12). Our studies confirmed that IL-11 enhances oligodendrocyte lineage cell number in vitro (9), but also revealed that IL-11 limits the function of antigen-presenting CD11c+ dendritic cells (DCs), restricting activation of encephalitogenic T lymphocytes (12).
Thus, IL-11 signaling is both neuroprotective and immunoregulatory, but the mechanisms underlying these effects are unknown. In a wider context, the processes determining the distinct actions of gp130 cytokines are incompletely characterized. In this report, we now identify differential regulation of apoptosis in Olig2+ oligodendrocytes versus CD11c+ DCs as an important mechanism underlying the effects of IL-11 in inflammatory demyelinating disease. These effects depend on lineage-specific activity of the transcription factors Stat1 and Stat3. In both cell types, Stat3 is anti-apoptotic, whereas Stat1 promotes cell death. However, following IL-11 treatment, activity of Stat3 predominates over its relative in oligodendrocytes, whereas the pro-apoptotic effects of Stat1 prevail in DCs. Collectively, these data reveal novel mechanisms underlying the actions of a neuroprotective and immunoregulatory member of the gp130 cytokine family. Our findings suggest new avenues to enhance oligodendrocyte survival and restrict CNS inflammation in MS.
Materials and Methods
Antibodies
The A2B5 hybridoma was obtained from the American Type Culture Collection (Manassas, VA), and supernatant (IgM) prepared. Other antibodies and dyes were: rat anti-CD11b, rat anti-GFAP, fluoromyelin (Invitrogen, Carlsbad, CA); hamster anti-CD11c (eBioscience, San Diego, CA); O4 mouse IgM (Dr. Peter Davies, AECOM); mouse anti-CNPase (IgG1), rabbit anti-Olig2 (Millipore Chemicon, Temecula, CA); mouse anti-myelin basic protein (MBP), mouse anti-neurofilament H nonphosphorylated (SMI 32, Covance Sternberger, Berkeley, CA); sheep anti-BrdU (Novus, Littleton, CO); mouse anti-PDGFRα (R&D Systems); rabbit anti-CD3 (Abcam, Cambridge, MA); mouse anti-actin (Santa Cruz, Santa Cruz, CA); rabbit caspase 9, cleaved caspase 3, PI3 kinase p85, phospho-Stat1(Y701), Stat1, phospho-Stat3(Y705), Stat3, phospho-Shp2(Y580), Shp2, phospho-p44/42(T202/Y204), phospho-Gsk3(S21/9), Cell Signaling (Beverly, MA).
Cytokines
Mouse IL-11 was from Peprotech (Rocky Hill, NJ) and was used at 1–100ng/ml, most commonly 100ng/ml based on our previous dose-response data (9, 12). Previous work in our laboratory has confirmed that mouse IL-11 is equally effective in mouse and rat cultures, and the mouse and rat IL-11 protein sequences are 97.5% similar (NCBI database). Rat TNFα was from Peprotech and was used at 10ng/ml. Salmonella minnesota LPS was from Alexis Biochemicals (Plymouth Meeting, PA). CpG was from Coley Pharmaceuticals (Wellesley, MA).
Inhibitors
A cell-permeable analog of the Stat3-SH2 domain-binding phosphopeptide containing a C-terminal membrane translocating sequence (Stat3CPI) was obtained from EMD (Gibbstown, NJ) and used at 100μM based on previous studies (15). Inactive non-phosphorylated control was from the same source. Pilot studies confirmed that 100μM Stat3CPI restricted activation of Stat3 but not Stat1 in CD11c+ DC cultures (data not shown)
Mice
All work was approved by the Institutional Animal Care and Use Committee. IL-11Rα−/−mice backcrossed onto C57BL/6 background for >12 generations were purchased from Jackson Laboratories (Bar Harbor, ME) and bred at MSSM (12). This genotype was generated by Drs. Lorraine Robb and C. Glenn Begley (Walter and Eliza Hall Institute, Melbourne, Australia) (16). Genotyping was by PCR as on the JAX website (http://jaxmice.jax.org) using primers: IL11Rα-F3 5′-GGCTCCCGTCATTACCTACA-3′, IL11Ra-R3 5′-AGCAGTCCTACCCGCTACAA-3′, IL11Rα-PGK1-R 5 5′-ACTTGTGTAGCGCCAAGTGC-3′. Stat1−/− mice (129S6/SvEv-Stat1tm1Rds) were generated by Dr. Robert Schreiber (Washington University, St. Louis, MO) and purchased from Taconic (Germantown, NY). Olig2+ cell numbers were similar in the CNS of unchallenged IL-11Rα−/− mice and wildtype littermates (Fig. 1e), and both groups exhibited normal CNS MBP immunoreactivity as previoualy shown (12). Light microscopic analysis of resin-embedded unlesioned adult (12wk) corpus callosal samples (4 per genotype) confirmed that myelin thickness and the ratio of axon to fiber diameter (G ratio) (17) were similar in IL-11Rα−/− mice and wildtype littermates (Table 1). Unchallenged IL-11Rα−/− mice showed no differences in CNS or peripheral immune system compared with wildtype controls, and no abnormalities in CD3+ or CD11c+ cell populations in splenic samples (12).
Figure 1. IL-11Rα−/− mice display exacerbated demyelination and inflammation in lysolecithin lesions.
(a) IL-11Rα−/− mice and wildtype littermates (12wk) were stereotactically microinjected with 1.5μl 1% lysolecithin into the left corpus callosum at 5.5 mm anterior to lambda, 1 mm lateral to bregma and 2.0mm deep, then sacrificed at 7–28dpi. Serial 20μm sections were stained for myelin (MBP), Olig2, GFAP, SMI-32 (transected axons) CD11b, CD11c and CD3, and morphometric data quantitated from Z-series projections (see Methods section). Panels (b,c,h,k,n) show entire lesions; higher magnification images of IL-11Rα−/− and wildtype littermate control samples are to the left in (e,g,j,m), and morphometric data to the right in (d,f,i,l,o). Data in panels (i,o) are from 14dpi. (b-d) Lysolecithin induced demyelination in the corpus callosum, with lesions larger at 7dpi (maximal demyelination) than 14dpi (onset of repair). Maximum cross-sectional MBP− area was greater in IL-11Rα−/− mice than controls at both 7 and 14dpi (d). Oligodendrocyte loss in lesions was increased in IL-11Rα−/− mice over wildtype littermates (e,f). Reactive astrogliosis was also more extensive (h,i) and the density of SMI-32+ transected axons higher in lesions in IL-11Rα−/− mice (g,i). (j-o) In IL-11Rα−/− animals, the inflammatory lesion was also markedly expanded, and infiltration of CD11b+ mononuclear phagocytes (j-l) and CD3+ T lymphocytes and CD11c+ dendritic cells (DCs) (m-o) was significantly increased. The latter were CD11c+CD11b+ myeloid DCs (j). Results are from 5 animals per genotype per condition per timepoint, 5 20x fields per animal. (d,i,l,o) Student’s t test, (f) ANOVA plus Bonferroni post test, *p<0.05, **p<0.01. Scalebars, (b,c,h,k,n) 90μm, (e,g,j,m) 15μm.
Table 1.
Normal myelin thickness and G ratio in corpus callosum of IL-11Rα−/− mice.
| Corpus Callosum | Wildtype | IL-11Rƒα−/− | P value |
|---|---|---|---|
| G Ratio (axon/fiber diameter) | 0.64+/−0.01 | 0.62+/−0.01 | ns |
| Myelin thickness (nm) | 152.3+/−8.3 | 174.2+/−11.1 | ns |
Random fields from epoxy-embedded sections of unlesioned corpus callosum from 12wk IL-11Rα−/− mice and wildtype littermates were captured by electron microscopy (at least 5 fields per animal, at least 4 mice per genotype), and axon and fiber diameter measured for 10 random axons per field by a blinded observer. Myelin thickness was calculated as (fiber diameter - axon diameter)/2. G ratio (myelin thickness relative to axon diameter) was calculated as (axon diameter)/(fiber diameter). Data were compared using Student’s t test. These parameters were similar in both genotypes, and no differences in myelin architecture were detected in unlesioned samples by light or electron microscopy.
Stereotactic microinjection
1.5μl 1% lysolecithin (Sigma) was delivered into the corpus callosum of 12wk mice by stereotactic microinjection, as described (18). Injected animals included IL-11Ra−/− mice and littermate wildtype controls (at least 5 per genotype per timepoint). Mice were perfused at 7–28dpi with 4% paraformaldehyde or glutaraldehyde, and brains processed for cryostat or epoxy-embedding.
Immunohistochemistry
Sections of cerebral cortex (20μm) were immunostained using described protocols (12). Stained sections were examined using a Leica TCS5 confocal microscope and stacks collected using 1μm on the Z axis and assembled into projections. To quantify demyelination, cross-sectional MBP− area was measured in stacks at 10x magnification using ImageJ software (NIH), and rostrocaudal size of MBP− area estimated in serial stacks. Cells positive for lineage and differentiation markers were quantified within the hypercellular lesion area by a blinded observer, at least 5 fields at 20x magnification per animal, at least 5 animals per timepoint.
