Abstract
Objectives
Owing to the challenges in obtaining pancreatic biopsies, pancreatic resection for presumed malignancy is often performed without histological confirmation. As a result, benign lesions are sometimes surgically removed. One such condition, which is poorly defined in the literature, is referred to as lipomatous pseudohypertrophy (LPH) of the pancreas.
Methods
Five cases of LPH were analyzed.
Results
Four patients underwent surgical resection, 3 of which were diagnosed preoperatively by radiology as having ductal adenocarcinoma. The fourth case was correctly interpreted by magnetic resonance imaging as LPH, but the patient underwent resection because of the intractable pain due to pancreatitis. The fifth patient has been placed on watchful waiting.
Two tumors were in the pancreatic head, one in the tail, one in the uncinate process, and one demonstrated diffuse involvement. Microscopically, they were characterized as having normal lipocytes without lipoblasts or inflammation. Within the adipose tissue, scattered microscopic foci of pancreatic parenchyma could be seen.
Conclusion
Lipomatous pseudohypertrophy of the pancreas is a distinct entity characterized by localized/diffuse replacement of pancreatic parenchyma with mature adipose tissue. It forms a pseudotumor that may be difficult to distinguish clinically from pancreatic adenocarcinoma. This entity should be considered when evaluating patients with a new diagnosis of a hypodense pancreatic neoplasm on imaging.
Keywords: lipomatous pseudohypertrophy, pancreas, neoplasm, pseudotumor
Lipomatous pseudohypertrophy (LPH) of the pancreas is a peculiar entity causing enlargement of the pancreas due to a significant increase in adipose tissue in patients who have no obesity or in whom there are no obvious findings of diabetes mellitus or pancreatitis. Examples of this uncommon lesion are reported in the literature as only a handful of individual case reports.1–21 Associations with rare childhood syndromes such as Shwachman-Diamond,12,19 Bannayan,20 or Johanson-Blizzard13 have been made. Lipomatous pseudohypertrophy often forms a benign mass mimicking cancer, resulting in unnecessary resection and morbidity.
Here, we present 5 new cases and review the clinical and pathological features.
MATERIALS AND METHODS
In the authors’ institutional and consultation files (Wayne State University and Karmanos Cancer Institute, Michigan, 1980–2007; Emory University Hospital, Georgia; Providence Hospital, Southfield, 1999–2007), the pathological materials, including the reports and routine formalin-fixed, paraffin-embedded, and hematoxylin-eosin–stained sections of 2446 cases, were reviewed. Four cases of pancreatic pseudotumor histologically characterized by mature adipose tissue replacing the pancreatic parenchyma were identified, and their clinicopathologic features analyzed. One case diagnosed using magnetic resonance imaging (MRI) scan has been placed on watchful waiting.
Patients’ age, sex, tumor size, and the macroscopic findings of the tumors were extracted from the reports (Table 1). Clinical findings of the cases and follow-up data were obtained through contact with the primary physicians or reviews of patients’ hospital charts.
TABLE 1.
Clinical Findings
| Case | Age, y | Sex | BMI, kg/m2 | Site | Size, cm | Clinical History | Coexisting Lesion |
|---|---|---|---|---|---|---|---|
| 1 | 68 | F | 38 | Head | 2.5 | Asymptomatic | None |
| 2 | 67 | F | N/A | Tail | 13 | Asymptomatic | Serous cystadenoma |
| 3 | 47 | M | 31 | Diffuse | 7 | Chronic pancreatitis, bile duct stricture | None |
| 4 | 59 | M | 28 | Head | 24 | Asymptomatic | None |
| 5* | 60 | F | 25 | Uncinate | 2.3 | Abdominal pain | None |
This case has been placed on watchful waiting.
F indicates female; M, male.
RESULTS
Clinical Findings
Mean patient age was 60 years (range, 47–68 years), 3 were female, and 3 cases were identified incidentally on imaging for unrelated concerns, and 2 were symptomatic. One patient presented with a history of chronic pancreatitis and biliary strictures. One was evaluated for new-onset upper abdominal pain. Four patients underwent surgical resection, 3 of which were diagnosed preoperatively as having ductal adenocarcinoma. The fourth case was correctly interpreted using MRI as LPH, but the patient underwent resection because of the intractable pain due to pancreatitis associated with the mass. The fifth patient has been placed on watchful waiting.
Mean patient body mass index (BMI) was 30 kg/m2 (range, 25–38 kg/m2). There was no history of alcoholism or diabetes mellitus, and none of the patients had evidence of Shwachman-Diamond, Bannayan, or Johanson-Blizzard syndromes.
