Abstract
l-DOPA-induced dyskinesias or abnormal involuntary movements (AIMs) are a debilitating adverse complication associated with prolonged l-DOPA administration for Parkinson's disease. Few treatments are currently available for dyskinesias. Our recent data showed that nicotine reduced l-DOPA-induced AIMs in parkinsonian animal models. An important question is the nicotinic acetylcholine receptor (nAChR) subtypes through which nicotine exerts this beneficial effect, because such knowledge would allow for the development of drugs that target the relevant receptor population(s). To address this, we used β2 nAChR subunit knockout [β2(−/−)] mice because β2-containing nAChRs are key regulators of nigrostriatal dopaminergic function. All of the mice were lesioned by intracranial injection of 6-hydroxydopamine into the right medial forebrain bundle. Lesioning resulted in a similar degree of nigrostriatal damage and parkinsonism in β2(−/−) and wild-type mice. All of the mice then were injected with l-DOPA (3 mg/kg) plus benserazide (15 mg/kg) once daily for 4 weeks until AIMs were fully developed. l-DOPA-induced AIMs were approximately 40% less in the β2(−/−) mice compared with the wild-type mice. It is interesting to note that nicotine (300 μg/ml in drinking water) reduced l-DOPA-induced AIMs by 40% in wild-type mice but had no effect in β2(−/−) mice with partial nigrostriatal damage. The nicotine-mediated decline in AIMs was much less pronounced in wild-type mice with near-complete degeneration, suggesting that presynaptic nAChRs on dopaminergic terminals have a major influence. These data demonstrate an essential role for β2* nAChRs in the antidyskinetic effect of nicotine and suggest that drugs targeting these subtypes may be useful for the management of l-DOPA-induced dyskinesias in Parkinson's disease.
Introduction
Loss of dopaminergic neurons in the nigrostriatal pathway leads to Parkinson's disease, a movement disorder characterized by rigidity, tremor, and bradykinesia. l-DOPA treatment alleviates these symptoms; however, its long-term administration results in the development of adverse effects, including debilitating abnormal involuntary movements (AIMs) or dyskinesias (Fahn, 2008; Poewe, 2009; Calabresi et al., 2010). Although the precise molecular alterations that underlie l-DOPA-induced dyskinesias remain elusive, their occurrence is associated with changes in dopamine receptors and their downstream signaling mechanisms. In addition, there are abnormalities in nondopaminergic neurotransmitter pathways linked to the nigrostriatal system, including the serotonergic, glutamatergic, adrenergic, opioid, and adenosine systems (Fox et al., 2008; Barroso-Chinea and Bezard, 2010; Cenci and Konradi, 2010). Converging evidence also suggests that the nicotinic cholinergic system plays a role, with recent data showing that nicotine administration reduced l-DOPA-induced AIMs in parkinsonian rats and monkeys (Quik et al., 2007; Bordia et al., 2008). These data suggest that drugs targeting the nicotinic cholinergic system may be of therapeutic benefit in the treatment of l-DOPA-induced dyskinesias.
Recent studies suggest that nicotine exerts its antidyskinetic effect through an interaction at nicotinic acetylcholine receptors (nAChRs). Evidence for this stems from studies showing that the nonselective nAChR blocker mecamylamine modulated l-DOPA-induced AIMs in rats (Bordia et al., 2010) and that nonselective nAChR agonists reduce l-DOPA-induced AIMs (Huang et al., 2011). The question that now arises is which central nervous system nAChR populations are involved, because such knowledge will allow for the development of drugs with optimal antidyskinetic effects and minimal adverse responses.
nAChRs are ligand-gated cation channels composed of α and β subunits. To date, five ligand binding α (α2, α3, α4, α6, and α7), two structural β (β2 and β4), and two accessory subunits (β3 and α5) have been identified in neuronal tissues (Albuquerque et al., 2009; Millar and Gotti, 2009). These receptor subunits coassemble to form a diverse family of nAChRs. The most abundant subtypes in the nervous system are homomeric α7 nAChRs and heteromeric β2* nAChRs, where the asterisk indicates the possible presence of other nicotinic subunits in the receptor complex. There are two primary subpopulations of β2* nAChRs. One of these are the α4β2* nAChRs, which have a widespread localization and are abundantly expressed in the nigrostriatal pathway. The others are α6β2* nAChRs that are relatively restricted in the central nervous system, including their presence in the nigrostriatal system (Grady et al., 2007; Millar and Gotti, 2009; Quik et al., 2009). Homomeric α7 nAChRs also have been identified in the nigrostriatal system but only in that of the mouse, making their relevance to nigrostriatal function uncertain.
Consistent with the receptor studies, the β2* nAChRs are the ones principally involved in regulating nigrostriatal dopaminergic function (Grady et al., 2007; Exley and Cragg, 2008; Barik and Wonnacott, 2009). Measurement of [3H]dopamine release from synaptosomes or endogenous dopamine release using cyclic voltammetry shows that β2* nAChRs are responsible for all of the nAChR-mediated release from the striatum in rodents and monkeys (Grady et al., 1992; McCallum et al., 2006; Exley et al., 2008; Meyer et al., 2008; Perez et al., 2008). This idea is further substantiated by studies showing that nicotine-induced dopamine release is abolished in β2 nAChR null mutant mice (Salminen et al., 2004).
In view of the importance of β2* nAChRs in nigrostriatal function, we initiated experiments to determine whether these subtypes also were involved in the nAChR-mediated decline in l-DOPA-induced dyskinesias. In this study, we used β2(−/−) mice, because this model offers the advantage that it specifically identifies the role of β2* nAChRs. We first verified the antidyskinetic effect of nicotine in control wild-type parkinsonian mice, because this had not been tested previously. Subsequent work to investigate the effect of nicotine in parkinsonian β2 knockout and wild-type mice showed that β2* nAChRs are the primary receptors that mediate the antidyskinetic effect of nicotine, without impeding the antiparkinsonian effect of l-DOPA. Drugs directed to β2* nAChRs thus may be optimal for reducing l-DOPA-induced dyskinesias.
Materials and Methods
6-Hydroxydopamine Lesioning.
Adult control mice were purchased from Charles River Laboratories, Inc. (Wilmington, MA). Wild-type and β2(−/−) C57BL/6 mice were bred at the University of Colorado, as described previously (Salminen et al., 2004), and then shipped to SRI International for further study. Mice were group-housed in a temperature- and humidity-controlled environment under a 12-h light/dark cycle with free access to food and water. After an acclimation period of 2 to 3 days, they were lesioned by injection of 6-hydroxydopamine (6-OHDA) into the medial forebrain bundle to produce nigrostriatal dopaminergic damage (Cenci and Lundblad, 2007). In brief, mice were anesthetized with isoflurane (3%) and then placed in a stereotaxic instrument (David Kopf Instruments, Tujunga, CA). A burr hole was drilled through the skull, and 3 μg of 6-OHDA (3 μg/μl) was injected stereotaxically at the site into the right ascending medial forebrain bundle. The coordinates for the position of the site were as follows relative to the Bregma and the dural surface: anteroposterior, −1.2; lateral, −1.2; ventral, 4.75. All of the procedures were approved by the Institutional Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Nicotine Treatment.