Histopathology
Epoxy sections of corpus callosum (1μm) were stained with toluidine blue and photographed on a Zeiss Axioplan2 microscope. For electron microscopy, serial sections were placed on 200 mesh copper grids, contrasted with lead citrate and uranyl acetate and scanned in a Hitachi HS600 microscope. For quantitation of myelin thickness and G ratio, random fields of lysolecithin lesion border (see text) or unlesioned corpus callosum were captured by electron microscopy (at least 5 fields per animal, at least 4 mice per genotype at 14dpi), and axon and fiber diameter measured for 10 random axons per field by a blinded observer using ImageJ software. Myelin thickness was calculated as (fiber diameter - axon diameter)/2. G ratio was calculated as (axon diameter)/(fiber diameter) (17, 18).
Oligodendrocyte progenitor cell (OPC) monocultures
OPCs were purified from cerebral cortices of P2 Sprague Dawley rats as described (18). OPCs were plated onto poly-D-lysine coated confocal dishes (Mat-Tek, Ashland, MA). After 24h, growth factor-free medium with 1–100 ng/ml human IL-11 or vehicle was added and cells grown for 5d. At plating these cultures are >95% Olig2+ A2B5+ PDGFRα+ CNPase− MBP− OPCs (12, 18). Oligodendrocyte lineage cells express IL-11Rα at all stages of differentiation (9). For siRNA studies, 5nM Stat1, Stat3, Shp2 or PI3 kinase siRNA (Dharmacon, Lafayette, CO) was nucleofected using an Amaxa Rat Oligodendrocyte Nucleofector Kit (Gaithersburg, MD). Controls included non-targeting siRNA and sham, and specificity was assessed by immunoblotting.
Modeling
To separate IL-11 effects on proliferation, apoptosis and differentiation, we adapted a previously-described mathematical model to our OPC culture system (19). The model quantitates Olig2+ cells positive or negative for BrdU at 12h timepoints, and separates the proliferation rate of Olig2+MBP− OPCs (α) and mature Olig2+MBP+ oligodendrocytes (γ), and the differentiation rate (β) from one to the other (Fig. 3f). It also measures the rate of apoptosis using TUNEL immunostaining. Apoptosis cannot be experimentally separated into rates for OPCs (δ) and mature cells (ε), thus the model is bounded by three possibilities, that all cell death is from only OPCs, or only mature cells, or that the death rates for both are equal (19). The model uses six equations to generate coefficients α through ε:
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
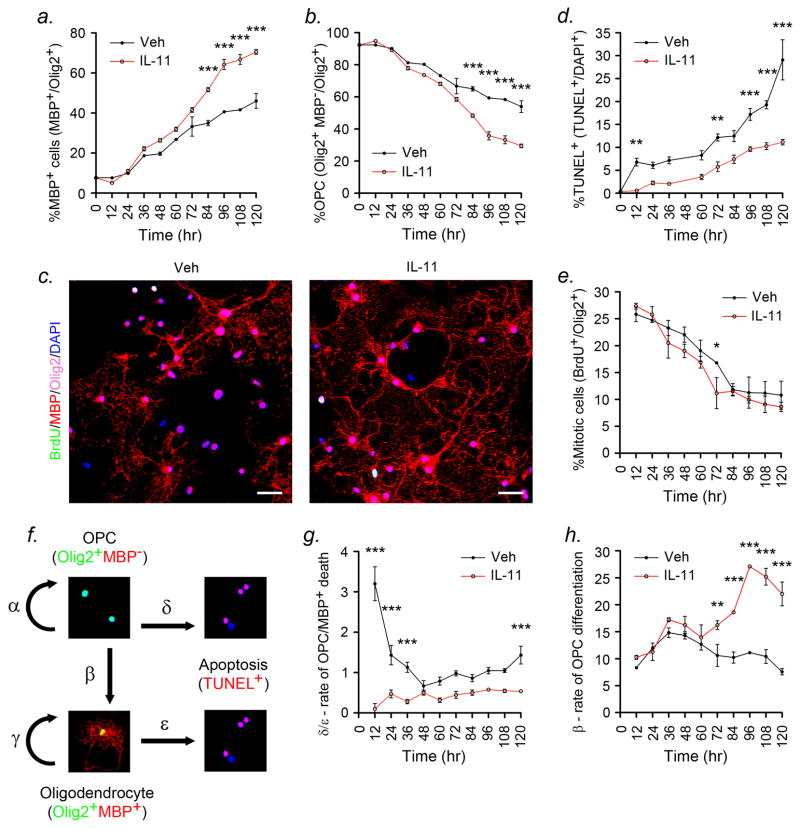
Figure 3. IL-11 potentiates oligodendrocyte progenitor survival and differentiation.
(a-e) Rat P2 OPCs grown in the presence of 100ng/ml IL-11 or vehicle for up to 120h were stained for Olig2, MBP, and BrdU or TUNEL. (a-c) In all cultures, the percentage of mature Olig2+MBP+ oligodendrocytes increased over time, while immature Olig2+MBP− OPCs declined. These changes were more pronounced in IL-11-treated cultures than controls, and reached significance from 84h (a,b). (d) The percentage of TUNEL+ apoptotic cells rose throughout the experiment in controls, but remained stable in IL-11-treated cultures. This difference was significant at 12h and became more pronounced towards the end of the study. In contrast, IL-11 had no effect on proliferation (e). (f) To understand the mechanism underlying these effects, we used a mathematical model to generate rates of proliferation for Olig2+MBP− OPCs (α) and mature Olig2+MBP+ cells (γ), differentiation from one to the other (β), and apoptosis for OPCs (δ) and mature oligodendrocytes (ε) (see Methods). (g,h) IL-11 greatly reduced the rate of apoptosis (δ+ε) in OPC cultures (g), and increased the rate of differentiation (β) (h). The reduction in rate of apoptosis was strongest within the first 36h (g), whereas the increase in differentiation rate was significant from 72h onwards (h). (a,b,d,e,g,h) ANOVA plus Bonferroni post test, *P<0.05, **P<0.01, ***P<0.001. (c) Scalebars, 20μm. Data are representative of three independent experiments on separate cultures.
The final two equations represent mitotic OPC and mature cell numbers between times i and i+1. The βi[αi/(1+αi)] NOPCi term represents OPCs that may have divided then differentiated. To validate the model, we compared values predicted for total cells and differentiation (equation 3) to measurements from 12h to 120h in untreated cultures. Predicted and measured values were not significantly different.
Myelinating co-cultures
Dorsal root ganglion (DRG) neurons were isolated from E16.5 rat embryos and cultured on collagen-coated confocal plates as described (20). Cells were alternately fed with fluorodeoxyuridine (Sigma) in medium supplemented with NGF at 50ng/ml to eliminate non-neuronal cells. Purified rat P2 OPCs were then plated onto to DRG neurons (20) and myelination induced by adding 1μg/ml TrkA-Fc (9). Co-cultures were grown in the presence of 1–100ng/ml IL-11 or vehicle, and medium replaced every other day. Cultures were fixed at 10–14d.
Dendritic cell cultures
Mouse CD11c+ dendritic cells (DCs) were cultured from bone marrow-derived monocytes as reported (21). Bone marrow was extracted from 8wk mouse femurs and tibias and erythrocytes lysed with ammonium chloride buffer, and MHC class II-expressing cells and lymphocytes removed using a cocktail of mAbs and rabbit complement as described (21). Purified monocytes were cultured in 24-well dishes in RPMI 1640 containing 10% FBS, 50 μg/ml gentamycin, 2 mM glutamine, 1 mM sodium pyruvate, and 50 U/ml GM-CSF (PeproTech) at 1×106 cells/ml. Purity was confirmed by immunostaining for CD11c. We have previously confirmed that CD11c+ DCs express IL-11Rα (12).
Macrophage cultures
For generation of peritoneal macrophages, mice were injected ip with 1ml thioglycollate (BD), then total cells harvested after 72h and plated at 106/ml in RPMI 1640 supplemented with 10% FCS and 1% penicillin/streptomycin (Invitrogen). After overnight culture, nonadherent cells were removed and purity of macrophages confirmed by F4/80 staining and flow cytometry.
Bromodeoxyuridine (BrdU) incorporation
To assess proliferation, 10μM BrdU (BD, San Diego, CA) was added to cells 24h prior to fixation. Staining for BrdU was according to manufacturer’s instructions.
TUNEL assay
To identify apoptotic cells, TUNEL labeling assays were performed using a commercial kit (Roche, Indianapolis, IN), according to the manufacturer’s instructions.
