INTRODUCTION
Gastrointestinal stromal tumor (GIST) is recognized as the most common mesenchymal tumor of the gastrointestinal tract. It constitutes 80% of all gastrointestinal mesenchymal tumors and approximately 20% of all small bowel malignancies, excluding lymphomas. It is believed that there are up to 5,000 new cases of GIST diagnosed each year in the United States.1 GIST first gained recognition as a distinct tumor type in the 1980s, before which it was considered a type of leiomyomatous tumor. Further research has demonstrated that approximately 90% of GISTs contain an activating KIT (CD117) mutation, while 5% carry a mutation in platelet-derived growth factor receptor alpha (PDGFRα). Accordingly, the treatment of GIST has changed radically with the evolution of targeted therapies in oncology. Six prospective, randomized controlled trials have been published on the treatment of this disease during the brief period since its molecular and pathologic characterization.
SURGERY AND RADIATION
There are no data from randomized controlled trials regarding the surgical management of GIST. By consensus, surgical resection with negative microscopic margins remains the primary treatment and is the only modality that appears to offer a significant chance of cure. Aggressive surgical therapy to debulk progressive, non-localized disease or to resect metastatic disease is advocated in select circumstances, but these approaches have not been evaluated in prospective, controlled studies.
Similarly, no randomized controlled trials have been published on the use of radiation therapy for GIST. In fact, retrospective data are sparse. Current applications for this treatment modality remain limited.
CHEMOTHERAPY AND METASTATIC DISEASE
In general, traditional chemotherapeutic agents, including doxorubicin, alone or with gemcitabine, or ifosfamide with etoposide or temozolomide, have not been efficacious in treating patients with GIST.2–7 The application of imatinib mesylate (Gleevec; Novartis Pharmaceutical, Basel, Switzerland) in the treatment of GIST reflects a major advance in the treatment of solid tumors with specific, therapeutic, molecular targeting. Imatinib, a product of rational drug development, demonstrates inhibitory activity against BCR-ABL, PDGFRα, and KIT kinases. By binding to the ATP-binding pocket of these proteins, imatinib blocks the transfer of a phosphate group to the substrate molecule and leads to inhibition of KIT or PDGFRα signal transduction.9 Given these effects, and regardless of the KIT or PDGFRα mutation status, imatinib has become the first-line medical treatment for metastatic, unresectable, or recurrent GIST. Surgery alone for metastatic disease often has limited value. The initial starting dose of imatinib is 400 mg/day administered orally, although some studies have compared this starting dose to an 800 mg/day dose. The data from these randomized trials will be discussed below, but 400 mg/day remains the standard initial therapy in most patients. The task of characterizing tumor mutation status (KIT/ PDGFRα), assessing disease progression, and integrating surgery and targeted therapy has led to the necessity for a multidisciplinary approach to treating patients with GIST. A team including radiologists, pathologists, medical oncologists, and surgeons is required for successful care of these patients.8
All of the currently available randomized controlled trials in the treatment of GIST address the use of targeted biologic agents, primarily in advanced (surgically unresectable or metastatic) disease but more recently in the adjuvant setting as well. Although initially advanced for use in chronic myelogenous leukemia, the dramatic benefits of imatinib mesylate in the treatment of GIST were demonstrated by Demetri and colleagues with an initial case report in 200110 followed by report of their phase II trial in 2002.11 This latter study demonstrated unprecedented early improvements in progression-free and overall survival in patients treated with either 400 mg/day or 600 mg/day of imatinib. A subsequent long-term follow-up study12 demonstrated a median survival of 57 months in patients for whom a 20-month median survival would have traditionally been estimated.
Subsequent studies of imatinib in GIST have addressed some of the questions regarding dosing and duration of therapy. Verweij and colleagues, reporting in 2004 for the European Organization for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group, the Italian Sarcoma Group (ISG) and the Australasian Gastrointestinal Trials Group (AGITG), demonstrated a small but statistically significant improvement in progression-free survival among patients receiving 800 mg/day of imatinib over those receiving 400 mg/day.7 However, a concurrent study of similar design published by Blanke and colleagues (SWOG S0033) failed to demonstrate significant improvements in the higher dose group.13 Nevertheless, some clinical benefit, in the form of delayed progression of disease, was identified in both trials among patients who crossed over to high-dose therapy after progressing on conventional-dose imatinib.13,16 In 2007, Van Glabbeke and colleagues presented results of the MetaGIST analysis, which combined 1640 patients from the SWOG S0033 and the EORTC-ISG-AGITG phase III trials.15 This meta-analysis affirmed a small but statistically significant improvement in progression-free survival (PFS) in the higher dose arm (800 mg/day), although no difference in overall survival was demonstrated. In particular, patients with exon 9 KIT mutations demonstrated worse PFS, which may have been somewhat improved by higher dose imatinib. However, since the original trials were not stratified for mutation location, the results of this post-hoc subgroup analysis require further validation.