Pathological Findings
Macroscopic Findings
In 2 cases, the tumor was located in the head, one was in the tail, and one was in the uncinate process, and there was diffuse involvement of the pancreas in 1 case. The tumors were 24, 13, 7, 2.5, and 2.3 cm in largest dimension (Table 1). In all cases, there was a sharply demarcated, lobulated, yellow tumor, which is composed of adipose tissue (Fig. 1). In 1 case, part of the common bile duct appeared to be dilated because of the mass effect.
FIGURE 1.
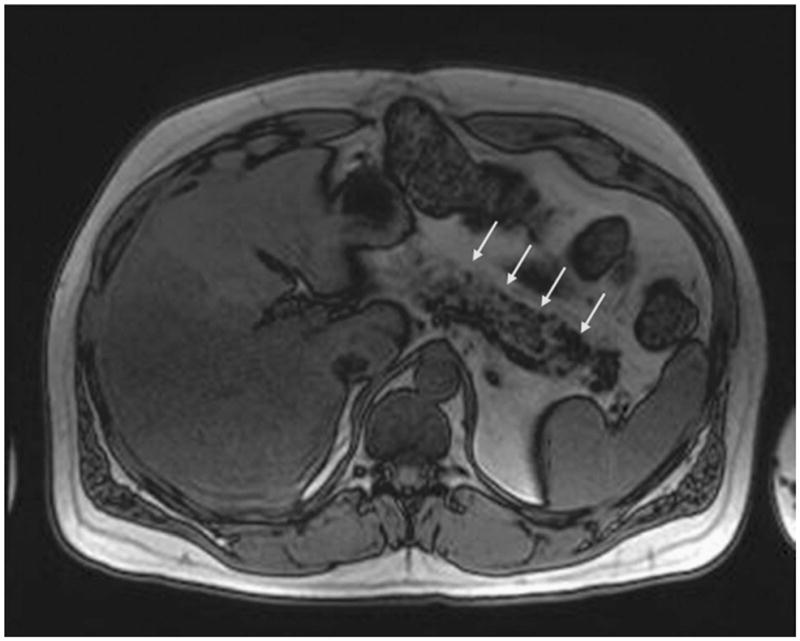
The pancreas is predominantly replaced by fatty tissue.
Microscopic Findings
The tumors were composed of mature adipose tissue replacing the pancreatic parenchyma, leaving only scattered clusters of pancreatic elements (Figs. 2–5), without showing any stigmata of chronic pancreatitis. Pancreatic elements were identified in the remote peripheral edges of the lesions, and in many areas, the tumor was sharply demarcated from the surrounding tissue (Figs. 2 and 3). The acini were extensively replaced by the adipose tissue, with only scattered islets and rare ducts remaining amid the fat (Figs. 4 and 5). Lipocytes were entirely normal (Figs. 4 and 5). No lipoblasts were identified. There was no significant inflammation.
FIGURE 2.
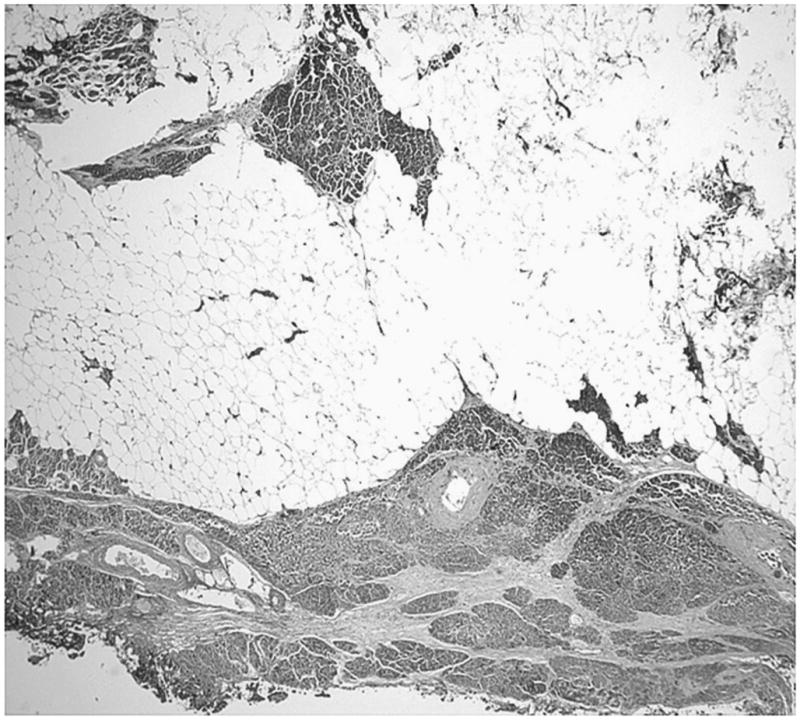
Tumor pushing into the adjacent pancreas is seen.