Immunoblotting
Western blotting was carried out as reported (9). For densitometry, nonsaturated films were scanned using a Canon LiDE scanner (Lake Success, NY), and mean pixel density of each band measured using ImageJ software as described (22). Measurements were standardized to actin loading control, and fold change versus control calculated.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde and immunostained using antibody combinations described in the text (9, 12, 18). Cultures were photographed using a Leica TCS5 confocal microscope and Z series stacks collected using 1μm on the Z axis and assembled into projections. Cells positive for each marker were counted in at least 5 stacks per condition per marker in each experiment by a blinded observer using ImageJ software. Results were expressed as percent of total (DAPI+) cells, and as number of positive cells, and were compared by statistics (see below). In DRG-OPC co-cultures, myelin formation was quantified in stacks immunostained for MBP and NF. Myelin segments were identified as MBP+ linear profiles extending along NF+ axons, and their number and length were quantified by a blinded observer in at least 5 stacks per experiment (9, 18).
Statistics
For multiple comparisons, one-way ANOVA followed by Bonferroni post-test was used. Two-way ANOVA plus Bonferroni post test was used to compare two treatment groups over multiple timepoints. Student’s t test was used to compare two groups of matched samples. P<0.05 was considered significant.
Results
Previously, we demonstrated that IL-11 receptor-deficient mice (IL-11Rα−/−) display exacerbated disease in MOG35–55-induced EAE (12). Compared with wildtype controls, IL-11Rα−/− mice exhibited a worsened clinical course, more extensive demyelinated lesions and increased CNS inflammation (12). Here, to clarify the roles of IL-11 in restricting myelin loss versus facilitating repair, we used a focal model of de- and remyelination in vivo (Figures 1,2, Table 1, Suppl. Figure 1). We then defined the mechanism of action of IL-11 in oligodendrocyte progenitors versus inflammatory cells in complementary studies in primary cultures (Figures 3–6, Suppl. Figures 2,3).
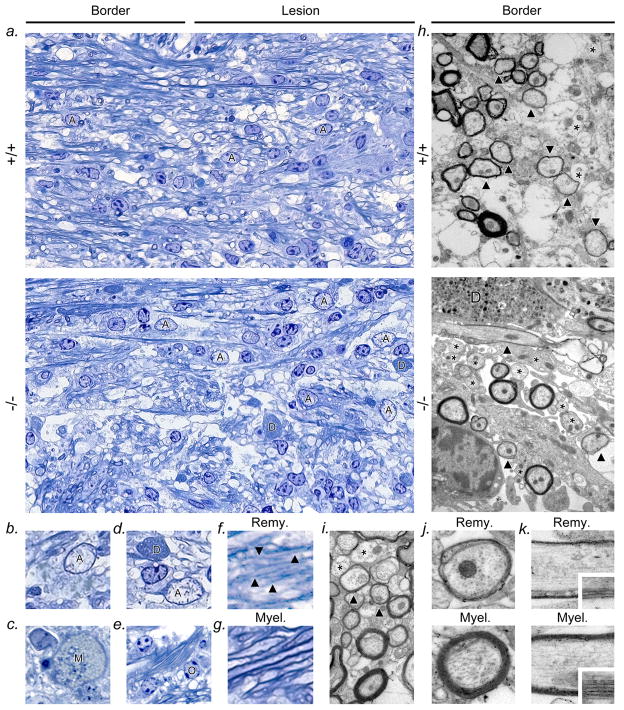
Figure 2. Delayed remyelination in IL-11Rα−/− mice.
(a) Light microscopy of epoxy sections stained with toluidine blue, showing representative pathology from 14dpi lysolecithin lesions in an IL-11Rα−/− mouse (lower panel) and wildtype littermate (upper panel). Individual cell types or details of pathology are illustrated at higher magnification in panels (b-g) (all IL-11Rα−/−genotype). (a) At 14dpi, the lesion center in both genotypes was hypercellular and demyelinated (right side of panels), and contained hypertrophic astrocytes (marked A, example shown in b), lipid-containing mononuclear phagocytes (c, marked M), and dystrophic transected axons (marked D, example shown in d). The lesion center was edematous in IL-11Rα−/− mice compared with controls, and compatible with data from samples immunostained for GFAP and SMI-32, reactive astrogliosis was more pronounced and dystrophic axons more numerous (a, lower panel). (e) Normal myelin and oligodendrocytes (O) were present outside the lesion in both genotypes (see also Table 1). Adjacent to the lesion center in both genotypes was a distinct border (a, left side of panels) containing axons wrapped by disproportionately thin myelin characteristic of remyelination (examples shown in f, compare with normal myelin in g). Panels (h-k) illustrate remyelination at the lesion border in both genotypes using electron microscopy of serial grids. Panel (h) compares the lesion border at 14dpi in an IL-11Rα−/− mouse (lower panel) and wildtype littermate (upper panel), showing demyelinated (asterisks) and remyelinated axons (arrowheads). Remyelinated axons were less abundant in IL-11Rα−/− samples than controls. Calculations of G ratio (axon diameter versus fiber diameter, a measurement of myelin thickness) for individual fibers within the lesion border in IL-11Rα−/− and wildtype mice (see Methods section) confirmed that a significantly smaller percentage of axons were remyelinated (G ratio >0.7) in IL-11Rα−/− mice than in wildtype littermates (IL-11Rα−/− 12.5+/−1.6 vs. wildtype 20.4+/−1.9, P=0.011, Student’s t test). De- and remyelinated axons are highlighted in lesion border (wildtype) in (i), and are compared in (j-k), in transverse (j) and longitudinal section (k, higher magnification inset). Images are representative of data from 4 animals per genotype per condition, 5 fields per animal at x2000, 10 axons per field. Magnification, (a) x300, (b-g) x750, (h,i) x2000, (j,k) x5000, inset x10000.
Figure 6. IL-11 induction of dendritic cell apoptosis is Stat1-dependent.
(a-c) CD11c+ DC cultures were pretreated with a Stat3 cell-permeable inhibitor (Stat3CPI, 100μM) or inactive analog control for 1h, then exposed to IL-11 or vehicle for 72h and protein extracts immunoblotted for caspase 9 and cleaved caspase 3 (a). Blots were quantified by densitometry, and data from multiple experiments compared (b,c). IL-11-induced cleavage of caspases 9 and 3 was exacerbated in Stat3CPI-treated cultures. Stat3CPI also caused a trend towards increased caspase cleavage even in the absence of IL-11. (d-f) DC cultures from Stat1−/− mice or wildtype littermates were exposed to IL-11 or vehicle for 72h, and cleavage of caspases 9 and 3 assayed by immunoblotting and densitometry as above. The IL-11-induced upregulation of caspase cleavage seen in wildtype cultures was abrogated in cultures from Stat1−/− mice. (b,c,e,f) Student’s t test, *P<0.05. Data are representative of three independent experiments on separate cultures.
Demyelination and oligodendrocyte death are exacerbated in IL-11Rα−/− mice
We used stereotactic microinjection of lysolecithin (18) to induce focal demyelinating lesions in the corpus callosum of 12wk IL-11Rα−/− mice and wildtype littermates, then followed the timecourse of lesion formation and resolution over a 4 week period using confocal imaging (Figure 1, Suppl. Figure 1) and light and electron microscopy (Figure 2, Table 1). In confocal imaging experiments, we stained serial 20μm sections of cerebral cortices at 7, 14 and 28dpi for myelin (MBP and fluoromyelin) and the lineage markers Olig2 (oligodendrocytes), CD11b (mononuclear phagocytes) and CD3 (T lymphocytes), and for CD11c+ antigen-presenting cells. We also used GFAPimmunoreactivity to measure astrogliosis, and SMI-32 for transected axons.
Lysolecithin microinjection into the left side of the corpus callosum (Fig. 1a) (18) resulted in demyelination in both genotypes, with lesions larger at 7dpi than at 14dpi (Figs. 1b-d). Importantly, the maximal cross-sectional MBP/fluoromyelin negative demyelinated area was significantly larger in the brains of IL-11Rα−/− mice than controls at both timepoints (Fig. 1d). Rostrocaudal lesion size was also increased in IL-11Rα−/− mice, at both 7dpi (IL-11Rα−/− 608μm vs. wildtype 455μm, P<0.05, Student’s t test) and 14dpi (IL-11Rα−/− 498μm vs. wildtype 280μm, P<0.0001). Moreover, numbers of Olig2+ cells were reduced to a much greater extent in lesions in IL-11Rα−/− mice than controls at both timepoints (Figs. 1e,f). The extent of GFAP+ reactive astrogliosis was also increased (Figs. 1g-i) and neuronal damage was exacerbated, as shown by increased density of SMI-32+ transected axons in lesions in IL-11Rα−/− mice (Figs. 1g,i). Lesions had resolved in both genotypes by 28dpi (data not shown).
CNS inflammation was also increased in lysolecithin lesions in IL-11Rα−/− animals (Figs. 1j-o). In both IL-11Rα−/− and wildtype mice, inflammatory infiltrates extended beyond the borders of the demyelinated area (Suppl. Figure 1), and contained activated CD11b+ phagocytes (Figs. 1j,k) and smaller numbers of infiltrating CD3+ T lymphocytes and CD11c+ antigen-presenting dendritic cells (DCs) (Figs. 1m,n), which further analysis identified as CD11b+ CD11c+ myeloid DCs (Figs. 1j,k). In receptor null mice, the inflammatory lesion was markedly expanded, and infiltration of all three cell types was much more extensive than in controls at both 7 and 14dpi (Figs. 1l,o).
