Abstract
Excessive synthesis of reactive oxygen species contributes to the pathology of many human diseases and originates from changes in the expression and posttranslational regulation of the transmembrane NADPH oxidases (Noxes). Nox5 is a novel Nox isoform whose activity is regulated by intracellular calcium levels. We have reported that the activity and calcium-sensitivity of Nox5 can also be modulated by direct phosphorylation. However, the kinases that phosphorylate Nox5 have not been identified, and thus, the goal of this study was to determine whether calcium-activated kinases such as calcium/calmodulin-dependent kinase II (CAMKII) are involved. We found that Nox5 activity in bovine aortic endothelial cells was suppressed by two doses of the CAMKII inhibitor 2-(N-[2-hydroxyethyl])-N-(4-methoxybenzenesulfonyl)amino-N-(4-chlorocinnamyl)-N-methylamine (KN-93). In cotransfected COS-7 cells, wild-type and constitutively active CAMKII, but not a dominant-negative, robustly increased basal Nox5 activity. The ability of CAMKII to increase Nox5 activity was also observed with fixed calcium concentrations in an isolated enzyme activity assay. CAMKII did not elevate intracellular calcium or activate other Nox enzymes. In vitro phosphorylation assays revealed that CAMKII can directly phosphorylate Nox5 on Thr494 and Ser498 as detected by phosphorylation state-specific antibodies. Mass spectrometry (MS) analysis revealed the phosphorylation of additional, novel sites at Ser475, Ser502, and Ser675. Of these phosphorylation sites, mutation of only Ser475 to alanine prevented CAMKII-induced increases in Nox5 activity. The ability of CAMKIIα to phosphorylate Ser475 in intact cells was supported by the binding of Nox5 to phosphoprotein-affinity columns and via MS/MS analysis. Together, these results suggest that CAMKII can positively regulate Nox5 activity via the phosphorylation of Ser475.
Introduction
NADPH oxidases (Noxes) are major sources of reactive oxygen species (ROS) including superoxide (O2⨪) and hydrogen peroxide (H2O2) in mammalian cells (Beckman and Ames, 1998; Lambeth, 2004). Because of their inherent reactivity, ROS production is tightly regulated to control the appropriate amount at the right time and place. In response to physiological stimuli, ROS are important contributors to cellular processes such as host defense, cell signaling, smooth muscle contraction, differentiation, and the formation of otoconia (Lambeth, 2007; Nakano et al., 2008). In contrast, the excessive or inappropriate production of ROS has been shown to underlie many disease processes such as asthma, cancer, atherosclerosis, hypertension, and diabetes (Lambeth, 2007). Strategies to suppress ROS using antioxidants have proven largely ineffective in the treatment of cancer and heart disease. The selective blockade of pathological or excessive ROS production, without targeting all ROS, may be a more effective approach. To achieve this, a better understanding of the mechanisms controlling Nox activity is necessary.
Nox enzymes can be functionally divided into an N-terminal transmembrane domain that spans the membrane six times and supports two heme residues and a C-terminal reductase domain that binds FAD and NADPH. The Nox family of enzymes is composed of seven members, designated Nox1 to 5 and Duox1 and 2 that are found in both phagocytic and nonphagocytic cells (Bedard and Krause, 2007). The mechanisms governing the activity of these isoforms is incompletely understood and an area of intensive investigation (Bedard and Krause, 2007). Activation of Nox1 to 3 requires the translocation of a number of cytosolic subunits. In contrast, Nox4 and Nox5 do not require cytosolic subunits to synthesize ROS, and Nox4 has been shown to constitutively produce H2O2 instead of O2⨪ (Martyn et al., 2006).
Nox5 was the last Nox isoform to be identified. Although originally described in testes and lymph nodes, it is also expressed in vascular tissue, in which it promotes the proliferation of vascular smooth muscle cells (Jay et al., 2008; Montezano et al., 2010). The expression and activity of Nox5 are dramatically elevated in coronary arteries with advanced lesions, which suggests that Nox5 may contribute to vascular disease (Guzik et al., 2008). Nox5 contains a unique N terminus that is characterized by four EF-hands that bind calcium and regulate its activity (Fulton, 2009). In addition, the activity of Nox5 can be also regulated by direct phosphorylation in response to PMA (Jagnandan et al., 2007; Serrander et al., 2007). Protein kinase C is believed to mediate the phosphorylation of Nox5 on Thr494 and Ser498, but this is based on the use of pharmacological inhibitors, and the isoforms involved remain to be identified (Jagnandan et al., 2005). Calmodulin has also been shown to activate Nox5 at lower levels of intracellular calcium (Tirone and Cox, 2007). However, the role of other kinase(s) in the phosphorylation and activation of Nox5, particularly those sensitive to calcium-calmodulin, are poorly understood.
Elevation of intracellular calcium influences a multitude of cellular functions (Berridge et al., 2003). Some of these effects are mediated by multifunctional calcium/calmodulin dependent protein kinases (CAMKs), a family of serine/threonine kinases that includes CAMKII (Soderling, 1999). CAMKII is activated by cytosolic calcium, which promotes calmodulin binding and autophosphorylation of Thr286 (Griffith, 2004). A number of studies have identified close relationships between CAMKII signaling, ROS production, and vascular function. Elevated ROS can render CAMKII constitutively active via oxidation of methionines 281/282 (Erickson et al., 2008). In human macrophages, zymosan-induced ROS production, predominantly generated by Nox2, is dependent on CAMKII signaling pathways (Kelly et al., 2010). CAMKII has also been shown to mediate redox-sensitive gene regulation in vascular endothelium (Cai et al., 2001). Interestingly, H2O2 has been shown to positively regulate Nox5 activity via a Ca2+-mediated, redox-dependent signaling pathway (El Jamali et al., 2008).
The ability of calcium-dependent kinases to regulate Nox5 activity is not yet known. A previous study reported that CAMKII inhibitors do not alter Nox5 activity in response to PMA (Serrander et al., 2007). However, the inhibitor used was an inactive structural analog 2-[N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine, phosphate (KN-92) and PMA does not elevate intracellular calcium. Therefore, whether an active CAMKII inhibitor 2-(N-[2-hydroxyethyl])-N-(4-methoxybenzenesulfonyl)amino-N-(4-chlorocinnamyl)-N-methylamine (KN-93) can influence Nox5 activity or whether CAMKII is important after calcium-mobilization remain unanswered questions. Therefore, the goals of this study were to investigate whether CAMKII can influence the phosphorylation and activity of Nox5.