FIGURE 5.
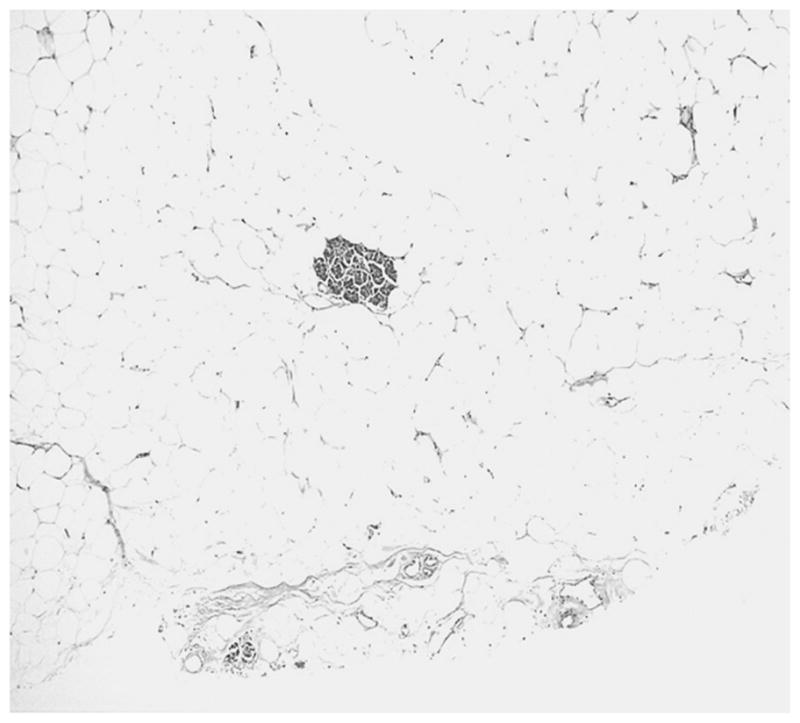
Scattered acini within the adipose tissue.
FIGURE 3.
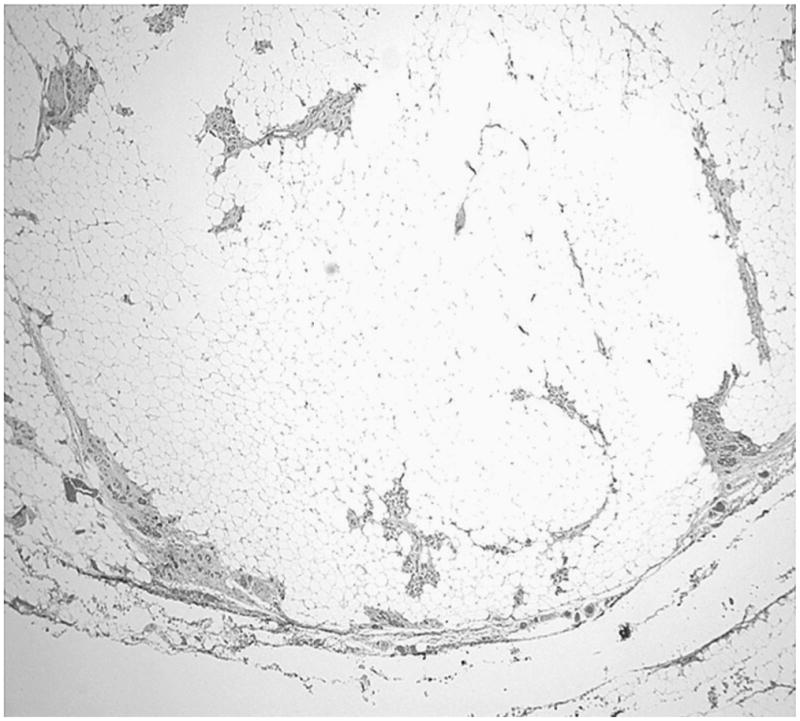
The tumor is well circumscribed, separated from the adjacent normal adipose tissue by a thin rim of fibrous pancreatic tissue.
FIGURE 4.