As for the question of therapy duration, Blay and colleagues, reporting for the French Sarcoma Group in 2007, clearly demonstrated the risks of interrupting imatinib therapy after one year.14 Eighty-one percent of patients with stable or responsive disease who interrupted imatinib therapy demonstrated prompt disease progression. In summary, these results suggest that initiating therapy with conventional-dose imatinib is appropriate for the majority of patients with surgically unresectable disease, and interruption in therapy should be avoided in patients with responsive or stable disease.
For patients whose disease progresses on imatinib or who are unable to tolerate the drug, Demetri and colleagues demonstrated the viability of sunitinib (Sutent; Pfizer, New York, USA) as a second-line treatment.17 While the response rates in this group are not nearly as dramatic as with first-line imatinib, sunitinib clearly offers improvement in progression-free survival over no treatment.
Finally, DeMatteo and colleagues, reporting for the American College of Surgeons Oncology Group, published in 2009 the results of the first randomized controlled trial addressing the role of imatinib in the adjuvant setting18. Early results from this trial demonstrated that imatinib 400 mg daily, administered for one year after complete resection of localized, primary GIST, provided a 15% absolute reduction in recurrence events compared to placebo at one year. Reductions in risk of recurrence were observed among all subgroups as stratified by tumor size. The impact on overall survival will require longer follow-up to establish but is not different at this time.
At the time of this writing, no randomized trials evaluating the neoadjuvant use of imatinib have been reported, although phase II data support its safety.19 In the absence of randomized data, retrospective data would suggest that patients can be treated in a neoadjuvant setting in order to improve resectability or reduce the extent of the operation.
ONGOING TRIALS
A number of active and recently closed trials should help answer important questions regarding the optimal use of imatinib in adjuvant or neoadjuvant settings. The Scandinavian Sarcoma Group has completed enrollment and is currently in the data collection phase on a randomized, controlled trial evaluating adjuvant therapy with imatinib (400 mg/day) administered as a short (12 months) versus long (36 months) course of treatment, following complete gross resection of GIST at high risk for recurrence. Recurrence-free survival is the primary endpoint. The EORTC recently closed their trial (protocol 62024) comparing two years of adjuvant therapy with imatinib versus observation alone in patients undergoing complete resection of localized primary GIST. Since overall survival is the primary endpoint, results are not expected for about 9 years. Additionally, the EORTC is accruing subjects for a phase III randomized study (protocol 62063) evaluating resection of residual disease in patients with metastatic GIST responding to imatinib.
LEVEL Ia EVIDENCE: RANDOMIZED CLINICAL TRIALS IN GIST
1. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors.
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CD, Joensuu H. N Engl J Med. 2002 Aug 15;347(7):472–80.
Hypothesis: Imatinib, evaluated at two different dose regimens, improves progression-free survival in patients with unresectable or metastatic GIST beyond historical expectations.
| # Patients Randomized |
Study Groups |
Stratification | Significance Demonstrated |
% Change Identified in Trial |
|---|---|---|---|---|
| 147 | 400 mg imatinib daily n = 73 600 mg imatinib daily n = 74 |
None | No difference in progression-free survival | p = NS |
Published Abstract: BACKGROUND: Constitutive activation of KIT receptor tyrosine kinase is critical in the pathogenesis of gastrointestinal stromal tumors. Imatinib mesylate, a selective tyrosine kinase inhibitor, has been shown in preclinical models and preliminary clinical studies to have activity against such tumors. METHODS: We conducted an open-label, randomized, multicenter trial to evaluate the activity of imatinib in patients with advanced gastrointestinal stromal tumor. We assessed antitumor response and the safety and tolerability of the drug. Pharmacokinetics were assessed in a subgroup of patients. RESULTS: A total of 147 patients were randomly assigned to receive 400 mg or 600 mg of imatinib daily. Overall, 79 patients (53.7 percent) had a partial response, 41 patients (27.9 percent) had stable disease, and for technical reasons, response could not be evaluated in 7 patients (4.8 percent). No patient had a complete response to the treatment. The median duration of response had not been reached after a median follow-up of 24 weeks after the onset of response. Early resistance to imatinib was noted in 20 patients (13.6 percent). Therapy was well tolerated, although mild-to-moderate edema, diarrhea, and fatigue were common. Gastrointestinal or intraabdominal hemorrhage occurred in approximately 5 percent of patients. There were no significant differences in toxic effects or response between the two doses. Imatinib was well absorbed, with pharmacokinetics similar to those reported in patients with chronic myeloid leukemia. CONCLUSIONS: Imatinib induced a sustained objective response in more than half of patients with an advanced unresectable or metastatic gastrointestinal stromal tumor. Inhibition of the KIT signal-transduction pathway is a promising treatment for advanced gastrointestinal stromal tumors, which resist conventional chemotherapy. (Copyright © [2002] Massachusetts Medical Society. All rights reserved.)