Materials and Methods
Cell Culture and Transfection
COS-7 cells and bovine aortic endothelial cells (BAECs) were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) containing l-glutamine, penicillin, streptomycin, and 10% (v/v) fetal bovine serum. Cells were transfected using Lipofectamine 2000 reagent (Invitrogen). KN-93 [2-(N-[2-hydroxyethyl])-N-(4-methoxybenzenesulfonyl)amino-N-(4-chlorocinnamyl)-N-methylamine] was obtained from Cayman Chemical (Ann Arbor, MI). Control and human CAMKIIα-specific siRNA were experimentally verified sequences that were obtained from QIAGEN (Valencia, CA) (Krueger et al., 2007) and checked for homology to all other sequences of the genome using a nonredundant database and designed with HP OnGuard (QIAGEN), which provides asymmetry (Schwarz et al., 2003), 3′-UTR/seed region analysis (Grimson et al., 2007), sodium nitroprusside avoidance, and interferon motif avoidance (Judge et al., 2005) to maximize target specificity. Human aortic vascular smooth muscle cells were obtained and cultured in SMbM media from Lonza Walkersville, Inc. (Walkersville, MD).
Immunoprecipitation and Immunoblotting
COS-7 cells were lysed in ice-cold lysis buffer containing 50 mM Tris-HCl, pH 7.4, 100 mM NaF, 15 mM Na4P2O7, 1 mM Na3VO4, 1% (v/v) Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml pepstatin A, and 5 μg/ml aprotinin. Lysates were be centrifuged at 10,000g to concentrate insoluble material. Nox5 was extracted from detergent-resistant microdomains by the addition of 1% SDS and subsequently diluted 1:10 in lysis buffer. Protein extracts were precleared by incubation with Protein A/G-agarose for 2 h at 4°C with rocking. Agarose beads were then pelleted by centrifugation at 1000g. HA-Nox5 in precleared lysates was immunoprecipitated by incubation with preconjugated agarose/anti-HA antibody overnight at 4°C with rocking. Immunoprecipitated proteins were eluted from the beads by boiling for 5 min in 2× sample buffer. Immunoprecipitates or cell lysates were immunoblotted with various antibodies.
Purification of Phosphorylated Proteins from Intact Cells
COS-7 cells were cotransfected with WT or S475A Nox5 in the presence of CAMKIIα, and 48 h later, cells were lysed in radioimmunoprecipitation assay buffer. Lysates were diluted in binding buffer in the absence of metal chelators and reducing agents, and phosphoproteins were purified using phosphoaffinity columns (Thermo Fisher Scientific, Waltham, MA).
In Vitro Phosphorylation
Nox5 was isolated by immunoprecipitation from COS-7 cells transduced with HA-Nox5 adenovirus and incubated with 50 ng of active CAMKII (Cell Signaling Technology, Danvers, MA) for 20 min at 30°C in buffer containing 20 mM HEPES, pH 7.4, 10 mM MgCl2, 10 mM MnCl2, 0.5 mM CaCl2, 1 μg of CAM, and 1 mM dithiothreitol with or without 100 μM ATP. The reaction was terminated by the addition of SDS sample buffer. Incorporation of phosphate into Nox5 was determined using by SDS-PAGE followed by immunoblotting using phosphorylation state-specific antibodies that recognize phosphorylated Nox5 at Ser490, Thr494, and Ser498 as characterized previously (Jagnandan et al., 2007).
MS Analysis
In Vitro Phosphorylated Nox5.
Immunoprecipitated Nox5 was phosphorylated in vitro as described above, size-fractionated by SDS-PAGE, and proteins visualized by silver staining. The band of interest was excised from the gel and digested with trypsin (0.1 μg) for 45 min at 4°C. Peptides were then extracted from the gel at room temperature and analyzed by MS using a LTQ Orbitrap (Thermo Fisher Scientific). Protein identification was obtained from the MS/MS spectra using Mascot analysis software (Matrix Science, Boston, MA).
Phosphorylated Nox5 in Intact Cells.
HA-NOX5β was purified using immunoprecipitation from COS-7 cells that were transfected with NOX5β and CAMKIIα. Immune complexes were subjected to SDS-PAGE (7% acrylamide). Proteins were visualized using the Imperial protein stain (Thermo Fisher Scientific), and a band corresponding to the molecular weight of NOX5β (∼82kDa) was excised, destained, and subjected to overnight in-gel digestion with trypsin (25 ng/μl in 25 mM ammonium bicarbonate buffer, pH 7.8). Peptides were extracted with 0.1% TFA/75% acetonitrile and evaporated to near dryness. Peptide calibration standards and matrix CHCA were purchased from Applied Biosystems (Foster City, CA). All spectra were taken on an ABSciex 5800 MALDI-TOF Mass Spectrometer in positive reflector mode (10 kV) with a matrix of CHCA. At least 1000 laser shots were averaged to get each spectrum. Masses were calibrated to known peptide standards. Aliquots (5 μl) of the extracted tryptic digest were purified using C18 ZipTip columns (Millipore Corporation, Billerica, MA) according to the manufacturer's instructions. Bound peptides were desalted with two 5-μl washes of 0.1% TFA and then eluted with 2.5 μl of aqueous, acidic acetonitrile (75% CH3CN, 0.1% TFA). The eluate was mixed with 2.5 μl of freshly prepared CHCA stock solution (20 mg/ml CHCA in aqueous acetonitrile), and 1.5-μl aliquots were spotted onto a MALDI sample plate for air-drying. MS/MS of 1822.74 m/z peak was done in positive reflector mode without collision-induced dissociation. MS and MS/MS spectra were analyzed using the Mascot Distiller software package (Matrix Science).
Measurement of Reactive Oxygen Species.
