Abstract
Ethanolic extracts of diploid Artemisia dracunculus L. (wild tarragon) from populations in the U.S., and polyploid tarragon from a variety of sources, were screened for the anti-diabetic compounds davidigenin; sakuranetin; 2′,4′-dihydroxy-4-methoxydihydrochalcone; 4,5-di-O-caffeoylquinic acid; 5-O-caffeoylquinic acid and 6-demethoxycapillarisin using LC-MS. Only decaploid plants contained all six target compounds and were the only plants that contained davidigenin and 2,4-dihydroxy-4-methoxydihydrochalcone. These results exhibit the importance of germplasm selection and provenance when studying plants for medicinal activity. Relying only on the “right species” for consistent medicinal activities may not be sufficient, as intraspecific variation may be highly significant.
Keywords: Artemisia dracunculus, wild tarragon, polyploidy, germplasm, diabetes, sakuranetin
1. Introduction
1.1 Artemisia dracunculus L
Artemisia dracunculus L. (tarragon) has a long history of human use and like many other species in the genus Artemisia, tarragon produces a wide array of phytochemicals including monoterpenoids, sesquiterpenoids, flavonoids, coumarins, isocoumarins, polyacetylenes, and alkaloids [1-7]. The uniquely fragrant variety, French tarragon (Artemisia dracunculus var. sativa Besser), is used as a culinary herb and wild or Russian tarragon (Artemisia dracunculus, numerous varieties) has been utilized as a medicine throughout its native range (western North America, Asia and Eastern Europe) for the treatment of a wide variety of diseases [8-12]. French tarragon and Russian tarragon differ dramatically in their smell and flavor. French tarragon is favored for its spicy, licorice-like flavor, which has been attributed to high amounts (60-81%) of estragole (methyl chavicol, 1-allyl-4-methoxybenzene) in its essential oil, while in most strains of Russian tarragon, estragole is nearly absent [13-21]. The species is notable for abundant polyploidy with chromosome cytotypes of 2×-10× being documented [22]. French tarragon is a sterile tetraploid that is reproduced clonally, while Russian tarragon has been found at all ploidy levels.
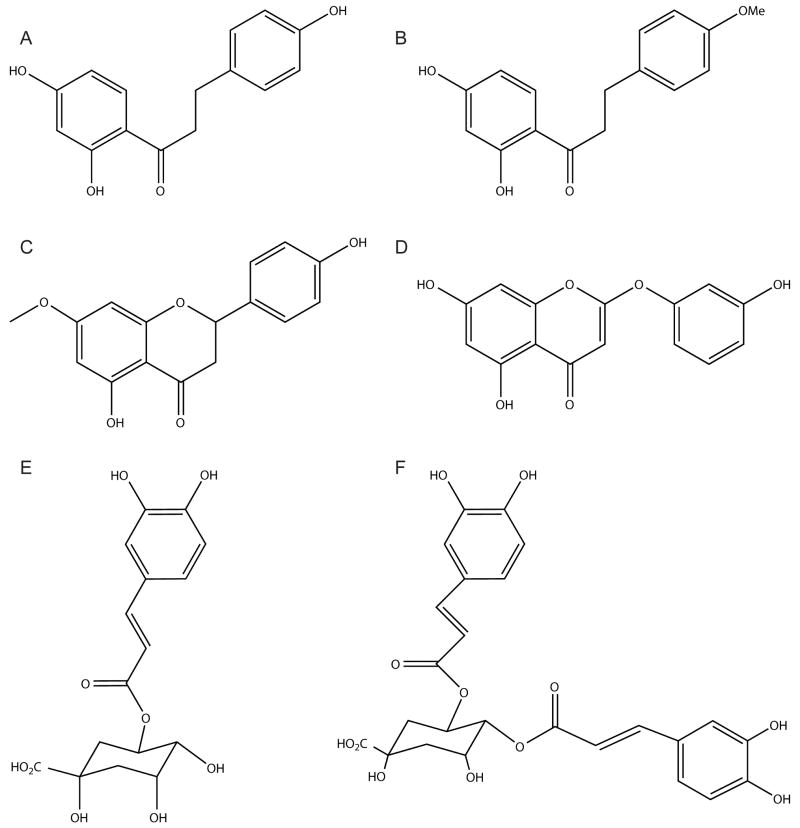
Extracts of wild tarragon have been shown to be active in a number of different pathways associated with the diabetic condition (see section 1.4), and the bioactive compounds davidigenin (A), 2′,4′- dihydroxy-4-methoxydihydrochalcone (B), sakuranetin (C), 6-demethoxycapillarisin (D), 5-O-caffeoylquinic acid (E), and 4,5-di-O-caffeoylquinic acid (F) have been previously isolated via bioassay-guided fractionation (Figure 1) [23-30]. The primary focus of this study was to assess for the presence or absence of these specific anti-diabetic compounds in A. dracunculus extracts prepared from diploid populations found throughout the western United States, as well as clones from these populations and polyploid plants (obtained from a variety of sources) grown in a common garden site to eliminate regional climatic differences. Two congeneric species were analyzed as well.
Figure 1.
Structures of bioactive compounds screened for in Artemisia dracunculus: davidigenin (A), 2′,4′-dihydroxy-4-methoxydihydrochalcone (B), sakuranetin (C), 6-demethoxycapillarisin (D), 5-O-caffeoylquinic acid (E), and 4,5-di-O-caffeoylquinic acid (F).
1.2. Intraspecific variation of phytochemicals
Variation and quality control of active phytochemicals is currently one of the main concerns within the area of medicinal plant use [31]. Both variations in genetic controls of gene expression by differing genotypes, as well as environmental influence on the expression of those genes, can affect the production of various secondary metabolites [32]. For example, flavonoid synthesis in plants can be influenced by a variety of biotic and abiotic factors including UV light radiation, drought, ozone, nutrient availability, diseases, herbivory and developmental stage [33-44]. In addition to variation in chemical production resulting from environmental and temporal conditions, many studies have looked at the variation of chemical groups within genetically related taxa as well. For example, polyacetylenes and related compounds have been shown to segregate various infrageneric groups within Artemisia [45,46]. In a chemotaxonomic study of flavonoids found in Betula species, Lahtinen et al. [47] found that diploid species did not contain any of the flavanones that were present in the leaves of other polyploid species. Because a number of the biologically active compounds in wild tarragon are flavonoids, and due to the high amount of polyploidy in wild tarragon, a similar finding could have a profound effect on the bioactivity of the extract associated with specific compounds.
Variation in chemical production by con-specific individuals has also been documented in wild tarragon. Both French and wild tarragon have been analyzed to determine if there are differences in their phytochemical compositions. In addition to distinct difference in essential oil profiles [13,15,16,19,48] (see section 1.3), chemical analyses of these tarragon varieties have shown marked qualitative variation. Flavonoids within varieties of A. dracunculus have also been shown to exhibit distinct segregation. Vienne et al. [49] investigated the presence of various flavonols in wild tarragon and French tarragon and found that both types of tarragon contained quercetin glycosides, but only the Russian tarragon contained patuletin glycosides. Chemical variation between cytotypes has also been noted. Using root extracts prepared from different A. dracunculus cytotypes from various geographic sources, Greger [2] conducted an analysis of polyacetylene content and showed that the diploid and decaploid cytotypes had similar qualitative profiles, while hexaploid and octoploids had unique chemical constituents. This within-species variation is particularly important to document because differences in the chemical content of A. dracunculus collections is likely to impact bioactivity.
