Abstract
The hippocampal N-methyl-d-aspartate receptor (NMDAR) activity plays important roles in cognition and is a major substrate for ethanol-induced memory dysfunction. This receptor is a glutamate-gated ion channel, which is composed of NR1 and NR2 subunits in various brain areas. Although homomeric NR1 subunits form an active ion channel that conducts Na+ and Ca2+ currents, the incorporation of NR2 subunits allows this channel to be modulated by the Src family of kinases, phosphatases, and by simple molecules such as ethanol. We have found that short-term ethanol application inhibits the NMDAR activity via striatal enriched protein tyrosine phosphatase (STEP)-regulated mechanisms. The genetic deletion of the active form of STEP, STEP61, leads to marked attenuation of ethanol inhibition of NMDAR currents. In addition, STEP61 negatively regulates Fyn and p38 mitogen-activated protein kinase (MAPK), and these proteins are members of the NMDAR super molecular complex. Here we demonstrate, using whole-cell electrophysiological recording, Western blot analysis, and pharmacological manipulations, that neurons exposed to a 3-h, 45 mM ethanol treatment develop an adaptive attenuation of short-term ethanol inhibition of NMDAR currents in brain slices. Our results suggest that this adaptation of NMDAR responses is associated with a partial inactivation of STEP61, an activation of p38 MAPK, and a requirement for NR2B activity. Together, these data indicate that altered STEP61 and p38 MAPK signaling contribute to the modulation of ethanol inhibition of NMDARs in brain neurons.
Introduction
Ethanol application inhibits N-methyl-d-aspartate receptor (NMDAR) activity (Lovinger et al., 1989), and subsequent studies have shown that the NMDAR in hippocampal brain slices develops resistance to the short-term effects of ethanol during a 5- to 15-min ethanol (100 mM) exposure (Grover et al., 1994; Miyakawa et al., 1997). However, precise mechanisms that regulate the development of this resistance to the inhibitory effects of short-term ethanol exposure on the NMDAR are not fully understood. Because NMDARs can undergo both rapid and delayed activity-dependent changes in adult hippocampal neurons (Heynen et al., 2000), we hypothesized that time- and ethanol dose-dependent adaptive changes of the NMDAR also may occur during ethanol exposure under ex vivo conditions, and such changes may underlie the mechanisms necessary for the functional adaptation of these receptors to ethanol inhibition. Previous studies also showed that adaptive changes can occur as a result of increased NMDAR expression (Snell et al., 1996; Roberto et al., 2004; Lack et al., 2007) and/or by selective phosphorylation/dephosphorylation of NMDARs after chronic ethanol treatment (Clapp et al., 2010; Wu et al., 2010). Here, we demonstrate that the development of adaptation of the NMDAR to ethanol inhibition involves the striatal enriched protein tyrosine phosphatase (STEP), the p38 mitogen-activated protein kinase (MAPK), and the activity of the NMDA NR2B receptor subunit.
Functional activity of the NMDAR is increased by protein tyrosine kinases (Salter and Kalia, 2004), but its activity is also regulated by protein tyrosine phosphatases (Wang et al., 1996; Pelkey et al., 2002; Snyder et al., 2005; Paul et al., 2007). STEP61 is a brain-specific protein tyrosine phosphatase and is found in both membrane-bound and cytosolic fractions (Boulanger et al., 1995; Goebel-Goody et al., 2009). Moreover, it coimmunoprecipitates with NMDARs (Pelkey et al., 2002; Braithwaite et al., 2006), suggesting a strong physical association between these two molecules as a signaling unit (Xu et al., 2009). Inhibition of STEP61, the only active isoform of STEP expressed in the hippocampus, has been shown to enhance NMDAR function (Pelkey et al., 2002) and to attenuate ethanol inhibition of the NMDA receptor (Hicklin et al., 2011) in rodent hippocampus.
Short-term ethanol application has been shown to decrease the level of the Tyr1472 phosphorylation site of the NR2B subunit without altering the levels of this protein (Alvestad et al., 2003; Wu et al., 2010). Tyr1472 is a site in the C-terminal tail of the NR2B in which STEP61 has been shown to act (Paul et al., 2003; Braithwaite et al., 2006), suggesting that short-term ethanol treatment activates STEP61, which is involved in dephosphorylating the Tyr1472 site. In addition, we have shown that ethanol inhibition of NMDA excitatory postsynaptic currents (EPSCs) was attenuated by microdialysis of STEP(C/S), a dominant-negative STEP mutant, into CA1 pyramidal neurons, and by animals having a STEP gene deletion (Hicklin et al., 2011). Here, we report that changes in STEP-regulated mechanisms may alter responses of the NMDAR to the inhibitory effects of ethanol.
Materials and Methods
Materials.
All drugs used to make up the artificial cerebrospinal fluid (aCSF) and internal recording solutions were purchased under the Fluka brand (Sigma-Aldrich, St. Louis, MO). Glutamatergic receptor antagonists d-(−)-2-amino-5-phosphonopentanoic acid (d-APV) and 6-cyano-7-nitroquinoxaline-2,3-dione and the GABAA receptor antagonist bicuculline methiodide also were purchased from Sigma-Aldrich. The GABAB antagonist 3-[[[(3,4-dichlorophenyl methyl] amino] propyl] diethoxymethyl) phosphinic acid (CGP-52432), the NMDA NR2B antagonist ifenprodil, and the Fyn kinase inhibitor PP2, were purchased from Tocris Cookson Inc. (Ellisville, MO). The MAPK inhibitor 4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)-1H-imidazole (SB202190) and the protein synthesis inhibitors anisomycin and cycloheximide were also purchased from Tocris Cookson. The protein tyrosine phosphatase inhibitor bpV(phen) was purchased from Calbiochem (San Diego, CA). An 8.0 M ethanol solution (in deionized water) was prepared immediately before each experiment from a 95% stock solution (Aaper, Shelbyville, KY) kept in a glass storage bottle at 4°C. Selective antibodies to NR2A and NR2B were produced in our laboratory (Snell et al., 1996), and the antibody to the NR1 subunit was purchased from BD Pharmingen (San Diego, CA). Anti-phospho-p38 antibody was purchased from Thermo Fisher Scientific (Waltham, MA), and the anti-STEP antibody was a gift from Dr. Lombroso (Yale University, New Haven, CT).
Animals.
Young adult (6–9 weeks old; 160–220 g body weight) male Sprague-Dawley rats were purchased from Harlan (Indianapolis, IN) and were housed three per cage with a 7:00 AM to 7:00 PM light cycle and with free access to food and water. The animals were maintained in a National Institutes of Health-accredited facility at the University of Colorado (Denver, CO), and the animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All of the experimental procedures were approved by the Institute for Animal Care and Use Committee at the University of Colorado Denver.