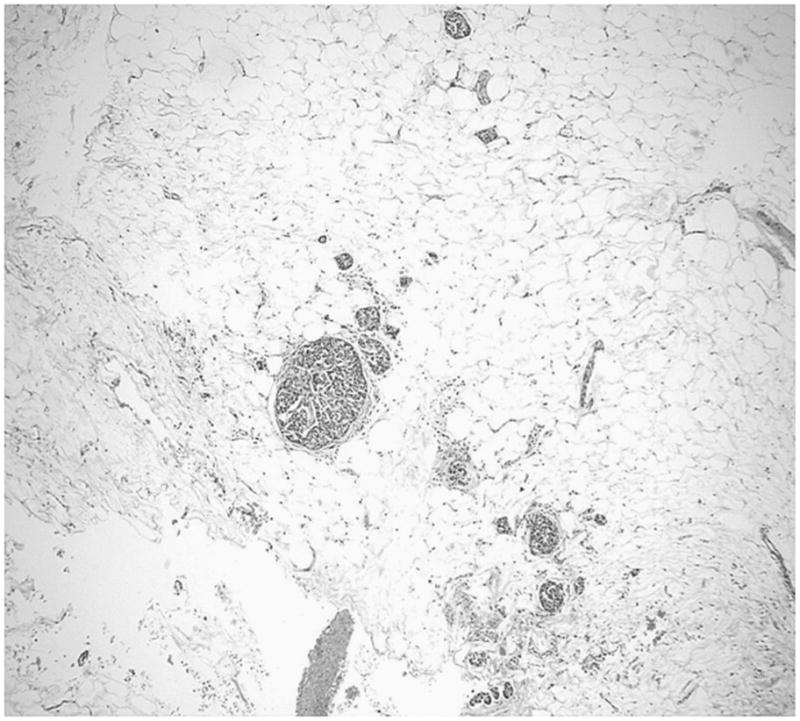
Scattered islets amid the adipose tissue.
One patient (case 2) had an incidental separate serous cystadenoma.
REVIEW OF THE LITERATURE
When the data reported in the literature1–21 are combined with the 5 cases here, the following general characteristics become apparent (Tables 2A and B): mean age is 41 years (range, 6 days to 80 years). There is no distinct difference in the sex distribution (male-female ratio, 1.2:1). Approximately in 70% of the cases, there is a diffuse involvement of the pancreas; in 20%, the tumor is located in the head, and in 10%, in the body and tail. Most common symptom at presentation is abdominal pain. In some cases, LPH seems to be associated with Shwachman-Diamond,12,19,22 Bannayan,20 and Johanson-Blizzard13 syndromes and juvenile variant of Parkinson disease.10 In a few cases, it is reported in conjunction with squamous cell carcinoma,8 leiomyosarcoma,23 and carcinoma arising in an LPH.14,17 Among our patients, one had a separate serous cystadenoma, which is probably of no particular association, because serous cystadenomas are notorious for coexisting with other lesions.
Table 2A.
Review of the Literature
| Reference | No. Cases | Age, y | Sex | Location | Clinical History | Clinical Findings | Pathological Findings |
|---|---|---|---|---|---|---|---|
| Hantelmann15 | 1 | 9 | M | N/A | Hodgkin disease, amyloidosis of liver, spleen, and kidneys | LPH of the pancreas | |
| Hoyer16 | 1 | 9 mo | M | Diffuse | Steatorrhea and weight loss | Lack of exocrine parenchyma, preservation of islets and overcompensation by fat | |
| Lumb and Beautyman21 | 2 | 9 mo; 6 d | F; M | N/A; diffuse | Steatorrhea | F: lipomatous pancreas M: a few minute areas of pancreatic tissue were found lying in fat tissue |
|
| Robson and Scott7 | 1 | 77 | M | Diffuse | Steatorrhea | Swelling of the ankles, legs, abdomen, cerebrovascular incident→Died | Abundance of mature adipose tissue with remnants of pancreas parenchyma |
| Beresford and Owen6 | 2 | 27; 23 | F; M | Both diffuse | F: scleroderma, pulmonary tuberculosis M: celiac disease |
F: →Died M: bronchopneumonia→Ex |
F: fatty infiltration and atrophy of the parenchyma M: fatty replacement of the acini with survival of the islets of Langerhans |
| Salm14 | 1 | 69 | M | Diffuse | Scirrhous carcinoma arising in LPH with widespread metastases and obstruction of the vena cava | ||
| Salm17 | 1 | 71 | F | Diffuse | Gangrene of the toes of the right foot and a palpable mass below the right costal margin | Pancreatic adenocarcinoma arising in LPH | |
| Siegler4 | 1 | 43 | M | Diffuse | Overwhelming pulmonary suppuration→Ex | Enlarged pancreas, replaced by fat; no visible exocrine portion, ducts, and islet tissue | |
| Nakamura et al9 | 1 | 43 | M | Body and tail | Abdominal mass | Marked fatty infiltration with scattered atrophied acinar cells associated with multiple cysts | |
| Okumura et al20 | 1 | 5 | F | Diffuse | Bannayan syndrome | Generalized subcutaneous lipomatosis, megalencephaly, and macrodactyly | Diffuse lipomatosis in the thoracic and abdominal cavity, and infiltration of fat tissue to the pancreas |
| Lozano et al27 | 2 | 25; 63 | F; M | N/A | Weight loss and massive steatorrhea | Lipomatosis of pancreas | |
| Henkinbrant et al3 | 1 | 70 | M | Diffuse | Abdominal pain, jaundice | Obstruction of the common bile duct | Enlarged pancreas, atrophy of the acinar lobules and the islets of Langerhans with marked fatty infiltration of the pancreas |