1.3. Regulatory Considerations and Safety of tarragon
Although originally classified as GRAS (Generally Recognised As Safe), a number of studies have shown that at high doses, estragole is carcinogenic and genotoxic (mostly due to the metabolization into 1′-hydroxyestragole). After reviewing the toxicological literature, the European Commission Scientific Committee on Food could not establish a safe exposure limit and recommended reductions in exposure and restrictions in use [50]. Estragole was also selected for toxicity testing by the National Toxicology Program (an interagency program between the National Institute of Environmental Health Sciences of the National Institutes of Health, the National Institute for Occupational Safety and Health of the Centers for Disease Control and Prevention, and the National Center for Toxicological Research of the Food and Drug Administration). The findings of this three month investigation were released as Toxicity Report Series no. 82 [51]. It stated that examination of reference literature showed no previous documentation of adverse health effects related to human exposure to estragole, but that the carcinogenicity of estragole and its known metabolites have been characterized in rodent bioassays. This report also presented the results of a 3-month study which showed that estragole caused carcinogenic effects in rats of the high dose group. Because rats and mice were exposed for only 3 months, these studies do not assess the full carcinogenic potential of estragole. Additionally, nonneoplastic effects were observed in numerous organs and tissues of study animals.
According to Smith et al. (2002)[52], studies have clearly shown that the conversion of estragole to 1′-hydroxyestragole is dose dependent and that the toxicological risk diminishes markedly at low levels of exposure. They also cite rodent studies that show that the metabolism, metabolic activation, and covalent binding implicated in toxicity and carcinogenicity of estragole are minimal in the dose range of 1–10 mg/kg body weight, which is approximately 100–1000 times the anticipated human exposure to these substances. As such, in their opinion, exposure to methyl eugenol and estragole resulting from food consumption, does not pose a significant cancer risk.
Most populations of Russian tarragon have repeatedly been shown to lack significant amounts of estragole and may or may not contain significant amounts of methyl eugenol (Eisenman et al., unpublished data) [15,16,19,48,49]. Ribnicky et al. (2004) [53] evaluated the toxicological and mutagenic effects of TARRALIN™, an ethanolic extract prepared from Russian tarragon plants. Their results showed that while small quantities of estragole were detected in living Russian tarragon plants, none was detected in either air-dried material, or in the extract prepared from frozen plant material. The loss of estragole was attributed to volatilization during the drying and extraction processes (extraction in ethanol at 80 °C and subsequent rotary evaporation). The extract contained a concentration of methyl eugenol similar to the fresh herb, however, the extract represented a potential 50-fold concentration of some of the herb's components since the yield of extract from fresh herb was 2–3%. Therefore, the overall methyl eugenol content of the extract was greatly decreased from its concentration within the fresh plant. Further analysis revealed that the extract was not active in the Ames test for mutagenicity and there were no histopathological findings that could be attributed to the administration of the extract to the test subjects.
1.4. Artemisia dracunculus and diabetes
Type 2 diabetes is a complex metabolic disorder causing elevated blood glucose levels. It is generally the result of a progressive decrease of insulin activity (insulin resistance), and decreased pancreatic insulin secretion, due to failure of insulin producing β-cells [54,55]. According to the World Health Organization more than 220 million people worldwide suffer from diabetes and an estimated 1.1 million people died from it in 2005 [56]. The National Center for Chronic Disease Prevention and Health Promotion has reported that 23.6 million people or 7.8% of the U.S. population have diabetes, and in adults, type 2 diabetes accounts for about 90 to 95 percent of all diagnosed cases of diabetes [57].
Swanston-Flatt [58] listed tarragon as a traditionally used plant medicine for the treatment of diabetes in the United Kingdom and early bioactivity research showed that tarragon significantly reduced hyperphagia and polydipsia associated with streptozotocin-induced diabetes in mice [59]. Tarragon also slowed body weight loss but did not significantly alter plasma glucose or insulin concentrations. More recent research has shown that an extract of commercially available wild tarragon reduced hyperglycemia in both streptozotocin-induced and genetically diabetic KK-Aγ mice and that the extract significantly reduced the expression of phosphoenolpyruvate carboxykinase (PEPCK) mRNA[25]. This enzyme catalyzes the first step in liver glucose production and is normally over-expressed in insulin-resistant diabetic rats. Using in vitro skeletal muscle cell cultures (from both human and obese Zucker rats), an alcoholic extract of A dracunculus was found to improve glucose disposal in these insulin sensitive tissues [27]. A bioassay-guided fractionation experiment using quantitative polymerase chain reaction (qPCR) to measure decreased PEPCK expression led to the isolation of two compounds, 6-demethoxycapillarisin and 2′,4′-dihydroxy-4-methoxydihydrochalcone [28]. The experiment showed that 6-demethoxycapillarisin exhibited stronger inhibitory activity on PEPCK expression and when combined with insulin it decreased PEPCK expression twice as much as insulin alone. Like insulin, 6-demethoxycapillarisin was shown to function by activating the PI3K pathway, in contrast to the effect of 2′,4′-dihydroxy-4-methoxydihydrochalcone, which was not associated with this pathway.
Additional bioassay guided fractionation experiments revealed that four compounds (4,5-di-O-caffeoylquinic acid, davidigenin [4,2′,4′-trihydroxydihydrochalcone], 6-demethoxycapillarisin, and 2′,4′-dihydroxy-4-methoxydihydrochalcone) contained in wild tarragon extract, inhibited aldose reductase activity [26]. In cells, excess glucose is converted to sorbitol by the aldose reductase enzyme which then accumulates in the cells causing a number of secondary diseases associated with the diabetic condition. In an in vitro analysis assessing the bioactivity of fractions derived from an alcoholic extract of A. dracunculus, three compounds showed inhibitory effects on protein tyrosine phosphatase-1B activity. Protein tyrosine phosphatase-1B is a key enzyme in the insulin signaling pathway and acts as a negative regulator and studies have shown that mice lacking the protein tyrosine phosphatase-1B (PTP-1B) have enhanced insulin sensitivity. The compounds with inhibitory effects on PTP-1B activities were identified as sakuranetin (4,2′,4′-trihydroxydihydrochalcone), 2′,4′-dihydroxy-4-methoxydihydrochalcone and its positional isomer 2′,4-dihydroxy-4′-methoxydihydrochalcone [23,60,61].