Brain Slice Preparation, Storage, and Whole-Cell Recordings.
Rats were quickly decapitated, and the brains were rapidly removed and immersed in ice-cold, sucrose-containing slicing buffer for 40 to 60 s to cool the interior of the brain. This sucrose-containing slicing buffer consisted of 87 mM NaCl, 2.5 mM KCl, 7 mM MgCl2, 0.5 mM CaCl2, 1.25 mM NaH2PO4, 25 mM d-glucose, 35 mM sucrose, and 25 mM NaHCO3. After removing one or both hippocampi from the brain, transverse slices 400 μm thick were made using a tissue chopper/slicer (TC-2 Tissue Sectioner; Sorvall, Newton, CT), and the slices were transferred to individual compartments in a storage unit (Proctor et al., 2006), where they were kept at 33°C in an aCSF solution that was gassed with 95% O2 and 5% CO2. This aCSF contained the following chemicals: 126 mM NaCl, 3.0 mM KCl, 1.5 mM MgCl2, 2.4 mM CaCl2, 1.2 mM NaH2PO4, 11 mM d-glucose, and 25.9 mM NaHCO3. The low-Mg2+ aCSF contained 0.2 mM MgCl2 instead of 1.5 mM.
Ex Vivo Ethanol Exposure.
Freshly prepared hippocampal slices were stored in aCSF for 1.5 h for recovery from the preparation procedure in a storage chamber made of individualized compartments with a bottom netting that was suspended in a 500-ml glass beaker. After the recovery, some of the brain slices were transferred to a drug-treatment unit, which is a miniature version of the storage chamber (100-ml glass beaker) that contained control aCSF or either ethanol (45 mM), ifenprodil (5 μM), anisomycin (20 μM), cycloheximide (60 μM), or SB202190 (0.5 μM) and was aerated with 95% O2 and 5% CO2. After an additional 3 h of exposure to control aCSF or drug treatment, whole-cell recordings were made from individual hippocampal CA1 pyramidal neurons while perfusing the slices with gassed aCSF. The baseline period, a short-term, ethanol challenge (80 or 120 mM ethanol) application, and washout period were monitored for evoked NMDA EPSC responses. In a separate group of slices, the brain slices were perfused with low-Mg2+ aCSF during the recording of NMDA EPSCs. In these ex vivo ethanol exposure experiments, 100 μl of the solution in the storage unit was removed and stored at −20°C for analysis of ethanol concentrations using the enzymatic method as described by Smolen and Smolen (1989). The ethanol concentration in the storage chamber was maintained at approximately 200 mg% (45 mM) during the 3-h exposure. Slices were then individually transferred from either the control or the drug-treatment storage unit to a recording chamber in which they were perfused with aCSF or low Mg2+ aCSF at 33°C for 20 min to allow for washout of the residual ethanol and/or excess Mg2+ before whole-cell recording began. After a baseline recording period (10–15 min), short-term ethanol (80 or 120 mM) was applied for 10 min, followed by a 15- to 30-min washout period.
Electrophysiological Recordings.
Whole-cell recordings were made at 33°C, and the slices were constantly superfused with oxygenated aCSF at 2 ml/min bulk flow rate. A Flaming/Brown electrode puller (Sutter Instruments, Novato, CA) was used to fabricate 6 to 9 MΩ whole-cell microelectrodes when filled with a K+-gluconate internal solution containing 130 mM K+-gluconate, 1 mM EGTA, 2 mM MgCl2, 0.5 mM CaCl2, 2.54 mM disodium ATP, 0.3 mM NaGTP, and 10 mM HEPES adjusted to pH 7.3 with KOH, and 280 to 290 mOsM. CA1 pyramidal neurons were recorded within the stratum pyramidale layer. Evoked NMDA synaptic responses (EPSCs) were obtained by stimulation in the stratum pyramidale layer with a twisted bipolar stimulating electrode made from 0.0010-in Formvar-coated Nichrome wire. This stimulating electrode was positioned to activate a few proximal presynaptic fibers that innervate on or near the soma of the recorded cell using brief electrical current pulses (200 μs). This stimulation paradigm routinely evokes a current responses of 1 to 3 nA and a pharmacologically isolated NMDAR current response of approximately 100 to 200 pA (average of 121.5 ± 6.3 pA, n = 101). Drugs were applied at 100-fold concentrations by bath superfusion at 1/100 bulk flow into the aCSF/low Mg2+ aCSF flow line via calibrated syringe pumps (Razel Scientific Instruments Inc., Stamford, CT).
Measurement of NMDA EPSCs.
CA1 pyramidal neurons were voltage-clamped at −60 mV (corrected for the liquid-junction potential) from the normal resting membrane potential (−65 to −74 mV; average, −67.6 ± 0.63 mV) to enhance the size of the current responses. The NMDA receptor-mediated EPSCs (NMDAR currents) were pharmacologically isolated using 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt (20 μM), bicuculline methyliodide (30 μM), and CGP-52432 (1 μM) to block α-amino-3-hydroxy-5-methyl-4-isoxzole propionate, GABAA, and GABAB receptor-mediated EPSCs, respectively. These isolated NMDA EPSCs were completely blocked by the NMDAR antagonist d-APV (25 μM).
Western Blot Determinations.
A separate group of hippocampal slices, CA1 mini slices (Coultrap et al., 2005), were prepared and exposed to aCSF or ethanol (45 mM) for 3 h under conditions identical with those used for the electrophysiological experiments. These slices were harvested and sonicated in buffer containing 10 mM Tris, 1 mM EDTA, and 1% SDS. After SDS-polyacrylamide gel electrophoresis, Western blotting was performed as described by Coultrap et al. (2005). A five-point dilution series of the CA1 hippocampal homogenate was included on each gel to increase the probability that samples were within the linear range of detection for each antibody. For the measurement of STEP33 and phospho-p38 (pp38) MAPK levels, the immune-reactivity was determined without a full five-point dilution standard because of the low levels of STEP33 and pp38 MAPK in control samples. Imaging of the blots was performed using the SuperSignal chemiluminescent substrate (Pierce, Rockford, IL), and an Alpha Innotech imaging system (Alpha Innotech, San Leandro, CA). The digital images were quantified using AlphaEase software (Alpha Innotech), and only values falling within the standard curve generated from the dilution series on each gel were incorporated into the final analysis.
Statistical Analysis.
Drug effects were quantified and presented as the percentage change of the NMDA EPSC amplitude after drug application, relative to the average of baseline values. Statistical analyses were carried out with the use of SigmaStat (Systat Software Inc., San Jose, CA) for Student's t test and ANOVA analyses. A Mann-Whitney rank sum test was used for nonparametric analysis of the STEP33 and pp38 MAPK data. The minimal significance level was set at p < 0.05.