| Yoshimura et al10 | 1 | 38 | M | Diffuse | Juvenile Parkinson disease | Enlargement of the pancreas due to massive fat replacement; the exocrine glandular elements showed marked atrophy and loss, whereas the islets were preserved; the liver exhibited a histology mimicking alcoholic hepatitis with diffuse Mallory bodies; the nervous system showed diffuse Lewy bodies | |
| Bom et al19 | 1 | 18 | F | Diffuse | Shwachman syndrome | Pancytopenia, short stature, recurrent infections | Pancreatic lipomatosis |
| MacMaster and Cummings12 | 1 | 34 | N/A | Diffuse | Shwachman syndrome | Pancreatic lipomatosis | |
| Shimizu et al23 | 1 | 49 | F | Body and tail | Epigastric pain, jaundice | Fatty replacement of the pancreatic body and tail associated with leiomyosarcoma in the pancreatic head | |
| Sasaki et al2 | 1 | 52 | F | Diffuse | Chronic hepatitis B | Cirrhosis→Ex | Massive fatty replacement of pancreas |
| Maunoury et al13 | 1 | 19 | M | N/A | Johanson-Blizzard syndrome | Pancreatic lipomatosis | |
| Bralet et al8 | 1 | 68 | F | Diffuse | Diabetes mellitus type 2, left upper abdominal pain, and weight loss | Massive fatty replacement of pancreas with coexisting squamous cell carcinoma | |
| Belkind-Gerson et al22 | 1 | 14 | F | N/A | Shwachman-Diamond syndrome | Neutropenia, short stature, skeletal abnormalities→Ex | Pancreatic lipomatosis |
| Kuroda et al1 | 1 | 80 | M | Diffuse | Hepatitis B | Cirrhosis→Ex | Massive fatty replacement of pancreas |
| Flohr et al5 | 1 | 32 | M | Head | Primary sclerosing cholangitis, cirrhosis | LPH |
N/A indicates not available.
Table 2B.
Summary of the Literature and Our Cases
| Case No. | Mean Age, y | Sex | Site | Coexisting Lesions | Associated Syndromes |
|---|---|---|---|---|---|
| 30 (25 + 5*) | 41 (range, <1–80) | M:F 1.2:1 | Head, n = 4 Body and tail, n = 3 Diffuse, n = 17 |
Carcinoma, n = 3 Leiomyosarcoma, n = 1 |
Shwachman-Diamond syndrome, n = 3 Bannayan syndrome, n = 1 Johanson-Blizzard syndrome, n = 1 |
Our cases.
DISCUSSION
Focal replacement of the exocrine pancreas with mature fat is a common histological change observed in the pancreas.24,25 It usually correlates directly with BMI and presence of diabetes mellitus and/or pancreatitis.26–29 Lipomatous pseudohypertrophy, by contrast, is the label applied to those cases associated with pseudotumor formation by fat, replacing almost an entire segment of exocrine parenchyma.8 Until now, this entity has been documented in the literature mostly by individual case reports1–20 (Tables 2A and B).
Lipomatous pseudohypertrophy typically comes to clinical attention incidentally during workup for other conditions, but the tumor is often large (our mean, 9.7 cm), up to 24 cm (as seen in case 4 of our study). Because it forms a mass, it is often mistaken for a malignancy. On the other hand, if proper radiological evaluation is performed, it can be recognized that the lesion is actually composed of fat, as it was in our case 3. The sheer size of the lesion may be disconcerting in some cases, as seen in one of our patients who received the preoperative diagnosis of liposarcoma. Endoscopic ultrasound–guided, fine-needle aspiration biopsy showing mature fat cells, without any atypia, might be helpful in such cases.30,31 Recently, we had another case, a 60-year-old woman who presented with lower abdominal pain, and we diagnosed this patient as having LPH, and the patient is currently on watchful waiting, with no changes in the radiological appearance of the lesion in 3-month intervals at 9 months after the original diagnosis.