2. Materials and methods
2.1. Plant material and extract preparation
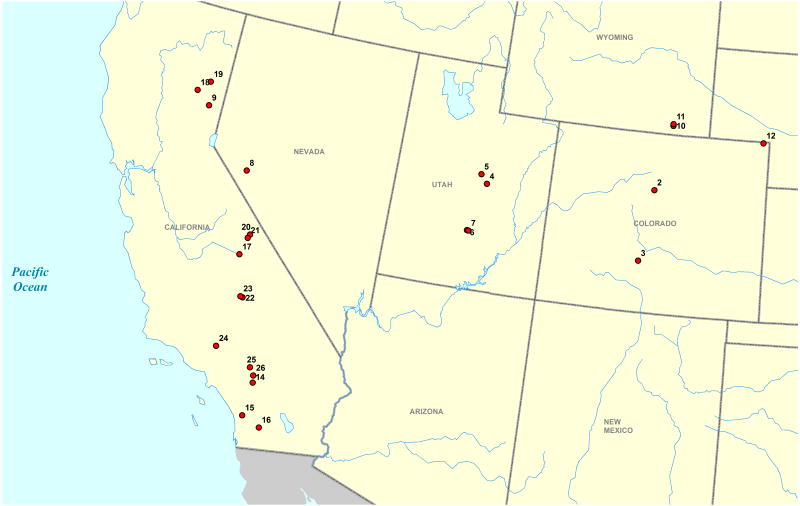
In order to collect all plants at the same time of year, to reduce potential variation due to difference in phenological stage, collection expeditions were conducted during the last week of August and the first week of September in 2006 and 2007. Sites were found by using locality records obtained via the Global Biodiversity Information Facility (GBIF) portal and the California Consortium of Herbaria database, as well as by direct examination of specimens from the herbaria BYU, CS, GH, KANU, NY, PH, RENO, RM, RSA, US and UT (abbreviations according to Index Herbariorum). In total, 115 extracts were collected from 26 sites throughout the western United States and an effort was made to sample from populations across an altitudinal gradient (Table 1; Figure 2). Field-collected samples consisted of diploid Artemisia dracunculus L. (104 samples), A. campestris L. subsp. caudata (Michaux) H. M. Hall & Clements (a closely related species in subgenus Dracunculus; 5 samples), A. ludoviciana Nutt. subsp. incompta (Nutt.) D. D. Keck (a distantly related species in subgenus Artemisia; 2 samples), A. ludoviciana Nutt. subsp. ludoviciana (3 samples), and A. ludoviciana Nutt. subsp. candicans (Rydberg) D. D. Keck (1 sample). Each sample was numbered using the site number followed by the unique individual identifier (eg., 26-220 is site 26, individual 220). Because plants can exhibit intraspecific variation of phytochemicals between genotypes, and can be influenced by environmental conditions, clones were made from a subset of the wild plants, and were cultivated in a common garden at Rutgers University (Table 2). For comparison within A. dracunculus, tetraploid French tarragon plants were purchased from Pantry Garden Herbs (Cleveland, MO), an octoploid plant was grown from seed collected in Kyrgyzstan, and three decaploid plants grown from purchased seeds (Sheffield's Seeds Co., Locke, NY) were also cultivated at Rutgers. In the results, these individuals are abbreviated FRENCH, KYRGYZ and RUGH, respectively.
Table 1.
Collection localities, coordinates (WGS84) and elevations. Artemisia dracunculus was collected at all sites except site 1(A. campestris subsp. caudata), site 4 (A. ludoviciana subsp. incompta and A ludoviciana subsp. ludoviciana), and site 5 (A. ludoviciana subsp. candicans).
| collection site | country | state | county | locality | latitude | long | Altitude (m) |
|---|---|---|---|---|---|---|---|
| site 01 | USA | Colorado | Kit Carson | ∼6.5 km E of Flagler. Growing in the dry bed of Sand Creek. Just S of frontage road bridge crossing creek and 300 m N of Route 70. | 39.28 | -102.99 | 1437 |
| site 02 | USA | Colorado | Clear Creek | Georgetown. ∼0.3 km north of Georgetown lake on NW side of Alvarado Rd. Rocky meadow, opposite a small graveyard. | 39.74 | -105.69 | 2566 |
| site 03 | USA | Colorado | Saguache | ∼12.2 km S of Villa Grove. Roadside drainage with Chrysothamnus nauseosus, Bromus sp. Bordering dry, sagebrush dominated rangeland. | 38.14 | -105.98 | 2384 |
| site 04 | USA | Utah | Emery | Huntington Canyon, ∼13 km NW of the town Huntington. N side of Huntington Creek at the turn-off towards Meetinghouse Canyon. | 39.39 | -111.09 | 1983 |
| site 05 | USA | Utah | San Pete | E side of Skyline Drive, ∼5 km south of the intersection with Route 31. Alpine meadow environment. | 39.58 | -111.31 | 3054 |
| site 06 | USA | Utah | Wayne | ∼7.0 km SE of Bicknell. N side of highway 24, ∼0.55 km W of State Road 117. Sage-brush meadow along edge of pasture. | 38.30 | -111.48 | 2113 |
| site 07 | USA | Utah | Wayne | W side of the town of Torrey. Roadside N of Route 24, ∼0.35 km W of the intersection of Route 24 and River Rd. | 38.30 | -111.44 | 2089 |
| site 08 | USA | Nevada | Mineral | ∼2.1 km S of the intersection between U.S. Route 95 and Colorado Drive. Along a dry wash, emptying into SW side of Walker Lake. | 38.62 | -118.74 | 1229 |
| site 09 | USA | California | Plumas | ∼3.5 km east of Portola. Immediately N of the junction of Big Grizzly Creek and the middle fork of the Feather River. Meadow area above riparian zone. | 39.82 | -120.43 | 1481 |
| site 10 | USA | Wyoming | Laramie | ∼34 km E of Laramie. W side of N Crow rd, 0.8 km N of the intersection between N Crow Rd and Route 210 (Happy Jack Rd). Dry sagebrush grassland. | 41.20 | -105.27 | 2337 |
| site 11 | USA | Wyoming | Laramie | ∼34 km E of Laramie. E side of N Crow rd, 5.0 km N of the intersection between N Crow Rd and Happy Jack Rd (Route 210). Edge of riparian area (North Branch Crow Creek) just E of Upper North Crow Reservoir. | 41.24 | -105.27 | 2264 |
| site 12 | USA | Colorado | Sedgwick | 1.7 km SE of Julesburg. E of Rt. 385 and just N of the South Platte River. Sandy area between dried-out braches of the river. | 40.97 | -102.25 | 1052 |
| site 14 | USA | California | San Bernardino | N of Yucaipa. Among grasses at the base of Crafton Hills. NW of the intersection of Oak Glen Rd and Creekside Drive. | 34.04 | -117.06 | 748 |
| site 15 | USA | California | San Diego | ∼20 km N of Escondido. SE side of intersection between Old Highway 395 and Nelson Way. Dry meadow area bordered by large trees. | 33.27 | -117.15 | 104 |
| site 16 | USA | California | San Diego | ∼1.25 km NE of Julian. NW of intersection between Rt. 78 and Whispering Pines Dr. Openly wooded area | 33.09 | -116.59 | 1244 |
| site 17 | USA | California | Inyo | ∼20.3 km west of the town Independence. Onion Valley campground, growing along the edge of a small stream. | 36.77 | -118.34 | 2802 |
| site 18 | USA | California | Plumas | ∼2.6 km south of Crescent Mills, amidst bear paths, just west of Indian Creek and south of Arlington Rd crossing. Just outside riparian zone. | 40.08 | -120.92 | 1064 |
| site 19 | USA | California | Lassen | ∼13.5 km south of Susanville. Wingfield Rd. Sagebrush dominated edge, outside of fenced pasture. | 40.34 | -120.57 | 1318 |
| site 20 | USA | California | Inyo | ∼19 km east of the town Big Pine. Westgard Pass. At the base of dry, rocky slopes. | 37.25 | -118.16 | 2054 |
| site 21 | USA | California | Inyo | Mojave desert region. ∼7.3 km due east of Big Pine. Along Waucoba Road/ Death Valley Rd. | 37.17 | -118.21 | 1366 |
| site 22 | USA | California | Inyo | ∼6.5 km W of Highway 395. S side of Nine Mile canyon Rd. Dry, steep sloped ravine. | 35.85 | -117.94 | 1222 |
| site 23 | USA | California | Tulare | ∼16.4 km W of Highway 395. S side of Nine Mile canyon Rd. Sage-brush meadow. | 35.86 | -118.01 | 1909 |
| site 24 | USA | California | Los Angeles | ∼0.75 km E of Elizabeth Lake, N side of Elizabeth lake Rd. Amid a mixed meadow of shrubby and herbaceous species. | 34.66 | -118.38 | 1018 |
| site 25 | USA | California | San Bernardino | Mojave Desert at foot of San Bernardino Mountains. SE side of the town Hesperia. In the dry basin of the Mojave River. ∼2.6 km mi north of the Mojave Forks Dam. | 34.37 | -117.24 | 898 |
| site 26 | USA | California | San Bernardino | S side of Rt 18 between Running Springs and Arrowbear Lake, immediately N of Hoffman Elementary School. In open areas of moderately wooded drainage area. | 34.20 | -117.09 | 1847 |
Figure 2.