Collectively, these results showed that demyelination, oligodendrocyte loss and axonal transection were all markedly exacerbated in lysolecithin lesions in IL-11Rα−/− mice. Moreover, CNS infiltration of inflammatory cells including CD11b+ mononuclear phagocytes, CD3+ T lymphocytes, and CD11c+ dendritic cells was strongly increased. Since myelin repair in lysolecithin lesions is known to begin at 10–14dpi (18, 23), the data from 7dpi implicated increased demyelination as a significant factor in determining lesion size in IL-11Rα−/− animals.
Remyelination is delayed in lysolecithin lesions in IL-11Rα−/− mice
To investigate remyelination, we analyzed epoxy-embedded samples by light and electron microscopy (Figure 2). Since myelin repair is first observed in lysolecithin lesions at 10–14dpi (18, 23), we focused on 14dpi samples in our analyses. These studies revealed that remyelination was delayed in IL-11Rα−/− mice.
In both IL-11Rα−/− mice and wildtype controls, at 14dpi the center of lysolecithin lesions was hypercellular and demyelinated (Fig. 2a), and contained hypertrophic astrocytes (Fig. 2b), lipid-containing mononuclear phagocytes (Fig. 2c), and dystrophic transected axons (Fig. 2d). The lesion center was edematous in IL-11Rα−/− mice compared with controls, and compatible with data from samples stained for GFAP and SMI-32, reactive astrogliosis was more pronounced and dystrophic axons were more numerous (Fig. 2a). Outside the lesion were oligodendrocytes and myelin (Fig. 2e), and myelin thickness and the ratio of axon to fiber diameter (G ratio) were similar in normal-appearing white matter in both genotypes (Table 1). In both IL-11Rα−/− mice and controls, between the lesion center and normal-appearing white matter was a distinct border which contained myelinated and demyelinated axons (Fig. 2a), and axons wrapped by disproportionately thin myelin, characteristic of remyelination (Figs. 2f,g) (24). For more detailed analysis of this area, we used electron microscopy (Figs. 2h-k). The presence of remyelinated axons was confirmed at the lesion border in electron micrographs of serial grids from both IL-11Rα−/− and wildtype samples (Figs. 2h-k), and importantly, fewer remyelinated axons were observed in IL-11Rα−/− samples than in controls (Fig. 2h).
To quantify numbers of remyelinated axons, we calculated the G ratio as a measurement of relative myelin thickness for individual fibers within the lesion border in IL-11Rα−/− and wildtype mice (see Methods section) (17). In both genotypes, the border contained axons with G ratio values above the normal range (G ratio >0.7), indicative of remyelination (illustrated in Figs. 2i-k). Notably, a smaller percentage of axons were remyelinated in IL-11Rα−/− mice than in wildtype littermates, and this difference was statistically significant (IL-11Rα−/− 12.5+/−1.6 vs. wildtype 20.4+/−1.9, P=0.011, Student’s t test). Thus, remyelination was delayed in IL-11Rα−/− animals.
IL-11 potentiates oligodendrocyte progenitor survival and differentiation
The results of these experiments in vivo revealed roles for IL-11 in limiting demyelination and oligodendrocyte loss, and in facilitating myelin repair. Moreover, these data also implicated IL-11 as immunoregulatory, restricting inflammation in demyelinated lesions. To investigate the mechanisms underlying these effects, we used mechanistic studies in primary cell cultures (Figures 3–6, Suppl. Figures 2,3).
Initially, we used modeling to analyze the impact of IL-11 on rates of proliferation, differentiation and apoptosis in primary cultures of oligodendrocyte progenitor cells (OPCs, Figure 3). These were purified from P2 rat cerebral cortices (18) then grown in the presence of 1–100ng/ml IL-11 or vehicle (most commonly 100ng/ml, see Methods) and harvested at 12h intervals for up to 120h (5d). Cultures were stained for Olig2 and the differentiation markers PDGFRα and A2B5 (OPCs), and CNPase and MBP (mature cells). TUNEL and BrdU labeling were used to identify apoptotic and mitotic cells.
These experiments showed that IL-11 enhanced oligodendrocyte maturation, and restricted apoptosis. All cultures, both IL-11-treated and controls, became progressively more differentiated over time during the experiment, with the percentage of mature Olig2+MBP+ oligodendrocytes increasing (Fig. 3a) and OPCs (Olig2+MBP−) declining (Fig. 3b). However, these changes were much more pronounced in IL-11-treated cultures than in controls, and these differences were significant from 84h onwards (Figs. 3a-c). Apoptosis was also strongly reduced in IL-11-treated cultures from as early as 12h, and this effect became more pronounced from 96h (Fig. 3d, P values shown). IL-11 had no effect on proliferation (Fig. 3e). Similar trends were seen using lower IL-11 concentrations, and data were the same in cultures stained for alternate markers for OPCs (PDGFRα+CNPase−) and mature cells (PDGFRα−CNPase+, data not shown).
To determine whether the shift towards differentiation in IL-11-treated cultures was due to enhanced maturation versus reduced apoptosis of mature cells, we adapted a previously-described mathematical model (Figs. 3f-h) (19). The model uses ordinary differential equations to define the rates of proliferation of Olig2+MBP− OPCs (α) and mature Olig2+MBP+ oligodendrocytes (γ), and the rate of differentiation of one to the other (β) (Fig. 3f and see Methods section). It also generates the combined rate of apoptosis for OPCs (δ) and mature cells (ε) (19). The model was validated in pilot studies using comparison of predicted values versus direct measurements for %Olig2+ cells in untreated cultures between 12 and 120h.
Generating rates α through ε from our raw data confirmed that IL-11 reduced the rate of apoptosis (δ+ε) in OPC cultures (Fig. 3g), and increased the rate of differentiation (β) (Fig. 3h). The reduction in apoptosis (δ+ε) was strongest within the first 36h of the experiment (Fig. 3g), whereas the increase in differentiation rate (β) reached statistical significance later, from 72h onwards (Fig. 3h). No effects were observed on other parameters (data not shown). Collectively, these findings showed that IL-11 acts on OPCs via a combination of supportive and instructive actions, enhancing both survival and maturation.
Differential actions of Stat1 and Stat3 underlie the effects of IL-11 on OPC cultures
To define the signaling events responsible for these effects, we used siRNA (Figure 4, Suppl. Figure 2). Gp130 family members signal via the transcription factors Stat1 and Stat3, and the Stat-independent SHP2 pathway through Ras-Erk and PI3kinase-Akt (Fig. 4a) (13). Immunoblotting with phospho-specific antibodies showed that in OPCs, IL-11 treatment resulted in phosphorylation of Stat1α/β at Tyr701 and Stat3α/β at Tyr705 (Suppl.Figs. 2a,b). Phosphorylation of Shp2 (Tyr580) and induction of Erk signaling (phospho-p44/42 at Thr202/Tyr204) were also seen, but no changes in PI3kinase-Akt pathway activation (phospho-GSK3 Ser21/9). Thus, IL-11 activated both Stat-dependent and independent signaling.
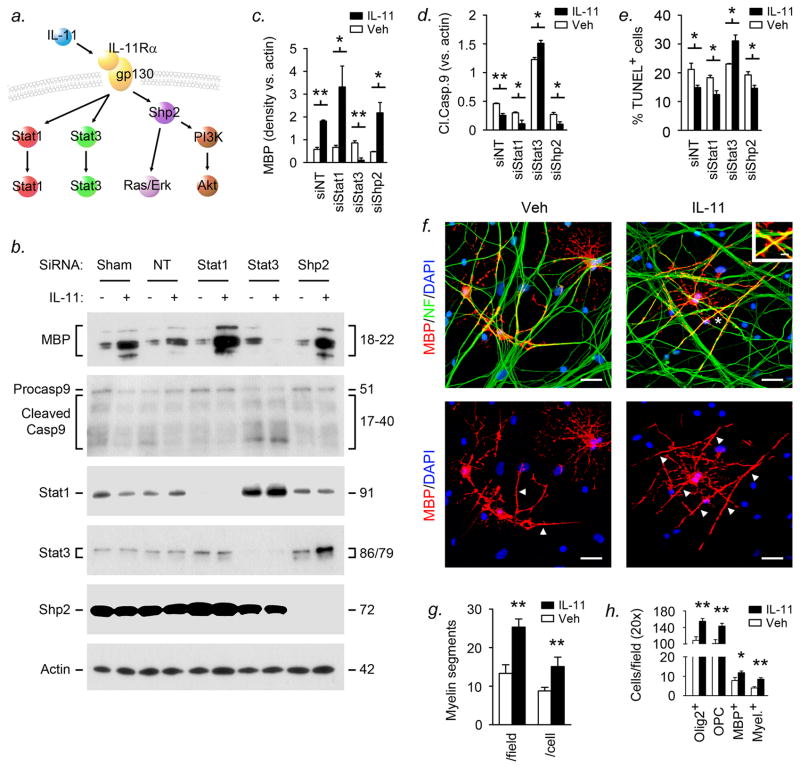
Figure 4. Differential actions of Stat1 and Stat3 underlie effects of IL-11 on OPC cultures.