Macroscopically, the appearance and consistency are those of adipose tissue. Histological examination of the pancreas shows massive replacement of pancreatic parenchyma by adipose tissue. The islets of Langerhans are relatively preserved, and typically there are scattered, small but well-preserved acinar elements. These acini do not show signs of injury such as cellular attenuation, inspissated secretions or tubular complex formation. No fibrosis or fat necrosis is noted. In other words, these pancreatic elements do not show any signs of either acute or chronic pancreatitis. Most interestingly, the pseudotumor typically has well-defined borders, although it does not appear to have a well-formed capsule. Neighboring pancreatic or soft tissue may show signs of compression; that is, these lesions do appear to have a mass effect. No significant infiltration of inflammatory cells or proliferation of ductal epithelial cells is identified. At the microscopic level, the main differential diagnosis is with a well-differentiated liposarcoma, which is reported in the literature as individual case reports.32–35 In fact, this was the working diagnosis of the primary pathologist in one of the cases. The findings that speak against this possibility are the perfect maturation of lipocytes, sharp demarcation of the lesion, lack of lipoblasts, and admixture of normal pancreatic parenchyma within the lesion.
The pathogenesis of replacement of the pancreatic tissue by fat is uncertain, and various possible causes have been postulated in the literature.2,4,6,7,9,10,14–16,26,27 Hoyer16 discussed the possibility of a congenital anomaly, in view of the incidence in childhood, but thought that the condition was more probably acquired, due to injury of the pancreatic parenchyma by infective or toxic agents.
Others have also argued that LPH can be an acquired condition due to obstruction of the ducts and secondary parenchymal damage and atrophy, replaced by adipose tissue.6,9,14,15 However, we have not observed any occlusive lesions of the ducts or any inciting factors that may have led to the selective atrophy of the pancreas in our cases. Furthermore, this would not explain the abundance of adipose tissue. Moreover, the scattered pancreatic elements in the lesion appear relatively uninjured.
It has been suggested that LPH may be a form of lipoma.7 Pancreatic lipomas are multilobed tumors that are sharply delineated from surrounding pancreatic tissue by a thin fibrous capsule.36–38 Lipomatous pseudohypertrophy, on the other hand, is characterized by an enlarged pancreas with massive replacement of pancreatic exocrine tissue by adipose tissue, and the shape of the pancreas is still preserved.6,39 More importantly, there are scattered atrophic pancreatic elements within the pseudotumor. Preservation of normal structures can occur in lipomas, such as in intramuscular lipomas; however, the distribution of pancreatic tissue within the lesions in our cases was not in keeping with an entrapment by a tumor, but rather appeared to be an integral part of the process.
In experimental animals, certain toxic agents such as ethionine or viruses have been observed to produce diffuse and massive fat replacement indistinguishable from LPH of the pancreas in humans, unassociated with any obvious inflammatory reaction.4,40 Yoshimura et al10 and Sasaki et al2 also reported that LPH might be related to chronic hepatitis B or other chronic advanced hepatic lesions that cause a fatty change and Mallory body formation. In fact, one of our patients had a fatty change in the right lobe of the liver, which was demonstrated by MRI. Therefore, the hypothesis that a specific underlying toxic agent may cause LPH of the pancreas may be a plausible explanation but still needs further investigation. On the other hand, relatively well preservation of the pancreatic elements and the pseudotumor formation by the process speak against this possibility.
In conclusion, LPH is a distinct entity of undetermined etiology and pathogenesis. Although some of the cases are easily diagnosed, in some, LPH may present as a pancreatic mass mimicking carcinoma, particularly if it is associated with massive enlargement of the pancreas. Because of mass effect, the lesion might even cause obstructive jaundice. It is important to remember the existence of this entity because it can be quite puzzling for the patients, their family, and the physicians to find out the absence of cancer after a pancreatectomy.
Acknowledgments
The authors thank Dr Al Braustein from Providence Hospital, Southfield, Mich, for providing 2 of the cases and information on them. The authors also thank Dr Sharon Weiss at Emory University Hospital for providing expert opinion on case 4.
This study was supported in part by the National Cancer Institute Specialized Program in Research Excellence, P50-CA62924.