Geographic distribution of Artemisia spp. collection sites across the western United States.
Table 2.
Molecular ions of target compounds (in negative ionization mode), and their retention times.
| Compound | Mass [M – H]− (m/z) | Approximate retention time (minutes) |
|---|---|---|
| davidigenin | 257 | 26 |
| 6-demethoxycapillarisin | 285 | 25 |
| sakuranetin | 285 | 30 |
| 2,4-dihydroxy-4-methoxydihydrochalcone | 271 | 31 |
| caffeoylquinic acids | 353 | 14 |
| di-O-caffeoylquinic acids | 515 | 20 |
To prepare the phytochemical extracts, 1 gram of fresh leaves was collected from the upper portion of each plant. The leaf sample was ground with a mortar and pestle in 10 mL of 95% ethanol and poured into a scintillation vial. An additional 10 mL of ethanol was used to rinse the mortar and added to the vial. The ground material was stored in the dark for 24 hours and then filtered through a folded Kimwipes EX-L (Kimberley-Clark) into a new scintillation vial. Upon returning to Rutgers University, the extracts were kept in the dark at 36° C until the analysis was conducted. Herbarium vouchers, collected from each plant sample, were deposited in the Rutgers University's Chrysler herbarium (CHRB).
Root cuttings from the plants sampled in the wild in 2006 were collected and planted in 7″ pots using Fafard Canadian Growing Mix 2 (Agawam, MA), and were cultivated in the Rutgers University research greenhouses in New Brunswick, NJ. In the spring and fall of 2007 all cultivated plants were trimmed back to just above the soil level to produce a new flush of stems. Root cuttings collected in 2007 were planted in the same manner as the 2006 material. During the 2007 collection trip, root cuttings were stored in dry potting mix rather than moistened paper towels. As a result, those collected in 2007 had a much higher survival rate. In April of 2008, all the surviving cultivated plants [53 wild-collected A. dracunculus plants, 3 A. dracunculus grown from purchased seed, 1 A. dracunculus from Kyrgyz seed, and French tarragon plants] were transplanted into 11″ pots, trimmed back to just above the level of the soil and placed in an outside growing area. At this time 9 g of Scotts Osmocote Classic 14-14-14 slow release fertilizer (Vero Beach, FL) was also applied to the surface of the soil of each pot. Each plant was subsequently watered twice per week and fertilized monthly with 5.5 g Scotts Peters Professional 20-20-20 fertilizer (Vero Beach, FL). To reduce environmental variability, all cultivated plants were watered thoroughly the day before they were harvested, and all were harvested on the same day in October 2007. The phytochemical extraction process for the cultivated plants was conducted in the same manner as for the wild-collected plant samples.
2.2. Phytochemical analysis
Five mL of each phytochemical extract was dried in a centrifugal evaporator and the dry solids were re-dissolved in a mixture of 2 mL Millipore H20 and 2 mL ethyl acetate (EA) using a vortex and sonicated at 20° C for 10 minutes. Next the samples were centrifuged for 5 minutes at 3000 rpm. The upper EA fraction was collected and placed in a new tube. The water was partitioned 2 more times with EA to obtain a total of 6 mL. The EA fraction was dried in the centrifugal evaporator and re-dissolved in 500μL of 95% ethanol. Each sample was centrifuged for 5 minutes at 3000 rpm and 20°C and 200 μL of the sample was put into a vial for liquid chromatography/mass spectroscopy (LC-MS) analysis.
The six investigated compounds were identified using liquid chromatography/mass spectroscopy (LC/MS). Each sample (30 μL injection volume) was analyzed using a LC-MS system consisted of a Waters (Milford, MA) solvent delivery system with a W616 pump and W600S controller, W717plus auto-sampler, and W996 photodiode array detector (PDA). UV data were collected and analyzed with the Waters Millennium® v. 3.2 software. After the 996 PDA detector the eluent flow was guided to a Varian 1200L (Varian Inc., Palo Alto, CA) triple quadrupole mass detector with electrospray ionization interface (ESI), operated in negative ionization mode. The electrospray voltage was -4.5 kV, heated capillary temperature was 240° C, sheath gas was air. The mass detector was set to scan from 65 to 1500 atomic mass units. Data from the Varian 1200L mass detector was collected, compiled and analyzed using Varian's MS Workstation, v. 6.41, SP2. Compounds were separated on a Phenomenex® Luna C-8 reverse phase column, size 250 × 4.6 mm, particle size 5 μm, pore size 100 Å, equipped with a Phenomenex® SecurityGuard™ pre-column. The mobile phase consisted of 2 components: Solvent A (0.5% ACS grade acetic acid in double distilled de-ionized water, pH 3-3.5), and Solvent B (100% Acetonitrile). The mobile phase flow was adjusted at 0.5 ml/min, and a gradient mode was used for all analyses. The initial conditions of the gradient were 85% A and 15% B; for 30 minutes the proportion reached 5% A and 95% B which was kept for the next 3 minutes; from minute 38 to minute 41 the gradient went back to initial conditions. A 10 minute equilibration interval was included between subsequent injections.
Identification of the compounds was based on deprotonated molecular ions in negative ionization mode and their retention times (Table 2). Due to the diversity of caffeoylquinic acid and chlorogenic acid derivatives in the extracts, and because of the crude nature of the extracts, distinct chromatogram peaks for 4,5-Di-O-caffeoylquinic acid and 5-O-caffeoylquinic acid could not be obtained. As a result, all plants were screened for a mixture of dicaffeoylquinates with a parent ion of m/z 515 and a retention time of 20 minutes, and a mix of chlorogenates with a parent ion of m/z 353 and a retention time of 14 minutes. The liquid chromatography protocol that was used did not sufficiently separate the isomers, 2′,4-dihydroxy-4′-methoxydihydrochalcone and 2′,4′-dihydroxy-4-methoxydihydrochalcone. Because of this, plants were screened only for the presence of the molecules and not the specific isomers.
3. Results and discussion
All target compounds were detected in the extracts of the decaploid Artemisia dracunculus plants grown from purchased seeds. Example chromatograms for the target compounds are shown in Figure 3. The decaploids were also the only plants in which davidigenin and 2,4-dihydroxy-4-methoxydihydrochalcone were detected. Sakuranetin was detected in 100% of the extracts prepared from diploid Artemisia dracunculus plants collected in North America, regardless of whether the extracts were prepared in the field from wild growing plants or from plants grown in the common garden. 6-demethoxycapillarisin, which was detected in 66% of the wild-prepared extracts of A. dracunculus, exhibited variation between wild growing plants and their clones cultivated in the common garden samples. In 32 of the North American plants, 6-demethoxycapillarisin was detected in the wild and not detected in the cultivated clones, and in one individual the compound was not detected in the wild yet was detected in the cultivated clone. This variation leads to the assumption that the production of 6-demethoxycapillarisin in these plants is subject to change depending on environmental conditions. Davidigenin, 2,4-dihydroxy-4-methoxydihydrochalcone, sakuranetin and 6-demethoxycapillarisin were not detected in Artemisia campestris, A. ludoviciana, the tetraploid French tarragon and the octoploid wild tarragon plant from Kyrgyzstan. This had one exception as 6-demethoxycapillarisin was detected in A. ludoviciana subsp. candicans. Table 3 presents the presence or absence of the target compounds for all the wild and cultivated samples. All extracts, from both wild-growing and cultivated samples of all species tested, were found to contain complexes of caffeoylquinic acids and dicaffeoylquinic acids. The nomenclature used for caffeoylquinic acids discussed in this paper is the recommended IUPAC numbering system [62,63].