COS-7 cells were transfected with cDNAs encoding Nox5 or control plasmids (RFP or lacZ), and 24 h later, cells were replated into white tissue culture-treated 96-well plates (Thermo Fisher Scientific) at a density of approximately 5 × 104 cells/well. The cells were incubated at 37°C in phenol-free Dulbecco's modified Eagle's medium (Sigma-Aldrich, St. Louis, MO) containing 400 μM concentration of the luminol analog 8-amino-5-chloro-7-phenylpyrido[3,4-d]pyridazine-1,4-(2H,3H) dione (L-012) (Wako Pure Chemicals, Tokyo, Japan) for a minimum of 20 min before the addition of agonists (Jagnandan et al., 2007). Luminescence was quantified over time using a Lumistar Galaxy (BMG Labtech, Durham, NC) luminometer. The specificity of L-012 for reactive oxygen species was confirmed by transfecting cells with a control plasmid such as green fluorescent protein or lacZ or by coincubation of a superoxide scavenger such as Tiron (5 mM). Both of these interventions yielded virtually undetectable levels of luminescence under control, PMA-, or ionomycin-stimulated conditions. Superoxide production is recorded as relative light units and as such, the absolute levels of ROS in separate experiments are not directly comparable. In human aortic smooth muscle cells, the calcium-dependent release of superoxide was measured as described by others using media with reduced calcium levels (Montezano et al., 2010).
Isolated Nox5 Activity Assay.
COS-7 cells coexpressing Nox5 and CAMKIIα were lysed in MOPS (30 mM, pH 7.2) based buffer containing KCl (100 mM), Triton (0.3%), and protease inhibitors (Sigma-Aldrich). Adherent cells were rocked gently, the lysis buffer was aspirated, and then the cells were washed three times with phosphate-buffered saline (4°C). Remaining cytoskeletal fractions were resuspended in MOPS, sonicated at low power, and spun down at 14,000 rpm (4°C). The supernatant was then aspirated, and the pellet was resuspended in MOPS buffer with mild sonication. The cell-free extract was aliquoted into buffers containing L-012 (400 μM), 1 mM MgCl2, 100 μM FAD (Sigma-Aldrich), and 26 μM concentration of calcium chloride. After a brief period of equilibration, reduced NADPH (Sigma-Aldrich) was injected to a final concentration of 100 μM, and the production of reactive oxygen species was monitored over time.
Calcium Measurements.
Change in calcium concentrations were measured under resting conditions and after exposure to ionomycin using Fluo4-NW (Invitrogen) as described previously (Church and Fulton, 2006).
Statistics.
All statistical analyses were performed using Instat software (GraphPad Software Inc., San Diego, CA) and were made using a two-tailed student's t test or analysis of variance with a post hoc test where appropriate. Differences are considered significant at p < 0.05.
Results
Endogenous CAMKII Positively Regulates Nox5 Activity.
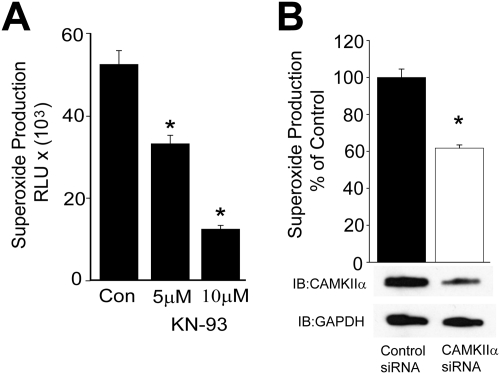
To determine whether CAMKII has a role in the regulation of Nox5 activity, we first used a pharmacological inhibitor of CAMKII, KN-93. BAECs were used as a source of endogenous CAMKII (Fleming et al., 2001) and were transduced with a Nox5 adenovirus, because these cells express low amounts of Nox5 compared with native blood vessels (D. Pandey, unpublished observations). As shown in Fig. 1A, pretreatment of BAEC with different doses of the CAMKII inhibitor KN-93 progressively reduced superoxide production from Nox5. We next investigated a role for CAMKII in the regulation of ROS production in human aortic vascular smooth muscle cells, which are known to endogenously express Nox5 (Jay et al., 2008). As shown in Fig. 1B, silencing CAMKIIα expression reduced calcium-dependent ROS production in human aortic vascular smooth muscle cells.
Fig. 1.
Endogenous CAMKIIα regulates Nox5 activity. A, BAECs were transduced with Nox5 adenovirus (multiplicity of infection of 50) and incubated with vehicle (CON) or increasing concentrations (5 and 10 μM) of the CAMKII inhibitor KN-93 for 30 min, and superoxide release was measured using L-012 chemiluminescence. B, human aortic vascular smooth muscle cells were transfected with 30 nmol control (nontargeting) siRNA or siRNA selective for CAMKIIα and 48 h later, calcium-dependent ROS production was measured using L-012 chemiluminescence. Results are presented as mean ± S.E.M., n = 5 to 7. *, p < 0.05 versus vehicle or control siRNA.
Active CAMKIIα Stimulates Nox5 Activity.
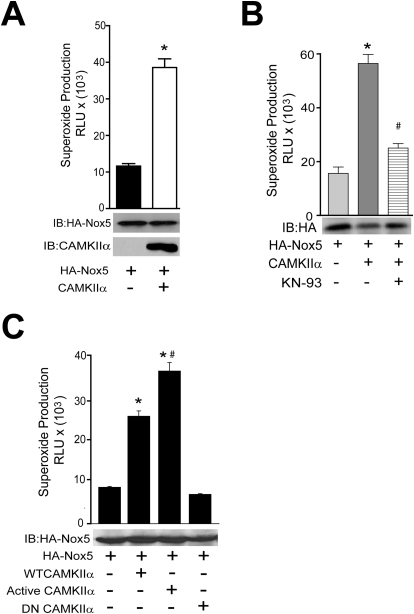
To complement results obtained using pharmacological inhibitors and siRNA, we next used a genetic approach to determine whether CAMKII is sufficient to increase Nox5 activity. COS-7 cells were cotransfected with Nox5 and either a control gene (RFP) or WT CAMKIIα and superoxide release was measured. As shown in Fig. 2A, cells expressing WT CAMKIIα released significantly more superoxide versus control cells. The equal expression level of Nox5 (bottom) in the presence of CAMKIIα suggests that the increase in activity results from a post-translational modification. The ability of CAMKII to increase Nox5-derived superoxide was sensitive to pharmacological inhibition with KN-93 (Fig. 2B). To further explore a relationship between Nox5 and CAMKII, we cotransfected COS-7 cells with Nox5 and either a control cDNA (RFP), WT, constitutively active, or a dominant-negative CAMKIIα. As shown in Fig. 2C, coexpression of WT CAMKII increases Nox5 activity and superoxide release. Coexpression of a constitutively active form of CAMKII (T286D), which mimics the persistent phosphorylation of Thr286, with Nox5 produces significantly higher levels of superoxide than the WT. Coexpression of a dominant-negative CAMKII (T305D), which mimics persistent inhibitory phosphorylation, does not elevate superoxide production above control levels.