Results
NMDARs Develop Resistance to the Effects of Short-Term Ethanol Application in Brain Slices.
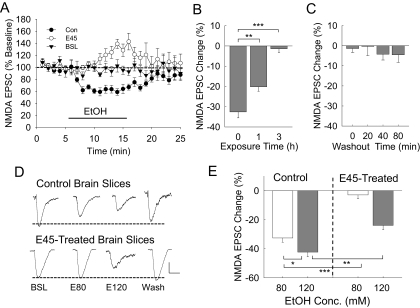
In control rodent brain slices, application of 80 mM ethanol typically inhibits synaptic NMDA EPSCs by 25 to 35% in CA1 hippocampal pyramidal neurons, and this inhibition is ethanol concentration-dependent with an estimated EC50 of 50 mM in rodent brains (Proctor et al., 2006). In routine experiments, we applied ethanol for a period of 10 min, which was followed by 10 to 30 min of washout, during which time NMDA EPSCs usually returned to baseline levels (Fig. 1A). However, in brain slices that were exposed to 3 h of 45 mM ethanol, we found that the short-term ethanol application resulted in very little inhibition but showed a time-dependent delayed facilitation of the NMDA EPSCs. During the first 2 to 5 min of short-term ethanol application, the inhibition of NMDA currents showed attenuation (resistance). After 5 to 10 min, the averaged NMDAR current responses show 13 to 17% enhancement. We found that significant attenuation of ethanol inhibition occurred after 1 h of 45 mM ethanol exposure, and a maximum attenuation was reached by 3 h of the 45 mM ethanol treatment (Fig. 1B). To determine whether this attenuated ethanol effect was reversible after removal of ethanol, we superfused the brain slices with normal aCSF for 20 to 80 min after their 3 h of 45 mM ethanol treatment, and the cells did not regain ethanol inhibition of the NMDA responses during that time (Fig. 1C). To demonstrate that the reduced short-term ethanol effect was not due to a substantial desensitization of NMDARs during the 45 mM ethanol exposure, we applied a higher short-term ethanol concentration (120 mM) challenge application. This increased concentration of ethanol still produced a significant inhibition of the NMDA EPSCs (Fig. 1, D and E). However, the ethanol inhibition was significantly reduced. Thus, the adaptive resistance of the NMDAR to the effects of ethanol (NMDAR resistance) was not due to complete desensitization of NMDARs to the action of ethanol in these neurons. To further determine that these ethanol-insensitive currents were indeed NMDA currents, we applied 20 μM d-APV to these neurons and found that d-APV completely blocked the current response.
Fig. 1.
Adaptive change of the NMDAR response to the effects of 80 and 120 mM ethanol application in hippocampal brain slices. A, time course of NMDAR current responses during whole-cell recordings from control (Con, n = 8) or 3-h 45 mM ethanol-treated (E45, n = 7) brain slices. To demonstrate the stability of the recordings, whole-cell patched CA1 neurons were superfused with normal aCSF for these baseline recordings (BSL, n = 6), without application of the 80 mM ethanol. B, brain slices were exposed to 45 mM ethanol ex vivo for 0, 1, or 3 h. The NMDAR current responses to short-term 80 mM ethanol application were recorded. Some resistance of NMDAR currents to 80 mM ethanol inhibition already occurred after 1 h of the 45 mM ethanol exposure, and maximum resistance was seen after 3 h of exposure. The number of cells recorded at 0, 1, and 3 h: 18, 7, and 16, respectively. C, after the 3-h 45 mM ethanol exposure, brain slices were superfused with normal aCSF for 0, 20, 40, or 80 min. The 80 mM ethanol inhibition of NMDAR currents was measured in these slices. Short-term ethanol application failed to significantly inhibit NMDAR currents even after 80 min of washout after the 3-h 45 mM ethanol exposure. The number of cells recorded at the 0-, 20-, 40-, and 80-min time periods: 18, 8, 8, and 6, respectively. D, representative traces of NMDAR current responses in neurons from control or 3-h 45 mM ethanol-treated brain slices that were recorded during the application of 80 mM (E80) or 120 mM (E120) ethanol. E, composite graph showing the average ethanol (80 or 120 mM) inhibition of NMDA EPSC amplitude responses from control or 45 mM ethanol-treated brain slices. The number of cells recorded from control neurons, 80 mM ethanol (n = 8); 120 mM (n = 6) and 45 mM ethanol-treated neurons, 80 mM ethanol (n = 7); and 120 mM ethanol (n = 6). One-way ANOVA shows significant differences between groups [F(3,23) = 39.328, p < 0.001]. Scale bars, 50 pA and 100 ms. Significance of the statistical analyses: *, p < 0.01; **, p < 0.005; ***, p < 0.001.
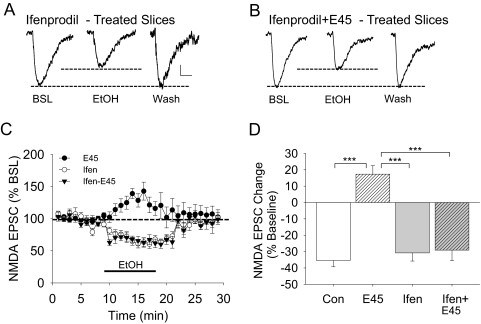
Reduced Ethanol Inhibition of NMDAR Currents Correlates with NR2B Inhibition.