Figure 3.
Representative chromatograms of targeted bioactive compounds in Artemisia dracunculus.
Table 3.
Results of the LC-MS analysis assessing for the presence of known anti-diabetic compounds in various Artemisia species and Artemisia dracunculus cytotypes.
| sample | Species/ploidya | davidigenin | chalconeb | 6-De-MeOc | sakuranetin | chlorogenates | caffeoylquinates | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wd | Ce | W | C | W | C | W | C | W | C | W | C | ||
| 1-1 | A. campestris | − | − | − | − | + | + | ||||||
| 1-2 | A. campestris | − | − | − | − | + | + | ||||||
| 1-3 | A. campestris | − | − | − | − | + | + | ||||||
| 1-4 | A. campestris | − | − | − | − | + | + | ||||||
| 1-5 | A. campestris | − | − | − | − | + | + | ||||||
| 2-11 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | + |
| 2-12 | A. dracunculus (2×) | − | − | − | − | + | + | + | + | + | + | + | + |
| 2-13 | A. dracunculus (2×) | − | − | − | − | − | − | + | + | + | + | + | + |
| 2-14 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 2-15 | A. dracunculus (2×) | − | − | + | − | + | + | ||||||
| 3-21 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 3-22 | A. dracunculus (2×) | − | − | − | − | + | + | ||||||
| 3-23 | A. dracunculus (2×) | − | − | − | − | + | + | ||||||
| 3-24 | A. dracunculus (2×) | − | − | − | − | + | + | ||||||
| 3-25 | A. dracunculus (2×) | − | − | − | − | + | + | ||||||
| 4-31 | A. ludoviciana | − | − | − | − | + | + | ||||||
| 4-32 | A. ludoviciana | − | − | − | − | + | + | ||||||
| 4-33 | A. ludoviciana | _ | _ | _ | _ | + | + | ||||||
| 4-34 | A. ludoviciana | − | − | − | − | + | + | ||||||
| 5-36 | A. ludoviciana | − | − | + | − | + | + | ||||||
| 6-37 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 6-38 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | + |
| 7-39 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 7-40 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 7-41 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 7-42 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 7-43 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 8-49 | A. dracunculus (2×) | − | − | − | − | + | + | + | + | + | + | + | + |
| 8-50 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | + |
| 8-51 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | + |
| 8-52 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 8-53 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | + |
| 9-54 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | + |
| 9-55 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | + |
| 9-56 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | + |
| 9-57 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | + |
| 9-58 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | + |
| 10-64 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | + |
| 10-65 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | + |
| 10-66 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 10-67 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 10-68 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 11-69 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 11-70 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 12-74 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 12-75 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 12-76 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 12-77 | A. dracunculus (2×) | − | − | + | − | + | + | ||||||
| 12-78 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | + |
| 14-100 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | + |
| 14-101 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | + |
| 14-102 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | + |
| 14-103 | A. dracunculus (2×) | − | − | − | − | + | + | + | + | + | + | + | |
| 14-104 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 15-110 | A. dracunculus (2×) | − | − | + | + | − | + | ||||||
| 15-111 | A. dracunculus (2×) | − | − | + | + | − | + | ||||||
| 15-112 | A. dracunculus (2×) | − | − | + | + | − | + | ||||||
| 15-113 | A. dracunculus (2×) | − | − | + | + | − | + | ||||||
| 15-114 | A. dracunculus (2×) | − | − | + | + | − | + | ||||||
| 16-120 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 16-121 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 16-122 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 16-123 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 16-124 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 17-130 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 17-131 | A. dracunculus (2×) | − | − | + | + | + | + | + | + | ||||
| 17-132 | A. dracunculus (2×) | − | − | + | + | + | + | + | + | ||||
| 17-133 | A. dracunculus (2×) | − | − | + | + | + | + | + | + | ||||
| 17-134 | A. dracunculus (2×) | − | − | + | + | + | + | + | + | ||||
| 18-140 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | |
| 18-141 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | |
| 18-142 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | + | |
| 18-143 | A. dracunculus (2×) | − | − | + | + | + | + | + | + | ||||
| 18-144 | A. dracunculus (2×) | − | − | + | + | + | + | + | + | ||||
| 19-150 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | ||
| 19-151 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | ||
| 19-152 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | ||
| 19-153 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | ||
| 19-154 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | ||
| 20-160 | A. dracunculus (2×) | − | − | − | − | − | − | + | + | + | + | ||
| 20-161 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 20-162 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 20-163 | A. dracunculus (2×) | − | − | − | − | − | + | + | + | + | |||
| 20-164 | A. dracunculus (2×) | − | − | − | − | − | + | + | + | + | |||
| 21-170 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 21-171 | A. dracunculus (2×) | − | − | + | − | + | + | + | + | ||||
| 21-172 | A. dracunculus (2×) | − | − | + | − | + | + | + | + | ||||
| 21-173 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 21-174 | A. dracunculus (2×) | − | − | + | − | + | + | + | + | ||||
| 22-180 | A. dracunculus (2×) | − | − | − | − | − | − | + | + | + | + | ||
| 22-181 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | ||
| 22-182 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | ||
| 22-183 | A. dracunculus (2×) | − | − | − | − | + | − | + | + | + | + | ||
| 22-184 | A. dracunculus (2×) | − | − | − | − | − | − | + | + | + | + | ||
| 23-190 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 23-191 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 23-192 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 23-193 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 23-194 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 24-200 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 24-201 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 24-202 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 24-203 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 24-204 | A. dracunculus (2×) | − | − | + | + | + | + | ||||||