Fig. 2.
Active CAMKIIα is sufficient for Nox5 activation. A, COS-7 cells were cotransfected with HA-Nox5 and either control (lacZ) or WT CAMKIIα cDNAs, and basal superoxide release was measured. Cell lysates were immunoblotted for total Nox5 and CAMKIIα (bottom). B, COS-7 cells were cotransfected with HA-Nox5 and either control (lacZ) or WT CAMKIIα in the presence and absence of KN-93 (10 μM). Basal superoxide release was measured with L-012. Cell lysates were immunoblotted for total Nox5 (bottom). Results are presented as the mean ± S.E.M., n = 5. *, p < 0.05 versus vehicle or control lacZ. C, COS-7 cells were cotransfected with HA-Nox5 and either control (lacZ), WT CAMKIIα, dominant-negative CAMKIIα (DN, T305D), or constitutively active-CAMKIIα (CA, T286D). Basal superoxide release was measured using L-012, and cell lysates were immunoblotted for total Nox5 (bottom). Results are presented as mean ± S.E.M. (n = 4–6); *, p < 0.05 versus control (lacZ); #, p < 0.05 versus WT CAMKIIα.
CAMKIIα Directly Modifies Nox5 Activity.
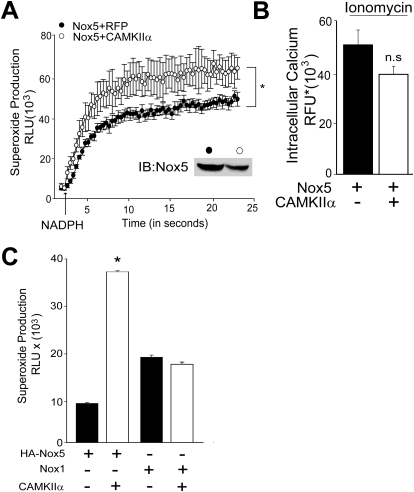
We next assessed whether CAMKII can directly influence Nox5 activity or alter other secondary events such as the level of intracellular calcium. To achieve this, we first performed an isolated Nox5 activity assay. Nox5 was purified from COS-7 cells coexpressing a control cDNA (RFP) or CAMKIIα and reconstituted with calcium, FAD, and superoxide production initiated with NADPH. As shown in Fig. 3A, Nox5 enzymatic activity from cells coexpressing CAMKII produced more superoxide than Nox5 extracted from control cells in response to NADPH. This occurred despite an apparently lower level of Nox5 expression in cell-free extracts from cells expressing CAMKIIα (inset). To determine whether CAMKII influences the level of intracellular calcium, we incubated COS-7 cells coexpressing Nox5 and either a control cDNA or CAMKIIα and measured intracellular calcium using the fluorescent calcium-reporter, Fluo-4. We found that expression of CAMKII did not modify basal or ionomycin-stimulated intracellular calcium levels (Fig. 3B).
Fig. 3.
CAMKIIα directly increases Nox5 activity in isolated activity assays but does not modify intracellular calcium levels or the activity of other Nox enzymes. A, superoxide release from Nox5 in an isolated activity assay. Nox5 was extracted from detergent-resistant microdomains of cells cotransfected with HA-Nox5 and a control (lacZ) or WT CAMKIIα. Nox5 was incubated in a buffer containing 100 nM CaCl2, 100 μM FAD, and superoxide initiated by injection of 100 μM NADPH (indicated by the arrow). B, measurement of intracellular calcium in intact COS-7 cells expressing Nox5 and CAMKIIα using Fluo4-NW. Changes in fluorescence intensity were monitored with a 485/10 nm excitation and a 520/10 nm emission filter. Results are presented as mean ± S.E.M., n = 6; *, p < 0.05 versus lacZ control. C, COS-7 cells were cotransfected with Nox5 or Nox1, NoxA1, or Nox01 with and WT CAMKIIα or control DNA (lacZ), and basal superoxide was measured using L-012 chemiluminescence. Results are presented as mean ± S.E.M. (n = 5–6); *, p < 0.05 versus control (lacZ).
CAMKII Does Not Stimulate ROS Production from Other Nox Enzymes.
To address whether CAMKII can increase the activity of other Nox enzymes, we coexpressed CAMKIIα with Nox1 and its regulatory proteins, NOXO1 and NOXA1. Although CAMKII can potently increase superoxide release from Nox5, it does not modify Nox1 activity (Fig. 3C).
Nox5 Is a Substrate for CAMKII Phosphorylation.
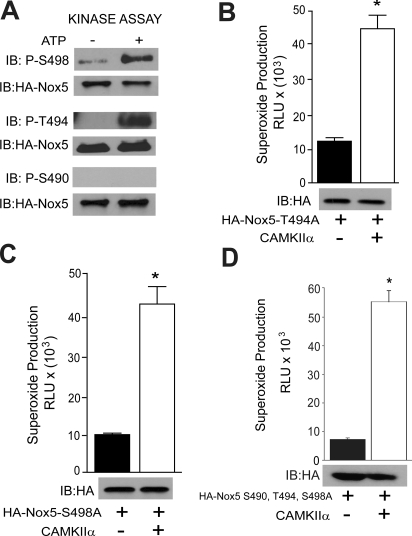
To determine whether CAMKII can directly phosphorylate Nox5, we performed an in vitro kinase assay using immunoprecipitated Nox5 as a substrate. As shown in Fig. 4A, in the presence of ATP, recombinant CAMKIIα robustly phosphorylated Nox5 on Ser498, Thr494, but not on Ser490. To identify whether these sites are functionally important we expressed single (S498A, T494A) or triple (S490A, T494A, S498A) phospho-null mutants of Nox5, together with CAMKIIα. As shown in Fig. 4, B to D, single mutation of Nox5 on Thr494 or Ser498 or mutation of all three phosphorylation sites did not prevent the ability of CAMKII to increase Nox5 activity.