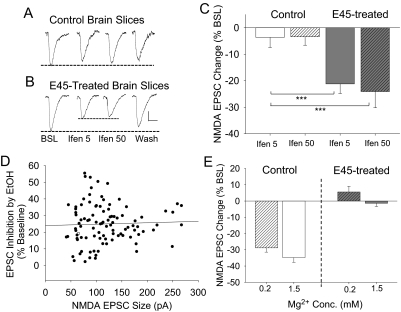
The NMDA NR2B subunit is known to be expressed in adult hippocampal neurons (Goebel-Goody et al., 2009). To determine the involvement of NR2B-containing NMDA receptors in these evoked synaptic NMDA EPSCs, we tested ifenprodil, a potent NR2B-selective antagonist, to inhibit NMDAR currents. The extent of inhibition by ifenprodil was used to estimate the contribution of active NR2B subunits in the evoked synaptic NMDA EPSC responses. In control brain slices, ifenprodil (5 or 50 μM) did not significantly inhibit NMDA EPSCs, but in 45 mM ethanol-treated brain slices, both 5 and 50 μM ifenprodil inhibited the NMDA EPSC amplitude by approximately 25% (Fig. 2). To determine whether the potency of ethanol inhibition of NMDA EPSCs is influenced by the size of the NMDA EPSCs, we tested this possibility by examining the correlation between NMDA EPSC amplitude and the percentage inhibition by short-term 80 mM ethanol application from 101 pyramidal neuronal recordings. The result showed no correlation between these parameters (correlation coefficient, 0.0681, p > 0.5, Pearson product moment correlation) (Fig. 2). In addition, these data showed that the overall baseline NMDA EPSC amplitude from control neurons was 121.5 ± 6.33 pA (n = 101), and that the neurons incubated in 45 mM ethanol was 119.9 ± 14.33 pA (n = 18; t = 0.092, p > 0.9). We also determined whether the NMDA EPSC amplitude was correlated with the resting membrane potential of each recorded neuron; the results indicated that there was no correlation between these two parameters (correlation coefficient, 0.165, p > 0.09, Pearson product moment correlation). Because α-amino-3-hydroxy-5-methyl-4-isoxzole propionate receptors are blocked for these determinations, it is possible that Mg2+ blockade of the NMDA receptors could be altered as a result of prolonged ethanol exposure. Thus, we also determined whether 45 mM ethanol exposure of brain slices could enhance NMDA EPSCs under reduced extracellular Mg2+ concentration. When we measured ethanol inhibition of NMDAR currents in low (0.2 mM) and normal (1.5 mM) Mg2+, short-term ethanol (80 mM) application inhibited NMDAR currents by 34.8 ± 2.9% in normal aCSF and 28.8 ± 2.6% in low-Mg2+ aCSF (t = 1.481, p > 0.16). In 45 mM ethanol-treated brain slices, short-term 80 mM ethanol did not significantly inhibit NMDAR currents in low Mg2+ aCSF (+5.6 ± 3.5%) and in normal Mg2+ aCSF (−1.4 ± 1.9%) [t = 1.830, p > 0.08] (Fig. 2E).
Fig. 2.
The adaptive change in functional NMDAR evoked responses to the effects of 45 mm ethanol treatment is accompanied by ifenprodil inhibition of NMDA EPSCs. Representative traces of NMDA EPSC amplitude during baseline (BSL), a 10-min application of ifenprodil (5 μM, Ifen 5; or 50 μM, Ifen 50), and washout of ifenprodil (Wash) from control (A) and 45 mm ethanol-treated (B) brain slices. The dashed lines indicated the relative sizes of the NMDA EPSC amplitude. C, the composite data show that ifenprodil only alters the cells in slices exposed to the 45 mm ethanol treatment. D, a scatterplot was used to determine whether there is a correlation between the NMDAR current inhibition by 80 mM short-term ethanol and the NMDA EPSC size (n = 101). The line indicates the linear regression of this plot (r2 = 0.00464, p > 0.49). The overall NMDA current amplitude was 121.5 ± 6.33 pA, and the ethanol caused an inhibition of 25.0 ± 1.17%. E, the effect of 80 mM ethanol on NMDA EPSCs recorded under low (0.2 mM Mg2+) or normal (1.5 mM Mg2+) conditions in neurons from control or 45 mM ethanol-treated brain slices. Scale bars indicate 25 pA and 50 ms. ***, p < 0.001.
NMDAR Resistance to the Inhibitory Effects of Ethanol Is Associated with STEP Inactivation.
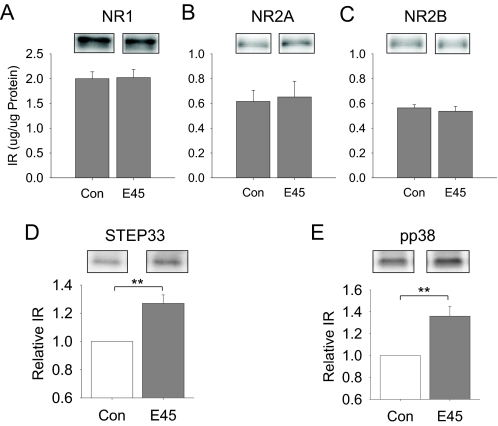
To determine whether the NMDA receptor subunit expression was increased during the 3-h ex vivo ethanol exposure, we used Western blot methods to measure protein levels of NR1, NR2A, and NR2B in CA1 mini-slices (Coultrap et al., 2005) from 3-h control or 45 mM ethanol-exposed hippocampal slices. These data showed that the levels of these subunit proteins in the CA1 region of the hippocampus did not differ significantly between control and 45 mM ethanol-exposed slices (Fig. 3, A–C). We found previously that ethanol inhibition of NMDAR activity was attenuated by inhibition of STEP activity (Alvestad et al., 2003; Hicklin et al., 2011). In addition, STEP61 was shown to be degraded to the inactive form of STEP33 (Xu et al., 2009). Therefore, we measured the levels of STEP33 after the 45 mM ethanol treatment. We also analyzed for changes in the levels of pp38 MAPK, because this protein kinase has been shown to be regulated by STEP61 (Xu et al., 2009). The data showed that there were significant increases in the levels of STEP33 (p < 0.05, Mann-Whitney rank sum test; Fig. 3D) and pp38 MAPK (p < 0.05, Mann-Whitney rank sum test; Fig. 3E) in the CA1 mini slices that were exposed to 45 mM ethanol ex vivo for 3 h compared with slices in control aCSF. These data suggest that there is an inactivation of STEP61 (to STEP33) and an activation of p38 MAPK, which are involved in the adaptive resistance of the NMDAR to the inhibitory effects of short-term ethanol application.
Fig. 3.
The adaptive change of the NMDAR response to the effects of ethanol is associated with increases in the levels of STEP33 and pp38 MAPK. A, the expression of NR1 subunit is not different between control (Con) and 45 mm ethanol-exposed (E45) CA1 mini slices. B, the expression of the NR2A subunit also is not altered by the 45 mm ethanol-exposure (E45) in CA1 mini slices compared with control (Con) slices. C, likewise, the expression of the NR2B subunit is not changed by 45 mm ethanol exposure (E45). These data are the average results of eight animals (n = 8). D, however, the level of STEP33 is increased in the 45 mm ethanol-treated (E45) hippocampal slices (n = 6) compared with those from control brain slices (n = 6). E, in addition, the level of pp38 also is significantly increased in 45 mM ethanol-exposed hippocampal slices (n = 6) compared with control-treated values (n = 6). **, p < 0.01.
bpV(phen) Attenuates Ethanol Inhibition of NMDA EPSCs.