Mice first were given a 2% saccharin solution for 2 days, and then nicotine (free base, 25 μg/ml) was administered in the drinking water for another 2 days. The dose of nicotine in the drinking water was increased gradually (nicotine titration) to 300 μg/ml over 10 days and subsequently maintained at that dose, as described previously (Sparks and Pauly, 1999; Pietilä and Ahtee, 2000; Lai et al., 2005).
l-DOPA Treatment and AIMs Measurement.
l-DOPA methyl ester and benserazide hydrochloride (a DOPA decarboxylase inhibitor given to inhibit the breakdown of l-DOPA in the periphery) were purchased from Sigma-Aldrich (St. Louis, MO). The indicated doses of l-DOPA were freshly dissolved in saline and injected subcutaneously once daily in combination with a fixed dose of 15 mg/kg benserazide throughout the study. AIMs were scored by a blinded rater using a modified mouse dyskinesia rating scale described previously (Cenci and Lundblad, 2007). In brief, mice were placed in separate cages and scored individually every 15 min (1-min monitoring periods) for 2 h 10 min after l-DOPA injection. The scale for different subtypes of AIMs (oral, forelimb, and axial) includes frequency and amplitude. Each AIM subtype was scored on a frequency scale from 0 to 4 (0 = no dyskinesia; 1 = occasional dyskinesia displayed for <50% of the observation time; 2 = sustained dyskinesia displayed for >50% of the observation time; 3 = continuous dyskinesia; 4 = continuous dyskinesia not interruptible by external stimuli). The amplitude was classified into two levels either “A” or “B.” “A” indicates oral AIMs without tongue protrusion, forelimb AIMs without the shoulder engaged, and axial AIMs with body twisting <60°. “B” indicates oral AIMs with tongue protrusion, forelimb AIMs with the shoulder engaged, or axial AIMs with body twisting ≥60°. The integrated scores for frequency and amplitude of AIMs used for data analysis were calculated as 1A = 1, 1B = 2, 2A = 2, 2B = 4, 3A = 4, 3B = 6, 4A = 6, and 4B = 8. This allows for scores for any one component (axial, oral, or forelimb) ranging from 0 to 8, with a maximum possible total score per time point of 24.
Limb Use Asymmetry Test.
The forelimb asymmetry (cylinder test) was used as a measure of parkinsonism (Schallert et al., 2000; Brooks and Dunnett, 2009). Exploratory activity was evaluated by a blinded rater for a 3-min period immediately before and 45 min after l-DOPA administration. Wall exploration was expressed in terms of the percentage use of the impaired forelimb (contralateral) compared with the total number of limb use movements.
Plasma Cotinine Levels.
The nicotine metabolite cotinine was determined as an index of nicotine intake. Cotinine is one of the primary metabolites of nicotine with an 18-h half-life and provides a good measure of nicotine intake (Lai et al., 2005; Matta et al., 2007). Blood (∼0.2 ml) from the ocular vein was drawn under isoflurane anesthesia. Cotinine was measured using an enzyme immunoassay kit according to the manufacturer's instructions (OraSure Technologies, Bethlehem, PA).
Dopamine Transporter Autoradiography.
Mice were killed by cervical dislocation 1 h after l-DOPA injection, and the brains were removed quickly and frozen in isopentane on dry ice. Eight-micrometer sections were cut at −15°C in a cryostat, thaw-mounted onto poly-l-lysine-coated slides, dried, and stored at −80°C. [125I]3β-(4-Iodophenyl)tropane-2β-carboxylic acid isopropyl ester (RTI-121) (specific activity, 2200 Ci/mmol; GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) autoradiography was performed as described previously (Quik et al., 2003). Brain sections were preincubated twice for 15 min in buffer [50 mM Tris-HCl (pH 7.4), 120 mM NaCl, and 5 mM KCl] and then incubated for 2 h with 100 pM [125I]RTI-121 in the same buffer also containing 0.025% bovine serum albumin and 1 μM fluoxetine. Sections were washed four times for 15 min in ice-cold buffer, once for 10 s in ice-cold doubly distilled H2O, dried, and exposed to Kodak MR film (Eastman Kodak, Rochester, NY) for 2 to 3 days along with 3H microscale standards (GE Healthcare). Nonspecific binding was determined in the presence of the dopamine uptake inhibitor nomifensine (100 μM).
[125I]Epibatidine Binding.
Striatal α4β2* nAChRs were measured using binding of [125I]epibatidine (specific activity, 2200 Ci/mmol; GE Healthcare) (Quik et al., 2003). Slides were preincubated in buffer containing 50 mM Tris-HCl, 120 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, and 1.0 mM MgCl2. Slides then were incubated in the same buffer also containing 0.015 nM [125I]epibatidine for 40 min. Nonspecific binding was assessed in the presence of nicotine (100 μM). Slides then were washed, air-dried, and exposed to Kodak MR film. Film was developed for image quantification using the ImageQuant system (GE Healthcare).
Data Analyses.
The ImageQuant program was used to determine optical density values from autoradiographic images. To evaluate specific binding, nonspecific binding was subtracted from total tissue binding. A calibration curve of radioactivity (nCi/mg wet weight tissue) versus optical density was generated using 3H microscale standards. The radioactivity in nanocuries per milligram then was converted to femtomoles per milligram of tissue. The 3H standards were calibrated for 125I autoradiography as described (Artymyshyn et al., 1990), including exposure time, section thickness, and concentration of radioactivity. Care was taken to ensure that the optical density readings of the samples were within the linear range of the film.
Statistical Analyses.
GraphPad Prism (GraphPad Software, Inc., San Diego, CA) was used for data analyses. Differences in ratings between treatment groups were determined using a Mann-Whitney U test or analysis of variance (ANOVA) followed by the appropriate post hoc test. A level of 0.05 was considered significant. Values are the mean ± S.E.M. of the indicated number of mice.
Results
Effect of Nicotine on l-DOPA-Induced AIMs at Varying l-DOPA Doses in Lesioned Control Mice.
Before initiation of work with nAChR knockout mice, experiments were done to investigate whether nicotine reduced l-DOPA-induced AIMs in control parkinsonian mice (Fig. 1). To approach this, we first measured AIMs induced by varying doses of l-DOPA in mice treated with saccharin only (n = 20 mice) or saccharin-containing nicotine (n = 19 mice) in the drinking water. Such studies were done to determine whether nicotine's ability to reduce AIMs is dependent on the magnitude of these abnormal movements. Mice were treated with 300 μg/ml nicotine, because our previous studies had shown that nicotine optimally modulated nAChR levels in mouse brain at this dose (Lai et al., 2005). One group of nicotine-treated mice (n = 10) then was injected once daily subcutaneously with 3 mg/kg l-DOPA plus 15 mg/kg benserazide for 2 weeks, a dose that was expected to result in the development of submaximal AIMs (L. Z. Huang and M. Quik, unpublished observations), and results were compared with those for l-DOPA-treated (3 mg/kg) mice receiving only saccharin in the drinking water (n = 10). Another group of nicotine-treated mice (n = 9) was injected once daily subcutaneously with 6 mg/kg l-DOPA plus 15 mg/kg benserazide (n = 10) for 2 weeks, a dose expected to lead to maximal AIMs (L. Z. Huang and M. Quik, unpublished observations), and results were compared with those for l-DOPA-treated (6 mg/kg) mice receiving only saccharin in the drinking water (n = 10). After 2 weeks of treatment l-DOPA-induced AIMs were tested. The nicotine-treated mice initially injected with 3 and 6 mg/kg l-DOPA then were given either 1 or 2 mg/kg l-DOPA, respectively; the saccharin-treated mice were randomized similarly into groups receiving either 1 or 2 mg/kg l-DOPA, respectively. After 2 weeks of treatment, the animals were rated for AIMs. Thus, for these studies, nicotine was given as a pretreatment, various doses of l-DOPA then were administered, and a nicotine dose response was conducted. This provided a measure of the ability of nicotine to prevent the development of AIMs.
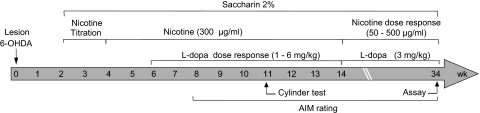
Fig. 1.
Treatment timeline to determine l-DOPA and nicotine dose-response effects. Mice were lesioned unilaterally with 6-OHDA injected into the right medial forebrain bundle at week 0. Two weeks later, they were given a 2% saccharin solution for 2 days. Nicotine treatment then was initiated in the drinking water, starting at 25 μg/ml for 2 days. The dose of nicotine in the drinking water was increased gradually (nicotine titration) to 300 μg/ml over 10 days and subsequently maintained at that dose. After 2 weeks of nicotine treatment at the final dose (300 μg/ml), a dose-response curve was done for l-DOPA (1–6 mg/kg) plus benserazide (15 mg/kg) administration, with l-DOPA plus benserazide administered on a once daily basis throughout the study. The effect of varying nicotine treatment (50–500 μg/ml) next was determined on AIMs using a fixed dose of l-DOPA (3 mg/kg). AIMs were rated throughout the study, and the cylinder test was performed as indicated.