Fig. 4.
CAMKIIα directly phosphorylates Nox5 on Ser498 and Ser494 in an in vitro kinase assay. A, Nox5 was immunoprecipitated from COS-7 cells transduced with HA-Nox5 adenovirus and subject to an in vitro kinase assay. Phosphorylated samples were immunoblotted for phosphorylated Ser490, Thr494, and Ser498 versus total Nox5. COS-7 cells were cotransfected with either T494A (B), S498A (C), or S490A, T494A, and S498A (D) triple mutants of HA-Nox5 together with WT CAMKIIα or control DNA (lacZ). Basal superoxide was measured by L-012. Relative expression of Nox5 was detected by immunoblotting (bottom). Results are presented as mean ± S.E.M. (n = 4–6); *, p < 0.05 versus control (lacZ).
Identification and Significance of Novel Nox5 Phosphorylation Sites.
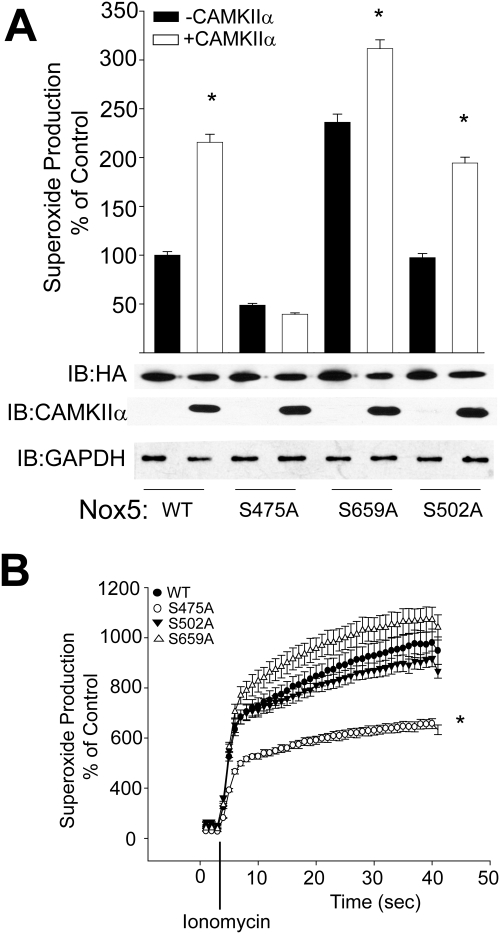
The ability of CAMKII to modify Nox5 activity seems to be due to a direct effect on the enzyme. However, the mutation of Ser490, Thr494, and Ser498 to nonphosphorylatable analogs did not prevent CAMKII-dependent changes in Nox5 activity. Therefore, we next examined whether CAMKII can phosphorylate Nox5 on other sites. Nox5 was phosphorylated in vitro using recombinant CAMKII, purified via SDS-PAGE, trypsin-digested, and subjected to MS/MS analysis (Duval et al., 2007). We found an additional three sites of phosphorylation, serines 475, 659, and 502 listed by rank of Mascot and Sequest scores (Table 1). To address the functional significance of these sites, we made point mutations of serine 475, 659, and 502 to the nonphosphorylatable analog alanine. As shown in Fig. 5A, the Nox5 mutant S475A was active but did not produce additional superoxide in the presence of CAMKII. The other identified sites, Ser659 and Ser502, were significantly activated by CAMKII, albeit to a reduced degree compared with the WT Nox5 (Fig. 5A). We also directly compared the calcium-dependent activity of S475A, S502A, S659A mutants versus that of WT Nox5 and found that the S475A Nox5 mutant produced less superoxide after stimulation with ionomycin (Fig. 5B).
TABLE 1.
Identification of novel CAMKIIα phosphorylation sites in Nox5
HA-Nox5β was immunoprecipitated from COS-7 cells transduced with HA-Nox5 adenovirus and subject to an in vitro kinase assay. Phosphorylated samples were run on SDS gel, silver-stained, and the band corresponding to the correct molecular weight of Nox5 was excised, trypsin-digested, and subjected to analysis by mass spectrometry.
| Peptide | Phospho Peptide | Mascot Score | Sequest Score |
|---|---|---|---|
| LYEpSFKASDPLGR | Ser475 | 46 | 3.4 |
| KDpSITGLQTR | Ser659 | 32 | 2.76 |
| SpSKGSEILLEK | Ser502 | N.D. | 0.84 |
N.D., not determined.
Fig. 5.
Mutation of S475A prevents CAMKIIα-dependent increases in Nox5 activity. A, COS-7 cells were cotransfected with either WT HA-Nox5 or S475A, S659A, or S502A mutants together with WT CAMKIIα or control DNA (lacZ), and basal superoxide was measured with L-012. Cell lysates were immunoblotted for relative expression of HA-Nox5 (bottom). Results are presented as mean ± S.E.M. (n = 4–5); *, p < 0.05 versus lacZ control. B, COS-7 cells were transfected with WT or S475A or S659A or S502A Nox5 and superoxide production was measured after stimulation with ionomycin (1 μM). Results are presented as mean ± S.E.M. (n = 5–6); *, p < 0.05 versus WT.
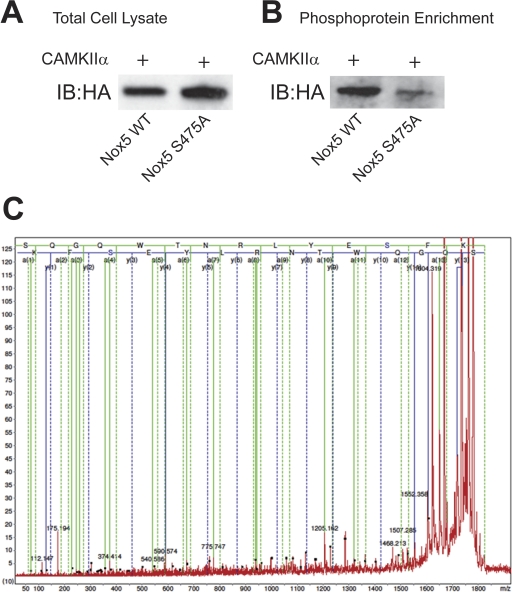
Identification of Nox5 Ser475 Phosphorylation in Intact Cells.