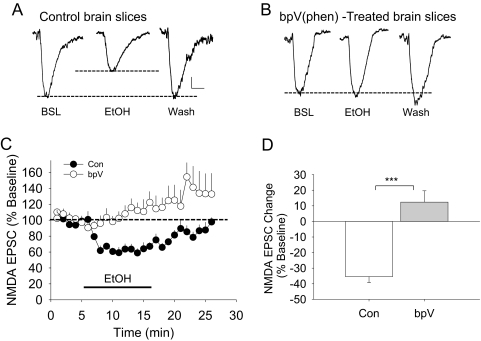
We next examined whether inhibition of protein tyrosine phosphatases, including STEP61, would mimic the adaptive resistance of NMDARs to the ethanol inhibition after prolonged ethanol treatment. Brain slices were exposed to 10 μM bpV(phen), a nonselective protein tyrosine phosphatase inhibitor, for 30 min before whole-cell recording of the NMDA EPSCs. Although short-term 80 mM ethanol application (10 min) produced a 32.3 ± 1.87% inhibition of synaptic NMDA EPSCs from control brain slices, this concentration of ethanol now enhanced synaptic NMDA EPSCs from bpV(phen)-treated brain slices (13.7 ± 7.70%). The effect of ethanol on NMDA EPSCs in bpV(phen)-treated neurons was significantly different from that of control neurons (t = 5.808, p < 0.001, Student's t test) (Fig. 4), so these results also indicate that inhibition of protein tyrosine phosphatase activity is involved in the adaptive resistance of NMDARs during prolonged exposure to ethanol in these neurons. However, in bpV(phen)-treated slices, short-term ethanol application enhanced NMDA EPSCs that persisted into the washout period, a finding similar to what we found in STEP KO mice (Hicklin et al., 2011) and those of Yaka et al. (2003) and Wang et al. (2007) during ethanol washout. To assess whether bpV(phen) treatment alone can increase the baseline NMDA EPSC amplitude, we measured the baseline NMDA current amplitude before (121.5 ± 6.33 pA) and after (121.6 ± 14.78 pA) (t = 0.008, p > 0.9) bpV(phen) exposure. These data indicated that bpV(phen) treatment alone did not significantly modify the basal NMDA current amplitudes.
Fig. 4.
Inhibition of protein tyrosine phosphatases can block ethanol inhibition of the NMDAR currents. Brain slices were treated with 10 μM bpV(phen) for 3 h and were tested for the effects of a short-term 80 mM ethanol challenge. Representative traces show that ethanol (80 mM, EtOH) inhibits NMDA EPSCs in control brain slices (A) compared with bpV(phen)-treated brain slices (B). Dotted lines represent baseline and the extent of ethanol inhibition. BSL, baseline; Wash, washout. C, time course of the average responses for synaptic NMDA EPSC amplitudes from control (Con) (n = 8) or bpV-treated brain slice preparations (n = 8). D, bar graphs show the averaged effects of 80 mM ethanol on NMDA EPSCs from control (Con, − 35.4 ± 3.9%) or from bpV(phen)-treated (12.4 ± 7.3%) slices. There is a significant difference between these two groups (t = 5.808, p < 0.001, Student's t test). Scale bars, 20 pA and 25 ms. ***, p < 0.001.
Prolonged Ifenprodil Treatment Blocks the Development of the NMDAR Resistance to Ethanol Inhibition.
We found that although ifenprodil (5 μM) had no effect on NMDA EPSCs from control slices, it inhibited NMDAR currents by approximately 25% in neurons from brain slices exposed to 45 mM ethanol for 3 h (Fig. 2). Therefore, we wanted to test whether NR2B subunit activity is necessary for the development of the adaptive resistance of NMDARs during the 3 h of ethanol exposure. For this study, brain slices were preincubated with or without ifenprodil (5 μM) for 30 min before and continuing throughout the 45 mM ethanol treatment. Incubation of brain slices with ifenprodil (5 μM) alone showed no change in the effect of 80 mM ethanol (an inhibition of NMDA EPSCs by 29.8 ± 4.7%) that was no different from control data (p > 0.4; Fig. 5). However, treatment with ifenprodil and 45 mM ethanol for 3 h significantly blocked the development of the adaptive resistance of NMDARs to the inhibitory effects of the short-term ethanol challenge (80 mM), resulting in a 27.3 ± 4.2% inhibition compared with a 17.3 ± 5.2% enhancement of ethanol-exposed slices without ifenprodil [F(3,28) = 28.765, p < 0.001; one-way ANOVA] (Fig. 5). These data indicate that NR2B subunit function is required for the adaptive resistance of NMDARs during the prolonged ethanol treatment and tested by the short-term ethanol challenge.
Fig. 5.
Ifenprodil blocks the adaptive change of the NMDAR response to the effects of 80 mM ethanol. Brain slices were pretreated with ifenprodil (5 μM, Ifen) for 30 min and then continued in the presence (Ifen + E45) or the absence (Ifen) of 45 mM ethanol for 3 h. Representative traces show NMDA EPSC responses during baseline (BSL), 80 mM ethanol application (EtOH), and during washout (Wash) from ifenprodil-treated brain slices only (A) or in ifenprodil + 45 mM ethanol-treated brain slices (B). Dotted lines show the baseline and the extent of ethanol inhibition. C, the time course of the NMDA EPSC responses during the experiment. The duration of the 80 mM ethanol application is shown by the solid line. D, plots of the average response change from control (Con), 45 mM ethanol (E45, 17.3 ± 5.2%), ifenprodil-treated (Ifen, −27.3 ± 4.2%, n = 10), and ifenprodil + E45-treated (Ifen + E45, −29.8 ± 4.7%, n = 7) slices. Scale bars in A and B, 25 pA and 25 ms. ***, p < 0.001.
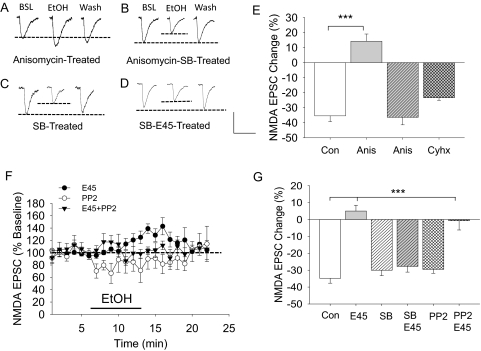
Activation of p38 MAPK Leads to Diminished Ethanol Inhibition of NMDA EPSCs.