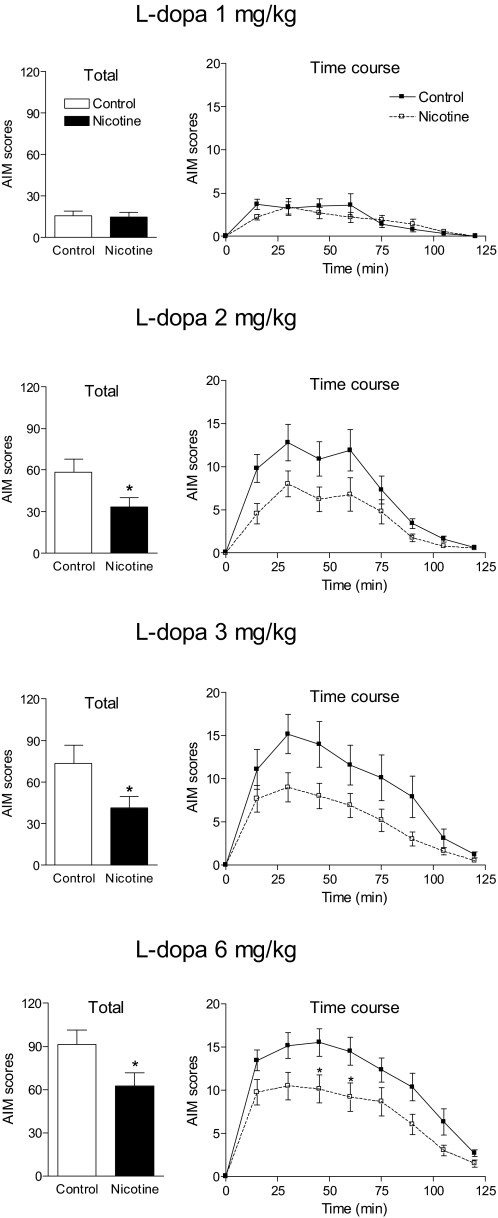
Results in Fig. 2 show that in saccharin-treated mice 1 mg/kg l-DOPA only induced minimal AIMs, which were not affected by nicotine treatment. In contrast, 2 mg/kg l-DOPA induced a more robust response in saccharin-treated mice, with significant AIM scores (∼60) that were reduced by approximately 40% with nicotine treatment (p < 0.05). The time course study at the 2 mg/kg l-DOPA dose shows a significant main effect of nicotine treatment on AIM scores using one-way repeated measures ANOVA (p < 0.05). Total AIM scores with 3 mg/kg l-DOPA were approximately 75 in saccharin-treated mice; these were reduced approximately 45% by nicotine (p < 0.05). Time course analyses with 3 mg/kg l-DOPA also showed a significant main effect of nicotine treatment on AIM scores using one-way repeated measures ANOVA (p < 0.05). In mice treated with 6 mg/kg l-DOPA, AIM scores were increased to approximately 90. In saccharin-treated mice, there was an approximately 30% reduction in total AIMs with nicotine treatment. There was a significant main effect of nicotine with time using one-way repeated measures ANOVA (p < 0.05), with a Bonferroni post hoc test indicating a significant effect of nicotine administration at 45 and 60 min after l-DOPA injection (p < 0.05).
Fig. 2.
Effect of nicotine on AIMs at varying l-DOPA doses in lesioned control mice. Two weeks after 6-OHDA lesioning, nicotine treatment was initiated as outlined in Fig. 1 and detailed under Results. Mice were maintained at the final dose of nicotine (300 μg/ml) for at least 2 weeks, after which various doses of l-DOPA (1, 2, 3, and 6 mg/kg) plus benserazide (15 mg/kg) were administered subcutaneously once daily each for several weeks. Animals were rated for axial, oral, and forelimb AIMs for 1 min every 15 min over a 2-h period, with the total AIMs representing the sum of these three components. Values are the mean ± S.E.M. of 9 to 14 mice. Significance of difference from control: *, p < 0.05. Data were analyzed by Mann-Whitney U test (total AIMs) or one-way repeated measures ANOVA followed by a Bonferroni post hoc test (time course).
Because robust AIM scores were obtained with the 3 mg/kg dose of l-DOPA and the reduction in AIMs scores was maximal with this dose, it was used for further study.
Nicotine at 300 μg/ml Is the Most Effective Dose to Reduce l-DOPA-Induced AIMs in Lesioned Control Mice.
Dose-response experiments then were done in the same group of mice described above to assess the nicotine dose that optimally reduced l-DOPA-induced AIMs in lesioned control mice. We tested a range of 50 to 500 μg/ml nicotine per dose in the drinking water, because previous work had shown that such nicotine doses effectively modulate varying biochemical and behavioral measures in mice (Sparks and Pauly, 1999; Pietilä and Ahtee, 2000; Lai et al., 2005). Before this second study, there was a 4-week nicotine washout period. A group of mice (n = 10) that had received initially only saccharin now were provided again only saccharin in the drinking water. The remainder of the mice were divided randomly into three groups receiving 50 μg/ml (n = 10), 150 μg/ml (n = 10), and 300 μg/ml (n = 9) nicotine in the drinking water for 2 weeks. Mice were injected subcutaneously with 3 mg/kg l-DOPA plus 15 mg/kg benserazide, and AIMs were rated subsequently. The three groups of mice subsequently were administered 300 μg/ml (n = 9), 400 μg/ml (n = 10), and 500 μg/ml (n = 9) nicotine, respectively. They were injected subcutaneously with 3 mg/kg l-DOPA plus 15 mg/kg benserazide, and AIMs were rated again.
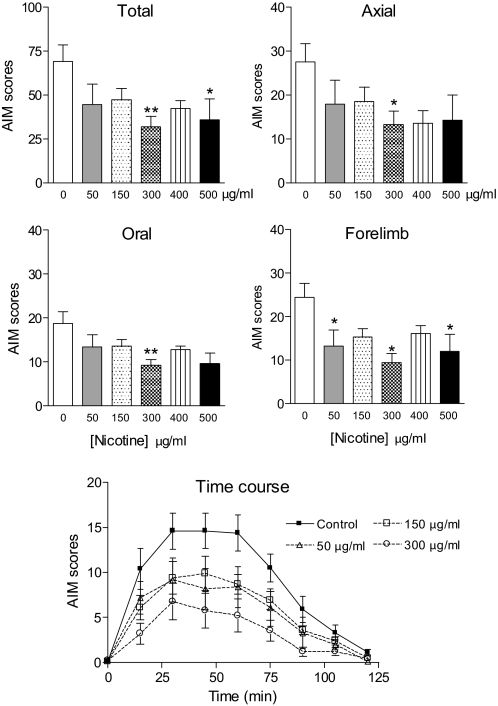
The results in Fig. 3 show that the 300 and 500 μg/ml nicotine doses led to significant decreases in total AIMs (p < 0.01 and 0.05, respectively). There were significant reductions in axial, oral, and forelimb AIMs with 300 μg/ml nicotine (p < 0.05, 0.01, and 0.05, respectively) and in forelimb AIMs with 500 μg/ml nicotine (p < 0.05). There were trends for declines in oral and axial AIMs with 500 μg/ml nicotine. Although total AIMs were not reduced significantly, the 50 μg/ml nicotine dose improved forelimb AIMs (p < 0.05). The 150 and 400 μg/ml nicotine did not have significant effects on total, axial, oral, or forelimb AIMs, although there were trends for the declines in all of the AIM components. Nicotine appeared to reduce AIMs most effectively at the 300 μg/ml dose. This may relate to a narrow therapeutic window, the relatively short treatment times per dose (2 weeks), or represent variability in responsiveness because there were declines at the other doses.