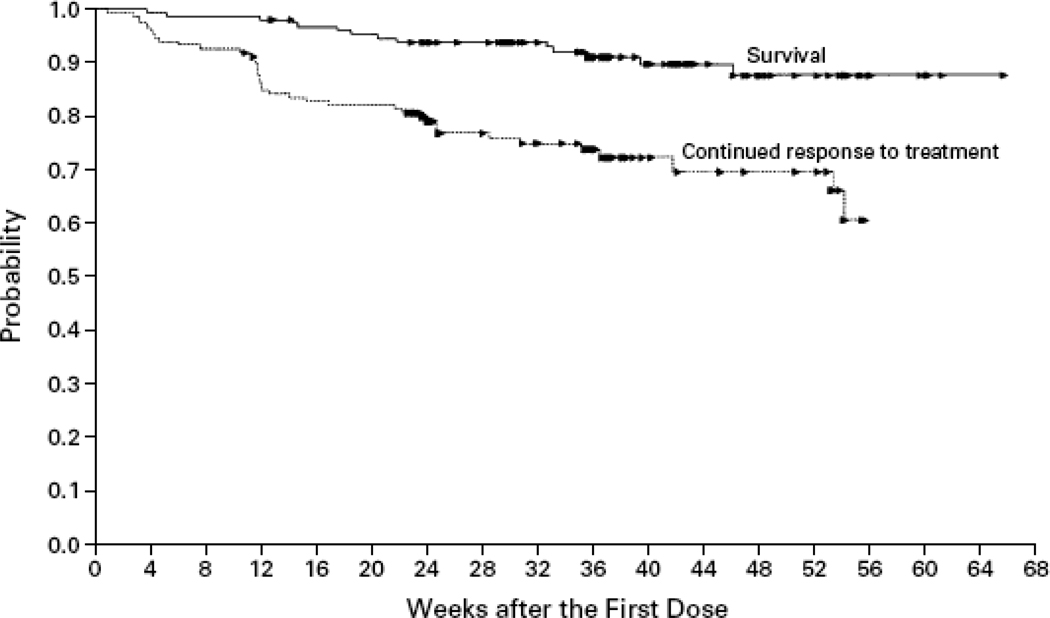
Editor’s Summary and Comments: In this seminal, multi-center, phase II study, Demetri and colleagues demonstrated the unprecedented efficacy of imatinib in unresectable or metastatic KIT-positive GIST. Beginning as a proof-of-concept study, promising early results prompted expanded enrollment. Following a 6 month follow-up of the first 100 patients, interim analysis demonstrated no difference in progression-free survival but illustrated a dramatic response over historical expectations. The study was closed as enrollment into a phase III trial was initiated. Of the 147 patients enrolled, 120 patients (81.6%) demonstrated either disease regression or stabilization. Although differences in efficacy were not observed between the two administered doses, the vast improvements in overall and progression-free survival seen in both arms over historical controls established the benefits of imatinib in advanced GIST. In a subsequent follow-up study,12 the investigators demonstrated the sustained benefit of imatinib administered at either dose. With longer follow-up, objective radiological responses, as determined by Response Evaluation Criteria In Solid Tumors (RECIST) criteria, increased from 54% to 68%. More importantly, survival was shown to be independent of the extent of response such that patients with partial responses or with stable disease experienced similar survival benefits. This may reflect the inadequacy of RECIST criteria in categorizing responses to molecular therapy. More specifically, changes in tumor density do not always correlate with alterations in tumor size.20 (figure 1)
Figure 1.
caption as embedded in figure, reprinted with permission from Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CD, Joensuu H. N Engl J Med. 2002 Aug 15;347(7):472–80.
Kaplan-Meier Estimates of Overall Survival and Time to Treatment Failure for All Patients.
Each arrowhead represents the point at which a patient’s data were censored.
2. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033.
Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, Raymond AK, Bramwell VH, Baker LH, Maki RG, Tanaka M, Hecht JR, Heinrich MC, Fletcher CD, Crowley JJ, Borden EC. J Clin Oncol. 2008 Feb 1;26(4):626–32
Hypothesis: High-dose imatinib (400 mg twice daily) achieves better progression-free and overall survival than conventional-dose imatinib (400 mg once daily).
| # Patients Randomized |
Study Groups |
Stratification | Significance Demonstrated |
% Change Identified in Trial |
|---|---|---|---|---|
| 746 | Imatinib 400 mg daily n = 345 Imatinib 400 mg twice daily n = 349 |
By Zubrod performance status (0–2 vs. 3) and by disease status (measurable vs. unmeasurable) | No significant differences in progression-free survival or overall survival | p = ns |
Published Abstract: PURPOSE: To assess potential differences in progression-free or overall survival when imatinib mesylate is administered to patients with incurable gastrointestinal stromal tumors (GIST) at a standard dose (400 mg daily) versus a high dose (400 mg twice daily). PATIENTS AND METHODS: Patients with metastatic or surgically unresectable GIST were eligible for this phase III open-label clinical trial. At registration, patients were randomly assigned to either standard or high-dose imatinib, with close interval follow-up. If objective progression occurred by Response Evaluation Criteria in Solid Tumors, patients on the standard-dose arm could reregister to the trial and receive the high-dose imatinib regimen. RESULTS: Seven hundred forty-six patients with advanced GIST from 148 centers across the United States and Canada were enrolled onto this trial in 9 months. With a median follow-up of 4.5 years, median progression-free survival was 18 months for patients on the standard-dose arm, and 20 months for those receiving high-dose imatinib. Median overall survival was 55 and 51 months, respectively. There were no statistically significant differences in objective response rates, progression-free survival, or overall survival. After progression on standard-dose imatinib, 33% of patients who crossed over to the high-dose imatinib regimen achieved either an objective response or stable disease. There were more grade 3, 4, and 5 toxicities noted on the high-dose imatinib arm. CONCLUSION: This trial confirms the effectiveness of imatinib as primary systemic therapy for patients with incurable GIST but did not show any advantage to higher dose treatment. It appears reasonable to initiate therapy with 400 mg daily and to consider dose escalation on progression of disease. (Copyright © 2008 American Society of Clinical Oncology. Reprinted with permission.)
Editor’s Summary and Comments: This multicenter, phase III trial was designed to investigate the appropriate dose of imatinib in patients with surgically incurable GIST, following on results of the earlier phase II trial. Imatinib was fairly well-tolerated at both doses, although dose reductions and grade 3–5 toxicities were more common in the high-dose arm. No significant differences were identified in progression-free survival or overall survival on intention-to-treat analyses. However, of the 133 patients that crossed over, 118 were able to be assessed for response, and among these, median progression-free survival was 5 months. These results suggest a possible benefit to increasing treatment dose consequent to conventional dose failure. Based on the results of this study, it is reasonable to presume that conventional dose imatinib is appropriate for most patients, with high-dose therapy reserved for patients progressing on a conventional dose.
3. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial.
Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M, Bertulli R, Judson I. Lancet. 2004 Sep 25-Oct 1;364(9440):1127–34.
Hypothesis: High-dose imatinib (400 mg twice daily) achieves better progression-free survival than low-dose imatinib (400 mg once daily).
| # Patients Randomized |
Study Groups | Stratification | Significance Demonstrated |
% Change Identified in Trial |
|---|---|---|---|---|
| 946 | 400 mg imatinib once daily n = 473 400 mg imatinib twice daily n = 473 |
None | 400 mg imatinib daily results in partial response of disease while a dose of 400 mg imatinib twice daily improves progression-free survival | Progression-free survival: Hazard ratio 0.82 (95% CI 0.69–0.98, p = 0.026) |
Published Abstract: BACKGROUND: Imatinib is approved worldwide for use in gastrointestinal stromal tumours (GIST). We aimed to assess dose dependency of response and progression-free survival with imatinib for metastatic GIST. METHODS: 946 patients were randomly allocated imatinib 400 mg either once or twice a day. Those assigned the once a day regimen who had progression were offered the option of crossover. The primary endpoint was progression-free survival. Analysis was by intention to treat. FINDINGS: At median follow-up of 760 days (IQR 644–859), 263 (56%) of 473 patients allocated imatinib once a day had progressed compared with 235 (50%) of 473 who were assigned treatment twice a day (estimated hazard ratio 0.82 [95% CI 0.69–0.98]; p=0.026). Side-effects arose in 465/470 (99%) patients allocated the once daily regimen compared with 468/472 (99%) assigned treatment twice a day. By comparison with the group treated once a day, more dose reductions (77 [16%] vs 282 [60%]) and treatment interruptions (189 [40%] vs 302 [64%]) were recorded in patients allocated the twice daily regimen, but treatment in both arms was fairly well tolerated. 52 (5%) patients achieved a complete response, 442 (47%) a partial response, and 300 (32%) stable disease, with no difference between groups. Median time to best response was 107 days (IQR 58–172). INTERPRETATION: If response induction is the only aim of treatment, a daily dose of 400 mg of imatinib is sufficient; however, a dose of 400 mg twice a day achieves significantly longer progression-free survival. (Copyright © Elsevier 2004)
Editor’s Summary and Comments: The European Organization for Research and Treatment of Cancer (EORTC) Soft Tissue and Bone Sarcoma Group, the Italian Sarcoma Group (ISG) and the Australasian Gastrointestinal Trials Group (AGITG) performed a worldwide, prospective, randomized trial to investigate once versus twice daily imatinib for metastatic GIST. At a median follow up of just over two years, only 6% fewer patients had disease progression with the twice daily dosing. While this did translate into a statistically significant increase in PFS, the effect was modest. Patients in the higher dose arm had slightly more dose reductions and treatment interruptions due to toxicities. Moreover, in the intention-to-treat analysis, treatment responses were remarkably similar between the two treatment groups. This trial both suggests that a lower dose of imatinib is adequate for treatment if a response is seen and establishes 400 mg as the standard dose of imatinib for treating advanced GIST.