In 45 mM ethanol-treated brain slices, there was an increased level of phospho-p38 MAPK (Fig. 3), suggesting an activation of this kinase. We tested whether a direct activation of p38 MAPK could produce similar adaptive changes of the NMDAR to the inhibitory effects of short-term ethanol application in brain slices after the 3-h 45 mM ethanol exposure. Brain slices were exposed to 20 μM anisomycin, a p38 MAPK activator, for 3 h, and then we performed whole-cell recordings to measure the effect of an 80 mM ethanol challenge on synaptic NMDA EPSCs. The results showed that anisomycin treatment alone significantly increased the basal NMDA current amplitude (154.4 ± 11.59 pA, t = 2.134, p < 0.05, compared with control) and produced an ethanol-induced enhancement of NMDA EPSCs (14.3 ± 4.80% increase, p < 0.001, Tukey test) (Fig. 6A), a result similar to that of the 3-h, 45 mM ethanol exposure. To test whether the activation of p38 MAPK was selectively involved, we added SB202190, a potent p38 MAPK inhibitor, to determine whether it blocked the anisomycin-induced acute ethanol enhancement of the NMDAR current responses. Indeed, the SB202190 (0.5 μM) pretreatment completely blocked the anisomycin-induced enhancement of NMDA EPSCs by short-term ethanol application (36.4 ± 5.0% inhibition, p < 0.001, Tukey test) (Fig. 6B). In addition to being a protein synthesis inhibitor, anisomycin also acts as a p38 MARK activator (Shifrin and Anderson, 1999). Therefore, we wanted to determine whether nonselective inhibition of protein synthesis may have a significant effect on the development of adaptive resistance of NMDARs to the inhibitory effects of short-term ethanol application. To separate the effect of anisomycin on p38 MAPK activation from protein synthesis inhibition, we tested the effects of pretreatment with cycloheximide (60 μM), a protein synthesis inhibitor with only weak stimulation of p38 MAPK (Iordanov et al., 1997), on the development of NMDAR resistance to the effects of ethanol. Cycloheximide enhanced the basal NMDA current amplitude (152.1 ± 9.51 pA, t = 2.187, p < 0.05 compared with that of control neurons), but it had no direct effect on the 80 mM ethanol inhibition of NMDA EPSCs, because these responses were similar to those from control slices: control, 34.3 ± 4.1% inhibition, and cycloheximide-treated, 28.4 ± 1.7% inhibition [t = 1.906, p > 0.08] (Fig. 6). Therefore, inhibition of protein synthesis cannot account for the anisomycin-induced ethanol enhancement of NMDA EPSCs. If the activation of p38 MAPK is involved in adaptive changes of the NMDA receptors, the inhibition of p38 MAPK should block the development of adaptation of NMDA receptors by 45 mM ethanol incubation. Our results show that the NMDA current amplitude was not affected by SB202190 treatment alone (148.1 ± 10.19 pA, t = 1.706, p > 0.05 compared with control), but SB202190 blocked the development of NMDA receptor adaptation (p < 0.001, post hoc Tukey analysis) (Fig. 6). To determine the involvement of the Src family of kinases (SFKs) in the adaptive changes of the NMDA receptor responses by the 3-h 45 mM ethanol exposure, we pretreated the brain slices with PP2, an SFK inhibitor, before 45 mM ethanol exposure. The results show that although PP2 did not alter the inhibition by ethanol application on NMDA EPSCs in control slices, it also did not block the adaptive resistance of NMDA EPSCs to the 3-h 45 mM ethanol-treated brain slices (Fig. 6). Although PP2 during the 45 mM ethanol treatment did not block the development of resistance of NMDAR currents to the 80 mM ethanol inhibition, it did block the late enhancement of NMDAR currents. These data indicate that SFKs are involved in late ethanol-induced increases in NMDAR currents. p38 MAPK, on the other hand, prevented the development of adaptive changes of the NMDA receptor.
Fig. 6.
Activation of p38 MAPK results in the development of resistance to the inhibition of the NMDAR response to short-term ethanol application. Brain slices were incubated with anisomycin (Anis; 20 μM), anisomycin (20 μM) + SB202190 (0.5 μM; SB + Anis), cycloheximide (Cyhx; 60 μM), SB202190 alone (SB), or SB202190 + E45 (SB+E45). The effects of an 80 mM ethanol challenge on NMDA EPSCs were measured. Representative traces show NMDA EPSC responses during baseline (BSL), 80 mM ethanol challenge (EtOH), or during washout (Wash) from anisomycin-treated brain slices (A), from anisomycin + SB-treated brain slices (B), from SB-treated brain slices (C), and from a 45 mM ethanol + SB-treated neuron (D). Dotted lines indicate the baseline and the extent of 80 mM ethanol inhibition. E, the average values show the effects of an 80 mM ethanol challenge on NMDA EPSCs in cells from the control (Con, −34.3 ± 4.1%, n = 9), anisomycin-treated (Anis, 14.3 ± 4.8%, n = 5), anisomycin + SB-treated (SB + Anis, −36.4 ± 5.0%, n = 6) brain slices, and from a subgroup of slices treated with cycloheximide (Cyhx, −23.4 ± 1.7%, n = 5). There are significant statistical differences among groups [F(3,21) = 28.087, p < 0.001, one-way ANOVA]. Post hoc Holm-Sidak analysis shows that the cycloheximide treatment does not differ significantly from control slices. F, time course of the NMDAR current responses during baseline, 80 mM ethanol application, and washout periods from brain slices treated with 45 mM ethanol (E45), PP2 (2 μM), or PP2 plus 45 mM ethanol (E45 + PP2) for 3 h. G, mean values for the effects of acute ethanol (80 mM) on NMDA currents recorded from control neurons (Con, −34.8 ± 2.9%, n = 8), E45-treated neurons (E45, 5.0 ± 3.4%, n = 18), SB202190-treated neurons (SB, −30.0 ± 3.2%, n = 12), neurons treated with SB and 45 mM ethanol (SB + E45, −27.8 ± 3.4%, n = 8), PP2-treated neurons (−28.8 ± 5.1%, n = 7), and PP2 + E45 treated neurons (−0.7 ± 5.5%, n = 6). Scale bar, 100 pA and 150 ms. ***, p < 0.001.
Discussion
This study, using the ex vivo brain slice model for a 3-h 45 mM ethanol exposure, provides new evidence for the molecular mechanisms regulating the functional adaptive changes of the NMDAR activity due to ethanol treatment. Previous studies (Grover et al., 1994; Miyakawa et al., 1997) showed that electrophysiological NMDA responses became partially resistant (tolerant) to the effects of 100 mM ethanol during 5 to 15 min of ethanol application in hippocampal brain slices. We show here that, in whole-cell recordings from CA1 pyramidal neurons, NMDARs also become resistant to inhibitory effects of short-term ethanol (80 mM) after 45 mM ethanol exposure ex vivo for 3 h. With this model system, we demonstrated that NMDA EPSCs become partially resistant to the inhibitory effects of ethanol within 1 h and reached a maximum by 3 h during the 45 mM ethanol exposure (Fig. 1). This resistance was not caused by an overall NMDAR desensitization or by an up-regulation of NMDA receptors in these CA1 hippocampal pyramidal neurons; the adaptive changes that occurred may be a result of subunit phosphorylation changes, as shown by STEP inactivation, p38 MAPK, and NR2B subunit activation.
The individual data points for the ethanol inhibition of NMDA EPSCs in the pyramidal neurons contain substantial scatter, and thus, we wanted to test whether the percentage inhibition correlates with the neuronal properties of each individual cell, including the size of the NMDAR current and/or the resting membrane potential. These results show that the magnitude of the ethanol inhibition was not significantly influenced by the size of the NMDAR current or the resting membrane potential. Therefore, our data are consistent with an earlier report showing that ethanol inhibition of NMDAR currents was noncompetitive (Peoples et al., 1997).