(a) Model of IL-11 signaling. Ligand-receptor binding activates STAT-dependent signaling via Stat1 and Stat3, and the Stat-independent SHP2 pathway, which stimulates Ras-Erk and PI3kinase-Akt. See also Suppl. Figs. 2a,b. (b-d) OPCs were nucleofected with siRNA for Stat1, Stat3, Shp2, or nontargeting (NT) control, or without siRNA (sham), then exposed to IL-11 or vehicle for 4–7d and protein extracts subjected to immunoblotting (b). Blots were quantified by densitometry, and data from multiple experiments compared (c,d). In sham and NT cultures, or Shp2-silenced cultures, IL-11 significantly increased MBP expression (b,c) and decreased caspase 9 cleavage (b,d). Similar data were also observed in the presence of PI3 kinase silencing (see Suppl. Fig. 2c). These effects were enhanced in the presence of Stat1 knockdown (b-d). Conversely, in cultures in which Stat3 was silenced, caspase 9 cleavage was increased, especially in the presence of IL-11 (b,d). Moreover, IL-11 treatment of Stat3-silenced cultures was associated with loss of detectable MBP (b,c). (e) OPCs nucleofected with siRNA for Stat1, Stat3 or Shp2 or controls as in (b-d) were exposed to IL-11 or vehicle for 7d, then apoptotic cells quantified by TUNEL assay. In NT cultures, or in the presence of Shp2 or Stat1 silencing, IL-11 significantly reduced the percentage of apoptotic cells. Conversely, IL-11 increased apoptosis in Stat3-silenced cultures. (f-h) OPCs were plated onto E16.5 DRG neurons and myelination initiated with 1μg/ml TrkA-Fc, then co-cultures were grown in the presence of IL-11 or vehicle. (f) After 14d, compact myelin segments were observed as MBP+ processes (arrowheads) extending along NF+ axons. Asterisked MBP+NF+ profiles are illustrated at higher magnification inset. (g) IL-11-treated-cultures contained significantly more segments per field and per cell than vehicle controls. (h) IL-11 treatment of co-cultures resulted in increased numbers of Olig2+ cells at all stages of maturation, including mature Olig2+MBP+ cells, myelinating cells (MBP+ with myelin segments), and Olig2+MBP− OPCs. (c-e,g,h) Student’s t test, *P<0.05, **P<0.01. (f) Scalebars, 10μm main panels, 1.5μm inset. Data are representative of three independent experiments on separate cultures.
SiRNA-mediated definition of the roles of these pathways in the effects of IL-11 produced striking findings (Figs. 4b-d, Suppl. Fig. 2c). OPCs were nucleofected with siRNA for Stat1, Stat3, Shp2, PI3 kinase or nontargeting (NT) control, or without siRNA (sham), then treated with IL-11 or vehicle and harvested at 4–7d for immunoblotting (Fig. 4b). Blots were quantified by densitometry (see Methods section), and data from multiple experiments compared (Figs. 4c,d). Suggesting interaction of different gp130 signaling pathways, Stat3 silencing induced a sixfold increase in Stat1 expression, while Shp2 silencing was associated with a fivefold increase in Stat3 in IL-11-treated cultures (Fig. 4b). Confirming effects of IL-11 on oligodendrocyte survival and maturation, IL-11 treatment increased expression of the differentiation marker MBP, and reduced levels of cleaved caspase 9, indicative of decreased apoptosis, in sham and NT-nucleofected cultures, or in the presence of Shp2 silencing (Figs. 4b-d). Similar data were also observed in the presence of PI3 kinase silencing (Suppl. Fig. 2c). Notably, both effects were enhanced in the presence of Stat1 knockdown (Figs. 4b-d). Conversely, in Stat3-silenced cultures, caspase cleavage was markedly increased, especially in the presence of IL-11 treatment (Figs. 4b,d). Most strikingly, IL-11 treatment of Stat3-silenced cultures was also associated with loss of detectable MBP (Figs. 4b,c).
To validate these effects, we used TUNEL immunostaining (Fig. 4e). These studies confirmed that in OPC cultures nucleofected with nontargeting siRNA, or siRNA for Shp2 or Stat1, IL-11 treatment reduced the percentage of TUNEL+ apoptotic cells. Conversely, IL-11 increased the percentage of apoptotic cells in Stat3-silenced cultures (Fig. 4e).
These results revealed that Stat1 and Stat3 exerted differential effects in IL-11-treated OPC cultures. Stat3 restricted apoptosis and enhanced maturation, and its activity prevailed over that of Stat1, producing a net positive outcome. Silencing of Stat3 allowed the effects of Stat1 to become visible, converting IL-11 from a survival and differentiation factor into a pro-apoptotic signal for OPCs.
To determine whether IL-11 also protected OPCs against apoptosis induced by inflammatory factors, we tested its effects in cultures exposed to TNFα, which has previously been implicated as an inducer of oligodendrocyte cell death in the inflamed CNS (25) (Suppl.Fig. 2d). Cells were treated with 10ng/ml TNFα plus IL-11 or vehicle control, then harvested at 5d for immunoblotting as above. In cultures exposed to TNFα, caspase cleavage was markedly increased, but this effect was rescued in the presence of IL-11 cotreatment.
IL-11 increases numbers rather than percentage of myelinating cells in CNS co-cultures
This work showed that IL-11 treatment of OPCs reduced apoptosis and enhanced maturation, with the net outcome determined by a balance in activity of Stat3 and Stat1. To define the roles of these mechanisms in the capacity for myelin formation, we examined co-cultures of OPCs and dorsal root ganglion (DRG) neurons (Figs. 4f-h). Previously, we found that IL-11 treatment increased numbers of myelin segments in vitro (9) but the mechanism was uncharacterized, although it was almost certainly oligodendrocyte-specific since more than 50% of OPCs express IL-11Rα, versus only 4.4% of DRG neurons (9).
Rat OPCs were plated onto E16.5 DRG neurons and myelination initiated with 1μg/ml TrkA-Fc (9, 20). IL-11 was added and left in the medium during myelination, which occurs over 10–14d (20), then cells were fixed and stained for neurofilament (NF, axons), MBP, and Olig2 (Figs. 4f-h and see Methods section). At 14d, all cultures contained mature MBP+ oligodendrocytes, and in those exposed to TrkA-Fc, MBP+ processes colocalized with NF+ axons, forming linear MBP+NF+ profiles which correspond to compact myelin segments (Fig. 4f) (9, 18, 20). Cultures exposed to IL-11 contained more segments per field and per cell than controls (Fig. 4g), and segment length was increased (23+/−2.8μm, vs. 14+/−1.5μm in controls, Student’s t test, P=0.013). Importantly, suggesting mechanism, IL-11 treatment increased numbers of Olig2+ cells at all stages of differentiation, including mature MBP+ cells and myelinating cells (MBP+ with myelin segments), as well as immature Olig2+MBP− OPCs (Fig. 4h). Mature and myelinating cells were not increased as a percentage of the total Olig2+ population in treated co-cultures, and myelin segments were not observed any earlier than in controls (data not shown).
These data suggested that the effect of IL-11 on myelination was likely the result of an overall increase in oligodendrocyte lineage cell viability in treated cultures. A smaller maturational effect could not be discounted, since treated cultures also displayed more segments per myelin-forming cell.
Stat1 activity underlies the pro-apoptotic effects of IL-11 on dendritic cell cultures
To investigate the mechanism of action of IL-11 in limiting CNS inflammation, we used primary monocultures of CD11c+ DCs, and CD11b+ macrophages (Figures 5,6, Suppl. Figure 3). Potential effects on CD3+ CD4+ T lymphocytes were discounted since we previously demonstrated that IL-11 exerts minimal or no direct actions on these cells (12). We examined cytokine and costimulatory molecule expression in DC cultures, and compared proliferation and apoptotic activity in DCs and in CD11b+ macrophages (Figure 5, Suppl. Figure 3). We then characterized the roles of Stat transcription factors in these events (Figure 6). Importantly, these studies revealed that IL-11 is strongly pro-apoptotic for antigen-presenting CD11c+ DCs, and that its differential actions on these cells versus Olig2+ oligodendrocytes are mediated via lineage-specific activity of Stat1 versus Stat3.
Figure 5. Pro-apoptotic effects of IL-11 on dendritic cells.