Fig. 3.
Nicotine at a dose of 300 μg/ml optimally decreased l-DOPA-induced AIMs in lesioned control mice. Mice were administered the indicated doses of nicotine or vehicle in the drinking water. After 2 weeks of the indicated dose of nicotine, l-DOPA (3 mg/kg) plus benserazide (15 mg/kg) was injected subcutaneously once daily for several weeks with the nicotine dosing continued. Animals then were rated for axial, oral, and forelimb AIMs for 1 min every 15 min over a 2-h period, with the total AIMs representing the sum of these three components. Values are the mean ± S.E.M. of 9 to 19 mice. Significance of difference from control: *, p < 0.05; **, p < 0.01. Data were analyzed by one-way ANOVA followed by a Dunnett's multiple comparison test (total, axial, oral, and forelimb AIMs) or one-way repeated measures ANOVA followed by a Bonferroni post hoc test (time course). Because nicotine at doses of 400 and 500 μg/ml had no significant effect on the time course of l-DOPA-induced AIMs, the data were omitted from the graph for clarity.
The time courses of the effect of nicotine on total AIMs at the different doses are shown in Fig. 3, bottom. There was an overall decline in AIM scores with the 150 and 300 μg/ml nicotine treatments (p < 0.05 and 0.01, respectively) but not with the 50 μg/ml dose, as assessed using one-way repeated measures ANOVA. Nicotine at doses of 400 and 500 μg/ml had no effect on the time course of l-DOPA-induced AIMs (L. Z. Huang and M. Quik, unpublished observations).
These results demonstrated that the 300 μg/ml nicotine dose most effectively reduced l-DOPA-induced AIMs in lesioned control mice. This dose therefore was selected for the experiments using β2(−/−) mice. Because the two higher doses of nicotine (400 and 500 μg/ml) did not result in a greater decline in AIMs than the 300 μg/ml dose, it is unlikely that the experimental design modified nicotine's effectiveness in reducing AIMs, that is, the fact that the these latter two doses were administered after the 150 and 300 μg/ml doses. In addition, the similar reduction in l-DOPA-induced AIMs with the 300 μg/ml dose in the experiments depicted in Figs. 2 and 3 indicate that the effect of nicotine was similar in preventing the development of AIMs and reducing existing AIMs, at least in mice.
Nicotine Treatment Does Not Affect Parkinsonism Either OFF or ON l-DOPA in Lesioned Control Mice.
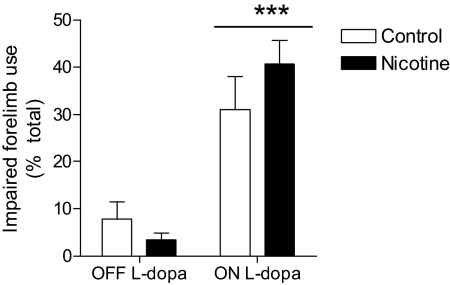
As a measure of parkinsonian behavior, we evaluated the effect of nicotine treatment on exploratory activity before and after l-DOPA treatment using the limb use asymmetry or cylinder test (Schallert et al., 2000; Brooks and Dunnett, 2009). The results in Fig. 4 show that the percentage of impaired forelimb use on the wall in the absence of l-DOPA (OFF l-DOPA) was not significantly different between control (7.8 ± 3.6%, n = 10) and nicotine-treated mice (3.4 ± 1.5%, n = 10). l-DOPA treatment (3 mg/kg) significantly improved impaired forelimb use (ON l-DOPA, p < 0.001) in control and nicotine-treated animals to a similar extent (31.0 ± 7.0%, n = 6, and 40.7 ± 5.0%, n = 8, respectively). These data suggest that nicotine treatment does not worsen behaviors associated with nigrostriatal dopamine damage, which is consistent with previous findings in monkeys and rats (Quik et al., 2007; Bordia et al., 2008).
Fig. 4.
Nicotine did not affect parkinsonism in lesioned control mice either OFF or ON l-DOPA. Mice were rated for forelimb use asymmetry (cylinder test) as an index of motor dysfunction. Impaired forelimb use was measured for 3 min 45 min after subcutaneous administration of l-DOPA (3 mg/kg) plus benserazide (15 mg/kg). Each value represents the mean ± S.E.M. of 6 to 10 mice. There was a significant main effect of l-DOPA treatment (***, p < 0.001). Data were analyzed by two-way ANOVA followed by a Bonferroni post hoc test.
Decline in l-DOPA-Induced AIMs in β2(−/−) Mice with Moderate Nigrostriatal Damage.
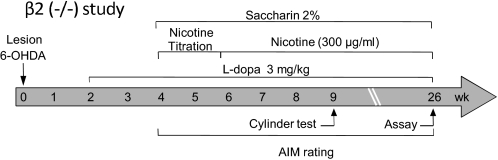
The β2(−/−) mice were used for these studies because β2* nAChR subtypes are the primary regulators of nAChR mediated nigrostriatal dopaminergic function. Wild-type and β2(−/−) mice were lesioned unilaterally with 6-OHDA and treated subsequently according to the timeline in Fig. 5.
Fig. 5.
Treatment timeline for the study using β2(−/−) nAChR and wild-type mice. All of the mice were lesioned unilaterally with 6-OHDA injected into the right medial forebrain bundle at week 0. l-DOPA (3 mg/kg) plus benserazide (15 mg/kg) was administered subcutaneously for 2 weeks. They next were given a 2% saccharin solution for 2 days. Nicotine treatment then was initiated in the drinking water, starting at 25 μg/ml for 2 days. The dose of nicotine in the drinking water was increased gradually (nicotine titration) to 300 μg/ml over 10 days and subsequently maintained at that dose. l-DOPA plus benserazide was administered once daily throughout the study. AIMs were rated, and the cylinder test was performed as indicated. Mice were killed at week 26.
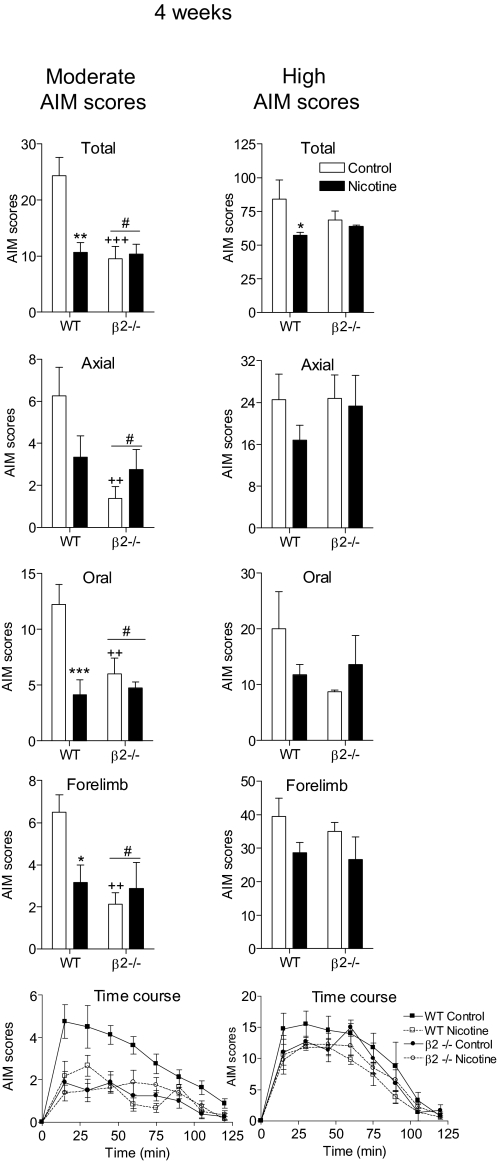
Experiments next were performed to assess whether l-DOPA treatment induced AIMs in lesioned wild-type and β2(−/−) mice. Mice were injected subcutaneously with 3 mg/kg l-DOPA plus 15 mg/kg benserazide and rated for axial, oral, and forelimb AIMs. Our previous data had shown that l-DOPA-induced AIM scores in mice exhibited considerable variability and that the scores tended to fall into two groups, with one group yielding mice with moderate AIMs and the other with high AIM scores. Because drug-induced improvements in l-DOPA-induced AIMs were dependent on AIM severity (Carta et al., 2007; Huang et al., 2011), the mice therefore were divided into a group with moderate AIM scores (<30) and another with high scores (>50). This subdivision into groups with moderate and high AIMs was feasible because there were an equal number of wild-type and β2(−/−) animals with such scores.