| 25-210 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 25-211 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 25-212 | A. dracunculus (2×) | − | − | − | − | − | + | + | + | + | + | ||
| 25-213 | A. dracunculus (2×) | − | − | − | − | − | − | + | + | + | + | ||
| 25-214 | A. dracunculus (2×) | − | − | − | − | − | − | + | + | + | + | ||
| 26-220 | A. dracunculus (2×) | − | − | − | − | − | − | + | + | + | + | ||
| 26-221 | A. dracunculus (2×) | − | − | − | − | − | − | + | + | + | + | ||
| 26-222 | A. dracunculus (2×) | − | − | − | − | − | − | + | + | + | + | ||
| 26-223 | A. dracunculus (2×) | − | − | − | + | + | + | ||||||
| 26-224 | A. dracunculus (2×) | − | − | − | − | − | − | + | + | + | + | ||
| RUGH-1 | A. dracunculus (10×) | + | + | + | + | + | + | ||||||
| RUGH-2 | A. dracunculus (10×) | + | + | + | + | + | + | ||||||
| RUGH-4 | A. dracunculus (10×) | + | + | + | + | + | + | ||||||
| French 1 | A. dracunculus (4×) | − | − | − | − | + | + | ||||||
| French 2 | A. dracunculus (4×) | − | − | − | − | + | + | ||||||
| French 3 | A. dracunculus (4×) | − | − | − | − | + | + | ||||||
| French 4 | A. dracunculus (4×) | − | − | − | − | + | + | ||||||
| French 5 | A. dracunculus (4×) | − | − | − | − | + | + | ||||||
| French 6 | A. dracunculus (4×) | − | − | − | − | + | + | ||||||
| French 7 | A. dracunculus (4×) | − | − | − | − | + | + | ||||||
| French 8 | A. dracunculus (4×) | − | − | − | − | + | + | ||||||
| French 9 | A. dracunculus (4×) | − | − | − | − | + | + | ||||||
| Kyrgyz | A. dracunculus (8×) | − | − | − | − | + | + | ||||||
Ploidy level is based on work by Eisenman and Struwe [55]
chalcone = 2,4−dihydroxy-4-methoxydihydrochalcone
6-De-MeO = 6-demethoxycapillarisin
W = extracts prepared from plants growing in their wild habitat
C = extracts prepared from plants cultivated in a common garden
Due to the simple preparation and partitioning of the extracts, and the specific liquid chromatography parameters, 5-O-caffeoylquinic acid (5-CQA) and 4,5-Di-O-caffeoylquinic acid (4,5-diCQA) were co-eluted with their related compounds and therefore did not produce isolated peaks. 3-O-caffeoylquinic acid (3-CQA) and 4-O-caffeoylquinic acid (4-CQA), isomers of 5-O-caffeoylquinic acid, have the same parent ion (m/z 353), as well as similar retention times. In a similar fashion, 3,4-Di-O-caffeoylquinic acid (3,4-diCQA) and 3,5-Di-O-caffeoylquinic acid (3,5-diCQA) share the same parent ion (m/z 515), and can have similar retention times as 4,5-diCQA acid. A revised liquid chromatography protocol with adjusted solvent flow rates would be necessary to adequately separate and quantify the 5-O-caffeoylquinic acid and 4,5-Di-O-caffeoylquinic acid from related compounds. Preliminary results from a revised LC protocol revealed that the wild prepared sample 14-104 contained primarily 4,5-diCQA, with lesser amounts of 3,5-diCQA and 3,4-diCQA, as well as an unknown diCQA in very small concentrations (J. Munafo, per. comm., data not shown). 1,3-diCQA and 1,5-diCQA were found in significant amounts in some Asteraceae such as artichoke (Cynara) and one of these could possibly be the unidentified compound [64]. Of the caffeoylquinic acids, 5-CQA was the most abundant, followed by 4-CQA, and very little 3-CQA (J. Munafo, per. comm., data not shown).
This study has shown that the target anti-diabetic compounds are not found ubiquitously in Artemisia species. More significantly, there was a distinct qualitative variation in the chemical content of the different A. dracunculus cytotypes. This variation is an extremely important factor to consider when selecting material for biological investigations. Although the target compounds davidigenin and 2′,4′-dihydroxy-4-methoxydihydrochalcone were not detected in North American populations, sakuranetin was detected in 100% of those samples.
The results suggest that the North American populations lack the capacity to produce some of these medicinal compounds or that they are produced in undetectable quantities, while decaploid plants contain all targeted compounds. These findings are similar to those of Greger [2], who also found that polyacetylene content in wild tarragon was associated with ploidy level, and that the decaploid cytotype had a unique assembly of compounds. It is also interesting that the tetra-, and octoploid plants of A. dracunculus lacked nearly all of the target compounds, while many of the diploids and all the decaploids screened contained the sakuranetin and 6-demethoxycapillarisin. This is also similar to Greger's findings in which a hexaploid and octoploid strain differed from the decaploid strain, while chemically, the diploid was more allied to the decaploid.
As documented in many different taxa, polyploidization can cause dramatic change in physiological processes and increase the production of secondary metabolites [65,66]. Based on the results of this study it seems quite possible that the production of 2,4-dihydroxy-4-methoxydihydrochalcone and davidigenin is unique to the decaploid strain. Since the decaploid strain is likely derived from diploids originating in Europe or Asia, it is possible that the diploid progenitors of this decaploid could produce all the target compounds [67]. An allopolyploid hybridization event in the formation of the decaploid A. dracunculus could have resulted in the introduction of novel genes and gene products, or the unique chemical profile may be the product of autopolyploidy and result from inter-specific variation. The latter may very well be the case, as it seems that this species has a propensity for genomic duplication as evidenced by widespread documentation of individuals within the polyploidy series [68].
With its diversity of ploidy levels, Artemisia dracunculus provides an interesting look into genomic evolution, and this genetic diversity is reflected in its variable chemistry. The findings of this study show that the proper selection of germplasm material for the development of botanical therapeutics is of the utmost importance, and that the provenance, and more specifically the cytotype and genotype, of the plant material are essential to developing an effective and consistent botanical therapeutic. Although the large series of polyploids known in A. dracunculus may be fairly unique, within-species genotypic variation remains an important consideration for other medicinal plants. Reliable germplasm, with plant material that is uniform in chemical composition, is critical for standardization of botanical products.
Acknowledgments
This research was supported by grant P50AT002776-01 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS) which funds the Botanical Research Center of Pennington Biomedical Research Center and the Biotech Center of Rutgers University, as well as by Fogarty International Center grant U01TW006674 (International Cooperative Biodiversity Groups - Central Asia). Field work was supported by the Rutgers University Graduate School and the C. Reed Funk Plant Biology Student Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Greger H, Bohlmann F. 8-Hydroxycapillarin. A new isocoumarin from Artemisia dracunculus. Phytochemistry. 1979;18:1244–1245. [Google Scholar]