A subsequent follow up study16 evaluated the feasibility, safety and efficacy of crossing-over from low-dose to high-dose imatinib upon progression of disease. Of the patients possible for cross-over, 55% (133/241) crossed over. While toxicities were often not high grade, slightly more than half of patients did not tolerate the dose escalation. Cross-over resulted in a 33% increase in time to progression. Based on this post-hoc analysis, cross-over to high-dose imatinib is feasible and safe in GIST patients who progress on low-dose therapy and it also appears to delay disease progression suggesting that there is therapeutic utility to an imatinib dose increase in patients with progression of disease.
4. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group.
Blay JY, Le Cesne A, Ray-Coquard I, Bui B, Duffaud F, Delbaldo C, Adenis A, Viens P, Rios M, Bompas E, Cupissol D, Guillemet C, Kerbrat P, Fayette J, Chabaud S, Berthaud P, Perol D. J Clin Oncol. 2007 Mar 20;25(9):1107–13.
Hypothesis: Continuous imatinib improves progression-free survival in patients with advanced GIST as compared with interrupted imatinib beyond one year of treatment.
| # Patients Randomized |
Study Groups |
Stratification | Significance Demonstrated |
% Change Identified in Trial |
|---|---|---|---|---|
| 58 | interrupted imatinib n = 32 continuous imatinib n = 26 |
None | Improvement in progression-free survival with continuous imatinib therapy versus interrupted imatinib therapy | Median PFS 18 months (95% CI 15.0–23.6) vs. 18 months (95% CI 3.5–9.7), p < 0.0001) |
Published Abstract: PURPOSE: Imatinib is the standard treatment of advanced GI stromal tumors (GISTs). It is not known whether imatinib may be stopped in patients in whom disease is controlled. METHODS: This prospective, randomized, multicentric phase III study was designed to compare continuous (CONT) compared with interrupted (INT) imatinib beyond 1 year of treatment in patients with advanced GIST. The primary end point was progression-free survival. Secondary end points included overall survival, response rate after reinitiation of imatinib, and quality of life. Early stopping rules in cases of rapid progression of disease were defined, with preplanned interim analyses. RESULTS: Between May 2002 and April 2004, 182 patients with advanced GIST were enrolled. Between May 2003 and April 2004, 98 patients in response or stable disease under imatinib reached more than 1 year of follow-up. Forty were not eligible for randomization, and 58 patients were randomly assigned, 32 and 26 patients in the INT and CONT arms, respectively. As of October 15, 2005, eight of 26 patients in the CONT group and 26 of 32 patients in the INT group had documented disease progression (P < .0001). Twenty-four of 26 patients with documented progression in the INT arm responded to imatinib reintroduction. No differences in overall survival or imatinib resistance were observed between the two arms. Quality of life evaluated 6 months after random assignment using the 30-item Quality of Life Questionnaire was not significantly different between the two groups of randomly assigned patients. CONCLUSION: Imatinib interruption results in rapid progression in most patients with advanced GIST, and cannot be recommended in routine practice unless patient experience significant toxicity. (Copyright © 2007 American Society of Clinical Oncology. Reprinted with permission.)