In the moderate AIM group, there was an approximately 60% decrease in AIM scores in β2 knockout mice compared with those in wild-type mice (Fig. 6, left). Analyses of the AIM subtypes showed that this was due to significant declines in axial, oral, and forelimb AIMs. In contrast, in knockout mice with high AIM scores, there were no significant declines in AIMs compared with those of their wild-type littermates (Fig. 6, right). These data suggest that β2* nAChRs play a significant role in the development of AIMs in mice with moderate AIM scores. Their reduced role in mice with high AIM scores could suggest that β2* nAChRs on nigrostriatal dopaminergic terminals play a critical role, because these most likely are lost with the extensive nigrostriatal damage generally present in mice with high AIM scores.
Fig. 6.
Nicotine treatment reduces l-DOPA-induced AIMs by interacting at β2* nAChRs. Lesioned mice were injected subcutaneously with l-DOPA (3 mg/kg) plus benserazide (15 mg/kg) for 2 weeks. They then were divided into two groups (moderate and high) based on the severity of the l-DOPA-induced AIM scores. Nicotine treatment was initiated as described, with the mice maintained on the final dose (300 μg/ml) for 4 weeks. Mice then were rated for axial, oral, and forelimb AIMs for 1 min every 15 min over a 2-h period, with the total AIMs representing the sum of these three components. Values are the mean ± S.E.M. of 3 to 8 mice. Significance of difference from control: *, p < 0.05; **, p < 0.01; ***, p < 0.001. Significance of difference from wild-type: #, p < 0.05. Significance of difference from the control wild-type group: ++, p < 0.01; +++, p < 0.001. Data were analyzed by two-way ANOVA (total, axial, oral, and forelimb AIMs) or one-way repeated measures ANOVA followed by a Bonferroni post hoc test (time course).
Nicotine Treatment Does Not Reduce l-DOPA-Induced AIMs in β2(−/−) Mice.
All of the mice subsequently were given 300 μg/ml nicotine in the drinking water. Nicotine treatment significantly decreased (∼60%) l-DOPA-induced total AIMs in wild-type mice with moderate AIM scores (p < 0.01; Fig. 6, left). This was due to a decline in oral (p < 0.001) and forelimb AIMs (p < 0.05), with a trend for a decline in axial AIMs. There was an overall main effect of nicotine in wild-type mice in the time course studies (p < 0.01). Particularly noteworthy is that total, axial, oral, and forelimb AIMs in the untreated β2(−/−) mice with moderate AIM scores were similar to those in the nicotine-treated wild-type animals. In addition, in β2(−/−) mice with moderate AIM scores, nicotine treatment did not result in a further reduction in l-DOPA-induced AIMs (Fig. 6, left). These observations suggest that β2* nAChRs are the major contributors to the nAChR-mediated decline in l-DOPA-induced dyskinesias.
We also evaluated the effect of nicotine in wild-type and β2(−/−) mice with high AIM scores (Fig. 6, right). Nicotine treatment decreased total AIMs in wild-type mice (p < 0.05), with a trend for a decline in the other AIM subtypes. Nicotine reduced l-DOPA-induced AIMs to a lesser extent in wild-type mice with high AIM scores compared with those in wild-type mice with moderate AIM scores, in agreement with our previous finding that nicotine decreases l-DOPA-induced dyskinesias more effectively in partially lesioned parkinsonian rats (Huang et al., 2011). Nicotine treatment did not affect AIM scores in β2 knockout mice compared with those in untreated knockout mice in the high AIM scores group.
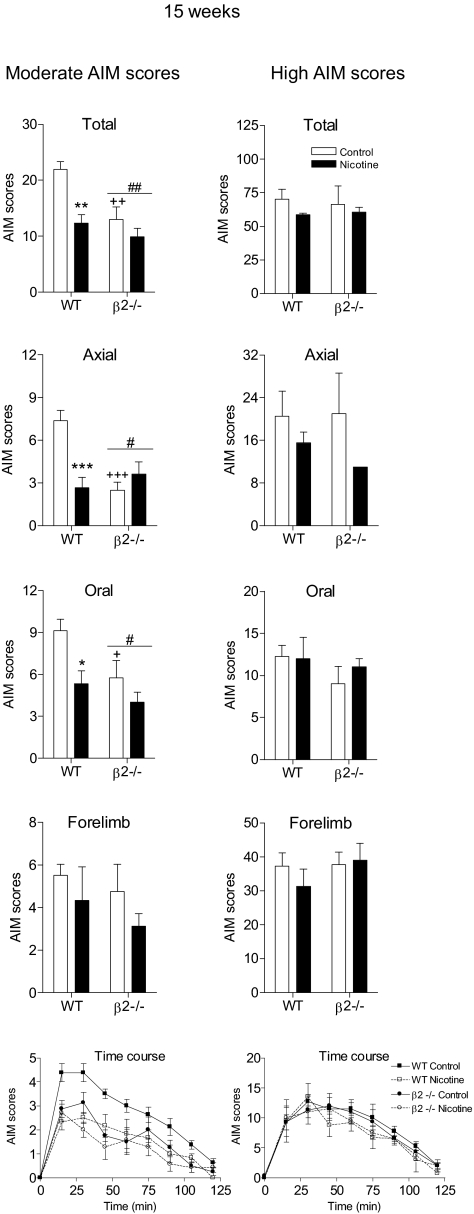
The Reduction in l-DOPA-Induced AIMs in Mice with Moderate AIM Scores Is Maintained with Continued Nicotine Treatment.
The results in Fig. 6 depict the effect of nicotine after 4 weeks of treatment. AIMs also were rated 15 weeks later in mice with moderate and high AIM scores. Similar reductions in total, axial, oral, and forelimb AIMs were obtained in wild-type mice with moderate AIM scores at 15 weeks compared with those at 4 weeks (Fig. 7). There also was a similar pattern of decline in AIM scores in wild-type and knockout mice with nicotine treatment in the time course data (p < 0.08). The β2(−/−) mice developed significantly fewer total AIMs (p < 0.01) compared with wild-type control mice, with no antidyskinetic effect of nicotine in β2(−/−) mice (Fig. 7). There were no significant decreases in l-DOPA-induced AIMs in wild-type or knockout mice with high AIM scores with or without nicotine treatment.
Fig. 7.
Reduction in l-DOPA-induced AIMs is maintained with continued nicotine treatment in mice with moderate AIM scores. Lesioned mice were injected subcutaneously with l-DOPA (3 mg/kg) plus benserazide (15 mg/kg) as outlined in Fig. 5. Nicotine treatment was initiated as described, with the mice maintained on the final dose (300 μg/ml) for 15 weeks. Mice then were rated for axial, oral, and forelimb AIMs for 1 min every 15 min over a 2-h period, with the total AIMs representing the sum of these three components. Values are the mean ± S.E.M. of 3 to 8 mice. Significance of difference from control: *, p < 0.05; **, p < 0.01; ***, p < 0.001. Significance of difference from wild-type: #, p < 0.05; ##, p < 0.01. Significance of difference from the control wild-type group: +, p < 0.05; ++, p < 0.01; +++, p < 0.001. Data were analyzed by two-way ANOVA (total, axial, oral, and forelimb AIMs) or one-way repeated measures ANOVA followed by a Bonferroni post hoc test (time course).
These data overall indicate that the effect of nicotine persists for at least 4 months. They further support the idea that β2* nAChRs are major contributors to the antidyskinetic effect of nicotine.