- 2.Greger H. Aromatic acetylenes and dehydrofalcarinone derivatives with the Artemisia dracunculus group. Phytochemistry. 1979;18:1319–1322. [Google Scholar]
- 3.Engelmeier D, Hadacek F, Hofer O, Lutz-Kutschera G, Nagl M, Wurz G, Greger H. Antifungal 3-butylisocoumarins from Asteraceae-Anthemideae. J Nat Prod. 2004;67:19–25. doi: 10.1021/np0301339. [DOI] [PubMed] [Google Scholar]
- 4.Bhutia TD, Valant-Vetschera KM. Chemodiversity of Artemisia dracunculus L. from Kyrgyzstan: Isocoumarins, coumarins, and flavonoids from aerial parts. Nat Prod Commun. 2008;3:1289–1292. [Google Scholar]
- 5.Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry. 2008;69:1732–1738. doi: 10.1016/j.phytochem.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Lutz-Kutschera G, Engelmeier D, Hadacek F, Werner A, Greger H, Hofer O. Synthesis of side chain substituted 3-butylisocoumarins and absolute configurations of natural isocoumarins from Artemisia dracunculus. Monatsch Chem. 2003;134:1195–1206. [Google Scholar]
- 7.Greger H. Anthemidae - Chemical Review. In: Heywood VH, Harborne JB, Turner BL, editors. The Biology and chemistry of the Compositae. Academic Press: London, New York; 1977. pp. 889–941. [Google Scholar]
- 8.Moerman DE. Native American Ethnobotany. Timber Press; Portland, Or: 1998. [Google Scholar]
- 9.Khalmatov KhKh, Kharlamov IA, Alimbayeva PK, Karriev MO, Khaetov IK. Osnovnuiye Lekarstvennuiye Rasteniya Srednei Azii. Meditsina; Tashkent: 1984. [Google Scholar]
- 10.Khodzhimatov M. Dikorastushchie Lekarstvennye Rasteniia Tadzhikistana. Glav Nauch Red Tadzhikskoi Sov Entsiklopedii; Dushanbe: 1989. [Google Scholar]
- 11.Uphof JCT. Dictionary of economic plants. 2nd. J Cramer; New York: 1968. [Google Scholar]
- 12.Steinmetz EF. Codex vegetabilis. 2d. The Author; Amsterdam: 1957. [Google Scholar]
- 13.Balza F, Jamieson L, Towers GHN. Chemical constituents of the aerial parts of Artemisia dracunculus. J Nat Prod. 1985;48:339–340. [Google Scholar]
- 14.Thieme H, Thitam N. Accumulation and composition of essential oils of Satureia hortensis, Satureia montana, and Artemisia dracunculus during ontogenesis. 2. Variations in oil content and composition. Pharmazie. 1972;27:324–331. [PubMed] [Google Scholar]
- 15.Albasini A, Bianchi A, Melegari M, Vampa G, Pecorari P, Rinaldi M. Studies of Artemisia dracunculus L. s.l. (tarragon) Fitoterapia. 1983;54:229–235. [Google Scholar]
- 16.Deans SG, Svoboda KP. Antibacterial activity of French tarragon (Artemisia dracunculus Linn.) essential oil and its constituents during ontogeny. J Hortic Sci. 1988;63:503–508. [Google Scholar]
- 17.Werker E, Putievsky E, Ravid U, Dudai N, Katzir I. Glandular Hairs, Secretory Cavities, and the Essential Oil in the Leaves of Tarragon (Artemisia dracunculus L.) J Herbs, Spices Med Plants. 1994;2:19. [Google Scholar]
- 18.Tomitaka Y, Kimura M, Asano K, Boo HO. Morphological characters and the essential oil in Artemisia dracunculus (French tarragon) and A. dracuncloides (Russian tarragon) Tokyo Nogyo Daigaku Nogaku Shuho. 1997;41:229–238. [Google Scholar]
- 19.Arabhosseini A, Padhye S, van Beek TA, van Boxtel AJB, Huisman W, Posthumus MA, Mueller J. Loss of essential oil of tarragon (Artemisia dracunculus L.) due to drying. J Sci Food Agric. 2006;86:2543–2550. [Google Scholar]
- 20.Lawrence BM. Progress in essential oils. Perfum Flavor. 1979;4:53–56. [Google Scholar]
- 21.Lawrence BM. Essential oils, 1979-1980. Allured Pub. Corp.; Wheaton, Ill: 1981. [Google Scholar]
- 22.Torrell M, Valles J. Genome size in 21 Artemisia L. species (Asteraceae, Anthemideae): Systematic, evolutionary, and ecological implications. Genome. 2001;44:231–238. [PubMed] [Google Scholar]
- 23.Kennedy BP, Ramachandran C. Protein tyrosine phosphatase-1B in diabetes. Biochem Pharmacol. 2000;60:877–883. doi: 10.1016/s0006-2952(00)00305-1. [DOI] [PubMed] [Google Scholar]
- 24.Wang ZQ, Ribnicky D, Zhang XH, Raskin I, Yu Y, Cefalu WT. Bioactives of Artemisia dracunculus L. enhance cellular insulin signaling in primary human skeletal muscle culture. Metab Clin Exp. 2008;57:S58–64. doi: 10.1016/j.metabol.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribnicky DM, Poulev A, Watford M, Cefalu WT, Raskin I. Antihyperglycemic activity of Tarralin™, an ethanolic extract of Artemisia dracunculus L. Phytomedicine. 2006;13:550–557. doi: 10.1016/j.phymed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Logendra S, Ribnicky DM, Yang H, Poulev A, Ma J, Kennelly EJ, Raskin I. Bioassay-guided isolation of aldose reductase inhibitors from Artemisia dracunculus. Phytochemistry. 2006;67:1539–1546. doi: 10.1016/j.phytochem.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Wang ZQ, Zhang XM, Ribnicky DM, Cefalu WT. Effect of a alcoholic extract of Artemisia dracunculus (Tarralin™) on glucose uptake in human skeletal muscle culture. In: Matschinsky FM, editor. 64th Scientific Sessions of the American Diabetes Association; Orlando, Florida, USA. Alexandria: American Diabetes Association; [June 4-8, 2004]. 2004. [Google Scholar]
- 28.Govorko D, Logendra S, Wang Y, Esposito D, Komarnytsky S, Ribnicky D, Poulev A, Wang Z, Cefalu WT, Raskin I. Polyphenolic compounds from Artemisia dracunculus L. inhibit PEPCK gene expression and gluconeogenesis in an H4IIE hepatoma cell line. Am J Physiol. 2007;293:E1503–E1510. doi: 10.1152/ajpendo.00420.2007. [DOI] [PubMed] [Google Scholar]
- 29.Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr. 2003;78:728–733. doi: 10.1093/ajcn/78.4.728. [DOI] [PubMed] [Google Scholar]
- 30.Andrade-Cetto A, Wiedenfeld H. Hypoglycemic effect of Cecropia obtusifolia on streptozotocin diabetic rats. J Ethnopharmacol. 2001;78:145–149. doi: 10.1016/s0378-8741(01)00335-x. [DOI] [PubMed] [Google Scholar]
- 31.Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N, Brinker A, Moreno DA, Ripoll C, Yakoby N, O'Neal JM, Cornwell T, Pastor I, Fridlender B. Plants and human health in the twenty-first century. Trends Biotechnol. 2002;20:522–531. doi: 10.1016/s0167-7799(02)02080-2. [DOI] [PubMed] [Google Scholar]
- 32.Lila MA. The nature-versus-nurture debate on bioactive phytochemicals: the genome versus terroir. J Sci Food Agric. 2006;86:2510–2515. [Google Scholar]
- 33.McDougal KM, Parks CR. Elevational variation in foliar flavonoids of Quercus rubra L. (Fagaceae) Am J Bot. 1984;71:301–308. [Google Scholar]
- 34.Tomas-Barberán FA, Msonthi JD, Hostettmann K. Antifungal epicuticular methylated flavonoids from Helichrysum nitens. Phytochemistry. 1988;27:753–755. [Google Scholar]
- 35.Coronado C, Zuanazzi J, Sallaud C, Quirion JC, Esnault R, Husson HP, Kondorosi A, Ratet P. Alfalfa Root Flavonoid Production Is Nitrogen Regulated. Plant Physiol. 1995;108:533–542. doi: 10.1104/pp.108.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Gitz DC, III, McClure JW. Effects of UV-B on flavonoids, ferulic acid, growth and photosynthesis in barley primary leaves. Physiol Plantarum. 1995;93:725–733. [Google Scholar]