Having shown that Ser475 is a site for CAMKIIα-mediated phosphorylation of Nox5 in vitro and that Ser475 is functionally relevant in mediating CAMKIIα-stimulated ROS production, we next sought evidence that this site is phosphorylated in intact cells. COS-7 cells expressing WT or S475A Nox5 together with CAMKIIα were lysed and phosphorylated proteins in the lysates were bound to phosphoprotein affinity columns. The relative expression of WT and S475A Nox5 in total cell lysates is shown in Fig. 6A. As shown in Fig. 6B, a Western blot on the eluate from the phosphoprotein affinity columns revealed greater binding of WT Nox5 compared with the S475A phospho-null mutant. These results suggest that in cells, the CAMKIIα-induced phosphorylation of Nox5 is diminished by mutation of Ser475. To obtain direct evidence that Ser475 is phosphorylated in intact cells, we next performed MS analysis. Nox5 was immunoprecipitated from cells expressing WT Nox5 and CAMKIIα and size-fractionated by SDS-PAGE. Bands corresponding to the MW of Nox5 were excised, trypsin-digested, and subjected to MS/MS analysis. As shown in Fig. 6C, using MALDI-TOF and MS/MS analysis we detected a phosphorylated peptide that corresponds to the region flanking Ser475.
Fig. 6.
CAMKII-dependent phosphorylation of Nox5 on Ser475 in intact cells. A, COS-7 cells were cotransfected with WT Nox5 and S475A Nox5 together with CAMKIIα, and cell lysates were probed for the relative expression of total HA-Nox5. B, phosphoproteins in cell lysates were bound to phosphoprotein affinity columns, and eluted proteins were probed for the expression of HA-Nox5. C, detection of phosphorylated amino acid modifications of Nox5 in COS-7 cells cotransfected with Nox5 and CAMKIIα. MS/MS spectra of parent ion m/z 1822.7 was obtained by MALDI-TOF-MS/MS fitted with peptide 464-SQGQWTNRLYES*FK-477 from the Nox5β sequence phosphorylated at Ser475.
Discussion
The major findings of this study are that CAMKII can directly increase the activity of Nox5 and promote superoxide release. The effect of CAMKII on superoxide release is selective for Nox5 because it does not modify the activity of Nox1. We found that in vitro CAMKIIα can phosphorylate Nox5 on at least five different sites: Thr494, Ser498, Ser502, Ser475, and Ser659. Of these sites, only Ser475 is functionally important for superoxide release. The ability of CAMKII to phosphorylate Nox5 on Ser475 in intact cells is suggested from the loss of binding to phosphoprotein affinity columns and is directly supported by the detection of a phosphorylated peptide containing Ser475 using mass spectrometry. Together, these results suggest that CAMKIIα can promote superoxide release from cells, at least in part, by regulating the activity of Nox5.
We showed previously that the PMA-dependent phosphorylation of Nox5 on Thr494 and Ser498 increases the calcium-sensitivity of Nox5 and permits elevated superoxide release at resting levels of intracellular calcium (Jagnandan et al., 2007). Although we found that CAMKII can increase the phosphorylation of Nox5 on Thr494 and Ser498 in vitro, the mutation of these sites to nonphosphorylatable residues had no effect on the ability of CAMKII to increase superoxide release from cells expressing Nox5. Thus, within the cell, it is possible that either CAMKII does not modify the phosphorylation of these sites or the phosphorylation of Ser475 can override these effects.
The mechanism by which the phosphorylation of Ser475 increases Nox5 activity is not yet known and the exact mechanisms regulating the calcium-dependent activity of Nox5 in general are not fully understood (Wei et al., 2010). This site lies in the C-terminal, cytoplasmic region of Nox5, just after the predicted FAD binding region and before the first NADPH binding site (Fulton, 2009). This region also contains, in close proximity, the other Nox5 phosphorylation sites Thr494, Ser498, and Ser502. Thus, given its location it is likely that phosphorylation of this site promotes electron flow from NADPH to FAD or FAD to the heme moieties.
Within the cell, Nox5 can be found primarily on membranes of the endoplasmic reticulum and also at the plasma membrane (Jagnandan et al., 2007; Serrander et al., 2007; Kawahara and Lambeth, 2008). The mechanism by which CAMKII interacts with Nox5 is not yet known. CAMKII is not intrinsically membrane-bound but can interact with substrates at the plasma membrane and also those that are present on the sarco/endoplasmic reticulum such as the ryanodine receptor (Wehrens et al., 2004; Hudmon et al., 2005). Some substrates of CAMKII have been proposed to contain a binding motif that can resemble part of the regulatory domain of CAMKII (Couchonnal and Anderson, 2008), but it is not yet known whether Nox5 binds tightly to CAMKII or participates in “kiss-and-run” interactions before phosphorylation.
CAMKII has been shown to regulate the levels of intracellular calcium by phosphorylating subunits of voltage gated calcium channels and also the ryanodine receptor (Wehrens et al., 2004; Hudmon et al., 2005). However, this action is unlikely to account for the ability of CAMKII to increase Nox5 activity in COS-7 cells for several reasons. First, in these cells, CAMKII did not modify intracellular calcium levels and the evidence that CAMKII can induce lasting increases in Nox5 activity in a cell-free activity assay in which the calcium concentration is fixed further supports this. Last, the loss of responsiveness of the S475A phospho-null mutant of Nox5 to CAMKII suggests that the mechanism by which CAMKII regulates Nox5 activity is not simply due to secondary changes in calcium. However, it is possible that in smooth muscle cells, which express both Nox5 and voltage-gated calcium channels, that CAMKII could promote the elevation of intracellular calcium and that this would serve to further increase Nox5 activity (Koch et al., 1990; Jay et al., 2008). The ability of CAMKII to regulate superoxide release from cells was also dependent on the presence of Nox5, because increased superoxide was not observed in control cells (without Nox5) or in cells expressing Nox1.