Nicotine Did Not Affect Parkinsonism in Lesioned Wild-Type and β2(−/−) Mice.
Experiments were done to determine whether nicotine affected parkinsonism in lesioned wild-type or knockout mice either OFF or ON l-DOPA using the forelimb use asymmetry or cylinder test (Table 1). The results in Table 1 show that the percentage of impaired forelimb use was similar in saccharin- and nicotine-treated wild-type mice with moderate AIM scores (OFF l-DOPA). l-DOPA administration significantly improved impaired forelimb use (ON l-DOPA). In the wild-type mice with high AIM scores, the percentage of impaired forelimb use was approximately 17% of total forelimb use (OFF l-DOPA). l-DOPA treatment significantly increased the impaired forelimb use (p < 0.001). However, nicotine did not affect parkinsonism in lesioned wild-type mice. Similar results were obtained for the β2(−/−) mice. l-DOPA injection significantly increased impaired forelimb use (p < 0.001), but nicotine had no effect on exploratory activity either ON or OFF l-DOPA.
TABLE 1.
Measurement of parkinsonism in lesioned wild-type and β2(−/−) mice treated with and without nicotine
Forty-five min after subcutaneous administration of l-DOPA (3 mg/kg) plus benserazide (15 mg/kg), mice were rated for forelimb use asymmetry (cylinder test) for 3 min. The 45-min time point was selected because the effect of l-DOPA is maximal. The values represent the mean ± S.E.M. of the indicated number of mice.
| Group | AIM Score Group | Treatment | No. of Mice | Impaired Forelimb Use |
|
|---|---|---|---|---|---|
| OFF l-DOPA | ON l-DOPA | ||||
| % total | |||||
| Wild-type | Moderate | Saccharin | 8 | 35.3 ± 5.1 | 66.5 ± 8.2* |
| Nicotine | 6 | 39.4 ± 5.6 | 63.7 ± 9.3 | ||
| High | Saccharin | 4 | 17.5 ± 6.9 | 90.8 ± 5.3** | |
| Nicotine | 3 | 17.4 ± 2.0 | 80.0 ± 20.0** | ||
| β2(−/−) | Moderate | Saccharin | 8 | 37.6 ± 8.5 | 57.1 ± 9.6 |
| Nicotine | 8 | 44.6 ± 8.0 | 60.8 ± 5.9 | ||
| High | Saccharin | 4 | 17.1 ± 8.3 | 71.8 ± 11.7** | |
| Nicotine | 3 | 15.7 ± 6.8 | 85.0 ± 22.1** | ||
Significance of difference from OFF l-DOPA:
p < 0.05;
p < 0.001. Data were analyzed by two-way ANOVA followed by a Bonferroni post hoc test.
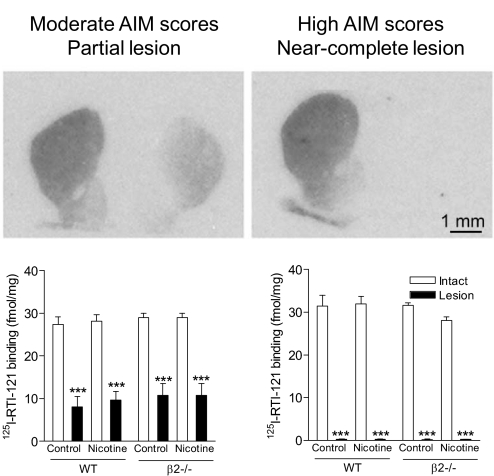
Nicotine Treatment Did Not Modify Striatal Dopamine Transporter Values in the Wild-Type and β2(−/−) Mice.
[125I]RTI-121 autoradiography was done to evaluate changes in the striatal dopamine transporter in lesioned wild-type and β2(−/−) mice with nicotine and/or l-DOPA treatments. In the moderate AIM scores group (Fig. 8, left), [125I]RTI-121 binding values were 28.4 ± 0.4 fmol/mg tissue on the intact side and 9.8 ± 0.6 fmol/mg tissue on the lesioned side, yielding approximately 65% reduction. In contrast, in mice with high AIM scores (Fig. 8, right), striatal dopamine transporter values were decreased by >99% in the lesioned side, with values of 30.8 ± 0.9 fmol/mg tissue on the intact side and 0.2 ± 0.08 fmol/mg tissue on the lesioned side. Nicotine treatment did not affect striatal dopamine transporter values in any group, nor did the absence of β2* nAChRs.
Fig. 8.
Striatal dopamine transporter levels in wild-type and β2(−/−) mice with moderate and high AIM scores. [125I]RTI-121 autoradiography was done to measure the dopamine transporter as described under Materials and Methods. The autoradiographic images (top) and quantitative analyses (bottom) indicate that mice with moderate AIM scores had a partial striatal lesion, whereas mice with severe AIM scores had a near-complete striatal dopamine lesion. Each value represents the mean ± S.E.M. of 6 to 8 mice in the groups with a partial lesion and 2 to 4 mice in the groups with a near-complete lesion. Significance of difference from the intact side: ***, p < 0.001. Data were analyzed by two-way ANOVA followed by a Bonferroni post hoc test.
Alterations in Striatal β2* nAChRs in 6-OHDA-Lesioned l-DOPA-Treated Mice.
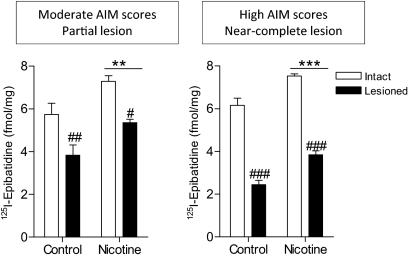
[125I]Epibatidine binding was decreased significantly with nigrostriatal damage in wild-type mice treated with or without nicotine. The declines in the striatum of mice with a partial lesion were 33 and 27% for control and nicotine-treated mice, respectively, and with near-complete lesion were 60 and 50%, respectively (Fig. 9). There also was a significant main effect of nicotine treatment in wild-type mice with both partial (p < 0.01) and near-complete (p < 0.001) lesion, with an increase in [125I]epibatidine binding. As expected, no [125I]epibatidine binding was detected in β2(−/−) mice (L. Z. Huang, S. R. Grady, and M. Quik, unpublished observations).
Fig. 9.
Effect of nicotine treatment (300 μg/ml) on striatal β2* nAChR expression in 6-OHDA-lesioned mice. [125I]Epibatidine was used to evaluate β2* nAChRs in mouse striatum. Binding was decreased with nigrostriatal damage and increased with nicotine treatment in wild-type mice. No binding was detected in the brains of β2(−/−) mice (data not shown). Each value represents the mean ± S.E.M. of 6 to 8 mice in the groups with a partial lesion and 2 to 4 mice in the groups with a near-complete lesion. Significance of difference from the control group: **, p < 0.01; ***, p < 0.001. Significance of difference from the intact side: #, p < 0.05; ##, p < 0.01; ###, p < 0.001. Data were analyzed by two-way ANOVA followed by a Bonferroni post hoc test.
Plasma Cotinine Levels.
The nicotine metabolite cotinine was measured as an index of nicotine intake. Cotinine levels were similar in β2(−/−) and wild-type mice, with values of 195.0 ± 44 ng/ml in wild-type littermates and 203.1 ± 58 ng/ml in β2(−/−) mice (Table 2). These values are similar to those in moderate smokers (Matta et al., 2007).
TABLE 2.