Editor’s Summary and Comments: This prospective, randomized, multicenter phase III trial was designed to determine whether imatinib therapy may be discontinued in patients whose disease is controlled following one year of treatment. The data suggested that therapy should be administered without interruption. During a two-year period, 182 patients were enrolled. Following one year of treatment with imatinib, patients with metastatic or unresectable GIST that had either stable or partially responsive disease were randomly assigned either to continue or to discontinue imatinib therapy. Patients who were randomized to interrupted therapy were able to cross over to the treatment arm if they had progression of disease. Of this arm, 81% (26/32) crossed over. Of these patients, 92% (24/26) responded to re-introduction of therapy. This suggested that even if there is no disease seen on a CT scan, viable microscopic disease remains after imatinib therapy. Lending further validity to this concept, no patients in the continuous treatment arm had progression of disease at undetectable disease sites. While there was no difference in overall survival, imatinib-resistance, or quality of life, continuous treatment resulted in a significantly longer progression-free survival. This trial affirmed that imatinib therapy should not be sporadic and demonstrated that for patients wherein imatinib was discontinued, early progression is expected.
5. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial.
Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG. Lancet. 2006 Oct 14;368(9544):1329–38.
Hypothesis: Sunitinib improves progression-free survival in patients with unresectable GIST who have failed imatinib therapy compared with placebo.
| # Patients Randomized |
Study Groups |
Stratification | Significance Demonstrated |
% Change Identified in Trial |
|---|---|---|---|---|
| 312 | Sunitinib n = 207 Placebo n = 105 |
Based on prior outcome on imatinib treatment: progression within 6 months vs progression beyond 6 months or intolerance to imatinib | Improvement in progression-free survival | Hazard ratio 0.33 (95% CI 0.23–0.47, p < 0.0001) |
Published Abstract: BACKGROUND: No effective therapeutic options for patients with unresectable imatinib-resistant gastrointestinal stromal tumour are available. We did a randomised, double-blind, placebo-controlled, multicentre, international trial to assess tolerability and anticancer efficacy of sunitinib, a multi-targeted tyrosine kinase inhibitor, in patients with advanced gastrointestinal stromal tumour who were resistant to or intolerant of previous treatment with imatinib. METHODS: Blinded sunitinib or placebo was given orally once daily at a 50-mg starting dose in 6-week cycles with 4 weeks on and 2 weeks off treatment. The primary endpoint was time to tumour progression. Intention-to-treat, modified intention-to-treat, and per-protocol analyses were done. This study is registered at ClinicalTrials.gov, number NCT00075218. FINDINGS: 312 patients were randomised in a 2:1 ratio to receive sunitinib (n=207) or placebo (n=105); the trial was unblinded early when a planned interim analysis showed significantly longer time to tumour progression with sunitinib. Median time to tumour progression was 27.3 weeks (95% CI 16.0–32.1) in patients receiving sunitinib and 6.4 weeks (95% CI 4.4–10.0) in those on placebo (hazard ratio 0.33; p<0.0001). Therapy was reasonably well tolerated; the most common treatment-related adverse events were fatigue, diarrhoea, skin discolouration, and nausea. INTERPRETATION: We noted significant clinical benefit, including disease control and superior survival, with sunitinib compared with placebo in patients with advanced gastrointestinal stromal tumour after failure and discontinuation of imatinib. Tolerability was acceptable. (Copyright © Elsevier 2006)
Editor’s Summary and Comments: In this international trial involving 56 centers, Demetri and colleagues validated the efficacy of sunitinib as a second-line therapy in advanced GIST. Patients that had failed imatinib therapy, either by disease progression or drug intolerance, were enrolled. Initially powered to detect a 50% improvement in median progression-free survival, the study was unblinded early after a planned interim analysis. Using intention-to-treat analysis, progression-free survival was superior in the sunitinib arm, extending the median time to progression by over 4 months. The threshold for difference in outcome was low since sunitinib was being compared to placebo. Although not designed to evaluate overall survival, early data suggested an overall survival advantage in the sunitinib arm (HR of death 0.49, 95% CI 0.29–0.83). Given the limited options for patients who fail imatinib therapy, this study affirmed the viability of sunitinib as a second-line therapy.
6. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial.
Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, Blackstein ME, Blanke CD, von Mehren M, Brennan MF, Patel S, McCarter MD, Polikoff JA, Tan BR, Owzar K; on behalf of the American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team. Lancet. 2009 Mar 28;373(9669):1097–104.
Hypothesis: Imatinib 400 mg daily administered for one year after complete resection of localized, primary GIST improves recurrence free survival over placebo.