(a,b) Mouse CD11c+ dendritic cells (DCs) were treated with 100ng/ml IL-11 or vehicle for 24h, then exposed to 5ng/ml LPS or vehicle for 6h. RNA was collected for QPCR (a), and supernatants for multiplex ELISA (b). See also Suppl. Fig. 3a. (a) LPS strongly induced mRNA for cytokines or their subunits including IL-23 p19, IL-12 p35, IL-12/23 p40, IL-6, TNFα, IL-1α and IFNβ, and costimulatory molecules including CD80. IL-11 pretreatment strongly inhibited induction of all of these transcripts. (b) Multiplex ELISA confirmed similar findings at the protein level. (c,d) CD11c+ cells were exposed to IL-11 or vehicle for 72h, then total cells and the percentage of TUNEL+ apoptotic cells and BrdU+ proliferating cells quantified. CD11c+ DCs were reduced by more than 60% in IL-11-treated cultures at 72h (c). Moreover, the percentage of apoptotic cells in treated cultures doubled to almost 40% while the percentage of proliferating cells was halved, to 15% (d). (e-g) CD11c+ DCs were exposed to IL-11 or vehicle for 72h, then immunoblotted for caspase 9 and cleaved caspase 3 (e). Blots were quantified by densitometry, and data from multiple experiments compared (f,g). Cleavage of both caspases was upregulated in IL-11-treated cultures at both timepoints tested, 36h and especially 72h (f,g). (a) ANOVA plus Bonferroni post test, (c,d,f,g) Student’s t test, *P<0.05, **P<0.01, ***P<0.001. Data are representative of three independent experiments on separate cultures.
Mouse CD11c+ DCs (22) were treated with 1–100ng/ml IL-11 (most commonly 100ng/ml) or vehicle for 24h, then exposed to TLR4 activation using 5ng/ml LPS for 6–24h. QPCR analysis showed that LPS strongly induced mRNA for cytokines implicated in Th17 (IL-23 p19, IL-6) and Th1 (IL-12 p35) differentiation, plus the shared IL-12/23 p40 subunit and other cytokines including TNFα, IL-1α, IFNβ and IL-10, but not TGFβ1 (Fig. 5a). Importantly, pretreatment with IL-11 strongly inhibited induction of almost all cytokines tested, including IL-23 p19, IL-12 p35, p40, IL-6, IL-1α, TNFα and IFNβ mRNA (Figs. 5a,b). Multiplex ELISA demonstrated similar findings at the protein level (Fig. 5b). IL-11 also strongly restricted induction of IL-10 protein (Fig. 5b), although effects on IL-10 mRNA did not reach significance (data not shown). Moreover, IL-11 inhibited LPS-induced expression of the costimulatory molecules CD80 and CD86 in CD11c+ DC cultures (Fig. 5a). Similar effects of IL-11 were observed in cultures subjected to TLR9 activation using 5ng/ml CpG (Suppl. Fig. 3a).
Subsequent experiments suggested that these effects of IL-11 were due to increased apoptosis in treated DC cultures, as opposed to a phenotypic shift. CD11c+ cells were treated with IL-11 or vehicle for 24–72h, then total cells and TUNEL+ apoptotic and BrdU+ proliferating cells counted (Figs. 5c,d). In marked contrast to our data from OPCs, numbers of CD11c+ DCs were reduced by more than 60% in IL-11-treated cultures at 72h (Fig. 5c). Importantly, the percentage of apoptotic cells in treated cultures doubled to 40%, while the percentage of proliferating cells was halved (Fig. 5d). Moreover, this effect appeared specific; treatment of primary CD11b+ CD11c− mouse peritoneal macrophages with 100ng/ml IL-11 for up to 7d had no effect on cell number or apoptosis (data not shown).
These results suggested pro-apoptotic effects of IL-11 on CD11c+ DCs as a significant mechanism underlying its immunoregulatory actions. To further test this hypothesis, we harvested protein extracts from IL-11-treated DC cultures and vehicle controls over 72h, then immunoblotted for caspase 9 and cleaved caspase 3, and quantified data by densitometry (Figs. 5e-g). Cleavage of both caspases was significantly potentiated in IL-11-treated cultures at both timepoints tested, 36h and especially 72h, confirming that apoptotic activity was increased in IL-11-treated DC cultures (Figs. 5e-g).
Next, we examined the roles of Stat1 and Stat3 in these effects (Figure 6, Suppl. Figure 3). Immunoblotting with phospho-specific antibodies confirmed that IL-11 activated both transcription factors in CD11c+ DC cultures at 30min, declining by 120min (Suppl. Fig. 3b). To inhibit Stat3 activity, we used a Stat3 cell-permeable inhibitor (CPI, 100μM, Figs. 6a-c) (15). Cultures were pretreated with Stat3CPI or inactive analog for 1h, then exposed to IL-11 for up to 72h. Importantly, IL-11-induced cleavage of caspases 9 and 3 was exacerbated in Stat3CPI-treated cultures (Figs. 6a-c). Stat3CPI also caused a trend towards increased caspase cleavage even in the absence of IL-11 (Figs. 6a-c).
Compatible with our results from oligodendrocyte cultures, inhibition of Stat1 activity produced the opposite effect. To investigate the role of Stat1 in IL-11-induced apoptosis, we examined caspase cleavage in DC cultures from Stat1−/− mice (Figs. 6d-f). Importantly, whereas the cleaved forms of caspases 9 and 3 were both strongly upregulated at 72h in IL-11-treated wildtype DCs compared with vehicle control, this increase was abrogated in Stat1−/− cultures (Figs. 6d-f). Thus, the pro-apoptotic effect of IL-11 in DC cultures was Stat1-dependent.
Collectively, our studies in vitro revealed that IL-11 differentially regulated apoptosis in Olig2+ oligodendrocytes versus CD11c+ DCs, and showed that the net outcome of IL-11 signaling in both cell types depended on activity of Stat1 versus Stat3 (Figure 7). In both lineages, Stat3 was anti-apoptotic, whereas Stat1 was pro-apoptotic. IL-11 treatment resulted in Stat3-dependent enhancement of survival in OPC cultures, but Stat1-mediated apoptosis in CD11c+ DCs. Our findings from lysolecithin lesions suggested relevance of these results to inflammatory demyelinating disease, implicating these differential effects of IL-11 as significant mechanisms underlying its neuroprotective and immunoregulatory actions in vivo.
Figure 7. Proposed mechanism underlying neuroprotective and immunoregulatory actions of IL-11.
IL-1β binds to its receptor IL-1R1 on astrocytes, activating signal transduction and IL-11 expression. Binding of IL-11 to IL-11Rα and gp130 activates STAT-dependent signaling in oligodendrocytes and dendritic cells. The latter may also be exposed to additional sources of IL-11 in the periphery. In both cell types, activation of S-tat1 is pro-apoptotic, while Stat3 activity is anti-apoptotic. In oligodendrocytes, exposure to IL-11 enhances activity of Stat3 over Stat1, promoting survival and maturation. In contrast, activity of Stat1 predominates in dendritic cells exposed to IL-11, resulting in apoptosis and damping of the inflammatory response.
Discussion
Members of the gp130 cytokine family have important functions in the hematopoietic, immune, reproductive and cardiovascular systems as well as in the CNS (13). IL-6 is immunoregulatory (26) and acts with TGFβ1 in driving Th17 effector cell differentiation (27), whereas IL-27 exerts both pro-inflammatory Th1-enhancing activity and anti-inflammatory functions (28). IL-6 and LIF regulate hematopoiesis, and hematopoietic progenitors are reduced in IL-6−/− and LIF−/− mice (29, 30), while IL-11 and IL-6 stimulate megakaryocytopoiesis (31, 32). LIF and IL-11 are important in pregnancy, and uterine decidualization or implantation are defective in IL-11Rα−/− or LIF−/− animals (16, 33). While these family members all signal in part via the common gp130 receptor subunit, the mechanisms responsible for their distinct patterns of activity are incompletely characterized.
Importantly, several members of the gp130 family are strongly implicated as neuroprotectants. Mice deficient in CNTF or CNTFR, and CT-1−/− mice, show loss of motoneurons (34, 35), and CNTF is protective in neurodegenerative primate models (36). CNTF and LIF also promote oligodendrocyte viability, and CNTF−/− or LIFR+/−gp130+/− mice with EAE display exacerbated disease and oligodendrocyte apoptosis, although LIF−/− mice show attenuated later signs (10, 11, 14). Recently, we demonstrated that IL-11Rα−/− mice also display exacerbated clinical signs and demyelinated lesions in EAE, but that in addition, they exhibit increased CNS inflammation (12). We found that IL-11 is trophic for oligodendrocytes (9), and our studies also revealed that IL-11 limits antigen-presenting CD11c+ DC function and thus encephalitogenic T cell activation (12). However, the mechanisms underlying these neuroprotective and immunoregulatory effects remained unknown.