Plasma cotinine levels in lesioned mice receiving chronic nicotine
Cotinine levels were measured in mouse plasma 16 weeks after the initiation of maintenance level of nicotine in the drinking water. Cotinine levels were not detectable in saccharin-treated mice. Values represent the mean ± S.E.M. of the indicated number of animals.
| Group | No. of Mice | Cotinine Level |
|---|---|---|
| ng/ml | ||
| Wild-type | 10 | 195.0 ± 44 |
| β2(−/−) | 10 | 203.1 ± 58 |
Discussion
The present results are the first to directly show that β2* nAChRs play a critical role in the nicotine-mediated decline in l-DOPA-induced AIMs in an animal model of dyskinesias. This contention is supported by our finding that l-DOPA-induced AIMs in β2 knockout mice are similar to those in nicotine-treated wild-type mice. Second, nicotine treatment did not further reduce AIMs in β2(−/−) mice. Our data also show that nicotine was more effective in decreasing AIMs in wild-type mice with partial nigrostriatal damage compared with those in wild-type mice with near-complete nigrostriatal damage. This suggests that β2* nAChRs on nigrostriatal dopaminergic neurons influence the occurrence of l-DOPA-induced AIMs. However, the declines in l-DOPA-induced AIMs in wild-type mice with near-complete nigrostriatal damage suggest that other populations of β2* nAChRs also are involved, albeit to a lesser extent. Altogether, these data provide support for the idea that drugs targeting β2* nAChRs may be useful in the treatment of l-DOPA-induced dyskinesias in Parkinson's disease.
The aim of the current study was to determine the role of β2* nAChR subtypes in l-DOPA-induced AIMs using knockout animals. Such studies require the use of mice; however, the antidyskinetic effect of nicotine only had been demonstrated previously in parkinsonian rats and monkeys. We therefore first tested whether nicotine reduced l-DOPA-induced AIMs in a parkinsonian mouse model. Mice with a unilateral 6-OHDA lesion of the medial forebrain bundle were used because they exhibit a parkinsonian phenotype very similar to that observed in the well characterized and validated 6-OHDA-lesioned rat model (Cenci and Lundblad, 2007). It is important to note that the mice used for the control studies were on the same genetic background (C57BL/6) as the nAChR knockout mice.
Lesioned mice developed AIMs in response to l-DOPA treatment in a time- and dose-dependent manner (Cenci and Lundblad, 2007). The time course of l-DOPA-induced AIMs in mice was somewhat shorter than that in rats and monkeys (Quik et al., 2007; Bordia et al., 2008), most likely because mice metabolize l-DOPA more quickly (Matta et al., 2007). The effect of nicotine on l-DOPA-induced AIMs also was dose-dependent with a maximal reduction at 300 μg/ml nicotine in the drinking water. This nicotine dosing regimen led to cotinine levels similar to those observed in moderate smokers (Matta et al., 2007). Nicotine treatment decreased oral, axial, and forelimb AIMs in parkinsonian mice, with the percentage declines very similar to those observed in parkinsonian rats (Bordia et al., 2008).
We next determined how the absence of β2* nAChRs influenced the occurrence of l-DOPA-induced AIMs. Our rationale for using β2(−/−) mice was based on studies showing that the predominant nAChR populations in the central nervous system are those containing the β2 subunit with little, if any, expression of the β2 subtype in the peripheral nervous system. In addition, β2* nAChRs are the primary subtypes in the nigrostriatal pathway. Parkinsonian behavior, assessed using forepaw placement, was similar in wild-type and β2(−/−) mice treated with or without nicotine. Thus, this behavior was not influenced by the absence of β2* nAChRs. However, there was an approximately 60% decrease in AIMs in knockout mice with moderate AIM scores, that is, mice with moderate nigrostriatal damage. The decreased level of l-DOPA-induced AIMs in knockout mice was very similar to that in wild-type mice treated with nicotine. In addition, nicotine treatment did not further reduce l-DOPA-induced AIMs in β2(−/−) mice with moderate nigrostriatal damage. These combined data suggest that β2* nAChRs on nigrostriatal dopaminergic terminals play an essential role in the occurrence of l-DOPA-induced AIMs.
Our previous studies had shown that nicotine decreases l-DOPA-induced dyskinesias most effectively in partially lesioned parkinsonian rats (Huang et al., 2011). Similar results were observed in the current experiments with parkinsonian mice. There were no consistent decreases in l-DOPA-induced AIMs with nicotine treatment in knockout mice with high AIM scores, that is, severe nigrostriatal damage. In addition, there were no significant declines in l-DOPA-induced AIMs in β2(−/−) mice with severe nigrostriatal damage compared with those in wild-type mice. This further supports the idea that presynaptic β2* nAChRs are involved in the occurrence of l-DOPA-induced AIMs.
The finding that the nicotine-mediated reduction in l-DOPA-induced AIMs was abolished in β2(−/−) mice suggests that the effect of nicotine is mediated entirely via β2 nAChRs. However, the demonstration that AIMs were reduced by only approximately 50% in β2(−/−) mice indicates that the nicotinic cholinergic system is involved only partially in the occurrence of AIMs. Future studies are planned to investigate the role of the α4β2* and α6β2* subtypes using α4 and/or α6 nAChR null mutant mice.
Our results demonstrate that β2* nAChRs are important in regulating aberrant locomotor activity, such as l-DOPA-induced AIMs. The idea that these receptors play a role in movement is consistent with previous work using β2* nAChR antagonists. Mecamylamine prevented the locomotor stimulant effects of nicotine, whereas intraventricular administration of the α7 nAChR blocker α-bungarotoxin was ineffective (Kempsill and Pratt, 2000). In addition, studies with β2(−/−) mice show that such mice are less active in a familiar environment, indicating that endogenous acetylcholine influences locomotion by interacting with β2* nAChRs (Picciotto et al., 1998). Nicotine-evoked increases in motor activity also do not occur in β2(−/−) mice (King et al., 2004). Altogether, these data suggest that β2* nAChRs have a major influence on certain types of motor activity.
Accumulating work shows that the nigrostriatal dopaminergic system contributes to the β2* nAChR modulation of motor control. Administration of nicotine to rats with unilateral nigrostriatal damage results in a circling movement that is blocked by mecamylamine (Lapin et al., 1987; Góngora-Alfaro et al., 1996). Furthermore, microinjection of cholinergic activating drugs into rat substantia nigra induces turning behavior, which can be blocked by mecamylamine (Góngora-Alfaro et al., 1996). Administration of nAChR agonists directly into the striatum also leads to an increase in movement that is inhibited by nAChR blockers (Abin-Carriquiry et al., 2010). This nAChR modulation of locomotor activity is blocked by dopamine receptor antagonists, supporting the idea that nAChRs on nigrostriatal terminals regulate dopamine receptor-mediated movement.
The mechanism of the antidyskinetic action of nicotine is currently not known; however, the present studies suggest that presynaptic β2* nAChRs are involved. The question then arises how an interaction at β2* nAChRs mediates a decline in l-DOPA-induced AIMs. As indicated in the Introduction, acute nicotine treatment stimulates dopamine release via activation of β2* nAChRs. Thus, one might anticipate a worsening of dyskinesias with acute nicotine treatment, because dyskinesias are associated generally with enhanced dopamine release after l-DOPA administration. However, our previous studies showed that dyskinesias were not reduced immediately after an acute nicotine injection but rather that a several day treatment regimen was required (Bordia et al., 2010). These data, coupled with work showing that a nicotinic antagonist reduces l-DOPA-induced dyskinesias, suggest that chronic nicotine administration exerts its antidyskinetic effect via a receptor desensitization block (Bordia et al., 2010). This idea is in agreement with work by others indicating that desensitization may be involved in antidepressant and addictive effects of nicotine (Mineur and Picciotto, 2010; Ortells and Arias, 2010).
Thus, a combination of studies suggests that the nicotinic cholinergic system can modulate locomotor activity under normal physiological conditions and in pathological states, that is, with nigrostriatal damage. The current results extend this work by showing that the nicotinic cholinergic system also plays a role in improving aberrant motor activity through an interaction at β2* nAChRs in the nigrostriatal pathway.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants NS47162, NS65851]; the National Institutes of Health National Institute on Drug Abuse [Grant DA015663]; and a fellowship from the Tobacco-Related Disease Research Program [Grant 18FT-0058A].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.182949.