References
- 1.Kuroda N, Okada M, Toi M, et al. Lipomatous pseudohypertrophy of the pancreas: further evidence of advanced hepatic lesion as the pathogenesis. Pathol Int. 2003;53(2):98–101. doi: 10.1046/j.1440-1827.2003.01437.x. [DOI] [PubMed] [Google Scholar]
- 2.Sasaki M, Nakanuma Y, Ando H. Lipomatous pseudohypertrophy of the pancreas in a patient with cirrhosis due to chronic hepatitis B. Pathol Int. 1998;48(7):566–568. doi: 10.1111/j.1440-1827.1998.tb03951.x. [DOI] [PubMed] [Google Scholar]
- 3.Henkinbrant A, Khalek W, Farchakh E, et al. Cholestatic jaundice complicating lipomatous pseudo-hypertrophy of the pancreas [in French] Acta Gastroenterol Belg. 1990;53(3):315–322. [PubMed] [Google Scholar]
- 4.Siegler DI. Lipomatous pseudohypertrophy of the pancreas associated with chronic pulmonary suppuration in an adult. Postgrad Med J. 1974;50(579):53–55. doi: 10.1136/pgmj.50.579.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flohr T, Bonatti H, Shumaker N, et al. Liver transplantation in a patient with primary sclerosing cholangitis suffering from lipomatous pseudohypertrophy of the pancreas. Transpl Int. 2008;21(1):89–91. doi: 10.1111/j.1432-2277.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 6.Beresford OD, Owen TK. Lipomatous pseudohypertrophy of the pancreas. J Clin Pathol. 1957;10(1):63–66. doi: 10.1136/jcp.10.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robson HN, Scott GB. Lipomatous pseudohypertrophy of the pancreas. Gastroenterology. 1953;23(1):74–81. [PubMed] [Google Scholar]
- 8.Bralet MP, Terris B, Bregeaud L, et al. Squamous cell carcinoma and lipomatous pseudohypertrophy of the pancreas. Virchows Arch. 1999;434(6):569–572. doi: 10.1007/s004280050385. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura M, Katada N, Sakakibara A, et al. Huge lipomatous pseudohypertrophy of the pancreas. Am J Gastroenterol. 1979;72(2):171–174. [PubMed] [Google Scholar]
- 10.Yoshimura N, Hayashi S, Fukushima Y. Diffuse Mallory bodies in the liver, diffuse Lewy bodies in the brain and diffuse fat replacement (lipomatous pseudohypertrophy) of the pancreas in a patient with juvenile Parkinson’s disease. Acta Pathol Jpn. 1992;42(11):826–831. doi: 10.1111/j.1440-1827.1992.tb01884.x. [DOI] [PubMed] [Google Scholar]
- 11.Adsay NV, Basturk O, Klimstra DS, et al. Pancreatic pseudotumors: non-neoplastic solid lesions of the pancreas that clinically mimic pancreas cancer. Semin Diagn Pathol. 2004;21(4):260–267. doi: 10.1053/j.semdp.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 12.MacMaster SA, Cummings TM. Computed tomography and ultrasonography findings for an adult with Shwachman syndrome and pancreatic lipomatosis. Can Assoc Radiol J. 1993;44(4):301–303. [PubMed] [Google Scholar]
- 13.Maunoury V, Nieuwarts S, Ferri J, et al. Pancreatic lipomatosis revealing Johanson-Blizzard syndrome [in French] Gastroenterol Clin Biol. 1999;23(10):1099–1101. [PubMed] [Google Scholar]
- 14.Salm R. Scirrhous adenocarcinoma arising in a lipomatous pseudohypertrophic pancreas. J Pathol Bacteriol. 1960;79:47–52. doi: 10.1002/path.1700790106. [DOI] [PubMed] [Google Scholar]
- 15.Hantelmann W. Fettsucht und Atrophie der Bauch specicheldruse bei Jungendlichen. Virchows Arch. 1931;282:630–642. [Google Scholar]
- 16.Hoyer A. Lipomatous pseudohypertrophy of the pancreas with complete absence of exocrine tissue. J Pathol Bacteriol. 1949;61:93–100. [Google Scholar]
- 17.Salm R. Carcinoma arising in a lipomatous pseudohypertrophic pancreas. Br Med J. 1968;3(5613):293. doi: 10.1136/bmj.3.5613.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitz-Moormann P, Pittner PM, Heinze W. Lipomatosis of the pancreas. A morphometrical investigation. Pathol Res Pract. 1981;173(1–2):45–53. doi: 10.1016/S0344-0338(81)80006-4. [DOI] [PubMed] [Google Scholar]
- 19.Bom EP, van der Sande FM, Tjon RT, et al. Shwachman syndrome: CT and MR diagnosis. J Comput Assist Tomogr. 1993;17(3):474–476. [PubMed] [Google Scholar]
- 20.Okumura K, Sasaki Y, Ohyama M, et al. Bannayan syndrome—generalized lipomatosis associated with megalencephaly and macrodactyly. Acta Pathol Jpn. 1986;36(2):269–277. doi: 10.1111/j.1440-1827.1986.tb01479.x. [DOI] [PubMed] [Google Scholar]