Our data now reveal that these actions of IL-11 are mediated via differential regulation of apoptosis in Olig2+ oligodendrocyte lineage cells versus CD11c+ dendritic cells, and depend on cell type-specific activity of the transcription factors Stat1 and Stat3. In primary OPC cultures, IL-11 restricted caspase 9 cleavage and apoptosis and enhanced maturation, resulting in increased oligodendrocyte numbers and myelin formation in OPC-DRG co-cultures (Figures 3, 4, Suppl. Figure 2). Notably, siRNA silencing of Stat1 augmented the effects of IL-11 in OPC cultures, whereas IL-11 induced apoptosis in the presence of Stat3 knockdown (Figure 4). Conversely, in CD11c+ DCs, IL-11 treatment potentiated caspase activation and increased cell death (Figure 5, Suppl. Figure 3). The same preparation of IL-11 was used in both OPC and DC cultures, ruling out nonspecific toxicity as a potential cause of the effects observed in the latter cell type. Moreover, the pro-apoptotic effects of IL-11 in DC cultures were increased by Stat3 inhibition, but were ablated in Stat1−/− DC cultures (Figure 6). Suggesting relevance to MS, demyelination and inflammation were exacerbated in the lysolecithin model in IL-11Rα−/− mice (Figure 1, Suppl. Figure 1), and remyelination was delayed (Figure 2, Table 1). Lesions in IL-11Rα−/− mutants showed increased oligodendrocyte loss and axonal transection, and expanded infiltration of CD11b+ mononuclear phagocytes, CD3+ T lymphocytes and CD11c+ DCs. IL-11 effects on CD11c+ DCs were likely significant in determining the latter phenotype, since IL-11 did not display strong effects in CD11b+ macrophage cultures and has minimal direct impact on encephalitogenic CD3+ T cells (12). Collectively, our findings suggest that distinct effects of IL-11 on oligodendrocytes versus dendritic cells may underlie its neuroprotective and immunoregulatory actions in demyelinating disease (Figure 7).
Members of the Stat family (1–4, 5α andβ, 6) are selectively activated by different cytokines via phosphorylation of conserved C-terminal tyrosine and serine residues by Janus (JAK) and MAP kinases, inducing dimerization and nuclear translocation (13). Structure is conserved, particularly for Stat1 and Stat3, but the functions of the two are distinct. Stat1−/− mice are susceptible to infection with microbial pathogens due to defects in interferon signaling (37). Stat1 has also been implicated in apoptotic cell death, and Stat1-deficient cell lines are less susceptible to TNFα-induced apoptosis than controls (38). Conversely, Stat3 has anti-apoptotic functions, both in healthy cells and especially in malignancies where it is constitutively activated (39). Stat3 has been shown to transform fibroblasts and cause tumors in nude mice, while dominant negative or antisense Stat3 constructs induce apoptosis in tumor cell lines (40). Thus, despite structural similarity, in some contexts Stat1 and Stat3 differentially regulate viability. A precedent for differential roles in the same cell type has also been reported. For example, although type I interferons are antiproliferative in wildtype CD4+ or CD8+ T cells, they are mitogenic and anti-apoptotic in Stat1−/− mice, and both effects are Stat3-dependent (41). Importantly, our data now reveal that Stat1 and Stat3 mediate distinct outcomes of the same cytokine in different lineages, and moreover suggest relevance of these differential actions to the same inflammatory demyelinating disorder in vivo. Interestingly, we also observed increased Stat1 protein in the presence of Stat3 silencing, compatible with previous findings suggesting reciprocal inhibition by Stat1 and Stat3 of one another’s activation and/or expression (42, 43).
We are investigating mechanisms determining the discordant outcomes of IL-11-driven Stat1/3 activation in OPCs versus DCs, and are examining the roles of the suppressors of cytokine signaling Socs1 and Socs3 in these effects. Socs family members have been implicated in fine control of Stat activation by interferons and gp130 cytokines, and Socs1 and Socs3 have reciprocal functions in IL-6 and IFNγ regulation (44). Socs3 deficiency results in prolonged activation of Stat1 and Stat3 by IL-6, but normal Stat1 activation after IFNγ treatment (45). Conversely, while IL-6 stimulation of Stat1 and Stat3 is normal in Socs1-deficient cells, Stat1 activation by IFNγ is prolonged (45). We are currently testing whether members of the Socs family also define cell type-specific outcomes of gp130 cytokine signaling.
Downstream of Stat activation, we are also examining effectors determining pro- versus anti-apoptotic outcomes. Stats regulate programmed cell death through both transcription-dependent and independent means. Transcriptional mechanisms include induction/suppression of caspase, Bcl/Bax, and cell cycle arrest genes, while transcription-independent events encompass interactions with molecules such as p53 and histone deacetylases (46). Previous reports have shown that different Stats have opposite effects on activity of anti-apoptotic members of the Bcl family such as Bcl-2 and Bcl-XL, versus pro-apoptotic molecules such as Bax and Bid (46). The latter molecules form pores in the outer mitochondrial membrane resulting in leakage of cytochrome c into the cytoplasm, binding to Apaf-1 and caspase 9, and initiation of apoptosis. Bcl-2 and Bcl-XL antagonize Bax, thus a shift towards their expression restricts programmed cell death (47). Importantly, in some systems Stat1 negatively regulates the Bcl-XL promoter whereas Stat3 positively modulates it (47), and Stat3 activation has been shown to upregulate Bcl-XL and reduce apoptosis in CNS neurons (48).
While our data demonstrate relevance of IL-11 signaling to both de- and remyelination, defining contributions of neuroprotective versus immunoregulatory outcomes to inflammatory demyelinating disease will require inducible lineage-specific models, for example using oligodendrocyte- versus dendritic cell-specific activation or inactivation of Stat3 or Stat1. Since the signaling events underlying the exacerbated EAE phenotypes of CNTF and LIF mutants have also not yet been fully characterized (10, 11), lineage-specific targeting of individual pathways is likely to contribute to our wider understanding of the neuroprotective roles of gp130 cytokines in vivo. Interestingly, selective improvement of oligodendrocyte viability may also allow investigation of the extent to which axonal integrity depends on an intact myelinating oligodendrocyte, and whether neuronal and oligodendrocyte pathology can be uncoupled.
Directly targeting oligodendrocyte protection or myelin repair represents a therapeutic approach for MS that could be used in combination with available immunoregulatory treatments, for additive or perhaps synergistic clinical outcomes. The gp130 cytokine family has been widely suggested as a potential avenue for neuroprotection in a variety of conditions, including MS, and our data now reveal novel mechanisms underlying the actions of a family member that is both neuroprotective and anti-inflammatory. These findings increase our understanding of the properties of IL-11, and of the gp130 family more widely. They suggest that cell type-specific manipulation of the component pathways of gp130 signaling may represent a means to enhance oligodendrocyte survival and restrict CNS inflammation in MS.
Supplementary Material
Acknowledgments
The authors thank Dr. Victor Friedrich, Rumana Huq and Timothy Kang of the MSSM Microscopy Shared Resource Facility, and Dr. Ronald Gordon of the MSSM Electron Microscopy Facility, for assistance with sample preparation and imaging. We also thank Drs. Leif Sander and Julie Margarian Blander (MSSM Immunology Institute) for help with macrophage cultures, and Drs. Celia Brosnan and Carmen Melendez-Vasquez for advice regarding the manuscript.
Abbreviations used in this paper
- CNTF
ciliary neurotrophic factor
- CT-1
cardiotrophin-1
- DAPI
4′,6′-diamidino-2-phenylindole
- EAE
experimental autoimmune encephalomyelitis
- gp130
glycoprotein 130
- IL-11Rα
interleukin-11 receptor alpha
- LIF
leukemia inhibitory factor
- MBP
myelin basic protein
- MOG35–55
myelin oligodendrocyte glycoprotein 35–55
- MS
multiple sclerosis
- OPC
oligodendrocyte progenitor cell
- OSM
oncostatin-M
- PDGFRα
platelet-derived growth factor receptor-α
- TNFα
tumor necrosis factor-α
Footnotes
This work was supported by U.S. Public Health Service Grants R01 NS046620, R01 NS062703, R01 NS056074 and ARRA supplement NS056074-02S1 (GRJ), R01 NS08952 (CSR), and AI083284, AI080917 and AI083481 (CBL). Support for BTG came from USPHS Training Grant T32GM008553-13, and for ATA from T32NS051147-03. This work was also supported by National MS Society Research Grants RG3874, RG4127 (GRJ), RG1001 (CSR) and Fellowship Grant FG1739 (YZ), and by the Jayne and Harvey Beker Foundation (GRJ). The MSSM Analytical Imaging Facility is supported in part by by NIH/NCI Shared Resources Grant R24CA095823.
Disclosures
The authors declare no conflicts of interest.