- AIM
- abnormal involuntary movement
- nAChR
- nicotinic acetylcholine receptor
- 6-OHDA
- 6-hydroxydopamine
- RTI-121
- 3β-(4-iodophenyl)tropane-2β-carboxylic acid isopropyl ester
- ANOVA
- analysis of variance.
Authorship Contributions
Participated in research design: Huang, Grady, and Quik.
Conducted experiments: Huang.
Contributed new reagents or analytic tools: Grady.
Performed data analysis: Huang and Quik.
Wrote or contributed to the writing of the manuscript: Huang, Grady, and Quik.
References
- Abin-Carriquiry JA, Urbanavicius J, Scorza C, Rebolledo-Fuentes M, Wonnacott S, Cassels BK, Dajas F. (2010) Increase in locomotor activity after acute administration of the nicotinic receptor agonist 3-bromocytisine in rats. Eur J Pharmacol 634:89–94 [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89:73–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artymyshyn R, Smith A, Wolfe BB. (1990) The use of 3H standards in 125I autoradiography. J Neurosci Methods 32:185–192 [DOI] [PubMed] [Google Scholar]
- Barik J, Wonnacott S. (2009) Molecular and cellular mechanisms of action of nicotine in the CNS. Handb Exp Pharmacol 192:173–207 [DOI] [PubMed] [Google Scholar]
- Barroso-Chinea P, Bezard E. (2010) Basal Ganglia circuits underlying the pathophysiology of levodopa-induced dyskinesia. Front Neuroanat 4:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, Campos C, Huang L, Quik M. (2008) Continuous and intermittent nicotine treatment reduces l-3,4-dihydroxyphenylalanine (l-DOPA)-induced dyskinesias in a rat model of Parkinson's disease. J Pharmacol Exp Ther 327:239–247 [DOI] [PubMed] [Google Scholar]
- Bordia T, Campos C, McIntosh JM, Quik M. (2010) Nicotinic receptor-mediated reduction in L-DOPA-induced dyskinesias may occur via desensitization. J Pharmacol Exp Ther 333:929–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SP, Dunnett SB. (2009) Tests to assess motor phenotype in mice: a user's guide. Nat Rev Neurosci 10:519–529 [DOI] [PubMed] [Google Scholar]
- Calabresi P, Di Filippo M, Ghiglieri V, Tambasco N, Picconi B. (2010) Levodopa-induced dyskinesias in patients with Parkinson's disease: filling the bench-to-bedside gap. Lancet Neurol 9:1106–1117 [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Björklund A. (2007) Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain 130:1819–1833 [DOI] [PubMed] [Google Scholar]
- Cenci MA, Konradi C. (2010) Maladaptive striatal plasticity in L-DOPA-induced dyskinesia. Prog Brain Res 183:209–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA, Lundblad M. (2007) Ratings of L-DOPA-induced dyskinesia in the unilateral 6-OHDA lesion model of Parkinson's disease in rats and mice. Curr Protoc Neurosci Chapter 9:Unit 9.25 [DOI] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. (2008) Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology 33:2158–2166 [DOI] [PubMed] [Google Scholar]
- Exley R, Cragg SJ. (2008) Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol 153:S283–S297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S. (2008) How do you treat motor complications in Parkinson's disease: medicine, surgery, or both? Ann Neurol 64:S56–S64 [DOI] [PubMed] [Google Scholar]
- Fox SH, Chuang R, Brotchie JM. (2008) Parkinson's disease–opportunities for novel therapeutics to reduce the problems of levodopa therapy. Prog Brain Res 172:479–494 [DOI] [PubMed] [Google Scholar]
- Góngora-Alfaro JL, Hernández-López S, Martínez-Fong D, Flores G, Aceves J. (1996) Circling behavior elicited by cholinergic transmission in the substantia nigra pars compacta: involvement of nicotinic and muscarinic receptors. Neuroscience 71:729–734 [DOI] [PubMed] [Google Scholar]
- Grady S, Marks MJ, Wonnacott S, Collins AC. (1992) Characterization of nicotinic receptor-mediated [3H]dopamine release from synaptosomes prepared from mouse striatum. J Neurochem 59:848–856 [DOI] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, Marks MJ. (2007) The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol 74:1235–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LZ, Campos C, Ly J, Ivy Carroll F, Quik M. (2011) Nicotinic receptor agonists decrease L-dopa-induced dyskinesias most effectively in partially lesioned parkinsonian rats. Neuropharmacology 60:861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Kempsill FE, Pratt JA. (2000) Mecamylamine but not the alpha7 receptor antagonist alpha-bungarotoxin blocks sensitization to the locomotor stimulant effects of nicotine. Br J Pharmacol 131:997–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SL, Caldarone BJ, Picciotto MR. (2004) Beta2-subunit-containing nicotinic acetylcholine receptors are critical for dopamine-dependent locomotor activation following repeated nicotine administration. Neuropharmacology 47:132–139 [DOI] [PubMed] [Google Scholar]
- Lai A, Parameswaran N, Khwaja M, Whiteaker P, Lindstrom JM, Fan H, McIntosh JM, Grady SR, Quik M. (2005) Long-term nicotine treatment decreases striatal alpha6* nicotinic acetylcholine receptor sites and function in mice. Mol Pharmacol 67:1639–1647 [DOI] [PubMed] [Google Scholar]
- Lapin EP, Maker HS, Sershen H, Hurd Y, Lajtha A. (1987) Dopamine-like action of nicotine: lack of tolerance and reverse tolerance. Brain Res 407:351–363 [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, et al. (2007) Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 190:269–319 [DOI] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Bordia T, Fan H, Tyndale RF, Langston JW, McIntosh JM, Quik M. (2006) Increases in alpha4* but not alpha3*/alpha6* nicotinic receptor sites and function in the primate striatum following chronic oral nicotine treatment. J Neurochem 96:1028–1041 [DOI] [PubMed] [Google Scholar]
- Meyer EL, Yoshikami D, McIntosh JM. (2008) The neuronal nicotinic acetylcholine receptors alpha 4* and alpha 6* differentially modulate dopamine release in mouse striatal slices. J Neurochem 105:1761–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar NS, Gotti C. (2009) Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 56:237–246 [DOI] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR. (2010) Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci 31:580–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortells MO, Arias HR. (2010) Neuronal networks of nicotine addiction. Int J Biochem Cell Biol 42:1931–1935 [DOI] [PubMed] [Google Scholar]
- Perez XA, Bordia T, McIntosh JM, Grady SR, Quik M. (2008) Long-term nicotine treatment differentially regulates striatal alpha6alpha4beta2* and alpha6(nonalpha4)beta2* nAChR expression and function. Mol Pharmacol 74:844–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP. (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177 [DOI] [PubMed] [Google Scholar]
- Pietilä K, Ahtee L. (2000) Chronic nicotine administration in the drinking water affects the striatal dopamine in mice. Pharmacol Biochem Behav 66:95–103 [DOI] [PubMed] [Google Scholar]
- Poewe W. (2009) Treatments for Parkinson disease–past achievements and current clinical needs. Neurology 72:S65–S73 [DOI] [PubMed] [Google Scholar]
- Quik M, Cox H, Parameswaran N, O'Leary K, Langston JW, Di Monte D. (2007) Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Ann Neurol 62:588–596 [DOI] [PubMed] [Google Scholar]
- Quik M, Huang LZ, Parameswaran N, Bordia T, Campos C, Perez XA. (2009) Multiple roles for nicotine in Parkinson's disease. Biochem Pharmacol 78:677–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Sum JD, Whiteaker P, McCallum SE, Marks MJ, Musachio J, McIntosh JM, Collins AC, Grady SR. (2003) Differential declines in striatal nicotinic receptor subtype function after nigrostriatal damage in mice. Mol Pharmacol 63:1169–1179 [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. (2004) Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol 65:1526–1535 [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. (2000) CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39:777–787 [DOI] [PubMed] [Google Scholar]
- Sparks JA, Pauly JR. (1999) Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57Bl/6 mice. Psychopharmacology 141:145–153 [DOI] [PubMed] [Google Scholar]