- 21.Lumb G, Beautyman W. Hypoplasia of the exocrine tissue of the pancreas. J Pathol Bacteriol. 1952;64(4):679–686. doi: 10.1002/path.1700640402. [DOI] [PubMed] [Google Scholar]
- 22.Belkind-Gerson J, Ontiveros-Nevares P, Ocampo-Roosens V, et al. Shwachman-Diamond syndrome in a Mexican family. Arch Med Res. 2001;32(4):318–323. doi: 10.1016/s0188-4409(01)00293-4. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu M, Hirokawa M, Matsumoto T, et al. Fatty replacement of the pancreatic body and tail associated with leiomyosarcoma of the pancreatic head. Pathol Int. 1997;47(9):633–636. doi: 10.1111/j.1440-1827.1997.tb04554.x. [DOI] [PubMed] [Google Scholar]
- 24.Stamm BH. Incidence and diagnostic significance of minor pathologic changes in the adult pancreas at autopsy: a systematic study of 112 autopsies in patients without known pancreatic disease. Hum Pathol. 1984;15(7):677–683. doi: 10.1016/s0046-8177(84)80294-4. [DOI] [PubMed] [Google Scholar]
- 25.Olsen TS. Lipomatosis of the pancreas in autopsy material and its relation to age and overweight. Acta Pathol Microbiol Scand. 1978;86A(5):367–673. doi: 10.1111/j.1699-0463.1978.tb02058.x. [DOI] [PubMed] [Google Scholar]
- 26.Dupont C, Sellier N, Chochillon C, et al. Pancreatic lipomatosis and duodenal stenosis or atresia in children. J Pediatr. 1989;115(4):603–605. doi: 10.1016/s0022-3476(89)80293-8. [DOI] [PubMed] [Google Scholar]
- 27.Lozano M, Navarro S, Perez-Ayuso R, et al. Lipomatosis of the pancreas: an unusual cause of massive steatorrhea. Pancreas. 1988;3(5):580–582. doi: 10.1097/00006676-198810000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Seifert G. Lipomatous Atrophy and Other Forms. London, UK: Churchill Livingstone; 1984. pp. 27–31. [Google Scholar]
- 29.So CB, Cooperberg PL, Gibney RG, et al. Sonographic findings in pancreatic lipomatosis. Am J Roentgenol. 1987;149(1):67–68. doi: 10.2214/ajr.149.1.67. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki R, Irisawa A, Hikichi T, et al. Pancreatic lipoma diagnosed using EUS-FNA. A case report. J Pancreas. 2009;10(2):200–203. [PubMed] [Google Scholar]
- 31.Di Matteo FM, Shimpi L, Pandolfi M, et al. EUS diagnosis of pancreatic lipoma: a case report. Gastrointest Endosc. 2006;64(1):146–148. doi: 10.1016/j.gie.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Dodo IM, Adamthwaite JA, Jain P, et al. Successful outcome following resection of a pancreatic liposarcoma with solitary metastasis. World J Gastroenterol. 2005;11(48):7684–7685. doi: 10.3748/wjg.v11.i48.7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milano C, Colombato LA, Fleischer I, et al. Liposarcoma of the pancreas. Report of a clinical case and review of the literature [in Spanish] Acta Gastroenterol Latinoam. 1988;18(2):133–138. [PubMed] [Google Scholar]
- 34.Elliott TE, Albertazzi VJ, Danto LA. Pancreatic liposarcoma: case report with review of retroperitoneal liposarcomas. Cancer. 1980;45(7):1720–1723. doi: 10.1002/1097-0142(19800401)45:7<1720::aid-cncr2820450733>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 35.Choux R, Andrac L, Rodriguez M, et al. Liposarcoma of the pancreas. Study of a case including ultrastructure [in French] Ann Anat Pathol. 1979;24(3):251–259. [PubMed] [Google Scholar]
- 36.Bigard MA, Boissel P, Regent D, et al. Intrapancreatic lipoma. First case in the literature. Gastroenterol Clin Biol. 1989;13(5):505–507. [PubMed] [Google Scholar]
- 37.Boglino C, Inserra A, Silvano A, et al. Intrapancreatic lipoma: a case report [in Italian] Pediatr Med Chir. 1993;15(4):397–399. [PubMed] [Google Scholar]
- 38.Merli M, Fossati GS, Alessiani M, et al. A rare case of pancreatic lipoma. Hepatogastroenterology. 1996;43(9):734–736. [PubMed] [Google Scholar]
- 39.Katz DS, Hines J, Math KR, et al. Using CT to reveal fat-containing abnormalities of the pancreas. Am J Roentgenol. 1999;172(2):393–396. doi: 10.2214/ajr.172.2.9930790. [DOI] [PubMed] [Google Scholar]
- 40.Kojima K. Current Encyclopedia of Pathology. 13B. Tokyo, Japan: Nakayama-Shoten; 1983. Metabolic Disturbances of the Pancreas; pp. 183–195. [Google Scholar]