Previous studies (Alvestad et al., 2003; Wu et al., 2010; Hicklin et al., 2011) showed that ethanol inhibition of NMDAR currents required STEP, because in the absence of STEP activity, ethanol not only failed to inhibit NMDAR currents, but it produced a late enhancement of the NMDAR current. This late enhancement was found to be mediated by SFKs, because it can be blocked by PP2, an SFK inhibitor (Yaka et al., 2003; Wang et al., 2007; Hicklin et al., 2011). These observations prompted the hypothesis that ethanol inhibition of NMDAR currents may be mediated both by ethanol-induced inhibitory and facilitatory processes, and the net effect of short-term ethanol application on these currents depends on the balance of these processes. However, we did not observe that PP2 reversed or enhanced the inhibitory effect of 80 mM ethanol, suggesting that if there is an ethanol-induced facilitation, it is a minor component under these control conditions. Furthermore, if the adaptive resistance of NMDAR currents to the ethanol inhibition is due to a selective increase of ethanol-induced facilitation processes, then PP2, which blocks the ethanol-induced facilitation, should be able to reverse the ethanol-induced late-phase enhancement of the NMDAR currents. Instead, PP2 and 45 mM ethanol treatment of the brain slices resulted in only blocking the facilitation of NMDAR currents by short-term ethanol application (Fig. 6). Therefore, the adaptive resistance of NMDAR currents to short-term ethanol application, after prolonged ethanol treatment, is not likely to be due to a selective increase in the ethanol-induced facilitation. In addition, the late ethanol-induced facilitation was reversible during a 15- to 20-min washout of ethanol, but short-term ethanol exposure still failed to inhibit NMDAR currents after an 80-min washout of ethanol after the 45 mM ethanol treatment (Fig. 1). Thus, it is unlikely that adaptive resistance of NMDAR currents is due to increased ethanol-induced facilitation, which masks the ethanol-induced inhibition.
We also tested the hypothesis that the adaptive changes of NMDAR current responses to the ethanol inhibition was due to alteration of the Mg2+ blockade after a 3-h 45 mM ethanol exposure. The data show that acute ethanol inhibition of NMDAR currents did not significantly differ in low Mg2+ and control Mg2+ recording conditions, consistent with results reported by Peoples et al. (1997). Therefore, altered Mg2+ blockade of NMDAR currents is not likely to explain the adaptive changes of NMDAR currents by 45 mM ethanol exposure.
Because ethanol failed to inhibit NMDAR currents when STEP was impaired (Alvestad et al., 2003; Hicklin et al., 2011), additional experiments were conducted to further our understanding of the underlying molecular mechanisms of a 3-h ethanol exposure. We found that increased levels of STEP33 and pp38 MAPK were correlated with the failure of short-term ethanol application to inhibit NMDAR currents (Wu et al., 2010). Here we find that adaptive resistance of NMDAR currents to ethanol inhibition correlated with the impairment of STEP-mediated mechanisms, because inhibition of STEP activity with bpV(phen) prevented the adaptation of the NMDAR currents to the inhibitory effects of short-term ethanol. STEP has been shown to regulate endocytosis of NMDA receptors by dephosphorylation of the phospho-NR2B Tyr1472 site (Paul et al., 2007), and short-term ethanol exposure enhanced dephosphorylation of Tyr1472 pNR2B (Wu et al., 2010; Hicklin et al., 2011). We reasoned that the adaptive resistance of NMDAR currents to the ethanol inhibition probably involves NR2B subunit activity. Our results show that although a 3-h 45 mM ethanol exposure did not alter the total NR2B protein expression, this treatment resulted in a 25% increase in ifenprodil inhibition of the NMDAR currents, suggesting an increased NR2B functional contribution to NMDAR currents by activation of NR2B subunit proteins. It is unlikely that an increase in NR2B function directly altered the ethanol sensitivity of these NMDAR currents, because ifenprodil did not alter the ethanol inhibition of recombinant NR2A or NR2B subunit-containing NMDAR currents (Lovinger, 1995). Moreover, pretreatment of brain slices with ifenprodil could block the development of adaptive resistance of the NMDAR currents to ethanol, suggesting that the functional activity of the NR2B subunit is involved in the adaptive resistance. However, ifenprodil does not directly alter ethanol sensitivity of the NMDAR currents. In another study, our preliminary data indicate that changes in NR2B-mediated synaptic Ca2+ concentration and Ca2+/calmodulin-dependent protein kinases II activity may be necessary for the adaptive resistance.
After the 3-h 45 mM ethanol exposure, there was a 30 to 40% increase in the level of phospho-p38 MAPK, indicating an increase in p38 MAPK activity (Xu et al., 2009). If activation of p38 MAPK is involved in the adaptive resistance of NMDAR currents, then the p38 MAPK activator anisomycin also should be able to produce resistance of NMDAR currents to the inhibitory effects of short-term ethanol application. Our results show that anisomycin treatment did indeed result in the development of resistance of the NMDAR currents to the ethanol inhibition, and this effect of anisomycin was effectively blocked by pretreatment of brain slices with the p38 MAPK inhibitor, SB202190. Finally, we also showed that pretreatment of brain slices with SB202190 could block the development of adaptive resistance of NMDAR currents by the 3-h 45 mM ethanol treatment. Xu et al. (2009) reported that the increase in the levels of STEP33 and pp38 MAPK are related to the excitotoxicity of these neurons via activation of extrasynaptic NMDARs. Our data show that adaptive resistance of NMDAR currents can be mimicked by STEP inhibition and p38 MAPK activation, suggesting that adaptive resistance of NMDAR currents could be associated with ethanol-induced excitotoxicity. Future studies will be focused on delineating the mechanisms of ethanol exposure-induced neuronal excitotoxicity.
Studies in humans have shown that people with low initial sensitivity (high resistance) to ethanol effects on cognition are also at higher risk for becoming alcohol-dependent (Schuckit and Smith, 2001). However, Newlin and Renton (2010) showed that people who develop greater ethanol tolerance (greater adaptive changes to the effects of ethanol) also have a greater risk for alcohol-dependence. Our findings that STEP61 and p38 MAPK activities can modulate ethanol inhibitory sensitivity of the NMDAR activity indicates that these proteins are important modulators for short- and long-term actions of ethanol in the brain. Moreover, this work suggests that NR2B subunit antagonists are likely to be effective in regulating the acquisition of functional tolerance to the inhibitory effects of ethanol.
Acknowledgments
We thank Dr. Richard Deitrich for comments on the manuscript.
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grants AA015086, AA018328] and VA Merit Review grants.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.068643.