In response to PMA, a recent study has ruled out CAMKII as a regulator of Nox5 activity based primarily on the lack of effect of the putative CAMKII inhibitor KN-92 on ROS production (Serrander et al., 2007). However, it should be noted that KN-92 does not effectively inhibit CAMKII and instead is often used an inactive structural analog of the active inhibitor, KN-93. In contrast, we found that superoxide release from BAEC expressing Nox5 and from COS-7 cells cotransfected with Nox5, and WT CAMKII was strongly inhibited by KN-93. PMA has also been shown to regulate the activity of voltage-sensitive calcium channels (Hudmon et al., 2005), and thus, it is possible that in cell types expressing these channels, PMA could indeed promote increased CAMKII activity through the secondary elevation of calcium. However, this has not been shown to be important for Nox5 activity (Jagnandan et al., 2007).
Because CAMKII is a calcium-calmodulin (Couchonnal and Anderson, 2008) regulated kinase, it is interesting that under resting conditions or in the absence of a calcium-transient, the activity of the WT kinase approached that of the constitutively active CAMKII. Although a fraction of the CAMKII pool is believed to be active, this is unlikely to account for the high basal activity observed. One explanation for this is that ROS are a potent stimulus for increased activity of CAMKII (Howe et al., 2004; Trebak et al., 2010), and the elevated levels of ROS derived from the coexpression of Nox5 may be the cause of the increased activity of WT CAMKII. Indeed, a recent study has shown that CAMKII can be directly modified by the NADPH oxidase-dependent oxidation of two methionine residues, which renders the enzyme constitutively active (Erickson et al., 2008). The ability of ROS to stimulate CAMKII-activity has important functional consequences. Elevated ROS have been shown to potently up-regulate the expression of endothelial nitric-oxide synthase in endothelial cells in a CAMKII-dependent manner (Cai et al., 2001). ROS-stimulated CAMKII also contributes to apoptosis of cardiac myocytes, impaired cardiac function, and increased mortality after myocardial infarction (Erickson et al., 2008). More relevant to the current study, ROS in the form of H2O2 have been shown to positively regulate Nox5 activity via a c-Abl kinase, Ca+2-mediated, redox-dependent signaling pathway (El Jamali et al., 2008). However, it is not yet known whether CAMKs participate in this process. The ability of ROS to stimulate CAMKII activity and then secondarily increase Nox5 activity also suggests the existence of a feed-forward mechanism that could lead to excessive ROS production via reciprocal stimulation.
The activities of Nox5 and CAMKII are strongly influenced by agonists that promote the mobilization of intracellular calcium, and thus, it is likely that both enzymes are coactivated when expressed in the same cell. Angiotensin II has been shown to stimulate CAMKII activity in vascular smooth muscle cells to promote hypertrophy (Li et al., 2010), and not surprisingly, angiotensin II is also a potent regulator of Nox5 (Montezano et al., 2010). Likewise, CAMKII and Nox5 are abundant proteins in sperm in which they are important for motility and capacitation (Sabeur and Ball, 2007; Schlingmann et al., 2007). CAMKII plays an important role in cardiac myocyte excitation contraction coupling, calcium handling, and apoptosis (Couchonnal and Anderson, 2008). It is not yet known whether Nox5 is expressed in human cardiac myocytes, although its expression has been documented in cardiac fibroblasts (Cucoranu et al., 2005).
In conclusion, the ability of CAMKII to regulate Nox5 activity may be significant in the regulation of ROS production that occurs downstream of calcium-mobilizing agonists such as angiotensin II. The ability of both proteins to reciprocally regulate the activity of each other could possibly contribute to excessive ROS production. Further studies are needed to identify the contribution of CAMKII to ROS production in diseases associated with excessive oxidative stress.
This work was supported in part by the Cardiovascular Discovery Institute at the Medical College of Georgia; the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL085827, HL092446, HL60190, HL67841]; and an American Heart Association Established Investigator Award.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.070193.
- Nox
- NADPH oxidase
- CAMKII
- calcium/calmodulin-activated kinase II
- ROS
- reactive oxygen species
- BAEC
- bovine aortic endothelial cell
- MS
- mass spectrometry
- MS/MS
- tandem mass spectrometry
- PMA
- phorbol 12-myristate 13-acetate
- siRNA
- small interfering RNA
- PAGE
- polyacrylamide gel electrophoresis
- TFA
- trifluoroacetic acid
- WT
- wild type
- HA
- hemagglutinin
- RFP
- red fluorescent protein
- MOPS
- 3-(N-morpholino)propanesulfonic acid
- MALDI-TOF
- matrix-assisted laser desorption ionization/time of flight
- KN-93
- 2-(N-[2-hydroxyethyl])-N-(4-methoxybenzenesulfonyl)amino-N-(4-chlorocinnamyl)-N-methylamine
- KN-92
- 2-[N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine, phosphate
- CHCA
- α-cyano-4-hydroxycinnamic acid
- L-012
- 8-amino-5-chloro-7-phenylpyrido[3,4-d]pyridazine-1,4-(2H,3H) dione.
Authorship Contributions
Participated in research design: Pandey and Fulton.
Conducted experiments: Pandey, Gratton, Rafikov, Black, and Fulton.
Contributed new reagents or analytic tools: Pandey, Rafikov, and Black.
Performed data analysis: Pandey, Gratton, Rafikov, Black, and Fulton.
Wrote or contributed to the writing of the manuscript: Pandey, Gratton, Rafikov, Black, and Fulton.