References
- 1.Frohman EM, Racke MK, Raine CS. Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 2.McDonald WI, Sears TA. Effect of demyelination on conduction in the central nervous system. Nature. 1969;221:182–183. doi: 10.1038/221182a0. [DOI] [PubMed] [Google Scholar]
- 3.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 4.Smith EJ, Blakemore WF, McDonald WI. Central remyelination restores secure conduction. Nature. 1979;280:395–396. doi: 10.1038/280395a0. [DOI] [PubMed] [Google Scholar]
- 5.Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- 6.DeAngelis T, Lublin F. Multiple sclerosis: new treatment trials and emerging therapeutic targets. Curr Opin Neurol. 2008;21:261–271. doi: 10.1097/WCO.0b013e328300c70d. [DOI] [PubMed] [Google Scholar]
- 7.Louis JC, Magal E, Takayama S, Varon S. CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science. 1993;259:689–692. doi: 10.1126/science.8430320. [DOI] [PubMed] [Google Scholar]
- 8.Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Taveggia C, Melendez-Vasquez C, Einheber S, Raine CS, Salzer JL, Brosnan CF, John GR. Interleukin-11 potentiates oligodendrocyte survival and maturation, and myelin formation. J Neurosci. 2006;26:12174–12185. doi: 10.1523/JNEUROSCI.2289-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linker RA, Maurer M, Gaupp S, Martini R, Holtmann B, Giess R, Rieckmann P, Lassmann H, Toyka KV, Sendtner M, Gold R. CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nat Med. 2002;8:620–624. doi: 10.1038/nm0602-620. [DOI] [PubMed] [Google Scholar]
- 11.Butzkueven H, Zhang JG, Soilu-Hanninen M, Hochrein H, Chionh F, Shipham KA, Emery B, Turnley AM, Petratos S, Ernst M, Bartlett PF, Kilpatrick TJ. LIF receptor signaling limits immune-mediated demyelination by enhancing oligodendrocyte survival. Nat Med. 2002;8:613–619. doi: 10.1038/nm0602-613. [DOI] [PubMed] [Google Scholar]
- 12.Gurfein BT, Zhang Y, Lopez CB, Argaw AT, Zameer A, Moran TM, John GR. IL-11 regulates autoimmune demyelination. J Immunol. 2009;183:4229–4240. doi: 10.4049/jimmunol.0900622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst M, Jenkins BJ. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet. 2004;20:23–32. doi: 10.1016/j.tig.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Linker RA, Kruse N, Israel S, Wei T, Seubert S, Hombach A, Holtmann B, Luhder F, Ransohoff RM, Sendtner M, Gold R. Leukemia inhibitory factor deficiency modulates the immune response and limits autoimmune demyelination: a new role for neurotrophic cytokines in neuroinflammation. J Immunol. 2008;180:2204–2213. doi: 10.4049/jimmunol.180.4.2204. [DOI] [PubMed] [Google Scholar]
- 15.Turkson J, Ryan D, Kim JS, Zhang Y, Chen Z, Haura E, Laudano A, Sebti S, Hamilton AD, Jove R. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J Biol Chem. 2001;276:45443–45455. doi: 10.1074/jbc.M107527200. [DOI] [PubMed] [Google Scholar]
- 16.Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4:303–308. doi: 10.1038/nm0398-303. [DOI] [PubMed] [Google Scholar]
- 17.Hildebrand C, Hahn R. Relation between myelin sheath thickness and axon size in spinal cord white matter of some vertebrate species. J Neurol Sci. 1978;38:421–434. doi: 10.1016/0022-510x(78)90147-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Argaw AT, Gurfein BT, Zameer A, Snyder BJ, Ge C, Lu QR, Rowitch DH, Raine CS, Brosnan CF, John GR. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc Natl Acad Sci U S A. 2009;106:19162–19167. doi: 10.1073/pnas.0902834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K, Gage FH. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J Cell Biol. 2004;164:111–122. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan JR, Watkins TA, Cosgaya JM, Zhang C, Chen L, Reichardt LF, Shooter EM, Barres BA. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 2004;43:183–191. doi: 10.1016/j.neuron.2004.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106:1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nait-Oumesmar B, Decker L, Lachapelle F, Avellana-Adalid V, Bachelin C, Van Evercooren AB. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- 24.Prineas JW, Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979;5:22–31. doi: 10.1002/ana.410050105. [DOI] [PubMed] [Google Scholar]
- 25.D’Souza S, Alinauskas K, McCrea E, Goodyer C, Antel JP. Differential susceptibility of human CNS-derived cell populations to TNF-dependent and independent immune-mediated injury. J Neurosci. 1995;15:7293–7300. doi: 10.1523/JNEUROSCI.15-11-07293.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samoilova EB, Horton JL, Hilliard B, Liu TS, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol. 1998;161:6480–6486. [PubMed] [Google Scholar]
- 27.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 28.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 29.Bernad A, Kopf M, Kulbacki R, Weich N, Koehler G, Gutierrez-Ramos JC. Interleukin-6 is required in vivo for the regulation of stem cells and committed progenitors of the hematopoietic system. Immunity. 1994;1:725–731. doi: 10.1016/s1074-7613(94)80014-6. [DOI] [PubMed] [Google Scholar]
- 30.Escary JL, Perreau J, Dumenil D, Ezine S, Brulet P. Leukaemia inhibitory factor is necessary for maintenance of haematopoietic stem cells and thymocyte stimulation. Nature. 1993;363:361–364. doi: 10.1038/363361a0. [DOI] [PubMed] [Google Scholar]
- 31.Teramura M, Kobayashi S, Hoshino S, Oshimi K, Mizoguchi H. Interleukin-11 enhances human megakaryocytopoiesis in vitro. Blood. 1992;79:327–331. [PubMed] [Google Scholar]
- 32.Ishibashi T, Kimura H, Uchida T, Kariyone S, Friese P, Burstein SA. Human interleukin 6 is a direct promoter of maturation of megakaryocytes in vitro. Proc Natl Acad Sci U S A. 1989;86:5953–5957. doi: 10.1073/pnas.86.15.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 34.Masu Y, Wolf E, Holtmann B, Sendtner M, Brem G, Thoenen H. Disruption of the CNTF gene results in motor neuron degeneration. Nature. 1993;365:27–32. doi: 10.1038/365027a0. [DOI] [PubMed] [Google Scholar]
- 35.Oppenheim RW, Wiese S, Prevette D, Armanini M, Wang S, Houenou LJ, Holtmann B, Gotz R, Pennica D, Sendtner M. Cardiotrophin-1, a muscle-derived cytokine, is required for the survival of subpopulations of developing motoneurons. J Neurosci. 2001;21:1283–1291. doi: 10.1523/JNEUROSCI.21-04-01283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emerich DF, Winn SR, Hantraye PM, Peschanski M, Chen EY, Chu Y, McDermott P, Baetge EE, Kordower JH. Protective effect of encapsulated cells producing neurotrophic factor CNTF in a monkey model of Huntington’s disease. Nature. 1997;386:395–399. doi: 10.1038/386395a0. [DOI] [PubMed] [Google Scholar]
- 37.Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 38.Kumar A, Commane M, Flickinger TW, Horvath CM, Stark GR. Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science. 1997;278:1630–1632. doi: 10.1126/science.278.5343.1630. [DOI] [PubMed] [Google Scholar]
- 39.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niu G, Shain KH, Huang M, Ravi R, Bedi A, Dalton WS, Jove R, Yu H. Overexpression of a dominant-negative signal transducer and activator of transcription 3 variant in tumor cells leads to production of soluble factors that induce apoptosis and cell cycle arrest. Cancer Res. 2001;61:3276–3280. [PubMed] [Google Scholar]
- 41.Tanabe Y, Nishibori T, Su L, Arduini RM, Baker DP, David M. Cutting edge: role of STAT1, STAT3, and STAT5 in IFN-alpha beta responses in T lymphocytes. J Immunol. 2005;174:609–613. doi: 10.4049/jimmunol.174.2.609. [DOI] [PubMed] [Google Scholar]
- 42.Qing Y, Stark GR. Alternative activation of STAT1 and STAT3 in response to interferon-gamma. J Biol Chem. 2004;279:41679–41685. doi: 10.1074/jbc.M406413200. [DOI] [PubMed] [Google Scholar]
- 43.Costa-Pereira AP, Tininini S, Strobl B, Alonzi T, Schlaak JF, Is’harc H, Gesualdo I, Newman SJ, Kerr IM, Poli V. Mutational switch of an IL-6 response to an interferon-gamma-like response. Proc Natl Acad Sci U S A. 2002;99:8043–8047. doi: 10.1073/pnas.122236099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells. 2001;19:378–387. doi: 10.1634/stemcells.19-5-378. [DOI] [PubMed] [Google Scholar]
- 45.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 46.Shen Y, Devgan G, Darnell JE, Jr, Bromberg JF. Constitutively activated Stat3 protects fibroblasts from serum withdrawal and UV-induced apoptosis and antagonizes the proapoptotic effects of activated Stat1. Proc Natl Acad Sci U S A. 2001;98:1543–1548. doi: 10.1073/pnas.041588198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong F, Jaruga B, Kim WH, Radaeva S, El-Assal ON, Tian Z, Nguyen VA, Gao B. Opposing roles of STAT1 and STAT3 in T cell-mediated hepatitis: regulation by SOCS. J Clin Invest. 2002;110:1503–1513. doi: 10.1172/JCI15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kretz A, Happold CJ, Marticke JK, Isenmann S. Erythropoietin promotes regeneration of adult CNS neurons via Jak2/Stat3 and PI3K/AKT pathway activation. Mol Cell Neurosci. 2005;29:569–579. doi: 10.1016/j.mcn.2005.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.