| # Patients Randomized |
Study Groups |
Stratification | Significance Demonstrated |
% Change Identified in Trial |
|---|---|---|---|---|
| 713 | Imatinib 400 mg daily n = 359 Placebo n = 354 |
By tumor size: ≥3-<6 cm, ≥6-<10 cm, or ≥10 cm | Improvement in recurrence free survival | Hazard ratio 0.35 (95% CI 0.22–0.53, p<0.0001) |
Published Abstract: BACKGROUND: Gastrointestinal stromal tumour is the most common sarcoma of the intestinal tract. Imatinib mesylate is a small molecule that inhibits activation of the KIT and platelet-derived growth factor receptor alpha proteins, and is effective in first-line treatment of metastatic gastrointestinal stromal tumour. We postulated that adjuvant treatment with imatinib would improve recurrence-free survival compared with placebo after resection of localised, primary gastrointestinal stromal tumour. METHODS: We undertook a randomised phase III, double-blind, placebo-controlled, multicentre trial. Eligible patients had complete gross resection of a primary gastrointestinal stromal tumour at least 3 cm in size and positive for the KIT protein by immunohistochemistry. Patients were randomly assigned, by a stratified biased coin design, to imatinib 400 mg (n=359) or to placebo (n=354) daily for 1 year after surgical resection. Patients and investigators were blinded to the treatment group. Patients assigned to placebo were eligible to crossover to imatinib treatment in the event of tumour recurrence. The primary endpoint was recurrence-free survival, and analysis was by intention to treat. Accrual was stopped early because the trial results crossed the interim analysis efficacy boundary for recurrence-free survival. This study is registered with ClinicalTrials.gov, number NCT00041197. FINDINGS: All randomised patients were included in the analysis. At median follow-up of 19.7 months (minimum-maximum 0–56.4), 30 (8%) patients in the imatinib group and 70 (20%) in the placebo group had had tumour recurrence or had died. Imatinib significantly improved recurrence-free survival compared with placebo (98% [95% CI 96–100] vs 83% [78–88] at 1 year; hazard ratio [HR] 0.35 [0.22–0.53]; one-sided p<0.0001). Adjuvant imatinib was well tolerated, with the most common serious events being dermatitis (11 [3%] vs 0), abdominal pain (12 [3%] vs six [1%]), and diarrhoea (ten [2%] vs five [1%]) in the imatinib group and hyperglycaemia (two [<1%] vs seven [2%]) in the placebo group. INTERPRETATION: Adjuvant imatinib therapy is safe and seems to improve recurrence-free survival compared with placebo after the resection of primary gastrointestinal stromal tumour. FUNDING: US National Institutes of Health and Novartis Pharmaceuticals. (Copyright © Elsevier 2009)
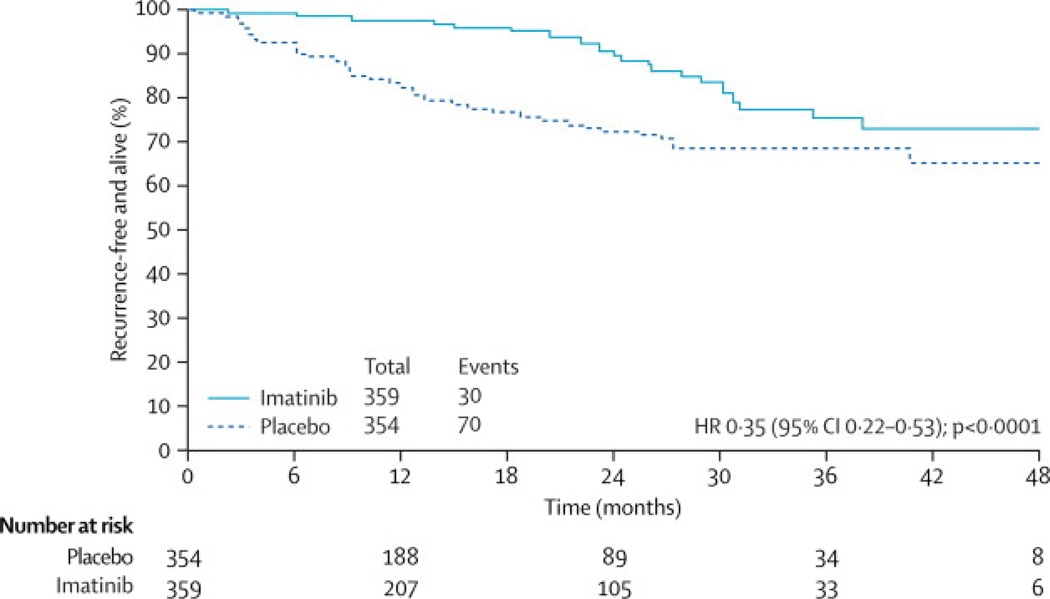
Editor’s Summary and Comments: While all other randomized controlled trials to date have explored the use of therapies in surgically incurable GIST, DeMatteo and colleagues evaluated the use of imatinib in an adjuvant setting, recognizing the significant risk of tumor recurrence following complete surgical resection. This trial was originally designed with overall survival as the primary endpoint, at a time when the efficacy of imatinib in advanced GIST had not yet been fully recognized. As the efficacy of imatinib became apparent, the study was redesigned to detect a 40% improvement in recurrence-free survival with a 90% power. The trial was closed early after interim analysis demonstrated efficacy in the therapy arm. With a median follow-up of nearly 20 months, a clear improvement in recurrence-free survival in the treatment arm was demonstrated. This effect was observed across all groups of the trial stratification. Differences in overall survival have not been demonstrated so far. In addition, the recurrence rate in the imatinib group was noted to increase appreciably around 18 months after surgery, raising the concern that one year of therapy may be inadequate for patients at high risk for recurrence. While this study clearly demonstrates that empiric adjuvant imatinib reduces the rates of early recurrence, it is not yet clear whether this strategy improves overall survival over a strategy of watchful waiting. Furthermore, optimal patient selection criteria remain to be determined. (FIG 2)
Figure 2.