- 37.Chaves N, Sosa T, Escudero JC. Plant growth inhibiting flavonoids in exudate of Cistus ladanifer and in associated soils. J Chem Ecol. 2001;27:623–631. doi: 10.1023/a:1010388905923. [DOI] [PubMed] [Google Scholar]
- 38.Cuadra P, Harborne JB, Waterman PG. Increases in surface flavonols and photosynthetic pigments in Gnaphalium luteo-album in response to UV-B radiation. Phytochemistry. 1997;45:1377–1383. [Google Scholar]
- 39.Cooper-Driver GA, Bhattacharya M. Role of phenolics in plant evolution. Phytochemistry. 1998;49:1165–1174. [Google Scholar]
- 40.Lavola A. Accumulation of flavonoids and related compounds in birch induced by UV-B irradiance. Tree Physiol. 1998;18:53–58. doi: 10.1093/treephys/18.1.53. [DOI] [PubMed] [Google Scholar]
- 41.Markham KR, Tanner GJ, Caasi-Lit M, Whitecross MI, Nayudu M, Mitchell KA. Possible protective role for 3′,4′-dihydroxyflavones induced by enhanced UV-B in a UV-tolerant rice cultivar. Phytochemistry. 1998;49:1913–1919. [Google Scholar]
- 42.Simmonds MSJ. Chemoecology: The legacy left by Tony Swain. Phytochemistry (Oxford) 1998;49:1183–1190. [Google Scholar]
- 43.Saleem A, Loponen J, Pihlaja K, Oksanen E. Effects of long-term open-field ozone exposure on leaf phenolics of European silver birch (Betula pendula Roth) J Chem Ecol. 2001;27:1049–1062. doi: 10.1023/a:1010351406931. [DOI] [PubMed] [Google Scholar]
- 44.Karlova K. Accumulation of flavonoid compounds in flowering shoots of Achillea collina Becker ex. Rchb. alba during flower development. Horticultural Science - UZPI (Czech Republic) 2006;33:158–162. [Google Scholar]
- 45.Greger H. New chemical markers within Artemisia (Compositae - Anthemideae) In: Margaris N, Koedam AA, Vokou D, editors. Aromatic Plants, Basic and Applied Aspects. Martin Nijhoff; The Hague: 1982. pp. 153–163. [Google Scholar]
- 46.Riggins CW, Clausen TP. Root acetylenes from Artemisia arctica. Biochem Syst Ecol. 2003;31:211–214. [Google Scholar]
- 47.Lahtinen M, Lempa K, Salminen J, Pihlaja K. HPLC analysis of leaf surface flavonoids for the preliminary classification of birch species. Phytochem Anal. 2006;17:197–203. doi: 10.1002/pca.906. [DOI] [PubMed] [Google Scholar]
- 48.Deans SG, Simpson EJM. Artemisia dracunculus. Med Aromat Plants--Ind Profiles. 2002;18:91–97. [Google Scholar]
- 49.Vienne M, Braemer R, Paris M, Couderc H. Chemotaxonomic study of two cultivars of Artemisia dracunculus L.: (“French” and “Russian” tarragon) Biochem Syst Ecol. 1989;17:373–374. [Google Scholar]
- 50.European Commission's Scientific Committee on Food. Opinion of the Scientific Committee on Food on Estragole (1-Allyl-4-methoxybenzene), SCF/CS/FLAV/FLAVOUR/6 ADD2 FINAL. [accessed 5 May 2011];2001 http://ec.europa.eu/food/fs/sc/scf/out104_en.pdf.
- 51.National Toxicology Program. NTP Technical Report on the 3-Month Toxicity Studies of Estragole (CAS No. 140-67-0) Administered by Gavage to F344/N Rats and B6C3F1 Mice. [accessed 5 May 2011];Toxicity Report Series Number 82 (NIH Publication No 11-5966) http://ntp.niehs.nih.gov/files/TS082peerreview_web%5B1%5D.pdf.
- 52.Smith RL, Adams TB, Doull J, Feron VJ, Goodman JI, Marnett LJ, Portoghese PS, Waddell WJ, Wagner BM, Rogers AE, Caldwell J, Sipes IG. Safety assessment of allylalkoxybenzene derivatives used as flavouring substances - methyl eugenol and estragole. Food and Chemical Toxicology. 2002;40:851–870. doi: 10.1016/s0278-6915(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 53.Ribnicky DM, Poulev A, O'Neal J, Wnorowski G, Malek DE, Jager R, Raskin I. Toxicological evaluation of the ethanolic extract of Artemisia dracunculus L. for use as a dietary supplement and in functional foods. Food Chem Toxicol. 2004;42:585–598. doi: 10.1016/j.fct.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: Scientific review. JAMA. 2002;287:360–372. doi: 10.1001/jama.287.3.360. [DOI] [PubMed] [Google Scholar]
- 55.Ashiya M, Smith RET. Non-insulin therapies for type 2 diabetes. Nat Rev Drug Discov. 2007;6:777–778. doi: 10.1038/nrd2354. [DOI] [PubMed] [Google Scholar]
- 56.World Health Organization. Diabetes, Fact sheet N°312. [accessed online 4 Oct 2010];2009 November; http://www.who.int/mediacentre/factsheets/fs312/en/index.html.
- 57.National Institute of Diabetes and Digestive and Kidney Diseases. NIH Publication No 08–3892. U.S. Department of Health and Human Services, National Institutes of Health; Bethesda: [accessed online 4 Oct 2010]. National Diabetes Statistics, 2007 fact sheet. http://diabetes.niddk.nih.gov/dm/pubs/statistics/ [Google Scholar]
- 58.Swanston-Flatt SK, Flatt PR, Day C, Bailey CJ. Traditional dietary adjuncts for the treatment of diabetes mellitus. Proc Nutr Soc. 1991;50:641. doi: 10.1079/pns19910077. [DOI] [PubMed] [Google Scholar]
- 59.Rakwal R, Agrawal GK, Kubo A, Yonekura M, Tamogami S, Saji H, Iwahashi H. Defense/stress responses elicited in rice seedlings exposed to the gaseous air pollutant sulfur dioxide. Environ Exp Bot. 2003;49:223–235. [Google Scholar]
- 60.Wang ZQ, Ribnicky D, Zhang XH, Raskin I, Yu Y, Cefalu WT. Bioactives of Artemisia dracunculus L. enhance cellular insulin signaling in primary human skeletal muscle culture. Metab, Clin Exp. 2008;57:S58–S64. doi: 10.1016/j.metabol.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang ZQ, Ribnicky DM, Logendra S, Poulev A, Ma J, Yang H, Kennelly EJ, Raskin I, Liu X, Cefalu WT. Bioactives from an Extract of Artemisia dracunculus L. exhibit potent inhibitory effects on PTB-1B activity in human skeletal muscle culture from subjects with type 2 diabetes. In: Matschinsky FM, editor. 66th Scientific Sessions of the American Diabetes Association; Washington, DC. Alexandria: American Diabetes Association; [June 9-13, 2006]. 2006. [Google Scholar]
- 62.IUPAC. Nomenclature of cyclitols. Biochem J. 1976;153:23–31. doi: 10.1042/bj1530023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clifford MN, Johnston KL, Knight S, Kuhnert N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J Agric Food Chem. 2003;51:2900–2911. doi: 10.1021/jf026187q. [DOI] [PubMed] [Google Scholar]
- 64.Schutz K, Kammerer D, Carle R, Schieber A. Identification and quantification of caffeoylquinic acids and flavonoids from artichoke (Cynara scolymus L.) heads, juice, and pomace by HPLC-DAD-ESI/MSn. J Agric Food Chem. 2004;52:4090–4096. doi: 10.1021/jf049625x. [DOI] [PubMed] [Google Scholar]
- 65.Levin DA. Polyploidy and novelty in flowering plants. Am Nat. 1983;122:1. [Google Scholar]
- 66.Lavania UC. Genomic and ploidy manipulation for enhanced production of phytopharmaceuticals. Plant Genet Resour. 2005;3:170–177. [Google Scholar]
- 67.Eisenman SW. PhD dissertation. Rutgers, The State University of New Jersey; 2010. Genetic and chemical variation in North American populations of the medicinal plant wild tarragon (Artemisia dracunculus L.) [Google Scholar]
- 68.Eisenman SW, Stuwe L. The global distribution of wild tarragon (Artemisia dracunculus L.; Asteraceae) cytotypes with twenty-seven new records from North America. Genet Resour Crop Ev. doi: 10.1007/s10722-010-9653-6. Published Online First: 26 January 2011. [DOI] [Google Scholar]