- NMDAR
- N-methyl-d-aspartate receptor
- aCSF
- artificial cerebral spinal fluid
- CGP-52432
- 3[[[(3,4-dichlorophenyl)methyl]amino]propyl](diethoxymethyl) phosphinic acid
- d-APV
- d-amino-phosphonovaleric acid
- PP2
- 3-(4-chlorophenyl)1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine
- EPSC
- excitatory postsynaptic current
- NMDA
- N-methyl-d-aspartate
- ANOVA
- analysis of variance
- pp38
- phospho-p38
- SFK
- Src family kinase
- MAPK
- mitogen-activated protein kinase
- STEP
- striatal enriched protein tyrosine phosphatase
- SB202190
- 4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)-1H-imidazole.
Authorship Contributions
Participated in research design: Wu and Proctor.
Conducted experiments: Wu and Coultrap.
Contributed new reagents or analytic tools: Browning and Proctor.
Performed data analysis: Wu and Coultrap.
Wrote or contributed to the writing of the manuscript: Wu, Couptrap, Browning, and Proctor.
References
- Alvestad RM, Grosshans DR, Coultrap SJ, Nakazawa T, Yamamoto T, Browning MD. (2003) Tyrosine dephosphorylation and ethanol inhibition of N-methyl-d-aspartate receptor function. J Biol Chem 278:11020–11025 [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Lombroso PJ, Raghunathan A, During MJ, Wahle P, Naegele JR. (1995) Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci 15:1532–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite SP, Paul S, Nairn AC, Lombroso PJ. (2006) Synaptic plasticity: one STEP at a time. Trends Neurosci 29:452–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp P, Gibson ES, Dell'acqua ML, Hoffman PL. (2010) Phosphorylation regulates removal of synaptic N-methyl-d-aspartate receptors after withdrawal from chronic ethanol exposure. J Pharmacol Exp Ther 332:720–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ, Nixon KM, Alvestad RM, Valenzuela CF, Browning MD. (2005) Differential expression of NMDA receptor subunits and splice variants among the CA1, CA3 and dentate gyrus of the adult rat. Mol Brain Res 135:104–111 [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. (2009) Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience 158:1446–1459 [DOI] [PubMed] [Google Scholar]
- Grover CA, Frye GD, Griffith WH. (1994) Acute tolerance to ethanol inhibition of NMDA-mediated EPSPs in the CA1 region of the rat hippocampus. Brain Res 642:70–76 [DOI] [PubMed] [Google Scholar]
- Heynen AJ, Quinlan EM, Bae DC, Bear MF. (2000) Bidirectional, activity-dependent regulation of glutamate receptors in the adult hippocampus in vivo. Neuron 28:527–536 [DOI] [PubMed] [Google Scholar]
- Hicklin TR, Wu PH, Radcliffe RA, Freund RK, Goebel-Goody SM, Correa PR, Proctor WR, Lombroso PJ, Browning MD. (2011) Alcohol inhibition of the NMDA receptor function, long-term potentiation, and fear learning requires striatal-enriched protein tyrosine phosphatase. Proc Natl Acad Sci USA 108:6650–6655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanov M, Bender K, Ade T, Schmid W, Sachsenmaier C, Engel K, Gaestel M, Rahmsdorf HJ, Herrlich P. (1997) CREB is activated by UVC through a p38/HOG-1-dependent protein kinase. EMBO J 16:1009–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läck AK, Diaz MR, Chappell A, DuBois DW, McCool BA. (2007) Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol 98:3185–3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. (1995) Developmental decrease in ethanol inhibition of N-methyl-d-aspartate receptors in rat neocortical neurons: relation to the actions of ifenprodil. J Pharmacol Exp Ther 274:164–172 [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. (1989) Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243:1721–1724 [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tsuboi K, Niki H. (1997) Fyn-kinase as a determinant of ethanol sensitivity: relation to NMDA-receptor function. Science 278:698–701 [DOI] [PubMed] [Google Scholar]
- Newlin DB, Renton RM. (2010) High risk groups often have higher levels of alcohol response than low risk: the other side of the coin. Alcohol Clin Exp Res 34:199–202; author reply 203–205 [DOI] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. (2003) NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci 6:34–42 [DOI] [PubMed] [Google Scholar]
- Paul S, Olausson P, Venkitaramani DV, Ruchkina I, Moran TD, Tronson N, Mills E, Hakim S, Salter MW, Taylor JR, et al. (2007) The striatal-enriched protein tyrosine phosphatase gates long-term potentiation and fear memory in the lateral amygdala. Biol Psychiatry 61:1049–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Askalan R, Paul S, Kalia LV, Nguyen TH, Pitcher GM, Salter MW, Lombroso PJ. (2002) Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron 34:127–138 [DOI] [PubMed] [Google Scholar]
- Peoples RW, White G, Lovinger DM, Weight FF. (1997) Ethanol inhibition of N-methyl-d-aspartate-activated current in mouse hippocampal neurones: whole-cell patch-clamp analysis. Br J Pharmacol 122:1035–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor WR, Diao L, Freund RK, Browning MD, Wu PH. (2006) Synaptic GABAergic and glutamatergic mechanisms underlying alcohol sensitivity in mouse hippocampal neurons. J Physiol 575:145–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. (2004) Acute and chronic ethanol alter glutamatergic transmission in rat central amygdala: an in vitro and in vivo analysis. J Neurosci 24:1594–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. (2004) Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci 5:317–328 [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. (2001) The clinical course of alcohol dependence associated with a low level of response to alcohol. Addiction 96:903–910 [DOI] [PubMed] [Google Scholar]
- Shifrin VI, Anderson P. (1999) Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis. J Biol Chem 274:13985–13992 [DOI] [PubMed] [Google Scholar]
- Smolen TN, Smolen A. (1989) Blood and brain ethanol concentrations during absorption and distribution in long-sleep and short-sleep mice. Alcohol 6:33–38 [DOI] [PubMed] [Google Scholar]
- Snell LD, Nunley KR, Lickteig RL, Browning MD, Tabakoff B, Hoffman PL. (1996) Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Mol Brain Res 40:71–78 [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, et al. (2005) Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci 8:1051–1058 [DOI] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. (2007) Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci 27:3593–3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Yu XM, Salter MW. (1996) Ca2+-independent reduction of N-methyl-d-aspartate channel activity by protein tyrosine phosphatase. Proc Natl Acad Sci USA 93:1721–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PH, Coultrap S, Browning MD, Proctor WR. (2010) Correlated changes in NMDA receptor phosphorylation, functional activity, and sedation by chronic ethanol consumption. J Neurochem 115:1112–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. (2009) Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci 29:9330–9343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Phamluong K, Ron D. (2003) Scaffolding of Fyn kinase to the NMDA receptor determines brain region sensitivity to ethanol. J Neurosci 23:3623–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]