Recurrence-free survival. Reprinted with permission from Ronald P DeMatteo, Karla V Ballman, Cristina R Antonescu et al, Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial, The Lancet, Volume 373, Issue 9669, 28 March 2009-3 April 2009, Pages 1097–1104,
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Dr. DeMatteo has received honoraria and served as a consultant for Novartis.
Contributor Information
Peter A. Learn, Email: learnp@mskcc.org.
Jason K. Sicklick, Email: sicklicj@mskcc.org.
Ronald P. DeMatteo, Email: dematter@mskcc.org.
REFERENCES
- 1.Katz SC, DeMatteo RP. Gastrointestinal stromal tumors and leiomyosarcomas. J Surg Oncol. 2008;97:350. doi: 10.1002/jso.20970. [DOI] [PubMed] [Google Scholar]
- 2.Dematteo RP, Heinrich MC, El-Rifai WM, et al. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002;33:466. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 3.Edmonson JH, Marks RS, Buckner JC, et al. Contrast of response to dacarbazine, mitomycin, doxorubicin, and cisplatin (DMAP) plus GM-CSF between patients with advanced malignant gastrointestinal stromal tumors and patients with other advanced leiomyosarcomas. Cancer Invest. 2002;20:605. doi: 10.1081/cnv-120002485. [DOI] [PubMed] [Google Scholar]
- 4.Trent JC, Beach J, Burgess MA, et al. A two-arm phase II study of temozolomide in patients with advanced gastrointestinal stromal tumors and other soft tissue sarcomas. Cancer. 2003;98:2693. doi: 10.1002/cncr.11875. [DOI] [PubMed] [Google Scholar]
- 5.Von Burton G, Rankin C, Zalupski MM, et al. Phase II trial of gemcitabine as first line chemotherapy in patients with metastatic or unresectable soft tissue sarcoma. Am J Clin Oncol. 2006;29:59. doi: 10.1097/01.coc.0000195088.28956.dd. [DOI] [PubMed] [Google Scholar]
- 6.Blair SC, Zalupski MM, Baker LH. Ifosfamide and etoposide in the treatment of advanced soft tissue sarcomas. Am J Clin Oncol. 1994;17:480. doi: 10.1097/00000421-199412000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 8.Kingham TP, DeMatteo RP. Multidisciplinary treatment of gastrointestinal stromal tumors. Surg Clin North Am. 2009;89:217. doi: 10.1016/j.suc.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nilsson B, Nilsson O, Ahlman H. Treatment of gastrointestinal stromal tumours: imatinib, sunitinib -- and then? Expert Opin Investig Drugs. 2009;18:457. doi: 10.1517/13543780902806400. [DOI] [PubMed] [Google Scholar]
- 10.Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 11.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 12.Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. Journal of Clinical Oncology. 2008;26:620. doi: 10.1200/JCO.2007.13.4403. [DOI] [PubMed] [Google Scholar]
- 13.Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. Journal of Clinical Oncology. 2008;26:626. doi: 10.1200/JCO.2007.13.4452. [DOI] [PubMed] [Google Scholar]
- 14.Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. Journal of Clinical Oncology. 2007;25:1107. doi: 10.1200/JCO.2006.09.0183. [DOI] [PubMed] [Google Scholar]
- 15.Van Glabbeke M, Owzar K, Rankin C, et al. Comparisonof two doses of imatinib for the treatment of gastrointestinal stromal tumors (GIST): a meta-analysis based on 1,640 patients. Journal of Clinical Oncology. 2007;25:546s. [Google Scholar]
- 16.Zalcberg JR, Verweij J, Casali PG, et al. Outcome of patients with advanced gastrointestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. European Journal of Cancer. 2005;41:1751. doi: 10.1016/j.ejca.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 17.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329. doi: 10.1016/S0140-6736(06)69446-4. [see comment] [DOI] [PubMed] [Google Scholar]
- 18.Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, doubleblind, placebo-controlled trial. Lancet. 2009;373:1097. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99:42. doi: 10.1002/jso.21160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]