References
- Beckman KB, Ames BN. (1998) The free radical theory of aging matures. Physiol Rev 78:547–581 [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4:517–529 [DOI] [PubMed] [Google Scholar]
- Cai H, Davis ME, Drummond GR, Harrison DG. (2001) Induction of endothelial NO synthase by hydrogen peroxide via a Ca2+/calmodulin-dependent protein kinase II/janus kinase 2-dependent pathway. Arterioscler Thromb Vasc Biol 21:1571–1576 [DOI] [PubMed] [Google Scholar]
- Church JE, Fulton D. (2006) Differences in eNOS activity because of subcellular localization are dictated by phosphorylation state rather than the local calcium environment. J Biol Chem 281:1477–1488 [DOI] [PubMed] [Google Scholar]
- Couchonnal LF, Anderson ME. (2008) The role of calmodulin kinase II in myocardial physiology and disease. Physiology (Bethesda) 23:151–159 [DOI] [PubMed] [Google Scholar]
- Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. (2005) NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97:900–907 [DOI] [PubMed] [Google Scholar]
- Duval M, Le Boeuf F, Huot J, Gratton JP. (2007) Src-mediated phosphorylation of Hsp90 in response to vascular endothelial growth factor (VEGF) is required for VEGF receptor-2 signaling to endothelial NO synthase. Mol Biol Cell 18:4659–4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Jamali A, Valente AJ, Lechleiter JD, Gamez MJ, Pearson DW, Nauseef WM, Clark RA. (2008) Novel redox-dependent regulation of NOX5 by the tyrosine kinase c-Abl. Free Radic Biol Med 44:868–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, et al. (2008) A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133:462–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. (2001) Phosphorylation of Thr(495) regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88:E68–E75 [DOI] [PubMed] [Google Scholar]
- Fulton DJ. (2009) Nox5 and the regulation of cellular function. Antioxid Redox Signal 11:2443–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith LC. (2004) Regulation of calcium/calmodulin-dependent protein kinase II activation by intramolecular and intermolecular interactions. J Neurosci 24:8394–8398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. (2007) MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 27:91–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, et al. (2008) Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 52:1803–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CJ, Lahair MM, McCubrey JA, Franklin RA. (2004) Redox regulation of the calcium/calmodulin-dependent protein kinases. J Biol Chem 279:44573–44581 [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. (2005) CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol 171:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagnandan D, Church JE, Banfi B, Stuehr DJ, Marrero MB, Fulton DJ. (2007) Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J Biol Chem 282:6494–6507 [DOI] [PubMed] [Google Scholar]
- Jagnandan D, Sessa WC, Fulton D. (2005) Intracellular location regulates calcium-calmodulin-dependent activation of organelle-restricted eNOS. Am J Physiol Cell Physiol 289:C1024–C1033 [DOI] [PubMed] [Google Scholar]
- Jay DB, Papaharalambus CA, Seidel-Rogol B, Dikalova AE, Lassègue B, Griendling KK. (2008) Nox5 mediates PDGF-induced proliferation in human aortic smooth muscle cells. Free Radic Biol Med 45:329–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. (2005) Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol 23:457–462 [DOI] [PubMed] [Google Scholar]
- Kawahara T, Lambeth JD. (2008) Phosphatidylinositol (4,5)-bisphosphate modulates Nox5 localization via an N-terminal polybasic region. Mol Biol Cell 19:4020–4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly EK, Wang L, Ivashkiv LB. (2010) Calcium-activated pathways and oxidative burst mediate zymosan-induced signaling and IL-10 production in human macrophages. J Immunol 184:5545–5552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch WJ, Ellinor PT, Schwartz A. (1990) cDNA cloning of a dihydropyridine-sensitive calcium channel from rat aorta. Evidence for the existence of alternatively spliced forms. J Biol Chem 265:17786–17791 [PubMed] [Google Scholar]
- Krueger U, Bergauer T, Kaufmann B, Wolter I, Pilk S, Heider-Fabian M, Kirch S, Artz-Oppitz C, Isselhorst M, Konrad J. (2007) Insights into effective RNAi gained from large-scale siRNA validation screening. Oligonucleotides 17:237–250 [DOI] [PubMed] [Google Scholar]
- Lambeth JD. (2004) NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4:181–189 [DOI] [PubMed] [Google Scholar]
- Lambeth JD. (2007) Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med 43:332–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li W, Gupta AK, Mohler PJ, Anderson ME, Grumbach IM. (2010) Calmodulin kinase II is required for angiotensin II-mediated vascular smooth muscle hypertrophy. Am J Physiol Heart Circ Physiol 298:H688–H698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. (2006) Functional analysis of Nox4 reveals unique characteristics compared with other NADPH oxidases. Cell Signal 18:69–82 [DOI] [PubMed] [Google Scholar]
- Montezano AC, Burger D, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, He Y, Callera GE, He G, Krause KH, et al. (2010) Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res 106:1363–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Longo-Guess CM, Bergstrom DE, Nauseef WM, Jones SM, Bánfi B. (2008) Mutation of the Cyba gene encoding p22phox causes vestibular and immune defects in mice. J Clin Invest 118:1176–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeur K, Ball BA. (2007) Characterization of NADPH oxidase 5 in equine testis and spermatozoa. Reproduction 134:263–270 [DOI] [PubMed] [Google Scholar]
- Schlingmann K, Michaut MA, McElwee JL, Wolff CA, Travis AJ, Turner RM. (2007) Calmodulin and CaMKII in the sperm principal piece: evidence for a motility-related calcium/calmodulin pathway. J Androl 28:706–716 [DOI] [PubMed] [Google Scholar]
- Schwarz DS, Hutvágner G, Du T, Xu Z, Aronin N, Zamore PD. (2003) Asymmetry in the assembly of the RNAi enzyme complex. Cell 115:199–208 [DOI] [PubMed] [Google Scholar]
- Serrander L, Jaquet V, Bedard K, Plastre O, Hartley O, Arnaudeau S, Demaurex N, Schlegel W, Krause KH. (2007) NOX5 is expressed at the plasma membrane and generates superoxide in response to protein kinase C activation. Biochimie 89:1159–1167 [DOI] [PubMed] [Google Scholar]
- Soderling TR. (1999) The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem Sci 24:232–236 [DOI] [PubMed] [Google Scholar]
- Tirone F, Cox JA. (2007) NADPH oxidase 5 (NOX5) interacts with and is regulated by calmodulin. FEBS Lett 581:1202–1208 [DOI] [PubMed] [Google Scholar]
- Trebak M, Ginnan R, Singer HA, Jourd'heuil D. (2010) Interplay between calcium and reactive oxygen/nitrogen species: an essential paradigm for vascular smooth muscle signaling. Antioxid Redox Signal 12:657–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Reiken SR, Marks AR. (2004) Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res 94:e61–70 [DOI] [PubMed] [Google Scholar]
- Wei CC, Motl N, Levek K, Chen LQ, Yang YP, Johnson T, Hamilton L, Stuehr DJ. (2010) Conformational States and kinetics of the calcium binding domain of NADPH oxidase 5. Open Biochem J 4:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]